Abstract
Asthma and cardiovascular disease (CVD) often co-exist. When a patient has both conditions, management requires an approach that addresses the unique challenges of each condition separately, while also considering their potential interactions. However, specific guidance on the management of asthma in patients with CVD and on the management of CVD in patients with asthma is still lacking. Nevertheless, health care providers need to adopt a comprehensive approach that includes both respiratory and CVD health. The management of CVD in patients with asthma requires a delicate balance between controlling respiratory symptoms and minimising potential cardiovascular (CV) risks. In the absence of specific guidelines for the management of patients with both conditions, the most prudent approach would be to follow established guidelines for each condition independently. Careful selection of asthma medications is essential to avoid exacerbation of CV symptoms. In addition, optimal management of CV risk factors is essential. However, close monitoring of these patients is important as there is evidence that some asthma medications may have adverse effects on CVD and, conversely, that some CVD medications may worsen asthma symptoms. On the other hand, there is also increasing evidence of the potential beneficial effects of asthma medications on CVD and, conversely, that some CVD medications may reduce the severity of asthma symptoms. We aim to elucidate the potential risks and benefits associated with the use of asthma medications in patients with CVD, and the potential pulmonary risks and benefits for patients with asthma who are prescribed CVD medications.
Key Points
| Managing both asthma and CVD in the same patient requires a careful approach to avoid worsening of either condition. This means that health care providers need to be cautious when choosing medications to ensure they don't negatively affect the other condition. |
| There are no specific guidelines for treating patients with both asthma and CVD, so health care providers should closely follow the established guidelines for each condition. Regular monitoring is important to detect any adverse effects early. |
| Some asthma medications may help with CVD, and some CVD medications may relieve asthma symptoms. An understanding of these potential benefits may have an impact on treatment strategies for patients with both conditions. |
Introduction
Although there is a growing consensus that allergic asthma is a risk factor for cardiovascular disease (CVD) [1–4], data on the relationship between these two conditions in both prospective and retrospective studies are controversial. Numerous investigations have indicated a notable correlation between asthma and a heightened incidence of CVD [5, 6]. Longitudinal analysis of data from the Framingham Offspring Cohort identified a link between asthma and lifetime risk of CVD [7]. Cox regression analysis showed an adjusted association between asthma and CVD incidence (hazard ratio [HR], 1.28; 95% CI 1.07–1.54) after adjustment for established CV risk factors, including age, sex, smoking status, dyslipidaemia, hypertension, and diabetes. The Nord-Trøndelag health study (HUNT) in Norway reported that adults with active asthma had an estimated 29% higher risk of developing acute myocardial infarction (MI) (adjusted HR, 1.29; 95% CI 1.08–1.54) compared with adults without asthma [8]. In other studies, the risk of CVD was reported to be between 32% [9] and 42% [10]. An analysis of 30 cohort studies involving 4,157,823 participants showed that patients with late-onset asthma had a higher risk than those with early-onset asthma (39 vs 26%) [11]. Among asthmatics, those who experience exacerbations have a higher risk of developing ischaemic heart disease or heart failure than those who remain free of exacerbations [12]. In addition, people with allergic asthma are more likely to develop CVD than those with non-allergic asthma [12]. In the HUNT study, there was a significant association between asthma control and acute MI risk, with highest risk in adults with uncontrolled asthma (adjusted HR, 1.73; 95% CI 1.13–2.66) compared to adults with controlled asthma (p for trend < 0.05) [8]. Other analyses have shown that the increased risk of CVD associated with asthma is restricted to certain subgroups, such as smokers [7] and women (39% in women vs 19% in men) [11]. Conversely, several studies, including the Atherosclerosis Risk in Communities study, which found that those who had ever had asthma had an adjusted relative risk of coronary heart disease of 0.87 (95% CI 0.66–1.14), while those who currently had asthma had an adjusted relative risk of 0.69 (95% CI 0.46–1.05) [13], have suggested that there is no significant correlation between asthma and CVD [14, 15]. Findings from the Italian College of General Practitioners database showed that although CVD and hypertension were more common in people diagnosed with asthma than in the general population, asthma seemed to have a weak association with these diseases [16]. In addition, the adjusted odds ratio (OR) for acute or previous MI was 0.98 (95% CI 0.90–1.07) [14].
Despite the conflicting data, it is commonly accepted that asthma and CVD can interact bidirectionally when they occur simultaneously [4]. Asthmatics are at increased risk of hypertension, coronary heart disease, stroke, and heart failure, while those with CVD often have poorer asthma control and more exacerbations [17]. A comprehensive approach that addresses their individual problems and potential interactions is needed to manage both conditions simultaneously.
This article aims to provide a thorough guide to the drugs used to treat patients with asthma and CVD, focusing on safety, efficacy, and the importance of an individualised treatment plan.
Understanding the Relationship Between Asthma and CVD
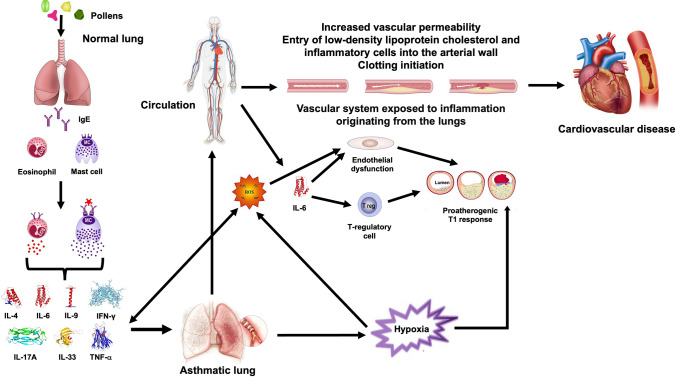
The relationship between asthma and CVD involves complex mechanisms [1, 17]. Chronic inflammation is common to both diseases [18]. Airway inflammation characterises asthma and is linked to airway hyperresponsiveness (AHR) [18]. Systemic inflammation is crucial in CVD, contributing to atherosclerosis, endothelial dysfunction, and plaque formation (Fig. 1) [18]. The MESA (Multi‐Ethnic Study of Atherosclerosis) has documented that subjects with persistent asthma have elevated carotid plaque scores and increased levels of inflammatory biomarkers compared with those without asthma [19]. The presence of a high carotid plaque score is a strong predictor of clot-induced strokes and major CV events (MACE), as the carotid artery runs along both sides of the neck. Poorly controlled asthma may increase systemic inflammation, potentially worsening CVD, while patients with CVD may have increased airway inflammation, leading to more frequent asthma exacerbations [4].
Fig. 1.
Potential pathogenetic mechanisms of the association between asthma and CVD chronic airway inflammation in asthma may contribute to systemic inflammation, suggesting that the vasculature is systemically exposed to inflammation generated in the lungs. Systemic inflammation is critical in CVD, contributing to atherosclerosis, endothelial dysfunction and plaque formation. IL-6 is a key effector cytokine in the atherosclerotic T1 process as it directly inhibits T regulatory cells, allowing the proatherogenic T1 response to occur. IL-6 also causes endothelial dysfunction, the first step in arterial damage and atherosclerotic plaque formation, propagates inflammation and predicts future atherosclerotic CVD events. Oxidative stress, resulting from an imbalance between ROS and antioxidants, is also implicated in both asthma and CVD. ROS can damage cellular components and promote inflammation, contributing to airway remodelling in asthma and endothelial dysfunction in CVD. Endothelial dysfunction, characterised by impaired vasodilation and pro-thrombotic properties, precedes atherosclerosis and CVD events. IL interleukin, IFN-γ interferon γ, IgE immunoglobulin E, ROS reactive oxygen species, T1 type 1, TNF-α tumour necrosis factor α
Immune dysregulation further complicates the asthma-CVD relationship, as immune cells and inflammatory mediators are involved in the pathogenesis of both conditions [2]. Allergic asthma is characterised by type (T)2 immune responses that drive eosinophilic inflammation and IgE production through specific cytokines [20]. Both clinical and preclinical studies suggest a potential pathogenic role for eosinophils in CVD. Patients with asthma are prone to develop atherosclerosis, vasospasm and acute MI, possibly due to excessive release of cysteinyl leukotrienes from eosinophils [21]. High levels of eosinophils in the blood and vasoconstriction have been associated with anginal attacks [22]. Nevertheless, it is interesting to note that whilst increased eosinophils in the asthmatic lung may contribute to the pathology, eosinophil-derived interleukin (IL)-4 and eosinophil cationic protein have been shown to have reparative properties in the diseased heart and aorta [2].
In addition, oxidative stress, resulting from an imbalance between reactive oxygen species (ROS) and antioxidants, is implicated in both asthma and CVD [23]. ROS can damage cellular components and promote inflammation, contributing to airway remodelling in asthma [21] and endothelial dysfunction in CVD [24]. Endothelial dysfunction, characterised by impaired vasodilation and pro-thrombotic properties, precedes atherosclerosis and CV events [24].
Sympathetic activation, triggered by stress, can worsen asthma symptoms due to bronchoconstriction and airway inflammation through β1- and α-adrenoceptor (AR) activation [25]. In CVD, it results in hypertension, vasoconstriction, and increased cardiac workload, increasing the risk of CV adverse events (AEs) [26].
Therefore, recognising the connections between asthma and CVD is important for effective disease management.
Diagnostic and Management Challenges
Evaluating patients with asthma and CVD is challenging due to shared characteristics, especially in older adults [4, 27, 28]. Accurate diagnosis is essential to differentiate between the two conditions as treatments for one can worsen the other. However, emerging evidence suggests that some drugs for asthma may benefit CVD, and vice versa.
Currently, asthma guidelines lack explicit recommendations for CV assessment in asthmatics, while CVD guidelines lack clear directives for pulmonary assessment in CVD patients. This ambiguity contributes to limited specific guidance on managing asthma in patients with CVD and vice versa.
Health care providers should adopt a comprehensive approach addressing both respiratory and CVD health. This involves optimising asthma management to reduce inflammation and improve symptom control and implementing strategies to reduce CV risk factors like hypertension, dyslipidaemia, and diabetes.
Cardiovascular Impact of Asthma Pharmacotherapy
Inhaled corticosteroids (ICS), adjusted according to disease severity, are the mainstay of asthma treatment [28, 29]. Other controller medications like inhaled long-acting β2-agonists (LABAs), inhaled long-acting muscarinic agents (LAMAs) and leukotriene modifiers can also be used [28, 29]. If symptoms persist or lung function does not improve on a low to medium dose of ICS alone, it may be necessary to add a LABA, preferably in a fixed-dose combination (FDC) [27, 28]. Short-acting β2-agonists (SABAs) provide rapid relief of asthma symptoms such as bronchoconstriction. However, treatment with SABA monotherapy alone is no longer recommended by the Global Initiative for Asthma (GINA) at any stage [29]. Instead, GINA suggests that a more effective rescue treatment strategy is to use the budesonide/formoterol combination rather than SABA alone [29]. The GINA also indicates that leukotriene receptor antagonists (LTRA) may be considered as a substitute for low-dose ICS in step 2 and as an adjunct to ICS in steps 3 and 4 [29]. If asthma is not adequately controlled on optimal therapy with a medium-dose ICS/LABA, it is appropriate to increase the ICS dose. If increasing the ICS dose does not improve asthma control, it is advisable to add a LAMA before considering oral corticosteroids (OCS) or a biologic agent [29, 30]. Occasionally, severe asthma can also be treated with macrolides, predominantly azithromycin [28].
While some asthma therapies may carry CV risks, they may also confer benefits for managing CVD (summarised in Table 1) [4].
Table 1.
Cardiovascular impact of asthma pharmacotherapy
| Anti-asthma agent | Potential risks | Potential benefits |
|---|---|---|
| Inhaled corticosteroids | Hypertension, venous thromboembolism, fluid retention, electrolyte imbalance, atherosclerotic changes and potential interaction with CV drugs metabolised by the CYP enzyme system | Reduced airway inflammation, prevention of asthma exacerbations, potential reduction in overall CV burden associated with severe asthma attacks and atherosclerosis incidence |
| β-agonists | Increased heart rate, palpitations, potential for arrhythmias, hypokalaemia, especially with high doses or prolonged use, peripheral arterial stiffness, risk of acute MI, congestive heart failure, cardiac arrest and sudden cardiac death, higher rates of hospitalisation for heart failure and increased risk of all-cause mortality in patients with left-ventricular dysfunction | Improved CV haemodynamic, increased cardiac output, reduced peripheral vascular resistance, and improved pulmonary capillary wedge pressure, reversal of bronchoconstriction often seen in patients with heart failure |
| Muscarinic receptor antagonists | Tachycardia, palpitations, and potential increase of the risk of CV AEs, particularly in patients with pre-existing CVD | |
| Theophylline | Tachycardia, arrhythmias and worsening of underlying cardiac conditions with the possibility of sudden death, especially in cases of overdose or drug-drug interactions | |
| Leukotriene modifiers | Acute MI, ischaemic stroke, atherosclerosis, aortic aneurysm, and intimal hyperplasia | Reduction in the risk of CV events and prevention of the progression of cardiac remodelling after MI with montelukast. Potent effects on platelet function, thrombosis, and integrin-mediated cell migration with zafirlukast |
| Macrolides | Prolongment of the QT interval, arrhythmias | |
| Oral corticosteroids | Hypertension, hyperglycaemia, dyslipidaemia, and increased risk of CV AEs | |
| Biologics | Hypertension, ventricular arrhythmias, and arterial and venous thromboembolic events with omalizumab. Risk of CV and thromboembolic AEs with mepolizumab, reslizumab and benralizumab | Dupilumab plays a CV protective role |
AEs adverse effects, CV cardiovascular, CYP cytochrome P450, MI myocardial infarction
Inhaled Corticosteroids
Inhaled corticosteroids reduce airway inflammation, which has a beneficial effect on airflow obstruction, AHR and exacerbations, and minimise the use of OCS that can cause significant systemic AEs and appear to increase the risk of CVD [31–34]. OCS can cause sodium and fluid retention issues, leading to hypertension or heart failure exacerbations [35].
Only a few published observational studies exist on the effects of ICS on CV risk in patients with asthma. These studies have shown that medium to high doses of ICS are generally associated with a lower CV risk compared with OCS. This is due to the lower systemic bioavailability of ICS [33, 34]. Furthermore, low-dose ICS do not increase this risk [36]. Higher doses of ICS may cause venous thromboembolism, albeit to a lesser extent than OCS [37], but have also been associated with reduced CV and all-cause mortality in women with asthma [38]. ICS may also reduce atherosclerosis by acting directly on the arterial wall through systemic absorption or indirectly by reducing airway inflammation and the spread of inflammatory mediators in the circulation [39].
The effect on the CV system associated with ICS varies depending on the ICS used and can be attributed to several factors, including systemic bioavailability, drug potency, pharmacokinetics and the specific safety profile of each corticosteroid [40, 41]. High systemic bioavailability can lead to systemic effects [40, 41]. Beclomethasone dipropionate, budesonide, flunisolide and triamcinolone acetonide have significant systemic bioavailability [40], which may result in a slightly higher risk of systemic AEs than corticosteroids with lower bioavailability. Conversely, fluticasone propionate, fluticasone furoate, mometasone furoate and ciclesonide have very low systemic bioavailability [40], which reduces the risk of systemic AEs. The amount of ICS available systemically is influenced by the efficiency of hepatic first-pass metabolism, which is high for fluticasone furoate, fluticasone propionate, mometasone furoate and ciclesonide, but low for budesonide, flunisolide, triamcinolone acetonide and beclomethasone dipropionate [42].
The potency of any ICS is influenced by several factors, such as its binding affinity to the glucocorticoid receptor. The hierarchy of potency among the available agents (from highest to lowest) is fluticasone furoate > mometasone > fluticasone propionate > beclomethasone > ciclesonide > budesonide > triamcinolone > flunisolide [41]. However, there is disagreement about the exact order of potency, and it has been suggested that the type of inhaler device used to administer the ICS can also play a role in determining relative potency [43]. Higher potency alone would result in more significant systemic effects, but the structural changes that allow for higher potency also facilitate efficacy at a lower dose, as well as lower rate and extent of bioavailability and high clearance [41]. In fact, the rate at which the drug is metabolised and cleared from the body also influences the duration of systemic exposure [40, 41]. Ciclesonide is a prodrug that is activated in the lungs and has low systemic bioavailability, which significantly reduces the risk of systemic AEs [44].
Unfortunately, comparisons of the safety profile of ICS remain indirect, limited, focused on therapies that also include ICS (ICS/LABA, triple therapy), and mainly on chronic obstructive pulmonary disease (COPD) [45].
In any case, although ICS are generally safe at recommended doses, health care providers should consider potential interactions with commonly prescribed CV drugs. ICS are metabolised by cytochrome P450 3A (CYP3A) enzymes, primarily CYP3A4 [46], which can be inhibited when multiple drugs compete for this enzyme, leading to increased systemic drug concentrations and potential toxicity [47]. Many CV drugs, such as β-blockers, calcium channel blockers and some antiarrhythmics, are also metabolised by the hepatic CYP system. Therefore, dose adjustment may be required, especially in elderly patients on polypharmacy.
Bronchodilators
Bronchodilators are essential for treating asthma symptoms and, when used with an ICS, help prevent exacerbations [31]. There are several classes of bronchodilators, each with different mechanisms and benefits. β2-Agonists are widely used, while muscarinic acetylcholine receptor (mAChR) antagonists and methylxanthines are appropriate for some patients [20]. However, bronchodilators, mainly β2-agonists, can cause CV AEs [4, 48], especially in regular users, because they can stimulate sympathetic nerve activity, potentially causing vasospasm [21], thereby compromising vascular health [49] and increasing CV risk [50].
β2-agonists
All β2-agonists carry a significant risk of CV AEs, particularly tachycardia and arrhythmias [51]. Individuals with asthma have an enhanced sympathetic response to β2-agonists, which may increase CV risk, as suggested by evidence that even a single clinical dose of inhaled salbutamol reduces flow-mediated dilation and increases peripheral arterial stiffness in asthmatic patients [52]. Salbutamol also increases plasma noradrenaline levels, which may be of concern in people with underlying CVD [53].
The risk of CV AEs stems from to the activation of β1 adrenergic receptors (β1-ARs) in the heart, resulting in increased heart rate and contractility [54]. β1-ARs dominate in the normal heart (77% compared to 23% for β2-ARs) [55], but β2-ARs, with a 2.5-fold higher density in the sinoatrial node compared to the right atrial myocardium, play a crucial role in regulating cardiac chronotropism [56]. Despite significant homology (65–70%) between β1- and β2-ARs [51], cardiac β2-ARs exhibit more efficient coupling to adenylyl cyclase than β1-ARs [57].
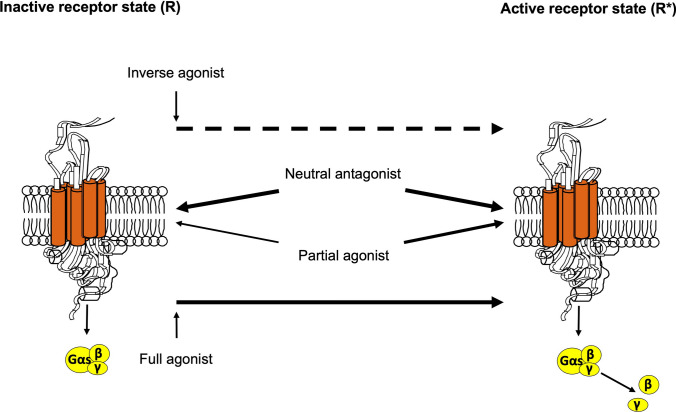
β2-Agonists are categorised into full agonists (e.g., isoproterenol, formoterol) that fully activate β2-ARs and partial agonists (e.g., salmeterol, vilanterol) that activate β2-ARs without eliciting maximal responses (Fig. 2) [54, 58]. While both types have similar downstream effects in airway smooth muscle (ASM), full agonists are preferred in acute exacerbations because β2-ARs may be desensitised in patients with asthma due to regular prior use of β2-agonists and as a result of inflammatory mediators characteristic of the disease [59]. Conversely, the heart has fewer β2-ARs than ASM, possibly explaining the improved safety profile of partial agonists [58].
Fig. 2.
β2-agonists can be divided into three groups: full agonists, such as isoproterenol or formoterol, which completely shift the balance towards the activated conformation, favouring active receptor state (R*); partial agonists, such as salmeterol or ultra-LABAs (vilanterol, indacaterol), which less frequently stabilise a different receptor conformation, leading to a relatively higher affinity for R* (although lower than that of a full agonist); and inverse agonists, which bind to the receptor in its inactive state, thus shifting the balance away from R* towards another conformation. A neutral antagonist has similar affinity for both inactive receptor state (R) and R* conformations, maintains the equilibrium unchanged, and inhibits the effects of both agonists and inverse agonists. LABAs long-acting β2-agonists
β2-Agonists can induce atrial fibrillation [60], arrhythmias [61], acute MI [48], congestive heart failure, cardiac arrest, and even sudden cardiac death [62]. These risks increase in individuals with long QT syndrome [62] and are aggravated by electrolyte imbalances, prevalent in up to 98% of severe asthmatics taking β2-agonists [63]. The increased risk of acute MI generally occurs primarily in the first 3 months of therapy and then decreases [64].
Activation of β2-ARs triggers the Na+–K+ ATPase pump [65], leading to potassium uptake into cells and potentially causing hypokalaemia. Hypokalaemia is associated with various arrhythmias, including torsades de pointes, polymorphic ventricular tachycardia, ventricular fibrillation, and ventricular ectopy [66]. It can also cause localised changes in conduction velocity and regional variability in action potential duration, fostering the formation of functional re-entry circuits [67]. Hypokalaemia contributes to arrhythmogenesis by reducing cardiac repolarisation reserve and increasing intracellular calcium levels [68], thereby promoting abnormal electrical activity and potentially life-threatening rhythm disturbances. Hypokalaemia has been associated with β2-agonist overdose in severe asthma or salbutamol abuse [69, 70], but some research suggests that the increased risk of atrial fibrillation in individuals with uncontrolled asthma cannot be solely attributed to β2-agonist use [71].
Observational studies link inhaled β2-agonists to an increased risk of acute MI [48, 72, 73], possibly due to increased heart rate and oxygen demand resulting from β-AR overstimulation, leading to myocardial injury and ischaemia [74]. Some researchers suggest that asthma-like symptoms may signal the onset of ischaemic heart disease [75]. However, other studies have not found this association [76, 77].
β-Agonists have been associated with higher rates of hospitalisation for heart failure and increased all-cause mortality in patients with left ventricular dysfunction [78], including intensive care unit (ICU) patients without chronic pulmonary disease [79]. However, the ABCHF study suggested that it is unlikely that previously occurring asthma or β-agonist use have a strong relationship to the development of idiopathic dilated cardiomyopathy [80]. A large study found no long-term mortality association after adjusting for various factors, suggesting that factors beyond β-agonist use, particularly heart failure severity, may influence mortality risk [81].
In heart failure patients experiencing dyspnoea, inhaled β2-agonists offer benefits like improved CV function, increased cardiac output, reduced peripheral vascular resistance, and better pulmonary capillary wedge pressures [82]. They also relieve bronchoconstriction, easing respiratory effort and thereby reducing cardiac workload [83]. However, caution is needed due to potential mild hypokalaemia and arrhythmias [61], particularly when hypoxaemia is present [84].
Rare cases of sudden death in patients with asthma have been linked to β2-agonist use [58], possibly due to exacerbation of underlying CVD and β2-agonist–induced cardiac arrhythmias [27].
However, a recent nested case–control analysis using the UK Clinical Practice Research Datalink of asthma patients showed that the use of SABA or ICS/LABA combination therapy was not associated with a higher risk of MACE such as stroke, heart failure, acute MI, arrhythmia or CV death compared with ICS (SABA vs ICS: HR 1.29; 95% CI 0.96–1.73; ICS/LABA vs ICS, HR 0.75; 95% CI 0.33–1.73) [85].
Muscarinic Acetylcholine Receptor Antagonists
Muscarinic acetylcholine receptor (mAChR) antagonists used to treat asthma are all non-selective against the 5 mAChRs subtypes and therefore, can antagonise cardiac M2 and M3 mAChRs if the plasma levels are sufficiently high, which raises concerns about potential CV risks of this drug class [86]. Activation of cardiac M2 mAChRs induces negative chronotropic and inotropic responses and blocking these receptors may cause CV AEs [87]. Cardiac M3 mAChRs play a role in modulating cardiac membrane repolarisation, regulating rhythm, and protecting against myocardial damage [88–90], especially in pathological conditions like ischaemia, hypertrophy, arrhythmia, and heart failure [91]. However, blocking M3 mAChRs eliminates this protective effect.
While short-acting muscarinic antagonists (SAMAs) exhibit non-selective binding properties, some LAMAs have longer residence times at M3 mAChRs and shorter residence times at M2 mAChRs, potentially reducing this risk [91].
In young patients with asthma, inhaled ipratropium bromide did not significantly affect heart function [92], but high doses were associated with a 69% increase in arrhythmia incidence [93]. However, clinical trials of LAMAs in asthma subjects showed that less than 2% of patients reported CV AEs with inhaled tiotropium bromide [94–96].
A study analysing CV AEs reported during treatment with aclidinium, tiotropium, glycopyrronium, and umeclidinium in patients mainly with COPD, but also in some subjects with asthma or bronchiectasis, found that tiotropium had fewer CV AE reports compared to ipratropium [97]. Other LAMAs were more frequently associated with CV AEs compared to tiotropium.
Women generally exhibit higher gene expression of M3 mAChRs compared to M2 mAChRs than men, resulting in a higher M3/M2 mAChR ratio [98, 99]. This increased M3/M2 selectivity raises concerns when using LAMAs in women with medical conditions that rely heavily on M3 mAChR function, such as cardiac ischaemia, pathological cardiac hypertrophy, arrhythmia, and heart failure [89].
LAMA monotherapy or replacing a LABA with a LAMA in combination with an ICS is not recommended for the management of asthma [20]. However, ICS/LABA/LAMA FDCs are currently endorsed for Step 4 and Step 5 asthma treatment [20]. A meta-analysis found no significant increase in severe CV AEs with triple FDCs compared with ICS/LABA FDCs, regardless of ICS doses [100]. Real-world evidence indicates that triple therapy actually reduces the likelihood of reported CV AEs compared to LAMA alone [97].
Methylxanthines
Theophylline is no longer recommended as an add-on treatment for asthma [20] due to its narrow therapeutic window and risk of causing tachycardia and severe arrhythmias, including sudden death, even at therapeutic levels [101]. However, theophylline is still used in some regions due to its affordability and availability in slow-release oral formulations. A recent meta-analysis suggests that doxofylline, a newer methylxanthine derivative that is attracting increasing attention for its distinct pharmacological properties and therapeutic advantages over traditional methylxanthines [102], could replace theophylline in the treatment of asthma due to its superior efficacy/safety profile [103]. The study showed that doxofylline was significantly more successful than theophylline in reducing daily asthma events and minimising the likelihood of AEs. It was also as effective as theophylline in improving forced expiratory volume in 1 second (FEV1).
Leukotriene Modifiers
Cysteinyl leukotrienes (CysLTs) are implicated in various CV conditions including acute MI, ischaemic stroke, atherosclerosis, aortic aneurysm, and intimal hyperplasia [104, 105]. Specifically, CysLT receptor 1 expression rises in response to pro-inflammatory cytokines released from coronary atherosclerotic lesions [106].
Recent experimental research suggests that montelukast, an orally active CysLT receptor antagonist, could prevent cardiac remodelling after MI and preserve cardiac function by regulating inflammation and preventing cardiomyocyte hypertrophy [107]. Another CysLT receptor antagonist, zafirlukast, inhibits platelet function, thrombosis, and integrin-mediated cell migration without causing bleeding, suggesting potential benefits in certain CV conditions [108]. However, information concerning the effects of CysLTs receptor antagonists on the heart remains limited.
A Swedish population-based study showed that CysLTR-1 antagonists may be beneficial in the prevention of secondary CVD, including ischaemic stroke [109]. In addition, a retrospective observational study found a significant association between the use of montelukast and a reduction in MACE in asthmatic patients [110]. In these individuals, ICS/LABA combinations reduced asthma exacerbations, but increased the risk of CV AEs compared with ICS+LTRA therapy. Furthermore, an interventional study in patients with asthma showed that montelukast can reduce levels of CVD-related inflammatory biomarkers, such as C-reactive protein, and lipid levels [111], thereby reducing heart problems.
Recent data have shown that montelukast could prevent heart problems in older asthmatics [112]. The study showed reduced efficacy in the management of acute symptoms as evidenced by increased use of SABA (rate ratio [RR], 1.58, p < 0.001), but the addition of LTRA treatment was associated with a lower likelihood of CV events compared to LABA (OR, 0.86, p = 0.006).
Macrolides
Macrolides, especially azithromycin, are occasionally used over the long term to reduce exacerbations in patients with asthma [113]. However, QTc (QT interval corrected for heart rate) prolongation may be of concern because it can lead to arrhythmias and potentially fatal torsades de pointes [114]. Macrolides can prolong the QT and QTc interval via their effect on the rapid delayed rectifier (IKr) potassium channel [115]. The risk is higher in the elderly (aged 60–80 years) [116] and in patients predisposed to CV events [114].
Regular assessment of the QT interval should be performed before starting macrolide therapy and periodically during treatment, to reduce the risk of serious CV AEs [115]. The British Thoracic Society guideline states that, for safety reasons, an ECG to assess the QTc interval should be performed before starting long-term macrolide therapy in adults with asthma [117]. If the QTc is > 450 ms for men and > 470 ms for women, this is considered a contraindication to starting macrolide therapy. In addition, an ECG should be done one month after starting treatment to check for new QTc prolongation. When detected, treatment should be stopped. Before starting low-dose macrolide therapy, it is important to review the patient's medical history for any evidence of heart disease, low serum potassium levels, slow pulse rate, family history of sudden death, or known prolonged QT interval. Patients with such a history should not be started on low-dose macrolide therapy without careful consideration and counselling regarding the increased risk of CV AEs. In addition, a comprehensive review of the patient's medication history should be performed to identify any medications that could potentially prolong the QTc interval, and patients taking such medications should not be prescribed low-dose macrolide therapy. Electrocardiogram assessment should always be repeated when starting a new drug that could potentially prolong the QTc interval, or when the dose is increased.
Biologics
Several studies have investigated the association between biologics and CV risk in asthma patients.
Some data suggest that elevated total serum IgE levels are associated with the presence of multivessel disease and may play a role in differentiating the severity of coronary artery disease independent of conventional CV risk factors [118]. Indeed, the EXCELS study reported higher rates of CV, cerebrovascular and arterial thromboembolic events in patients treated with omalizumab, a monoclonal antibody (mAb) targeting IgE [119], occasional case reports have linked omalizumab to arterial and venous thromboembolic events [120], and a French pharmacovigilance study found an association between omalizumab and hypertension, ventricular arrhythmias and venous thromboembolism [121].
However, a pooled analysis of 25 double-blind, placebo-controlled randomized controlled trials (RCTs) did not confirm an increased CV risk [122]. Discrepancies among studies may arise from factors like varying coronary heart disease burdens and contributions from other treatments in more severe asthmatics, particularly OCS [123]. Current recommendations and expert opinions support omalizumab use in individuals with a history of CVD [124].
There is currently no evidence linking novel biologics targeting interleukin (IL)-5 or its receptor (mepolizumab, reslizumab and benralizumab) to an increased risk of CVD [119], which is consistent with the lack of genetic evidence implicating IL-5 inhibition in CV and thromboembolic AEs [125]. For example, the aforementioned French pharmacovigilance study found that the association of IL-5 inhibitors with hypertension, ventricular arrhythmias and venous thromboembolism was much weaker than that of omalizumab [119]. However, this is in contrast to the hypothesis that IL-5 may protect against arterial plaque formation [126], so that IL-5 inhibition could theoretically increase the risk of CVD.
Dupilumab, a fully humanised mAb that inhibits the common receptor component for IL-4 and IL-13 signalling, may provide CV protection by reducing biomarkers associated with atherosclerosis and CV comorbidities [127]. This mAb has been reported to significantly reduce serum levels of CCL17 (or thymus and activation-regulated chemokine, TARC) [128], which is produced by conventional dendritic cells, signals through CCR4 on regulatory T (Treg) cells and drives atherosclerosis by suppressing Treg functions through yet undefined mechanisms [129] and is considered an extremely useful clinical biomarker not only for monitoring the efficacy of treatment but also for ensuring successful treatment outcomes [130]. Furthermore, dupilumab significantly affects the expression of atherosclerosis-related genes [131] and suppresses key immune and atherosclerosis/CV risk proteins [132].
In clinical trials, tezepelumab, a mAb blocking thymic stromal lymphopoietin, showed a numerical imbalance in cardiac events compared with placebo [133]. However, there is no evidence of a causal relationship between its use and these events in asthmatics.
Pulmonary Impact of CVD Pharmacotherapy
Standard therapy for various CVDs typically includes β-AR blockers, angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin II type 1 receptor blockers (AT-1RBs), statins, anti-platelet agents, calcium channel blockers and diuretics [28]. Recently, the focus has also been on proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9-Is), a relatively new targeted therapeutic modality for lowering low-density lipoprotein-cholesterol [134]. While some of these treatments may cause AEs in asthmatics, they may also potentially benefit the progression of asthma (Table 2).
Table 2.
Pulmonary impact of CVD pharmacotherapy
| Cardiovascular agent | Potential risks | Potential benefits |
|---|---|---|
| β2-AR blockers | Potential for bronchospasm in patients with pre-existing airway disease, reduced efficacy of β2-agonists and occurrence of moderate and severe asthma exacerbations, although cardioselective β-blockers may have a lower risk | Reduced risk of pulmonary congestion and subsequent exacerbations of lung disease in patients with heart failure or ischaemic heart disease. Increased β2-AR regulation in the lung with reduced need for β2-agonists. Inverse agonists effective in reducing AHR |
| Angiotensin-converting enzyme inhibitors | Dry cough and occasionally bronchospasm due to increased bradykinin levels. Worsening of asthma severity in patients with hypertension | Chronic reduction in ACE activity may be beneficial |
| Angiotensin II type 1 receptor antagonists | Are generally well tolerated but may cause cough in a small percentage of patients | Induce a small reduction in AHR. Attenuating Ang II/AT1 activation may be beneficial |
| Statins | Pharmacological interaction of atorvastatin, lovastatin, and simvastatin with corticosteroids at the level of CYP3A4, with a greater risk of muscle-related complications | Reduced frequency of exacerbations, asthma-related emergency department visits, hospitalizations, and systemic corticosteroid use. Improved anti-inflammatory effects of ICS even in smokers with asthma |
| Anti-platelet agents | Hypersensitivity reaction known as aspirin-exacerbated respiratory disease | Competitive thienopyridine P2Y12 receptor antagonists may reduce AHR. TxA2 synthetase inhibitors may reduce AHR but not in patients with acute asthma |
| Calcium channel blockers | Improvement in lung function among individuals with mild asthma, notably evident in cases of EIA. Reduction of the annual decline in lung function | |
| Diuretics | Enhancement of the effects of hypokalaemia with arrhythmia by concomitant use of β2 agonists or ICS and a thiazide. Metabolic alkalosis with compensatory hypoventilation and worsening of hypercapnia with high doses of loop diuretics | Effective in managing fluid overload conditions such as congestive heart failure and pulmonary oedema, thereby relieving symptoms of dyspnoea, and improving overall lung function |
| PCSK9 inhibitors | In the European population, genetically predicted inhibition of PCSK9 could significantly increase the risk of asthma |
ACE angiotensin-converting enzyme, AHR airway hyperresponsiveness, Ang II/AT1 Angiotensin II/Angiotensin II type 1 receptor, AR adrenoceptor, CYP cytochrome P450, EIA exercise-induced asthma, ICS inhaled corticosteroid, PCSK9 proprotein convertase subtilisin/kexin type 9, P2Y12 purinergic receptor P2Y G protein-coupled 12, TxA2 thromboxane A2
β-AR Blockers
β-AR blockers are effective in treating acute coronary syndromes and preventing coronary events by primarily blocking β1-ARs, which inhibits the action of endogenous adrenaline and noradrenaline on the heart [135]. This reduces myocardial demand by decreasing heart rate and force of contraction, resulting in lower mortality rates from CVD. β-AR blockers also benefit congestive heart failure, cardiac arrhythmias, and hypertension, and this also in asthmatic patients [28]. In those with heart failure or ischaemic heart disease and asthma, β-blockers may reduce the risk of pulmonary congestion and subsequent pulmonary exacerbations.
However, non-selective β-AR blockers, such as propranolol, nadolol, pindolol, labetalol, sotalol, carvedilol, and timolol, which act on both β1- and β2-ARs, may pose a risk to asthmatics due to their potential to induce bronchospasm and worsen respiratory symptoms [136–138]. They can also antagonise the effects of β2-agonists [136] and cause asthma exacerbations [138]. A meta-analysis of RCTs revealed that short-term oral non-selective β-blocker use in patients with asthma led to an average decrease in FEV1 of 10.2% in 11.1% of patients, along with a reduction in β2-agonist response in 20% of patients [136]. However, the impact on FEV1 differed according to the specific non-selective β-blocker employed. Administration of labetalol did not result in a statistically significant alteration in FEV1 compared to placebo (− 2.7%, p = 0.43), whereas propranolol did (− 17%, p < 0.001).
Cardioselective β1-AR blockers like metoprolol, atenolol, acebutolol, betaxolol, esmolol, bisoprolol and nebivolol do not increase the risk of asthma exacerbations in patients with CVD and asthma [137, 139]. However, they lack complete specificity for β1-ARs, and exhibit variable selectivity, potentially antagonising β2-ARs at therapeutic doses [139]. A systematic review of blinded, placebo-controlled RCTs examining the effects of single dose or continuous treatment cardio-selective β-blockers in patients with reversible airway disease] indicated that the first dose of active treatment resulted in a minor reduction in FEV1 (7.46%) compared to the placebo, without any associated respiratory symptoms [140]. Subsequent continued treatment, which lasted from three days to four weeks, did not demonstrate any significant difference in FEV1, symptoms, or frequency of inhaler use compared to the placebo. Meanwhile, the response to β2-agonists remained unchanged.
Recent research has found a significant association between the use of oral timolol or intravenous propranolol and an increased risk of asthma attacks, regardless of asthma history [141]. Conversely, oral celiprolol, a combination of celiprolol and propranolol, bisoprolol, and atenolol, intravenous practolol and intravenous sotalol were associated with a reduced likelihood of exacerbating asthma symptoms. No documented asthma-related deaths associated with cardioselective β1-blockers have been reported in the published literature, except one potential death in an asthmatic reported in the VigiBase dataset, although there was insufficient information to establish definitive causality [142]. However, the absence of reports of asthma deaths does not definitively rule out their occurrence.
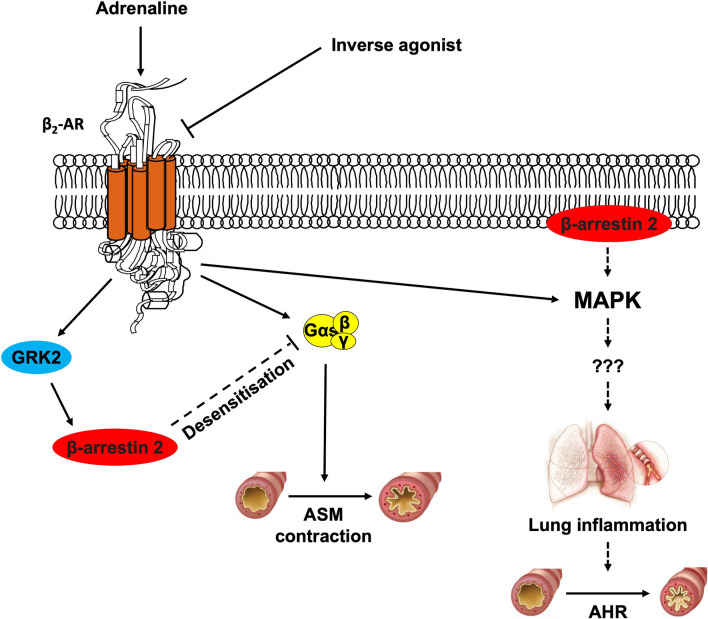
β-AR blockers can vary in their properties [143]. Some exhibit inverse agonist activity at different β-ARs and exert beneficial effects on airway epithelial and immune cells by inhibiting constitutive pro-inflammatory signalling via the non-canonical β-arrestin 2/extracellular signal-regulated kinase pathway (Fig. 3) [144]. Conversely, variations in the degree of intrinsic sympathomimetic activity (ISA) are associated with the downregulation of β2-ARs [54].
Fig. 3.
Chronically administered β2-AR inverse agonists, by deactivating spontaneously active β2-ARs, exert their beneficial effects on both airway epithelial and immune cells by suppressing constitutive pro-inflammatory signalling via non-canonical β-arrestin 2-mediated pathways. In addition, deactivation of β2-ARs by inverse agonists prevents phosphorylation of these receptors, thereby preventing desensitisation and down-regulation. AHR airway hyperresponsiveness, AR adrenoceptor, ASM airway smooth muscle, β2-ARs beta-2 adrenergic receptors, GRK2 G-protein coupled receptor kinase 2, IL interleukin, MAPK p38 mitogen-activated protein kinase
Guidelines recommend avoiding β-AR blockers with ISA for CVD treatment [145]. Conversely, chronic inverse agonist treatment may benefit asthmatics. Nadolol, a non-selective β-AR blocker acting as an inverse agonist, reduced AHR and did not neutralise the effect of salbutamol in subjects with mild to moderate asthma [146, 147]. Similarly, in patients with mild or moderate asthma taking regular bisoprolol, a cardioselective β1-AR blocker acting as an inverse agonist, the response to salbutamol after mannitol challenge was comparable to placebo [148]. However, large trials are needed to confirm these preliminary findings.
When deciding whether to prescribe β-blockers to patients with chronic airway diseases, it is important to make a thorough assessment of their clinical characteristics, airway disease severity and concomitant CVD [149]. A comprehensive risk-benefit assessment of the specific β-blockers to be prescribed to asthmatic patients with CVD comorbidities is needed. The potential benefits of β-blockers in the management of CVD should be carefully weighed against the potential risk of exacerbation of respiratory symptoms. Based on current evidence, it is advisable to prioritise selective β-blockers over non-selective β-blockers for the treatment of patients with asthma, as selective β1-ARs are less likely to worsen pulmonary symptoms compared with non-selective β-blockers that also act on β2-ARs [150]. This approach aims to minimise respiratory AEs while providing the necessary CV benefits. However, even when using selective β1-AR blockers, careful consideration of drug dose and timing is essential [149].
Co-administering β2-agonists and β-AR has shown promise in patients with asthma and CVD. Prolonged use of inhaled β2-agonists can cause reduced β2-AR responsiveness, known as desensitisation, affecting disease control [151]. This desensitisation involves several intracellular signalling pathways [58]. Patients with CVD and chronic airway disease often experience increased cardiac adrenergic activity [54], leading to further desensitisation of β2-ARs through G protein-coupled receptor kinase 2 (GRK2)-mediated phosphorylation, β-arrestin 2 binding and receptor internalisation, and reduced airway relaxation and bronchoprotection [152, 153]. Long-term use of β-blockers can reverse β2-AR desensitisation [151] potentially reducing the need for β2-agonists [154].
In an experimental model of congestive heart failure, combining the β2-agonist indacaterol with the β-blocker metoprolol effectively reversed cardiac remodelling, reduced infarct size, lowered blood pressure and heart rate, restored ejection fraction, and left ventricular dimensions, and normalised gene expression and cyclic adenosine monophosphate levels, while decreased cardiac GRK2 expression [155]. These findings suggest that stimulating β2-ARs while blocking β1-ARs may benefit failing heart treatment, possibly by promoting cardiac progenitor cell expansion.
Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Type 1 Receptor Blockers
ACE-Is and AT-1RBs are used to treat hypertension and heart failure. ACE-Is inhibit the conversion of angiotensin (Ang) I to Ang II, a potent vasoconstrictor, while AT-1RBs block Ang II effects by antagonising its receptors. Angiotensin-converting enzyme is also involved in the metabolism of bradykinin and ACE-Is are more efficient at inhibiting bradykinin degradation than Ang II synthesis [156].
ACE-Is are often associated with cough in patients with respiratory disease and can occasionally induce bronchospasm due to bradykinin accumulation in the airways [157]. The incidence of this particular side effect is not well defined, with percentages ranging from 2.8 to 40%, depending on factors such as ethnicity, genotype, pre-existing CVD, assessment techniques and the specific ACE-I prescribed [157]. Cough typically occurs within the first two weeks after initiating ACE-I treatment, although delayed onset is possible [157]. In hypertensive patients with asthma, ACE-Is have been associated with worse asthma outcomes, including increased need for rescue medication such as SABAs, more frequent emergency department visits or hospital admissions, and a greater reliance on systemic corticosteroids [158, 159].
Some evidence suggests that individuals with active asthma, particularly older women with a higher body mass index, may have reduced tolerance to ACE-Is [160]. However, not all studies have consistently observed a risk of bronchoconstriction or asthma exacerbation [161]. Given the variability in individual responses to ACE-Is, further investigation is warranted if a potential risk is identified [162].
Conversely, AT-1RBs do not usually induce cough even in patients intolerant to ACE-Is [163] and may even slightly reduce AHR [164]. Individuals with active asthma are at a higher risk of ACE-I intolerance, leading to a greater likelihood of switching to AT-1RBs, especially in severe asthma and older age groups [160].
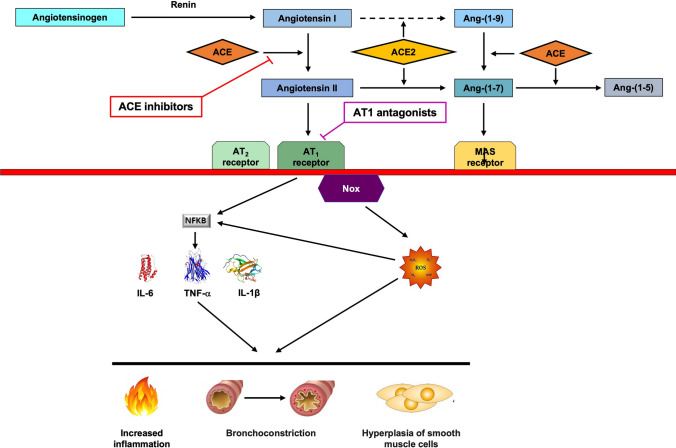
The renin-angiotensin system influences inflammation, potentially affecting asthma pathophysiology (Fig. 4) [165]. Two pathways, mediated by ACE/Ang II/AT1-R and ACE2/Ang-1-7/Mas receptor, are involved. Balancing these pathways is crucial for organ and system homeostasis. Increased ACE/ACE2 activity ratio favours pro-inflammatory effects by enhancing Ang II formation and Ang-(1–7) degradation [166]. Ang II over-activates AT1-Rs, inducing vasoconstriction and increasing pro-inflammatory cytokine levels, effects related to the ability of Ang II to activate nuclear factor (NF)-κB via AT1-Rs [167]. Conversely, Mas receptor activation by Ang-(1–7) promotes anti-inflammatory effects by inhibiting NF-κB signalling, potentially resolving inflammation [168].
Fig. 4.
Schematic view of the RAS cascade and proinflammatory effects of angiotensin II. Activation of the RAS results increased production of inflammatory cytokines (in this figure only a few are shown), which play pivotal roles in increasing pulmonary inflammation and promote hyperplasia of smooth muscle cells and bronchoconstriction. Chronic reduction of ACE activity and the use of pharmacological strategies aimed at attenuating Ang II/AT1 activation may have long-term benefits in the treatment of patients with asthma. ACE angiotensin converting enzyme, ACE2 angiotensin converting enzyme 2, Ang-(1–5) angiotensin-(1–5), Ang-(1–7) angiotensin-(1–7), Ang-(1–9) angiotensin-(1–9), AT1 AT1 receptor, AT2 AT2 receptor, IL interleukin, MAS Mas receptor (MAS1), NF-κB nuclear factor-κB, Nox nicotinamide adenine dinucleotide phosphate oxidase, ROS reactive oxygen species, TNF-α tumour necrosis factor α
Research indicates harmful effects of Ang II/AT1 activation on inflammation, pulmonary remodelling, and AHR in asthma [169]. Elevated circulating ACE levels are significantly associated with asthma development [170]. Patients with severe acute asthma often have elevated levels of Ang II [171]. Furthermore, Ang II exacerbates methacholine and endothelin-1–induced bronchoconstriction [172, 173] and provokes contraction of human ASM through the RhoA/Rho-associated coiled-coil–containing protein kinase 2 signalling pathway [174].
Chronic reduction of ACE activity and pharmacological strategies targeting Ang II/AT1 activation may offer long-term benefits in asthma. The AT1-R blockade may exert its effect in part by enhancing the Ang-(1–7)/Mas receptor pathway [169]. However, large RCTs are needed to validate these findings and to determine the safety and efficacy of ACE-Is and AT-1RBs in asthma.
Statins
Statins, commonly used for lowering cholesterol and preventing CVD [175], are now recognised for their potential benefits in lung health. Earlier concerns about statins causing drug-induced interstitial lung disease (ILD) [176] have been contradicted by recent findings showing a reduced risk of ILD and idiopathic pulmonary fibrosis with statin use, particularly at higher dosages [177]. Statins affect respiratory processes such as AHR, adaptive immunity, and T2 inflammation by targeting metabolites in the mevalonate pathway [178]. These anti-inflammatory and immunomodulatory effects hold promise for asthma management.
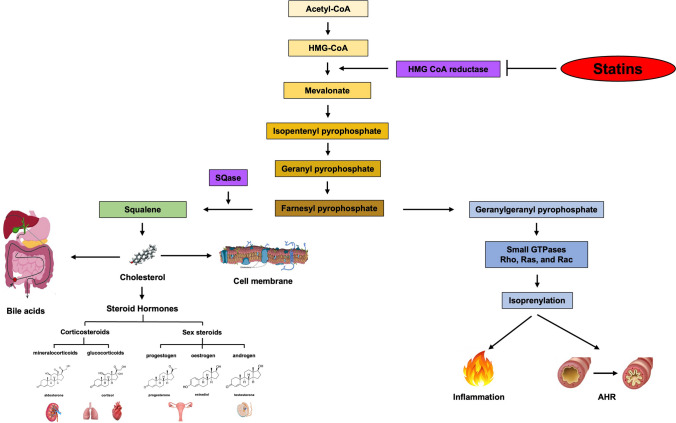
By inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme in the mevalonate pathway, statins modulate inflammatory cells involved in both lung and systemic inflammation, including eosinophils, neutrophils, macrophages, mast cells, T cells and dendritic cells, and can attenuate AHR (Fig. 5) [166, 179]. Statins can also reduce pro-inflammatory cytokines, inhibit lymphocyte proliferation, exert antioxidant effects, increase endothelial nitric oxide synthase expression, and suppress ASM proliferation [180]. However, their effectiveness as an asthma treatment is still uncertain [180], with conflicting data from trials possibly due to inadequate drug levels in the lungs [178].
Fig. 5.
Mevalonate pathway and mechanism of action of statins. The mechanism by which statins could be used for the treatment of asthmatic patients seems to be the same as that observed for cholesterol lowering. AHR bronchial hyperresponsiveness, CoA coenzyme A, SQase squalene synthase
Nevertheless, some studies suggest that statin treatment is associated with reduced asthma-related emergency visits, hospitalisations, and corticosteroid use [181], and a reduced risk of exacerbations in adult asthmatics [182]. Meta-analyses also suggest that statins may reduce exacerbations and improve asthma management, relieving symptoms and decreasing inflammatory markers without affecting lung function [183, 184]. Moreover, statins may enhance the anti-inflammatory effects of ICS even in smokers with asthma [185–187], although pharmacokinetic interactions between statins and corticosteroids at the level of CYP3A4 may increase systemic concentrations and AEs [188], such as muscle-related AEs [189, 190] and increases in blood glucose levels [191, 192].
Inhaled statins present a potential new option for asthma treatment, bypassing hepatic metabolism and allowing for lower doses with greater efficacy and reduced systemic AEs [178].
It is challenging to draw definitive conclusions regarding the efficacy and safety of statins for the management of asthma due to the limited strength of the available evidence. Given the anti-inflammatory capabilities of statins, which have the potential to improve asthma control and reduce asthma exacerbations, their use may be an option as additional therapy to conventional treatment. However, it is essential to carry out long-term prospective clinical trials in specific populations to establish the efficacy and safety of statins as anti-inflammatory agents for the treatment of asthma.
Anti-platelet Agents
Anti-platelet agents like aspirin, clopidogrel, and ticagrelor are essential for managing and preventing ischaemic heart disease and valvular heart disease [179]. Recent evidence suggests that platelets play a role in asthma [193], interacting with dendritic cells and contributing to allergen sensitisation [194]. Additionally, platelets are crucial for recruiting eosinophils and neutrophils to the lung, forming extracellular traps, and influencing the adaptive immune response by promoting Th2 cells and T2 innate lymphoid cell activation [195]. They also facilitate the transition from IgM to IgE antibodies, inhibit eosinophil apoptosis in the lung, and induce allergic reactions to innocuous environmental allergens. While interfering with platelet activation is a promising way to modulate inflammatory responses [28], the mechanisms involved in inflammation are different from those involved in blood clotting [196]. As a result, existing antiplatelet drugs are unlikely to provide any therapeutic benefit in the treatment of asthma.
Indeed aspirin, which targets cyclooxygenase-1 and inhibits thromboxane A2 synthesis in platelets [197], may exacerbate conditions like aspirin-exacerbated respiratory disease (AERD) [198]. Aspirin-exacerbated respiratory disease is characterised by symptoms like rhinorrhoea, bronchospasm, and hives due to an imbalance in arachidonic acid metabolism associated with excessive production of CysLTs, IL-33/thymic stromal lymphopoietin and prostaglandin (PG)D2, and reduced production of airway PGE2 [199]. Aspirin-exacerbated respiratory disease affects approximately 7% of asthmatics [200], with a higher prevalence in those with nasal polyposis [201]. The P2Y12 inhibitors, such as clopidogrel, prasugrel and ticagrelor, which prevent ADP-mediated platelet activation and aggregation, are alternative drugs for aspirin-intolerant individuals requiring anti-platelet therapy [202].
In asthmatics, prasugrel reduced AHR [203], whereas ticagrelor did not significantly affect lung function [204]. Combining clopidogrel with montelukast showed a synergistic effect [205]. As discussed above, platelet activation associated with inflammation is based on unidirectional agonism at P2Y1 receptors, which is distinct from the purinergic signalling involved in platelet aggregation [206]. Novel drugs that selectively inhibit platelet-dependent inflammation without causing bleeding have potential as anti-inflammatory agents [207].
Platelet derived thromboxane A2 (TxA2), a potent platelet agonist, can cause bronchoconstriction and airway inflammation [207]. While TxA2 synthetase inhibitors have shown benefit in asthma [207], their efficacy during acute asthma attacks is limited [208] due to the production of alternative bronchoconstrictors like PGD2 and PGF2 alpha because of alterations in the arachidonic acid pathway [209].
Calcium Channel Blockers
Calcium channel blockers inhibit the influx of calcium ions into cells, thereby relaxing smooth muscle in blood vessels and the heart, which is helpful in the treatment of hypertension, angina pectoris and some cardiac arrhythmias [210].
Research suggests that calcium plays an important role in the pathophysiology of asthma. Increased intracellular calcium levels in ASM cells contribute to bronchoconstriction, AHR and inflammation [211]. Blocking calcium channels can alleviate these effects and reduce ASM contraction, relieve bronchoconstriction induced by various stimuli and induce bronchodilation [212].
Oral calcium channel blockers do not worsen asthma and may even have beneficial effects on lung function, particularly in patients with mild asthma and exercise-induced asthma [213]. A retrospective observational study found that calcium channel blockers reduced the annual decline in lung function in asthmatics, probably because of their anti-inflammatory effects and inhibition of airway remodelling [214]. However, they must be used with caution as they can also induce angioedema, occasionally necessitating intubation [215].
Diuretics
Diuretics are essential for managing fluid retention and blood pressure in individuals with both asthma and CVD. However, their effect on respiratory function, particularly in asthmatics, requires careful consideration. Thiazides, loop diuretics, and potassium-sparing diuretics, each with distinct mechanisms of action, regulate fluid balance.
Thiazides may exacerbate hypokalaemia when used with β2-agonists or ICS [216], as β2-agonists can translocate potassium into cells and corticosteroids may increase urinary potassium excretion [217]. Hypokalaemia can weaken respiratory muscles [218] and potentially trigger cardiac arrhythmias [66]. Thiazides may also induce metabolic alkalosis, reducing ventilatory drive and worsening respiratory symptoms in asthmatics [219].
Loop diuretics, effective in treating fluid overload in heart failure, may cause electrolyte imbalances like hypokalaemia [220]. High doses can induce metabolic alkalosis by excessive loss of chloride and hydrogen ions in the urine, potentially worsening hypercapnia, particularly in those with respiratory impairment [221].
Potassium-sparing diuretics are less likely to cause hypokalaemia but should be used cautiously in individuals with renal impairment or at risk of hyperkalaemia, as elevated potassium levels may adversely affect respiratory function [222].
PCSK9 Inhibitors
PCSK9-Is have pleiotropic effects, such as anti-inflammatory and anti-allergic properties, beyond lipid control [223]. It has been postulated that PCSK9 expression may contribute to inflammation and that PCSK9-Is may reduce inflammation by downregulating PCSK9 expression [224]. PCSK9 inhibition was observed to suppress the development of AHR, monocytosis in bronchoalveolar lavage fluid and systemic pro-inflammatory mediators in the lungs of mice fed a high-fat diet [225]. However, a drug target Mendelian randomization analysis in the European population revealed that genetically predicted inhibition of PCSK9 could significantly increase the risk of asthma [226]. Given the genetic variations between different ethnic groups, it is important to recognize that the efficacy and side effects of PCSK9-Is may differ between populations. To achieve a more comprehensive conclusion, future studies should incorporate subgroup analyses involving diverse populations.
Conclusion
The concomitant management of asthma and CVD requires a comprehensive approach. Although currently there are no specific guidelines specifically addressing the optimal treatment of patients with both asthma and CVD, it is advisable to adhere to the existing guidelines for the treatment of asthma alongside with those for the treatment of CVD, carefully selecting asthma therapies to avoid worsening CV symptoms, controlling CV risk factors like hypertension, hyperlipidaemia, diabetes, obesity, and promoting smoking cessation.
Collaboration between pulmonologists and cardiologists is crucial for developing personalised treatment plans. Close monitoring and tailoring of treatment to individual patient factors and comorbidities, with regular follow-up and communication between health care providers, are essential for comprehensive management.
Continuing advancements in knowledge underscore the importance of remaining updated to provide optimal care for patients with asthma and CVD. There is a pressing need to enhance comprehension of the interplay between these two diseases and develop evidence-based guidelines and treatment protocols. Evaluating the efficacy, safety and long-term outcomes of different treatment strategies is essential to improve clinical practice. Future research should investigate the pathways linking the exacerbation of one disease to the other, considering both respiratory and CV parameters, to guide appropriate therapeutic interventions. Furthermore, advances in pharmacogenomics and precision medicine offer hope for personalised therapeutic approaches to asthma and CVD, using genetic profiles to tailor drug response and minimise AEs.
Declarations
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Conflict of interest
The authors have no financial or non-financial relationships or activities concerning this article.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors were involved in the initial conception of the manuscript. M.C. and C.P.P. led the drafting and coordinated the revisions of the manuscript among all authors. N.A.H., L.C., M.G.M., and P.R. critically revised the content on their areas of expertise. All authors approved the final draft.
References
- 1.Aggarwal K, Bansal V, Mahmood R, et al. Asthma and cardiovascular diseases: uncovering common ground in risk factors and pathogenesis. Cardiol Rev. 2023. 10.1097/CRD.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Guo J, Zhang Y, Liu T, et al. Allergic asthma is a risk factor for human cardiovascular diseases. Nat Cardiovas Res. 2022;1(5):417–30. 10.1038/s44161-022-00067-z. [DOI] [PubMed] [Google Scholar]
- 3.Dodd KE, Blackley DJ, Mazurek JM. Cardiovascular disease among adults with work-related asthma, 2012–2017. Am J Prev Med. 2023;64(2):194–203. 10.1016/j.amepre.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Cazzola M, Hanania NA, Rogliani P, et al. Cardiovascular disease in asthma patients: from mechanisms to therapeutic implications. Kardiol Pol. 2023;81(3):232–41. 10.33963/KP.a2023.0038. [DOI] [PubMed] [Google Scholar]
- 5.Wen LY, Ni H, Li KS, et al. Asthma and risk of stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2016;25(3):497–503. 10.1016/j.jstrokecerebrovasdis.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Gao S, Yu M, Sheng Z, Tan W. Association of asthma with coronary heart disease: a meta analysis of 11 trials. PLoS ONE. 2017;12(6): e0179335. 10.1371/journal.pone.0179335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Li ZF, An ZY, et al. Association between asthma and all-cause mortality and cardiovascular disease morbidity and mortality: a meta-analysis of cohort studies. Front Cardiovasc Med. 2022;9: 861798. 10.3389/fcvm.2022.861798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cepelis A, Brumpton BM, Laugsand LE, et al. Asthma, asthma control and risk of acute myocardial infarction: HUNT study. Eur J Epidemiol. 2019;34(10):967–77. 10.1007/s10654-019-00562-x. [DOI] [PubMed] [Google Scholar]
- 9.Hekking PP, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6(1):108–13. 10.1016/j.jaip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Rogliani P, Laitano R, Ora J, Beasley R, Calzetta L. Strength of association between comorbidities and asthma: a meta-analysis. Eur Respir Rev. 2023;32(167): 220202. 10.1183/16000617.0202-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollevick ME, Xu KY, Mhango G, et al. The relationship between asthma and cardiovascular disease: an examination of the Framingham Offspring Study. Chest. 2021;159(4):1338–45. 10.1016/j.chest.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi HG, Kwon MJ, Kim JH, et al. Association between asthma and cardiovascular diseases: a longitudinal follow-up study using a national health screening cohort. World Allergy Organ J. 2024;17(6): 100907. 10.1016/j.waojou.2024.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schanen JG, Iribarren C, Shahar E, et al. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60(8):633–8. 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazzola M, Calzetta L, Bettoncelli G, et al. Cardiovascular disease in asthma and COPD: a population-based retrospective cross-sectional study. Respir Med. 2012;106(2):249–56. 10.1016/j.rmed.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Valencia-Hernández CA, Del Greco MF, Sundaram V, Portas L, Minelli C, Bloom CI. Asthma and incident coronary heart disease: an observational and Mendelian randomisation study. Eur Respir J. 2023;62(5):2301788. 10.1183/13993003.01788-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazzola M, Calzetta L, Bettoncelli G, Novelli L, Cricelli C, Rogliani P. Asthma and comorbid medical illness. Eur Respir J. 2011;38(1):42–9. 10.1183/09031936.00140310. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Liu H, Yang P, et al. Exploring the genetic association of allergic diseases with cardiovascular diseases: a bidirectional Mendelian randomization study. Front Immunol. 2023;14:1175890. 10.3389/fimmu.2023.1175890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreslová M, Kirchnerová O, Rajdl D, et al. Bronchial asthma as a cardiovascular risk factor: a prospective observational study. Biomedicines. 2022;10(10):2614. 10.3390/biomedicines10102614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tattersall MC, Dasiewicz AS, McClelland RL, et al. Persistent asthma is associated with carotid plaque in MESA. J Am Heart Assoc. 2022;11(23): e026644. 10.1161/JAHA.122.026644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cazzola M, Page CP, Matera MG, et al. Revisiting asthma pharmacotherapy: where do we stand and where do we want to go? Eur Respir J. 2023;62(2):2300700. 10.1183/13993003.00700-2023. [DOI] [PubMed] [Google Scholar]
- 21.Hung MJ, Mao CT, Hung MY, Chen TH. Impact of asthma on the development of coronary vasospastic angina: a population-based cohort study. Medicine (Baltimore). 2015;94(42): e1880. 10.1097/MD.0000000000001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakata Y, Komamura K, Hirayama A, et al. Elevation of the plasma histamine concentration in the coronary circulation in patients with variant angina. Am J Cardiol. 1996;77(12):1121–6. 10.1016/s0002-9149(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 23.Bazan-Socha S, Wójcik K, Olchawa M, et al. Increased oxidative stress in asthma-relation to inflammatory blood and lung biomarkers and airway remodeling indices. Biomedicines. 2022;10(7):1499. 10.3390/biomedicines10071499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Incalza MA, D’Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21(8):993–9. 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshide S, Mogi M, Kario K. Sympathetic nervous activation and hypertension. Hypertens Res. 2023;46(7):1636–7. 10.1038/s41440-023-01319-6. [DOI] [PubMed] [Google Scholar]
- 27.Cazzola M, Rogliani P, Calzetta L, et al. Bronchodilators in subjects with asthma-related comorbidities. Respir Med. 2019;151:43–8. 10.1016/j.rmed.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Ora J, Cavalli F, Cazzola M. Management of patients with asthma or COPD and cardiovascular disease: risks versus benefits. In: Martínez-García MA, Pépin J-L, Cazzola M, editors. Cardiovascular complications of respiratory disorders (ERS Monograph). Sheffield: European Respiratory Society; 2020. p. 66–81. 10.1183/2312508X.10027419. [Google Scholar]
- 29.Levy ML, Bacharier LB, Bateman E, et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33(1):7. 10.1038/s41533-023-00330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agache I, Akdis CA, Akdis M, et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy. 2021;76(1):14–44. 10.1111/all.14425. [DOI] [PubMed] [Google Scholar]
- 31.Ora J, Calzetta L, Matera MG, et al. Advances with glucocorticoids in the treatment of asthma: state of the art. Expert Opin Pharmacother. 2020;21(18):2305–16. 10.1080/14656566.2020.1807514. [DOI] [PubMed] [Google Scholar]
- 32.Varas-Lorenzo C, Rodriguez LA, Maguire A, Castellsague J, Perez-Gutthann S. Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis. 2007;192(2):376–83. 10.1016/j.atherosclerosis.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–95. 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Ekström M, Nwaru BI, Hasvold P, et al. Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy. 2019;74(11):2181–90. 10.1111/all.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng MK, Celermajer DS. Glucocorticoid treatment and cardiovascular disease. Heart. 2004;90(8):829–30. 10.1136/hrt.2003.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suissa S, Assimes T, Brassard P, et al. Inhaled corticosteroid use in asthma and the prevention of myocardial infarction. Am J Med. 2003;115(5):377–81. 10.1016/s0002-9343(03)00393-0. [DOI] [PubMed] [Google Scholar]
- 37.Ayodele OA, Cabral HJ, McManus DD, et al. Glucocorticoids and risk of venous thromboembolism in asthma patients aged 20–59 years in the United Kingdom’s CPRD 1995–2015. Clin Epidemiol. 2022;14:83–93. 10.2147/CLEP.S341048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camargo CA, Barr RG, Chen R, et al. Prospective study of inhaled corticosteroid use, cardiovascular mortality, and all-cause mortality in asthmatic women. Chest. 2008;134(3):546–51. 10.1378/chest.07-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podgórski M, Kupczyk M, Grzelak P, et al. Inhaled corticosteroids in asthma: promoting or protecting against atherosclerosis? Med Sci Monit. 2017;23:5337–44. 10.12659/msm.904469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372–80. 10.1111/bcp.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye Q, He XO, D’Urzo A. A review on the safety and efficacy of inhaled corticosteroids in the management of asthma. Pulm Ther. 2017;3:1–18. 10.1007/s41030-017-0043-5. [Google Scholar]
- 42.Matera MG, Rinaldi B, Calzetta L, Rogliani P, Cazzola M. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids for asthma treatment. Pulm Pharmacol Ther. 2019;58: 101828. 10.1016/j.pupt.2019.101828. [DOI] [PubMed] [Google Scholar]
- 43.Raissy HH, Kelly HW, Harkins M, Szefler SJ. Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med. 2013;187(8):798–803. 10.1164/rccm.201210-1853PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi GA, Cerasoli F, Cazzola M. Safety of inhaled corticosteroids: room for improvement. Pulm Pharmacol Ther. 2007;20(1):23–35. 10.1016/j.pupt.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Rogliani P, Ora J, Cavalli F, Cazzola M, Calzetta L. Comparing the efficacy and safety profile of triple fixed-dose combinations in COPD: a meta-analysis and IBiS score. J Clin Med. 2022;11(15):4491. 10.3390/jcm11154491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore CD, Roberts JK, Orton CR, et al. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos. 2013;41(2):379–89. 10.1124/dmd.112.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevrioukova I. Interaction of human drug-metabolizing CYP3A4 with small inhibitory molecules. Biochemistry. 2019;58(7):930–9. 10.1021/acs.biochem.8b0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macie C, Wooldrage K, Manfreda J, et al. Cardiovascular morbidity and the use of inhaled bronchodilators. Int J Chron Obstruct Pulmon Dis. 2008;3(1):163–9. 10.2147/copd.s1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinenno FA, Jones PP, Seals DR, et al. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278(4):H1205–10. 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- 50.Lee CJ, Hwang J, Kang CY, et al. Asthma and increased risk of myocardial infarction and mortality among hypertensive Korean patients. Hypertens Res. 2023;46(7):1694–704. 10.1038/s41440-023-01257-3. [DOI] [PubMed] [Google Scholar]
- 51.Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187(7):690–6. 10.1164/rccm.201209-1739PP. [DOI] [PubMed] [Google Scholar]
- 52.Moore LE, Kapoor K, Byers BW, et al. Acute effects of salbutamol on systemic vascular function in people with asthma. Respir Med. 2019;155:133–40. 10.1016/j.rmed.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 53.Snyder EM, Wong EC, Foxx-Lupo WT, et al. Effects of an inhaled β2-agonist on cardiovascular function and sympathetic activity in healthy subjects. Pharmacotherapy. 2011;31(8):748–56. 10.1592/phco.31.8.748. [DOI] [PubMed] [Google Scholar]
- 54.Matera MG, Martuscelli E, Cazzola M. Pharmacological modulation of β-adrenoceptor function in patients with coexisting chronic obstructive pulmonary disease and chronic heart failure. Pulm Pharmacol Ther. 2010;23(1):1–8. 10.1016/j.pupt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Bristow MR, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59(3):297–309. 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 56.Matera MG, Calzetta L, Cazzola M. β-Adrenoceptor modulation in chronic obstructive pulmonary disease: present and future perspectives. Drugs. 2013;73(15):1653–63. 10.1007/s40265-013-0120-5. [DOI] [PubMed] [Google Scholar]
- 57.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51(4):651–90. [PubMed] [Google Scholar]
- 58.Matera MG, Panettieri RA Jr. β2-adrenoceptor modulation in COPD and its potential impact on cardiovascular comorbidities. In: Martínez-García MA, Pépin J-L, Cazzola M, editors. Cardiovascular complications of respiratory disorders (ERS Monograph). Sheffield: European Respiratory Society; 2020. p. 229–37. 10.1183/2312508X.10028519. [Google Scholar]
- 59.Hanania NA, Dickey BF, Bond RA. Clinical implications of the intrinsic efficacy of beta-adrenoceptor drugs in asthma: full, partial and inverse agonism. Curr Opin Pulm Med. 2010;16(1):1–5. 10.1097/MCP.0b013e328333def8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan WL, Yang KP, Chao TF, et al. The association of asthma and atrial fibrillation—a nationwide population-based nested case–control study. Int J Cardiol. 2014;176(2):464–9. 10.1016/j.ijcard.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 61.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309–21. 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 62.Thottathil P, Acharya J, Moss AJ, et al. Risk of cardiac events in patients with asthma and long-QT syndrome treated with β2-agonists. Am J Cardiol. 2008;102(7):871–4. 10.1016/j.amjcard.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammad HA, Abdulfttah MT, Abdulazez AO, et al. A study of electrolyte disturbances in patients with chronic stable asthma and with asthma attacks. Egypt J Chest Dis Tuberc. 2014;63(3):529–34. 10.1016/j.ejcdt.2014.03.010. [Google Scholar]
- 64.Zhang B, de Vries F, Setakis E, van Staa TP. The pattern of risk of myocardial infarction in patients taking asthma medication: a study with the General Practice Research Database. J Hypertens. 2009;27(7):1485–92. 10.1097/HJH.0b013e32832af68d. [DOI] [PubMed] [Google Scholar]
- 65.Clausen T. Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol. 2010;24(5):595–605. 10.1111/j.1472-8206.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 66.Skogestad J, Aronsen JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol. 2018;9:1500. 10.3389/fphys.2018.01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poelzing S, Veeraraghavan R. Heterogeneous ventricular chamber response to hypokalemia and inward rectifier potassium channel blockade underlies bifurcated T wave in guinea pig. Am J Physiol Heart Circ Physiol. 2007;292(6):H3043–51. 10.1152/ajpheart.01312.2006. [DOI] [PubMed] [Google Scholar]
- 68.Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10(3): e004667. 10.1161/CIRCEP.116.004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daubert GP, Mabasa VH, Leung VW, et al. Acute clenbuterol overdose resulting in supraventricular tachycardia and atrial fibrillation. J Med Toxicol. 2007;3(2):56–60. 10.1007/BF03160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng B, Yadav K. Acute salbutamol toxicity in the emergency department: a case report. World J Emerg Med. 2021;12(1):73–5. 10.5847/wjem.j.1920-8642.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cepelis A, Brumpton BM, Malmo V, et al. Associations of asthma and asthma control with atrial fibrillation risk: results from the Nord-Trøndelag Health Study (HUNT). JAMA Cardiol. 2018;3(8):721–8. 10.1001/jamacardio.2018.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Au DH, Curtis JR, Every NR, et al. Association between inhaled β-agonists and the risk of unstable angina and myocardial infarction. Chest. 2002;121(3):846–51. 10.1378/chest.121.3.846. [DOI] [PubMed] [Google Scholar]
- 73.Lee CH, Choi S, Jang EJ, et al. Inhaled bronchodilators and acute myocardial infarction: a nested case–control study. Sci Rep. 2017;7(1):17915. 10.1038/s41598-017-17890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robin ED, McCauley R. Sudden cardiac death in bronchial asthma, and inhaled beta-adrenergic agonists. Chest. 1992;101(6):1699–702. 10.1378/chest.101.6.1699. [DOI] [PubMed] [Google Scholar]
- 75.Squire I. Shortness of breath, prescription of bronchodilators and the risk of myocardial infarction. J Hypertens. 2009;27(7):1358–9. 10.1097/HJH.0b013e32832c4e06. [DOI] [PubMed] [Google Scholar]
- 76.Rossinen J, Partanen J, Stenius-Aarniala B, et al. Salbutamol inhalation has no effect on myocardial ischaemia, arrhythmias and heart-rate variability in patients with coronary artery disease plus asthma or chronic obstructive pulmonary disease. J Intern Med. 1998;243(5):361–6. 10.1046/j.1365-2796.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 77.Uddin MJ, Groenwold RH, de Boer A, et al. Evaluating different physician’s prescribing preference based instrumental variables in two primary care databases: a study of inhaled long-acting beta2-agonist use and the risk of myocardial infarction. Pharmacoepidemiol Drug Saf. 2016;25(Suppl 1):132–41. 10.1002/pds.3860. [DOI] [PubMed] [Google Scholar]
- 78.Au DH, Udris EM, Curtis JR, et al. Association between chronic heart failure and inhaled β-2-adrenoceptor agonists. Am Heart J. 2004;148(5):915–20. 10.1016/j.ahj.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Ling Y, Chen Q, et al. Inhaled beta2-agonists increase in-hospital mortality in ICU patients with heart failure. Int Heart J. 2021;62(5):1076–82. 10.1536/ihj.20-825. [DOI] [PubMed] [Google Scholar]
- 80.Sengstock DM, Obeidat O, Pasnoori V, et al. Asthma, β-agonists, and development of congestive heart failure: results of the ABCHF study. J Card Fail. 2002;8(4):232–8. 10.1054/jcaf.2002.127771. [DOI] [PubMed] [Google Scholar]
- 81.Bermingham M, O’Callaghan E, Dawkins I, et al. Are beta2-agonists responsible for increased mortality in heart failure? Eur J Heart Fail. 2011;13(8):885–91. 10.1093/eurjhf/hfr063. [DOI] [PubMed] [Google Scholar]
- 82.Maak CA, Tabas JA, McClintock DE. Should acute treatment with inhaled beta agonists be withheld from patients with dyspnea who may have heart failure? J Emerg Med. 2011;40(2):135–45. 10.1016/j.jemermed.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 83.Minasian AG, van den Elshout FJ, Dekhuijzen PN, et al. Bronchodilator responsiveness in patients with chronic heart failure. Heart Lung. 2013;42(3):208–14. 10.1016/j.hrtlng.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Burggraaf J, Westendorp RG, in’t Veen JC, et al. Cardiovascular side effects of inhaled salbutamol in hypoxic asthmatic patients. Thorax. 2001;56(7):567–9. 10.1136/thorax.56.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amegadzie JE, Gamble JM, Farrell J, et al. Association between inhaled β-agonists initiation and risk of major adverse cardiovascular events: a population-based nested case–control study. Int J Chron Obstruct Pulmon Dis. 2022;17:1205–17. 10.2147/COPD.S35892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stolz D, Cazzola M. Characterising the cardiovascular safety profile of inhaled muscarinic receptor antagonists. In: Martínez-García MA, Pépin J-L, Cazzola M, editors. Cardiovascular complications of respiratory disorders (ERS Monograph). Sheffield: European Respiratory Society; 2020. p. 238–50. 10.1183/2312508X.10028619. [Google Scholar]
- 87.Matera MG, Cazzola M. Muscarinic receptor antagonists. Handb Exp Pharmacol. 2017;237:41–62. 10.1007/164_2016_68. [DOI] [PubMed] [Google Scholar]
- 88.Patanè S. M3 muscarinic acetylcholine receptor in cardiology and oncology. Int J Cardiol. 2014;177(2):646–9. 10.1016/j.ijcard.2014.09.178. [DOI] [PubMed] [Google Scholar]
- 89.Hang P, Zhao J, Qi J, et al. Novel insights into the pervasive role of M3 muscarinic receptor in cardiac diseases. Curr Drug Targets. 2013;14(3):372–7. 10.2174/138945013804998963. [PubMed] [Google Scholar]
- 90.Saternos HC, Almarghalani DA, Gibson HM, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018;50(1):1–9. 10.1152/physiolgenomics.00062.2017. [DOI] [PubMed] [Google Scholar]
- 91.Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. 10.1124/pr.111.004580. [DOI] [PubMed] [Google Scholar]
- 92.Lehrer PM, Hochron SM, Rausch L, et al. Effects of aerosol ipratropium bromide on cardiac vagal tone. Chest. 1994;105(6):1701–4. 10.1378/chest.105.6.1701. [DOI] [PubMed] [Google Scholar]
- 93.Adimadhyam S, Schumock GT, Walton S, et al. Risk of arrhythmias associated with ipratropium bromide in children, adolescents, and young adults with asthma: a nested case–control study. Pharmacotherapy. 2014;34(4):315–23. 10.1002/phar.1336. [DOI] [PubMed] [Google Scholar]
- 94.Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–207. 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 95.Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367–76. 10.1016/S2213-2600(15)00031-4. [DOI] [PubMed] [Google Scholar]
- 96.Ohta K, Ichinose M, Tohda Y, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS ONE. 2015;10(4): e0124109. 10.1371/journal.pone.0124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matera MG, Calzetta L, Rogliani P, et al. Cardiovascular events with the use of long-acting muscarinic receptor antagonists: an analysis of the FAERS database 2020–2023. Lung. 2024. 10.1007/s00408-024-00677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Obeidat M, Zhou G, et al. Responsiveness to ipratropium bromide in male and female patients with mild to moderate chronic obstructive pulmonary disease. EBioMedicine. 2017;19:139–45. 10.1016/j.ebiom.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calzetta L, Puxeddu E, Rogliani P. Gender-related responsiveness to the pharmacological treatment of COPD: a first step towards the personalized medicine. EBioMedicine. 2017;19:14–5. 10.1016/j.ebiom.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogliani P, Cavalli F, Chetta A, et al. Potential drawbacks of ICS/LABA/LAMA triple fixed-dose combination therapy in the treatment of asthma: a quantitative synthesis of safety profile. J Asthma Allergy. 2022;15:565–77. 10.2147/JAA.S283489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bittar G, Friedman HS. The arrhythmogenicity of theophylline. A multivariate analysis of clinical determinants. Chest. 1991;99(6):1415–20. 10.1378/chest.99.6.1415. [DOI] [PubMed] [Google Scholar]
- 102.Cazzola M, Page CP, Calzetta L, Rogliani P, Matera MG. Doxofylline: advancing and empowering equitable asthma and COPD management beyond tradition. Adv Ther. 2024. 10.1002/adtp.202400103. [Google Scholar]
- 103.Calzetta L, Hanania NA, Dini FL, et al. Impact of doxofylline compared to theophylline in asthma: a pooled analysis of functional and clinical outcomes from two multicentre, double-blind, randomized studies (DOROTHEO 1 and DOROTHEO 2). Pulm Pharmacol Ther. 2018;53:20–6. 10.1016/j.pupt.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 104.Bäck M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23(1):41–8. 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Capra V, Bäck M, Barbieri SS, et al. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev. 2013;33(2):364–438. 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 106.Eaton A, Nagy E, Pacault M, et al. Cysteinyl leukotriene signaling through perinuclear CysLT1 receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J Mol Med (Berl). 2012;90(10):1223–31. 10.1007/s00109-012-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muluhie M, Castiglioni L, Rzemieniec J, et al. Montelukast, an available and safe anti-asthmatic drug, prevents maladaptive remodelling and maintains cardiac functionality following myocardial infarction. Sci Rep. 2024;14(1):3371. 10.1038/s41598-024-53936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou X, Cai J, Liu W, et al. Cysteinyl leukotriene receptor type 1 (CysLT1R) antagonist zafirlukast protects against TNF-α-induced endothelial inflammation. Biomed Pharmacother. 2019;111:452–9. 10.1016/j.biopha.2018.12.064. [DOI] [PubMed] [Google Scholar]
- 109.Ingelsson E, Yin L, Bäck M. Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J Allergy Clin Immunol. 2012;129(3):702-707.e2. 10.1016/j.jaci.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 110.Hoxha M, Tedesco CC, Quaglin S, et al. Montelukast use decreases cardiovascular events in asthmatics. Front Pharmacol. 2021;11: 611561. 10.3389/fphar.2020.611561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Allayee H, Hartiala J, Lee W, et al. The effect of montelukast and low-dose theophylline on cardiovascular disease risk factors in asthmatics. Chest. 2007;132(3):868–74. 10.1378/chest.07-0831. [DOI] [PubMed] [Google Scholar]
- 112.Altawalbeh SM, Thorpe CT, Zgibor JC, et al. Antileukotriene agents versus long-acting beta-agonists in older adults with persistent asthma: a comparison of add-on therapies. J Am Geriatr Soc. 2016;64(8):1592–600. 10.1111/jgs.14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2022;59:2102730. 10.1183/13993003.02730-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–90. 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Albert RK, Schuller JL, COPD Clinical Research Network. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med. 2014;189(10):1173–80. 10.1164/rccm.201402-0385CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Food and Drug Administration. FDA Drug Safety Communication: azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. 2013. http://www.fda.gov/drugs/drugsafety/ucm341822.htm. Accessed 30 Mar 2024.
- 117.Smith D, Du Rand IA, Addy C, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. BMJ Open Respir Res. 2020;7(1): e000489. 10.1136/bmjresp-2019-000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kounis NG, Hahalis G. Serum IgE levels in coronary artery disease. Atherosclerosis. 2016;251:498–500. 10.1016/j.atherosclerosis.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 119.Iribarren C, Rahmaoui A, Long AA, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol. 2017;139(5):1489–95. 10.1016/j.jaci.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 120.Oblitas CM, Galeano-Valle F, Vela-De La Cruz L, et al. Omalizumab as a provoking factor for venous thromboembolism. Drug Target Insights. 2019;13:1177392819861987. 10.1177/1177392819861987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quinta JB, Montastruc F, Sommet A, et al. Cardiovascular adverse effects of anti-IL-5/IL-5Rα therapies: a real-world study. J Allergy Clin Immunol Pract. 2021;9(3):1411–3. 10.1016/j.jaip.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 122.Iribarren C, Rothman KJ, Bradley MS, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: pooled analysis of patient-level data from 25 randomized, double-blind, placebo-controlled clinical trials. J Allergy Clin Immunol. 2017;139(5):1678–80. 10.1016/j.jaci.2016.12.953. [DOI] [PubMed] [Google Scholar]
- 123.Bleecker ER, Al-Ahmad M, Bjermer L, et al. Systemic corticosteroids in asthma: a call to action from World Allergy Organization and Respiratory Effectiveness Group. World Allergy Organ J. 2022;15(12): 100726. 10.1016/j.waojou.2022.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dorscheid DR, Lee JK, Ramesh W, et al. Guidance for administering biologics for severe asthma and allergic conditions. Can Respir J. 2022;2022:9355606. 10.1155/2022/9355606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alton P, Hughes DM, Zhao SS. Cardiovascular safety of genetically proxied interleukin-5 inhibition: a Mendelian randomization study. Respir Investig. 2023;61(2):149–52. 10.1016/j.resinv.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 126.Knutsson A, Björkbacka H, Dunér P, et al. Associations of interleukin-5 with plaque development and cardiovascular events. JACC Basic Transl Sci. 2019;4(8):891–902. 10.1016/j.jacbts.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kader HA, Azeem M, Jwayed SA, et al. Current insights into immunology and novel therapeutics of atopic dermatitis. Cells. 2021;10(6):1392. 10.3390/cells10061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107(3):535–41. 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 129.Döring Y, van der Vorst EPC, Yan Y, et al. Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis. Nat Cardiovasc Res. 2024;3:221–42. 10.1038/s44161-023-00413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kataoka Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J Dermatol. 2014;41(3):221–9. 10.1111/1346-8138.12440. [DOI] [PubMed] [Google Scholar]
- 131.Villani AP, Pavel AB, Wu J, et al. Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy. 2021;76(10):3107–21. 10.1111/all.14859. [DOI] [PubMed] [Google Scholar]
- 132.He H, Olesen CM, Pavel AB, et al. Tape-strip proteomic profiling of atopic dermatitis on dupilumab identifies minimally invasive biomarkers. Front Immunol. 2020;11:1768. 10.3389/fimmu.2020.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaplin S. Tezepelumab as add-on treatment for severe asthma. Prescriber. 2023;34(3):13–4. 10.1002/psb.2050. [Google Scholar]
- 134.Schonck WAM, Stroes ESG, Hovingh GK, Reeskamp LF. Long-term efficacy and tolerability of PCSK9 targeted therapy: a review of the literature. Drugs. 2024;84(2):165–78. 10.1007/s40265-024-01995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baker JG, Wilcox RG. β-Blockers, heart disease and COPD: current controversies and uncertainties. Thorax. 2017;72(3):271–6. 10.1136/thoraxjnl-2016-208412. [DOI] [PubMed] [Google Scholar]
- 136.Morales DR, Jackson C, Lipworth BJ, et al. Adverse respiratory effect of acute β-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145(4):779–86. 10.1378/chest.13-1235. [DOI] [PubMed] [Google Scholar]
- 137.Morales DR, Lipworth BJ, Donnan PT, et al. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med. 2017;15(1):18. 10.1186/s12916-017-0781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loth DW, Brusselle GG, Lahousse L, et al. β-Adrenoceptor blockers and pulmonary function in the general population: the Rotterdam study. Br J Clin Pharmacol. 2014;77(1):190–200. 10.1111/bcp.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144(3):317–22. 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev. 2002;1:CD002992. 10.1002/14651858.CD002992. [DOI] [PubMed] [Google Scholar]
- 141.Huang KY, Tseng PT, Wu YC, et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021;11(1):452. 10.1038/s41598-020-79837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bennett M, Chang CL, Tatley M, et al. The safety of cardioselective β1-blockers in asthma: literature review and search of global pharmacovigilance safety reports. ERJ Open Res. 2021;7(1):00801–2020. 10.1183/23120541.00801-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parra S, Bond RA. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol. 2007;7(2):146–50. 10.1016/j.coph.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 144.Walker JK, Penn RB, Hanania NA, et al. New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011;163(1):18–28. 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Coll Cardiol. 2015;65(18):1998–2038. 10.1016/j.jacc.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 146.Hanania NA, Singh S, El-Wali R, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther. 2008;21(1):134–41. 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanania NA, Mannava B, Franklin AE, et al. Response to salbutamol in patients with mild asthma treated with nadolol. Eur Respir J. 2010;36(4):963–5. 10.1183/09031936.00003210. [DOI] [PubMed] [Google Scholar]
- 148.Bennett MR, Chang CL, Tuffery C, et al. The impact of regular bisoprolol on the response to salbutamol in asthma: a double-blind randomized placebo-controlled crossover trial. Respirology. 2021;26(3):225–32. 10.1111/resp.13955. [DOI] [PubMed] [Google Scholar]
- 149.Matera MG, Rogliani P, Calzetta L, Cazzola M. An overview of the efficacy and safety of β2-adrenoceptor antagonists for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2024;23(7):833–44. 10.1080/14740338.2024.2362817. [DOI] [PubMed] [Google Scholar]
- 150.Tiotiu A, Novakova P, Kowal K, et al. Beta-blockers in asthma: myth and reality. Expert Rev Respir Med. 2019;13(9):815–22. 10.1080/17476348.2019.1649147. [DOI] [PubMed] [Google Scholar]
- 151.Oehme S, Mittag A, Schrödl W, et al. Agonist-induced β2-adrenoceptor desensitization and downregulation enhance pro-inflammatory cytokine release in human bronchial epithelial cells. Pulm Pharmacol Ther. 2015;30:110–20. 10.1016/j.pupt.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Rinaldi B, Capuano A, Gritti G, et al. Effects of chronic administration of β-blockers on airway responsiveness in a murine model of heart failure. Pulm Pharmacol Ther. 2014;28(2):109–13. 10.1016/j.pupt.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Cheung D, Timmers MC, Zwinderman AH, et al. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327(17):1198–203. 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 154.Lin R, Peng H, Nguyen LP, et al. Changes in β2-adrenoceptor and other signaling proteins produced by chronic administration of “beta-blockers” in a murine asthma model. Pulm Pharmacol Ther. 2008;21(1):115–24. 10.1016/j.pupt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rinaldi B, Donniacuo M, Sodano L, et al. Effects of chronic treatment with the new ultra-long-acting β2-adrenoceptor agonist indacaterol alone or in combination with the β1-adrenoceptor blocker metoprolol on cardiac remodelling. Br J Pharmacol. 2015;172(14):3627–37. 10.1111/bph.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taddei S, Bortolotto L. Unraveling the pivotal role of bradykinin in ACE inhibitor activity. Am J Cardiovasc Drugs. 2016;16(5):309–21. 10.1007/s40256-016-0173-3. [DOI] [PubMed] [Google Scholar]
- 157.Christiansen SC, Zuraw BL. Treatment of hypertension in patients with asthma. N Engl J Med. 2019;381(11):1046–57. 10.1056/NEJMra1800345. [DOI] [PubMed] [Google Scholar]
- 158.Lunde H, Hedner T, Samuelsson O, et al. Dyspnoea, asthma, and bronchospasm in relation to treatment with angiotensin converting enzyme inhibitors. BMJ. 1994;308(6920):18–21. 10.1136/bmj.308.6920.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Christiansen SC, Schatz M, Yang SJ, et al. Hypertension and asthma: a comorbid relationship. J Allergy Clin Immunol Pract. 2016;4(1):76–81. 10.1016/j.jaip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 160.Morales DR, Lipworth BJ, Donnan PT, et al. Intolerance to angiotensin converting enzyme inhibitors in asthma and the general population: a UK population-based cohort study. J Allergy Clin Immunol Pract. 2021;9(9):3431–9. 10.1016/j.jaip.2021.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Packard KA, Wurdeman RL, Arouni AJ. ACE inhibitor-induced bronchial reactivity in patients with respiratory dysfunction. Ann Pharmacother. 2002;36(6):1058–67. 10.1345/aph.1A332. [DOI] [PubMed] [Google Scholar]
- 162.Song WJ, Niimi A. Angiotensin-converting enzyme inhibitors, asthma, and cough: relighting the torch. J Allergy Clin Immunol Pract. 2021;9(9):3440–1. 10.1016/j.jaip.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2012;12(4):263–77. 10.1007/BF03261835. [DOI] [PubMed] [Google Scholar]
- 164.Myou S, Fujimura M, Kamio Y, et al. Effect of losartan, a type 1 angiotensin II receptor antagonist, on bronchial hyperresponsiveness to methacholine in patients with bronchial asthma. Am J Respir Crit Care Med. 2000;162(1):40–4. 10.1164/ajrccm.162.1.9907127. [DOI] [PubMed] [Google Scholar]
- 165.Tan WSD, Liao W, Zhou S, et al. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17. 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 166.Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev. 2018;98(1):505–53. 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matera MG, Calzetta L, Rinaldi B, et al. Treatment of COPD: moving beyond the lungs. Curr Opin Pharmacol. 2012;12(3):315–22. 10.1016/j.coph.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 168.Magalhães GS, Gregório JF, Cançado Ribeiro ATP, et al. Oral formulation of angiotensin-(1–7) promotes therapeutic actions in a model of eosinophilic and neutrophilic asthma. Front Pharmacol. 2021;12: 557962. 10.3389/fphar.2021.557962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gregório JF, Rodrigues-Machado MDG, Santos RAS, et al. Asthma: role of the angiotensin-(1–7)/Mas (MAS1) pathway in pathophysiology and therapy. Br J Pharmacol. 2021;178(22):4428–39. 10.1111/bph.15619. [DOI] [PubMed] [Google Scholar]
- 170.Hui Q, Hao Y, Ye F, et al. Genetically high angiotensin-converting enzyme concentrations causally increase asthma risk: a meta-analysis using Mendelian randomization. Front Med (Lausanne). 2022;9: 941944. 10.3389/fmed.2022.941944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu G, Chen Y, Wang Y, et al. Angiotensin II enhances group 2 innate lymphoid cell responses via AT1a during airway inflammation. J Exp Med. 2022;219(3): e20211001. 10.1084/jem.20211001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Millar EA, Nally JE, Thomson NC. Angiotensin II potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J. 1995;8(11):1838–41. 10.1183/09031936.95.08111838. [DOI] [PubMed] [Google Scholar]
- 173.Sakai H, Nishizawa Y, Nishimura A, et al. Angiotensin II induces hyperresponsiveness of bronchial smooth muscle via an activation of p42/44 ERK in rats. Pflugers Arch. 2010;460(3):645–55. 10.1007/s00424-010-0844-y. [DOI] [PubMed] [Google Scholar]
- 174.Li N, Cai R, Niu Y, et al. Inhibition of angiotensin II-induced contraction of human airway smooth muscle cells by angiotensin-(1–7) via downregulation of the RhoA/ROCK2 signaling pathway. Int J Mol Med. 2012;30(4):811–8. 10.3892/ijmm.2012.1080. [DOI] [PubMed] [Google Scholar]
- 175.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. 2016;316(19):1997–2007. 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 176.Andreikos D, Karampitsakos T, Tzouvelekis A, et al. Statins’ still controversial role in pulmonary fibrosis: what does the evidence show? Pulm Pharmacol Ther. 2022;77: 102168. 10.1016/j.pupt.2022.102168. [DOI] [PubMed] [Google Scholar]
- 177.Jang HJ, Lee DY, Loloci G, et al. Association between the use of statins and risk of interstitial lung disease/idiopathic pulmonary fibrosis: time-dependent analysis of population-based nationwide data. Eur Respir J. 2023;62(1):2300291. 10.1183/13993003.00291-2023. [DOI] [PubMed] [Google Scholar]
- 178.Zeki AA, Elbadawi-Sidhu M. Innovations in asthma therapy: is there a role for inhaled statins? Expert Rev Respir Med. 2018;12(6):461–73. 10.1080/17476348.2018.1457437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cazzola M, Calzetta L, Rinaldi B, et al. Management of chronic obstructive pulmonary disease in patients with cardiovascular diseases. Drugs. 2017;77(7):721–32. 10.1007/s40265-017-0731-3. [DOI] [PubMed] [Google Scholar]
- 180.Naing C, Ni H. Statins for asthma. Cochrane Database Syst Rev. 2020;7(7):CD013268. 10.1002/14651858.CD013268.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim JH, Wee JH, Choi HG, et al. Association between statin medication and asthma/asthma exacerbation in a national health screening cohort. J Allergy Clin Immunol Pract. 2021;9(7):2783–91. 10.1016/j.jaip.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 182.Park C, Jang JH, Kim C, et al. Real-world effectiveness of statin therapy in adult asthma. J Allergy Clin Immunol Pract. 2024;12(2):399–408. 10.1016/j.jaip.2023.10.029. [DOI] [PubMed] [Google Scholar]
- 183.Sunata K, Kabata H, Kuno T, et al. The effect of statins for asthma. A systematic review and meta-analysis. J Asthma. 2022;59(4):801–10. 10.1080/02770903.2021.1879850. [DOI] [PubMed] [Google Scholar]
- 184.Zhang QX, Zhang HF, Lu XT, et al. Statins improve asthma symptoms by suppressing inflammation: a meta-analysis based on RCTs. Eur Rev Med Pharmacol Sci. 2022;26(22):8401–10. 10.26355/eurrev_202211_30376. [DOI] [PubMed] [Google Scholar]
- 185.Thomson NC, Charron CE, Chaudhuri R, et al. Atorvastatin in combination with inhaled beclometasone modulates inflammatory sputum mediators in smokers with asthma. Pulm Pharmacol Ther. 2015;31:1–8. 10.1016/j.pupt.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 186.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, et al. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126(4):754–62. 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 187.Maneechotesuwan K, Kasetsinsombat K, Wamanuttajinda V, et al. Statins enhance the effects of corticosteroids on the balance between regulatory T cells and Th17 cells. Clin Exp Allergy. 2013;43(2):212–22. 10.1111/cea.12067. [DOI] [PubMed] [Google Scholar]
- 188.Bellosta S, Corsini A. Statin drug interactions and related adverse reactions: an update. Expert Opin Drug Saf. 2018;17(1):25–37. 10.1080/14740338.2018.1394455. [DOI] [PubMed] [Google Scholar]
- 189.Crisan E, Patil VK. Neuromuscular complications of statin therapy. Curr Neurol Neurosci Rep. 2020;20(10):47. 10.1007/s11910-020-01064-0. [DOI] [PubMed] [Google Scholar]
- 190.Owczarek J, Jasińska M, Orszulak-Michalak D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol Rep. 2005;57(1):23–34. [PubMed] [Google Scholar]
- 191.Ponziani MC, Karamouzis I, Mele C, et al. Baseline glucose homeostasis predicts the new onset of diabetes during statin therapy: a retrospective study in real life. Hormones (Athens). 2017;16(4):396–404. 10.14310/horm.2002.1760. [DOI] [PubMed] [Google Scholar]
- 192.Price DB, Voorham J, Brusselle G, et al. Inhaled corticosteroids in COPD and onset of type 2 diabetes and osteoporosis: matched cohort study. NPJ Prim Care Respir Med. 2019;29(1):38. 10.1038/s41533-019-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sullivan PJ, Jafar ZH, Harbinson PL, et al. Platelet dynamics following allergen challenge in allergic asthmatics. Respiration. 2000;67(5):514–7. 10.1159/000067466. [DOI] [PubMed] [Google Scholar]
- 194.Yue M, Hu M, Fu F, et al. Emerging roles of platelets in allergic asthma. Front Immunol. 2022;13: 846055. 10.3389/fimmu.2022.846055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luo L, Zhang J, Lee J, et al. Platelets, not an insignificant player in development of allergic asthma. Cells. 2021;10(8):2038. 10.3390/cells10082038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chebbo M, Duez C, Alessi MC, et al. Platelets: a potential role in chronic respiratory diseases? Eur Respir Rev. 2021;30(161): 210062. 10.1183/16000617.0062-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Badimon L, Vilahur G, Rocca B, et al. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc Res. 2021;117(9):2001–15. 10.1093/cvr/cvab003. [DOI] [PubMed] [Google Scholar]
- 198.Li KL, Lee AY, Abuzeid WM. Aspirin exacerbated respiratory disease: epidemiology, pathophysiology, and management. Med Sci (Basel). 2019;7(3):45. 10.3390/medsci7030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laidlaw TM, Boyce JA. Updates on immune mechanisms in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2023;151(2):301–9. 10.1016/j.jaci.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rajan JP, Wineinger NE, Stevenson DD, et al. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676–81. 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 201.Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004;328(7437):434. 10.1136/bmj.328.7437.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thakker RA, Salazar L, Jazar DA, et al. Coronary artery disease and aspirin intolerance: background and insights on current management. Cardiol Ther. 2022;11(2):175–83. 10.1007/s40119-022-00255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lussana F, Di Marco F, Terraneo S, et al. Effect of prasugrel in patients with asthma: results of PRINA, a randomized, double-blind, placebo-controlled, cross-over study. J Thromb Haemost. 2015;13(1):136–41. 10.1111/jth.12779. [DOI] [PubMed] [Google Scholar]
- 204.Butler K, Maya J, Teng R. Effect of ticagrelor on pulmonary function in healthy elderly volunteers and asthma or chronic obstructive pulmonary disease patients. Curr Med Res Opin. 2013;29(5):569–77. 10.1185/03007995.2013.781502. [DOI] [PubMed] [Google Scholar]
- 205.Trinh HKT, Nguyen TVT, Choi Y, et al. The synergistic effects of clopidogrel with montelukast may be beneficial for asthma treatment. J Cell Mol Med. 2019;23(5):3441–50. 10.1111/jcmm.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arkless KL, Pan D, Shankar-Hari M, et al. Stimulation of platelet P2Y1 receptors by different endogenous nucleotides leads to functional selectivity via biased signalling. Br J Pharmacol. 2024;181(4):564–79. 10.1111/bph.16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dogné JM, de Leval X, Benoit P, et al. Therapeutic potential of thromboxane inhibitors in asthma. Expert Opin Investig Drugs. 2002;11(2):275–81. 10.1517/13543784.11.2.275. [DOI] [PubMed] [Google Scholar]
- 208.Magazine R, Surendra VU, Chogtu B. Comparison of oral montelukast with oral ozagrel in acute asthma: a randomized, double-blind, placebo-controlled study. Lung India. 2018;35(1):16–20. 10.4103/lungindia.lungindia_226_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hernandez JM, Janssen LJ. Revisiting the usefulness of thromboxane-A2 modulation in the treatment of bronchoconstriction in asthma. Can J Physiol Pharmacol. 2015;93(2):111–7. 10.1139/cjpp-2014-0364. [DOI] [PubMed] [Google Scholar]
- 210.Godfraind T. Calcium channel blockers in cardiovascular pharmacotherapy. J Cardiovasc Pharmacol Ther. 2014;19(6):501–15. 10.1177/1074248414530508. [DOI] [PubMed] [Google Scholar]
- 211.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007;30(1):114–33. 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- 212.Twiss MA, Harman E, Chesrown S, et al. Efficacy of calcium channel blockers as maintenance therapy for asthma. Br J Clin Pharmacol. 2002;53(3):243–9. 10.1046/j.0306-5251.2001.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chiu KY, Li JG, Lin Y. Calcium channel blockers for lung function improvement in asthma: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119(6):518–23. 10.1016/j.anai.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 214.Tsuzuki R, To M, Yamawaki S, et al. Inhibitory effect of calcium channel blockers on the deterioration of lung function in adult-onset asthma. Ann Allergy Asthma Immunol. 2021;126(6):731–3. 10.1016/j.anai.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 215.Martinez Manzano JM, Lo KB, Jarrett SA, et al. Angioedema associated with the use of dihydropyridine calcium channel blockers. A case series. Ann Allergy Asthma Immunol. 2022;128(2):228–9. 10.1016/j.anai.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 216.Lipworth BJ, McDevitt DG, Struthers AD. Prior treatment with diuretic augments the hypokalemic and electrocardiographic effects of inhaled albuterol. Am J Med. 1989;86(6 Pt 1):653–7. 10.1016/0002-9343(89)90438-5. [DOI] [PubMed] [Google Scholar]
- 217.Chandy D, Aronow WS, Banach M. Current perspectives on treatment of hypertensive patients with chronic obstructive pulmonary disease. Integr Blood Press Control. 2013;6:101–9. 10.2147/IBPC.S33982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kardalas E, Paschou SA, Anagnostis P, et al. Hypokalemia: a clinical update. Endocr Connect. 2018;7(4):R135–46. 10.1530/EC-18-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vasileiadis I, Alevrakis E, Ampelioti S, et al. Acid-base disturbances in patients with asthma: a literature review and comments on their pathophysiology. J Clin Med. 2019;8(4):563. 10.3390/jcm8040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 2: loop diuretics and potassium-sparing agents. Expert Opin Pharmacother. 2014;15(5):605–21. 10.1517/14656566.2014.879117. [DOI] [PubMed] [Google Scholar]
- 221.Rutten FH, Cramer MJ, Lammers JW, et al. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J Heart Fail. 2006;8(7):706–11. 10.1016/j.ejheart.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 222.Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387–93. 10.1053/j.ajkd.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 223.Tang Z, Jiang L, Peng J, et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30(4):931–8. 10.3892/ijmm.2012.1072. [DOI] [PubMed] [Google Scholar]
- 224.Xu Y, Li Y. Association between lipid-lowering drugs and allergic diseases: a Mendelian randomization study. World Allergy Organ J. 2024;17(4): 100899. 10.1016/j.waojou.2024.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liang L, Chung SI, Guon TE, Park KH, Lee JH, Park JW. Statin administration or blocking PCSK9 alleviates airway hyperresponsiveness and lung fibrosis in high-fat diet-induced obese mice. Respir Res. 2024;25(1):213. 10.1186/s12931-024-02842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xie W, Li J, Du H, Xia J. Causal relationship between PCSK9 inhibitor and autoimmune diseases: a drug target Mendelian randomization study. Arthritis Res Ther. 2023;25(1):148. 10.1186/s13075-023-03122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]