Abstract
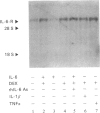
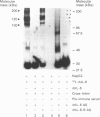
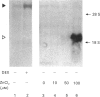
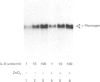
cDNAs coding for the human hepatic interleukin-6 receptor (IL-6-R) have been isolated from a library made from poly(A) RNA of dexamethasone-treated human hepatoma cells (HepG2). We found the hepatic IL-6-R to be identical to the one expressed by leucocytes. A polyclonal antiserum was raised in rabbits against the IL-6-R protein expressed in Escherichia coli. Although the entire IL-6-R protein was used for immunization, only antibodies to the cytoplasmic domain of the IL-6-R were obtained. It is demonstrated by affinity cross-linking and subsequent immunoprecipitation with antibodies against the ligand as well as against the receptor that the cloned cDNA codes for the functional IL-6-R on HepG2 cells. When the hepatic IL-6-R cDNA was overexpressed in HepG2 cells, these cells became more sensitive to low concentrations of IL-6 with respect to the induction of gamma-fibrinogen mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Geiger T., Hirano T., Northoff H., Ganter U., Bauer J., Kishimoto T., Heinrich P. C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. doi: 10.1016/0014-5793(87)80344-7. [DOI] [PubMed] [Google Scholar]
- Bomsztyk K., Sims J. E., Stanton T. H., Slack J., McMahan C. J., Valentine M. A., Dower S. K. Evidence for different interleukin 1 receptors in murine B- and T-cell lines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8034–8038. doi: 10.1073/pnas.86.20.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot F. J., van Eijk L., Broeders L., Aarden L. A., Löwenberg B. Interleukin-6 synergizes with M-CSF in the formation of macrophage colonies from purified human marrow progenitor cells. Blood. 1989 Feb;73(2):435–437. [PubMed] [Google Scholar]
- Caracciolo D., Clark S. C., Rovera G. Human interleukin-6 supports granulocytic differentiation of hematopoietic progenitor cells and acts synergistically with GM-CSF. Blood. 1989 Feb 15;73(3):666–670. [PubMed] [Google Scholar]
- Castell J. V., Geiger T., Gross V., Andus T., Walter E., Hirano T., Kishimoto T., Heinrich P. C. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988 Nov 1;177(2):357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Kilian P. L., Stern A. S., Nunes P., Parker K. P., Kaffka K. L., Chua A. O., Lugg D. K., Gubler U. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R. A., Song K., Onasch M. A., Fischer W. H., Chang D., Ringold G. M. Complementary DNA cloning of a receptor for tumor necrosis factor and demonstration of a shed form of the receptor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6151–6155. doi: 10.1073/pnas.87.16.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ikebuchi K., Hirai Y., Wong G. G., Yang Y. C., Clark S. C., Ogawa M. Synergism between interleukin-6 and interleukin-3 in supporting proliferation of human hematopoietic stem cells: comparison with interleukin-1 alpha. Blood. 1988 Jun;71(6):1759–1763. [PubMed] [Google Scholar]
- Lenstra J. A., Kusters J. G., Koch G., van der Zeijst B. A. Antigenicity of the peplomer protein of infectious bronchitis virus. Mol Immunol. 1989 Jan;26(1):7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Nordan R. P., Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986 Aug 1;233(4763):566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Dietrich A., Marks F. Molecular cloning of mouse protein kinase C (PKC) cDNA from Swiss 3T3 fibroblasts. Gene. 1988 Dec 30;74(2):465–471. doi: 10.1016/0378-1119(88)90179-5. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Hipp E., Lenz D., Legrés L. G., Korr H., Hirano T., Kishimoto T., Heinrich P. C. Structural and functional studies on the human interleukin-6 receptor. Binding, cross-linking, internalization, and degradation of interleukin-6 by fibroblasts transfected with human interleukin-6-receptor cDNA. J Biol Chem. 1991 Feb 25;266(6):3841–3846. [PubMed] [Google Scholar]
- Rose-John S., Schooltink H., Lenz D., Hipp E., Dufhues G., Schmitz H., Schiel X., Hirano T., Kishimoto T., Heinrich P. C. Studies on the structure and regulation of the human hepatic interleukin-6 receptor. Eur J Biochem. 1990 May 31;190(1):79–83. doi: 10.1111/j.1432-1033.1990.tb15548.x. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Taga T., Matsuda T., Hirano T., Kishimoto T., Kaziro Y. Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol. 1988 Aug;8(8):3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Schiel X., Rose-John S., Dufhues G., Schooltink H., Gross V., Heinrich P. C. Microheterogeneity of human interleukin 6 synthesized by transfected NIH/3T3 cells: comparison with human monocytes, fibroblasts and endothelial cells. Eur J Immunol. 1990 Apr;20(4):883–887. doi: 10.1002/eji.1830200425. [DOI] [PubMed] [Google Scholar]
- Schroeder J. J., Cousins R. J. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3137–3141. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Snyers L., De Wit L., Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P. G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988 Apr 1;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Vink A., Cayphas S., Uyttenhove C. Interleukin-HP1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987 Mar 1;165(3):641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]