Abstract
It has been hypothesized that human immunodeficiency virus type 1 (HIV-1) evolves toward increased cytopathicity in conjunction with disease progression in infected patients. A viral property known to evolve in some but not all patients is coreceptor utilization, and it has been shown that a switch in coreceptor utilization is sufficient for the development of increased cytopathicity. To test the hypothesis that the evolution of other viral properties also contributes to accelerating cytopathicity in vivo, we used human lymphoid tissue explants to assay the cytopathicity of a panel of primary HIV-1 isolates derived from various stages of disease characterized by the presence or absence of changes in coreceptor preference. We found no evidence of coreceptor-independent increases in cytopathicity in isolates obtained either before coreceptor preference changes or from patients who progressed to AIDS despite an absence of coreceptor evolution. Instead, the cytopathicity of all HIV-1 isolates was determined solely by their coreceptor utilization. These results argue that HIV-1 does not evolve toward increased cytopathicity independently of changes in coreceptor utilization.
Human immunodeficiency virus type 1 (HIV-1) is known to evolve throughout the course of disease in infected individuals (25, 28). To compare the cytopathicity and replication kinetics of clinical isolates from early and late stages of disease, various cell line-based assays have been used to show that late viruses typically are more cytopathic and can replicate faster in vitro (7, 23). The identification of HIV-1 coreceptors and of their expression on various cell lines has shed new light on these data. Virtually all HIV-1 isolates obtained from patients use one or both of two chemokine receptors, CCR5 (9, 11) and CXCR4 (12), as major coreceptors, along with CD4 (15), for entry into target cells (reviewed in reference 2). Viruses isolated early in the course of disease typically use CCR5 as a coreceptor (R5 viruses), whereas viruses isolated late in the course of disease commonly can use either CXCR4 alone (X4 viruses) or both CCR5 and CXCR4 (R5X4 viruses) (8). Typically, cell lines used for in vitro characterization express high levels of CXCR4 and low levels of CCR5 (29), and these facts explain why late X4 viruses characteristically replicate more vigorously and have greater cytopathic effects in such experiments. Likewise, using a novel experimental system based on ex vivo human lymphoid histocultures, it has been established that X4 viruses are more cytopathic than R5 viruses (13, 14, 18) and specifically that late X4 viruses are more cytopathic than early R5 viruses (22). An important remaining question is whether primary isolates from different stages of disease differ in their cytopathicity independently of coreceptor preference.
To determine whether HIV-1 cytopathicity corresponds to the stage of HIV-1 disease, we tested a variety of primary isolates and biological clones derived from HIV-1-infected patients using an ex vivo human lymphoid histoculture system (13, 14, 18, 21, 22). These experiments were carried out with either blocks (14, 18, 22) or dispersed cultures (D. A. Eckstein, M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. R. Roederer, M. P. Sherman, C. Klein, P. S. Chin, and M. A. Goldsmith, submitted for publication) of human tonsil specimens, and similar results were obtained in both assays. HIV-1 isolates or clones were first expanded, and then their titers were determined by end-point dilution on phytohemagglutinin-activated peripheral blood mononuclear cells pooled from two to four normal donors. The inoculum size was either 20 50% tissue culture infective doses per tissue block or 50 50% tissue culture infective doses per well of dispersed tissue. Histoculture infections typically were carried out for 2 weeks, with culture medium changes the day after infection and every 3 days thereafter. At the end of the experiment, the tissue was harvested and split into two equal samples for immunostaining and analysis by fluorescence-activated cell sorting. One sample was stained with antibodies to CD3, CD4, CD8, and CCR5 for analysis of depletion of both total CD4+ CD3+ lymphocytes (referred to hereafter as CD4+ T cells) and CCR5+ and CCR5− subsets of CD4+ T cells. The other sample was stained with antibodies to CD3, CD4, CD45RA, and CD62L for measurement of depletion of naive and memory CD4+ T cells (19, 20).
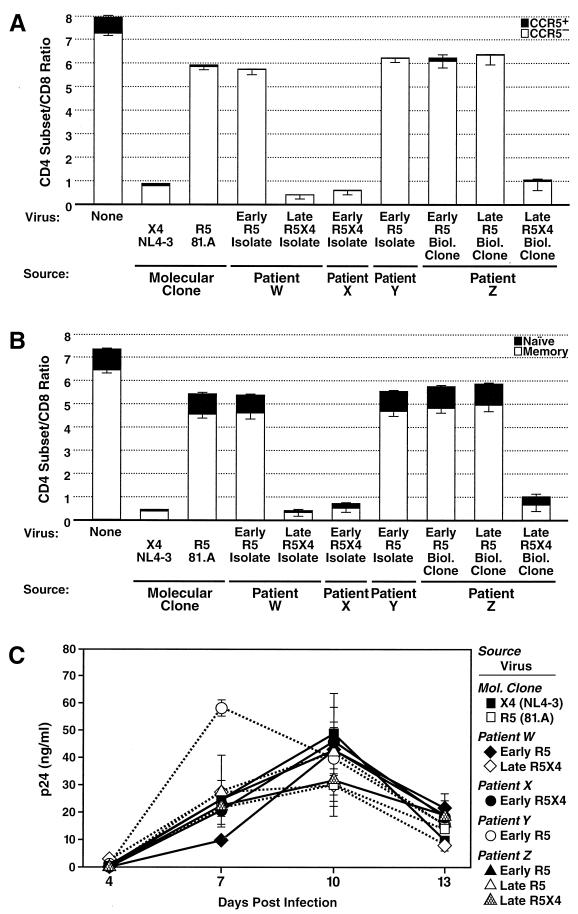
We first sought to determine whether X4 viruses present early after infection in some individuals differ in their cytopathicity from X4 viruses isolated late in disease. We compared the CD4+ T-cell depletion potential of an R5X4 isolate obtained from a patient within 90 days of infection (patient X) (Table 1 and Fig. 1) to that of an R5X4 isolate derived from a patient 6.5 years after seroconversion (patient W) (Fig. 1). As a positive control, we also assayed depletion by a highly cytopathic X4 molecular clone, NL4-3 (1, 13, 14, 18, 22). We found that the early R5X4 isolate depleted CD4+ T cells as potently as did both the late R5X4 isolate and the control virus, NL4-3 (Fig. 1A); each virus led to 85 to 90% depletion of all CD4+ T cells relative to the results obtained for uninfected samples. Furthermore, the CCR5+ and CCR5− subsets of CD4+ T cells were thoroughly depleted by all viruses tested here (Fig. 1A). In addition, depletion analysis of naive and memory subsets of CD4+ T cells was performed. As was observed previously with other X4 viruses, severe depletion of both CD4+ T-cell subsets was observed with all X4 and R5X4 viruses tested here (Fig. 1B). Finally, robust viral replication kinetics were observed for these viruses, based on measurements of HIV-1 p24 in the culture supernatants (Fig. 1C). These results, which are consistent with the fact that nearly all CD4+ T cells in human tonsils express CXCR4 (13, 14) and are thus potential targets for HIV-1, demonstrate that cytopathicity correlates well with the coreceptor preference of X4 isolates. Indeed, we have detected very little variability in the depletion behavior of a wide range of X4 and R5X4 isolates (data not shown).
TABLE 1.
Summary of primary HIV-1 isolates tested
| Patient | Virus | Reference(s) | Patient designation in reference(s) |
|---|---|---|---|
| W | Early R5 isolate | 7, 8, 22 | Patient C (1/85) |
| Late R5X4 isolate | 7, 8, 22 | Patient C (7/86) | |
| X | Early R5X4 isolate | None | None |
| Y | Early R5 isolate | 16 | MJM |
| Z | Early R5 biological clone | 25 | Patient 8 |
| Late R5 biological clone | 25 | Patient 8 | |
| Late R5X4 biological clone | 25 | Patient 8 | |
| A | Early R5 biological clone | 3, 4, 10, 24, 28 | ACH 424 |
| Late R5 biological clone | 3, 4, 10, 24, 28 | ACH 424 | |
| B | Early R5 biological clone | 4 | ACH 537 |
| Late R5 biological clone | 4 | ACH 537 | |
| C | Early R5 biological clone | 4, 10 | ACH 38 |
| Late R5 biological clone | 4, 10 | ACH 38 | |
| D | Early R5 biological clone | 4, 10 | ACH 617 |
| Late R5 biological clone | 4, 10 | ACH 617 |
FIG. 1.
Cytopathic potential of primary HIV-1 isolates correlates with coreceptor utilization but not stage of disease. (A) Dispersed human tonsil tissue in replicate microtiter wells (three for experimental viruses and two for NL4-3) was inoculated with the indicated viruses (Table 1). Biol. Clone, biological clone. Tissue was harvested, immunostained, and analyzed by fluorescence-activated cell sorting 13 days after infection as described previously (14, 22). The total height of the column in the graph represents the ratio of CD4+ T cells to CD8+ T cells. The standard error of the mean is represented by the error bars. (B) The samples shown in panel A were analyzed for depletion of memory and naive cells. Naive CD4+ T cells were defined as CD4+ T cells that were CD45RA+ CD62L+, and all other CD4+ T cells were defined as memory CD4+ T cells (19, 20). (C) Culture supernatant was assayed for HIV-1 p24 by an enzyme-linked immunosorbent assay to monitor viral replication. Experiments with different donor specimens were conducted twice with dispersed cultures and once with tissue blocks; data from a representative experiment are presented.
We next sought to determine if the behavior of R5 viruses was similarly independent of patient status. We compared the cytopathicity of an early R5 biological clone to that of a late R5 biological clone derived from the same patient (patient Z) (Fig. 1). In addition, we tested whether these clones differed from two other R5 isolates: one isolate was derived from a patient within 90 days of infection (patient Y) (Fig. 1), and the other was isolated 5 years after seroconversion from a patient who was asymptomatic at the time (patient W) (Fig. 1). As a positive control, we also tested a previously characterized R5 molecular clone, 81.A (26). All five R5 viruses were found to deplete CD4+ T cells equally. Each depleted approximately 15% of total CD4+ T cells (Fig. 1A) but nearly all CCR5+ CD4+ T cells (Fig. 1A). As demonstrated previously, the apparent decreased cytopathicity of R5 viruses compared with X4 viruses is due to a decreased target pool size resulting from the limited expression of CCR5 compared with that of CXCR4 (13, 14). Moreover, R5 viruses depleted a portion of memory CD4+ T cells, presumably the CCR5-expressing fraction, but did not deplete naive CD4+ T cells (Fig. 1B), due to a very low level of CCR5 expression (5, 27, 29). To establish that there was nothing unusual about the patient from whom these biological clones originated, we also tested an R5X4 biological clone that was isolated late in disease from patient Z at the same time as the previously tested late R5 biological clone. Indeed, the depletion profile of this R5X4 biological clone was similar to that of all other X4 and R5X4 viruses tested here (Fig. 1A and B). Substantial replication was evident for all R5 viruses and the control R5X4 clone (Fig. 1C). In summary, the results thus far revealed no evidence that any viral trait other than coreceptor preference regulates the cytopathicity of primary isolates in ex vivo cultures of human tonsils.
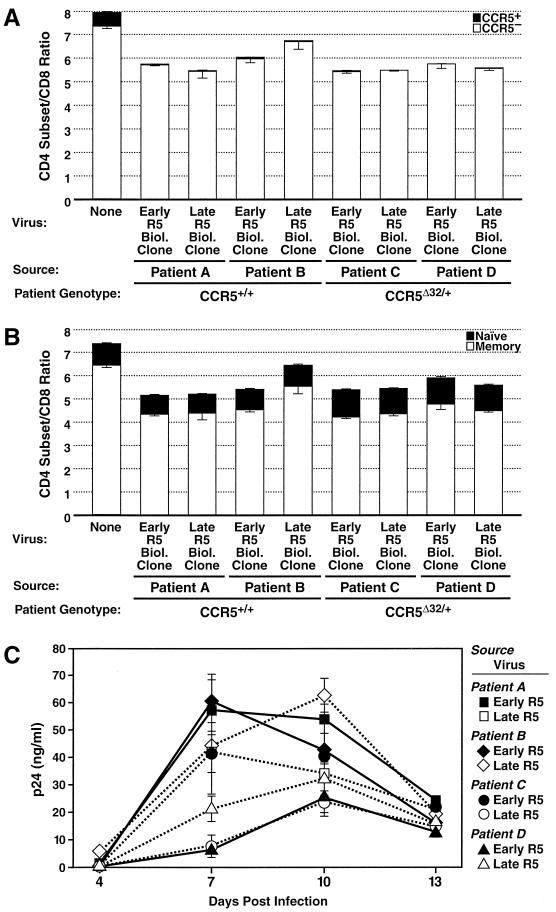
It is possible that the late R5 biological clone tested above was not especially cytopathic because it had experienced no selective pressure to acquire greater cytopathic properties in the context of highly cytopathic R5X4 viruses that were already systemic in the individual. To address this issue, we tested a panel of biological clones isolated longitudinally from four patients who exhibited significant disease progression but never developed detectable X4 viremia or who were treated with antiretroviral agents. Two patients were homozygous for the wild-type allele of CCR5 (patients A and B) (Table 1 and Fig. 2) and progressed to AIDS within ∼4 years of seroconversion, whereas the other two patients were heterozygous for the Δ32 allele of CCR5 (patients C and D) (Fig. 2) and progressed to AIDS in ∼8 to 10 years. The depletion patterns of these eight viruses were analyzed with particular interest in differences between early and late viruses within a given patient or between viruses from patients with different genotypic patterns. However, the results obtained for these eight viruses showed no such differences. Each infection yielded moderate depletion of CD4+ T cells, with profound depletion of the CCR5+ subset of CD4+ T cells and sparing of the CCR5− subset (Fig. 2A). Moreover, memory CD4+ T cells were depleted moderately by each of these isolates, while naive CD4+ T cells were not (Fig. 2B). Again, substantial viral replication kinetics were seen throughout the course of the experiment for these isolates (Fig. 2C). These results demonstrate that R5 viruses isolated in either the absence or the presence of systemic X4 viremia are equally cytopathic, even R5 viruses causing severe disease progression in the absence of evolution to the X4 phenotype. These data argue against a model of HIV-1 evolution that posits selective pressure during the course of disease on HIV-1 to acquire cytopathic traits other than expanded target cell range via coreceptor evolution.
FIG. 2.
Primary R5 HIV-1 isolates from advanced disease in the absence of X4 viremia retain selective cytopathicity for CCR5+ T cells. Experiments were performed with the indicated viruses (Table 1) as described in the legend to Fig. 1. (A) Depletion of CD4+ T cells, including total CD4+ T cells, and CCR5+ and CCR5− subsets. (B) Depletion of memory and naive subsets of CD4+ T cells. (C) HIV-1 replication kinetics. Experiments with different donor specimens were conducted twice with dispersed cultures and once with tissue blocks; data from a representative experiment are presented.
In summary, we have shown that the cytopathicity for tissue lymphocytes of a diverse set of primary isolates from various stages of disease is entirely restricted by coreceptor utilization and does not typically display coreceptor-independent evolution during the progression of disease. This finding likely has implications for disease pathogenesis, but the possibility that there may be subtle, coreceptor-independent evolution of pathogenicity in vivo that is not reflected in this ex vivo culture system cannot be excluded. Likewise, we cannot exclude the possibility that the propagation of virus isolates may have diminished virulence differences, a potential problem with any functional survey of primary isolates. Given the range of sources of viruses and the uniformity of our results, this report nonetheless establishes the general principle that the ability of HIV-1 to deplete CD4+ T cells in histocultures is a predictable event based on coreceptor usage of the virus and coreceptor expression of the target tissue.
Our results indicating equal degrees of cytopathicity of early and late R5 viruses from patients who progressed to AIDS but lacked X4 viremia are in agreement with one but not another study of similar isolates tested in the SCID-hu Thy-Liv xenotransplant model (3, 24). Berkowitz et al. (3) analyzed two late-stage R5 biological clones, including one from patient A, and did not find increased cytopathicity relative to that of control viruses. In contrast, Scoggins et al. (24) tested the cytopathicity of early-, middle-, and late-stage disease biological clones derived from some of the same patient isolates as those tested here and found significant depletion of CD4+ CD8+ thymocytes in some implants with a single late-stage clone but not with clones from earlier in disease. It is important to note the differences between the SCID-hu model and histocultures with regard to interpretation of the above results. The human tissue in SCID-hu xenografts originates from thymic tissue and thus represents a system to test the effects of HIV-1 on immature and developing thymocytes (6, 17). In fact, the bulk of this tissue is CD4+ CD8+ thymocytes, of which more than 90% would be eliminated by thymic selection normally. In contrast, the experimental explants used in the present study are derived from mature lymphoid tissue that is populated by T cells that have survived thymic selection. Thus, the depletion properties observed here are indicative of the cytopathic capabilities of HIV-1 for mature CD4+ T cells.
In the context of disease progression, the data regarding the cytopathicity of early and late R5 viruses indicate that HIV-1 need not experience an increase in cytopathicity over time to cause severe disease in infected people. An R5 virus that successfully infects and eliminates the entire CCR5-expressing pool of CD4+ T cells is apparently cytopathic enough to deplete the immune system sufficiently to cause AIDS, presumably through attrition of cells that dynamically express CCR5 at various stages of the cellular life cycle (5). We hypothesize that as the immune system seeks to replenish the CCR5-expressing fraction of CD4+ T cells to restore homeostasis in the context of peripheral destruction of such cells, an R5 virus will continually find new target cells until too few CD4+ cells remain to maintain a functional immune system.
Acknowledgments
We thank Bruce Cheesbro for kindly providing plasmids and members of the surgical staff at Kaiser hospitals (San Rafael and San Francisco) for generous assistance in obtaining posttonsillectomy samples. We acknowledge the technical assistance of Valerie Stepps, Marty Bigos, and Kathleen Kulka and the assistance of Heather Gravois and John Carroll in the preparation of the manuscript. Some viruses used in this study were obtained from the Amsterdam Cohort Studies, a collaboration among the Academic Medical Center, the Municipal Health Service, and the CLB in Amsterdam, The Netherlands, and others were obtained from the Multicenter AIDS Cohort Study (MACS; http://www.statepi.jhsph.edu/macs/macs.html).
J.F.K. was supported by the National Science Foundation and the Biomedical Science Graduate Program at UCSF, D.K. was supported by the Dutch Council for Scientific Research (N.W.O. grant 901-02-214), B.S. was supported by the Boehringer Ingelheim Fund, R.C. was supported by an NIH grant (AI41373) and the Irene Diamond Fund, J.I.M. and A.B.V. were supported by an NIH grant (AI37984), and A.B.V. was also supported by a grant from the American Foundation for AIDS Research (70531-28-RF). This work was supported by NIH grants to M.A.G. (AI43695 and CA86814) and by the J. David Gladstone Institutes.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, van't Wout A B, Kootstra N A, Moreno M E, Linquist-Stepps V D, Bare C, Stoddart C A, Schuitemaker H, McCune J M. R5 strains of human immunodeficiency virus type 1 from rapid progressors lacking X4 strains do not possess X4-type pathogenicity in human thymus. J Virol. 1999;73:7817–7822. doi: 10.1128/jvi.73.9.7817-7822.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaak H, Brouwer M, Ran L J, de Wolf F, Schuitemaker H. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J Infect Dis. 1998;177:600–610. doi: 10.1086/514219. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 7.Connor R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.de Roda Husman A M, van Rij R P, Blaak H, Broersen S, Schuitemaker H. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J Infect Dis. 1999;180:1106–1115. doi: 10.1086/314987. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13.Grivel J C, Margolis L B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 14.Grivel J C, Penn M L, Eckstein D A, Schramm B, Speck R F, Abbey N W, Herndier B, Margolis L, Goldsmith M A. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 16.Mariani R, Wong S, Mulder L C, Wilkinson D A, Reinhart A L, LaRosa G, Nibbs R, O'Brien T R, Michael N L, Connor R I, Macdonald M, Busch M, Koup R A, Landau N R. CCR2–64I polymorphism is not associated with altered CCR5 expression or coreceptor function. J Virol. 1999;73:2450–2459. doi: 10.1128/jvi.73.3.2450-2459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 18.Penn M L, Grivel J C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Buck D, Terstappen L W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 20.Roederer M, Raju P A, Mitra D K, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schramm B, Penn M L, Palacios E H, Grant R M, Kirchhoff F, Goldsmith M A. Cytopathicity of human immunodeficiency virus type 2 (HIV-2) in human lymphoid tissue is coreceptor dependent and comparable to that of HIV-1. J Virol. 2000;74:9594–9600. doi: 10.1128/jvi.74.20.9594-9600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schramm B, Penn M L, Speck R F, Chan S Y, De Clercq E, Schols D, Connor R I, Goldsmith M A. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J Virol. 2000;74:184–192. doi: 10.1128/jvi.74.1.184-192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scoggins R M, Taylor J R, Jr, Patrie J, van't Wout A B, Schuitemaker H, Camerini D. Pathogenesis of primary R5 human immunodeficiency virus type 1 clones in SCID-hu mice. J Virol. 2000;74:3205–3216. doi: 10.1128/jvi.74.7.3205-3216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankarappa R, Margolick J B, Gange S J, Rodrigo A G, Upchurch D, Farzadegan H, Gupta P, Rinaldo C R, Learn G H, He X, Huang X L, Mullins J I. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology. 1995;213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- 27.van Rij R P, Blaak H, Visser J A, Brouwer M, Rientsma R, Broersen S, de Roda Husman A M, Schuitemaker H. Differential coreceptor expression allows for independent evolution of non-synctium-inducing and syncytium-inducing HIV-1. J Clin Investig. 2000;106:1039–1052. doi: 10.1172/JCI7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van 't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]