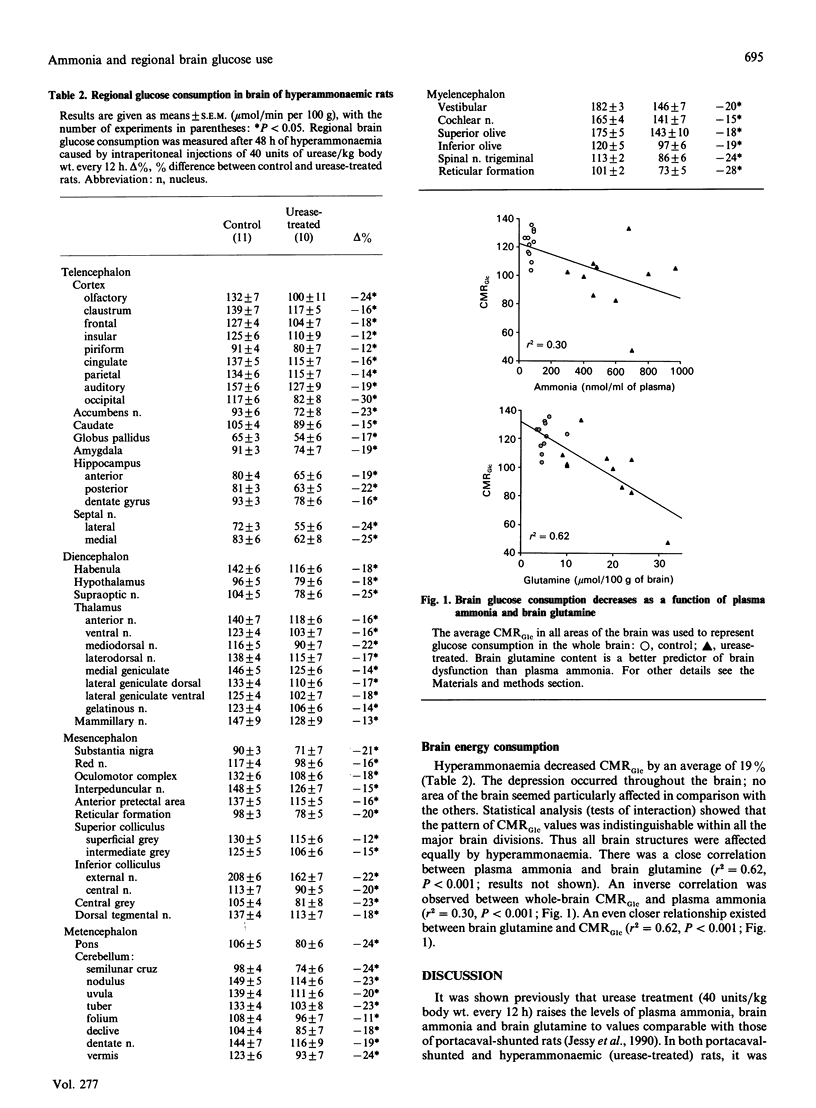
Abstract
Recent studies showed that hyperammonaemia caused many of the metabolic changes in portacaval-shunted rats, a model of hepatic encephalopathy. These changes included a depression in the cerebral metabolic rate of glucose (CMRGlc), an indication of decreased brain function. 2. The purpose of the present experiments was to determine whether the depression of CMRGlc caused by ammonia is confined to certain brain structures, or whether the depression is an overall decrease in all structures, such as occurs in portacaval-shunted rats. To accomplish this objective, rats were made hyperammonaemic by giving them intraperitoneal injections of 40 units of urease/kg body wt. every 12 h; control rats received 0.154 m-NaCl. CMRGlc was measured 48 h after the first injection, by using quantitative autoradiography with [6-14C]glucose as a tracer. 3. The experimental rats had high plasma ammonia concentrations (control 70 nmol/ml, experimental 610 nmol/ml) and brain glutamine levels (control 5.4 mumol/ml). Hyperammonaemia decreased CMRGlc throughout the brain by an average of 19%. CMRGlc showed an inverse correlation with plasma ammonia, but a stronger correlation with the brain glutamine content. 4. Hyperammonaemia led to a decrease in CMRGlc throughout the brain that was indistinguishable from the pattern seen in portacaval-shunted rats. This is taken as further evidence that the cerebral depression found in portacaval-shunted rats is a consequence of hyperammonaemia. The observation that depression of CMRGlc correlated more closely with brain glutamine content than with plasma ammonia suggests that metabolism of ammonia is an important step in the pathological sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth R. F., Giguère J. F., Michaud J., Lavoie J., Layrargues G. P. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987 Feb-Apr;6(1-2):1–12. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- Cooper A. J., Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987 Apr;67(2):440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- DeJoseph M. R., Hawkins R. A. Glucose consumption decreases throughout the brain only hours after portacaval shunting. Am J Physiol. 1991 Apr;260(4 Pt 1):E613–E619. doi: 10.1152/ajpendo.1991.260.4.E613. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Jessy J. Hyperammonaemia does not impair brain function in the absence of net glutamine synthesis. Biochem J. 1991 Aug 1;277(Pt 3):697–703. doi: 10.1042/bj2770697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Mans A. M., Davis D. W., Viña J. R., Hibbard L. S. Cerebral glucose use measured with [14C]glucose labeled in the 1, 2, or 6 position. Am J Physiol. 1985 Jan;248(1 Pt 1):C170–C176. doi: 10.1152/ajpcell.1985.248.1.C170. [DOI] [PubMed] [Google Scholar]
- Hourani B. T., Hamlin E. M., Reynolds T. B. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch Intern Med. 1971 Jun;127(6):1033–1036. [PubMed] [Google Scholar]
- Jessy J., Mans A. M., DeJoseph M. R., Hawkins R. A. Hyperammonaemia causes many of the changes found after portacaval shunting. Biochem J. 1990 Dec 1;272(2):311–317. doi: 10.1042/bj2720311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSTEN E., GEREZ C., KIRSTEN R. [An enzymatic microdetermination method for ammonia, specifically for extracts of animal tissues and fluids. Determination of NH4 ions in blood]. Biochem Z. 1963;337:312–319. [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Davis D. W., Bryan R. M., Hawkins R. A. Regional cerebral glucose utilization in rats with portacaval anastomosis. J Neurochem. 1983 Apr;40(4):986–991. doi: 10.1111/j.1471-4159.1983.tb08082.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Davis D. W., Biebuyck J. F., Hawkins R. A. Failure of glucose and branched-chain amino acids to normalize brain glucose use in portacaval shunted rats. J Neurochem. 1986 Nov;47(5):1434–1443. doi: 10.1111/j.1471-4159.1986.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., DeJoseph M. R., Davis D. W., Viña J. R., Hawkins R. A. Early establishment of cerebral dysfunction after portacaval shunting. Am J Physiol. 1990 Jul;259(1 Pt 1):E104–E110. doi: 10.1152/ajpendo.1990.259.1.E104. [DOI] [PubMed] [Google Scholar]
- Oei L. T., Kuys J., Lombarts A. J., Goor C., Endtz L. J. Cerebrospinal fluid glutamine levels and EEG findings in patients with hepatic encephalopathy. Clin Neurol Neurosurg. 1979;81(1):59–63. doi: 10.1016/s0303-8467(79)80008-6. [DOI] [PubMed] [Google Scholar]
- Vergara F., Plum F., Duffy T. E. Alpha-ketoglutaramate: increased concentrations in the cerebrospinal fluid of patients in hepatic coma. Science. 1974 Jan 11;183(4120):81–83. doi: 10.1126/science.183.4120.81. [DOI] [PubMed] [Google Scholar]


