Abstract
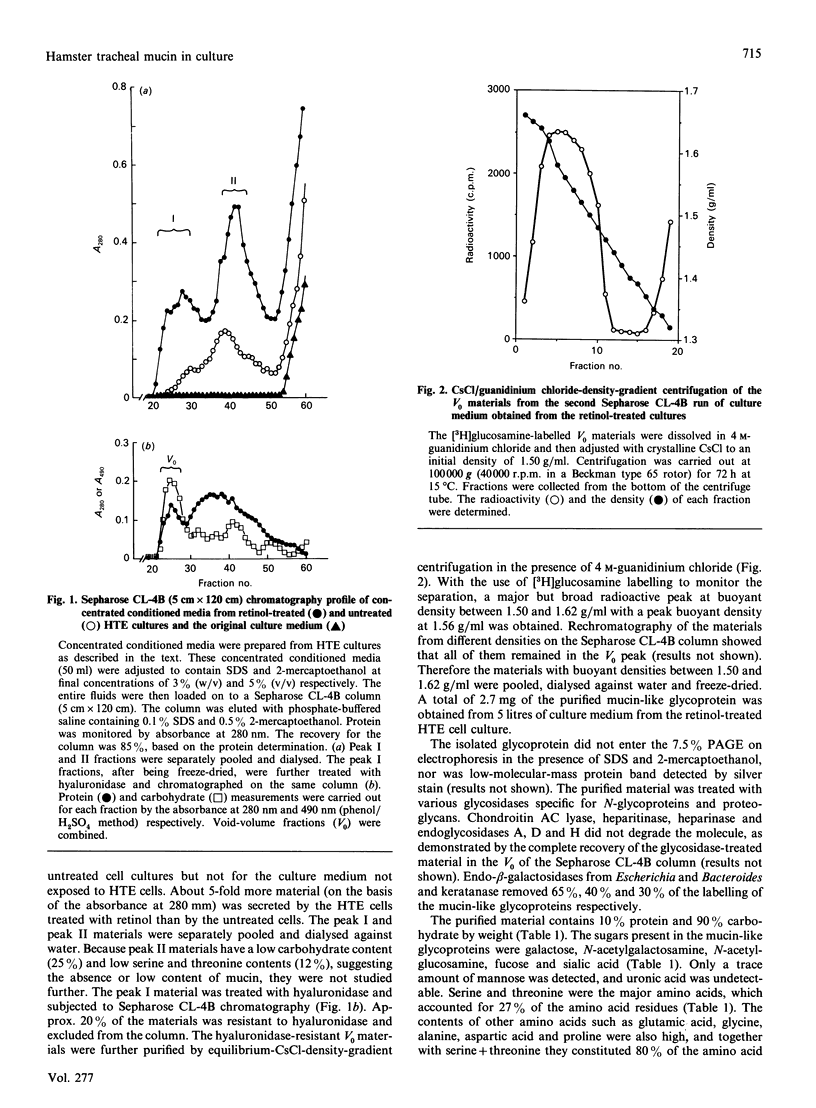
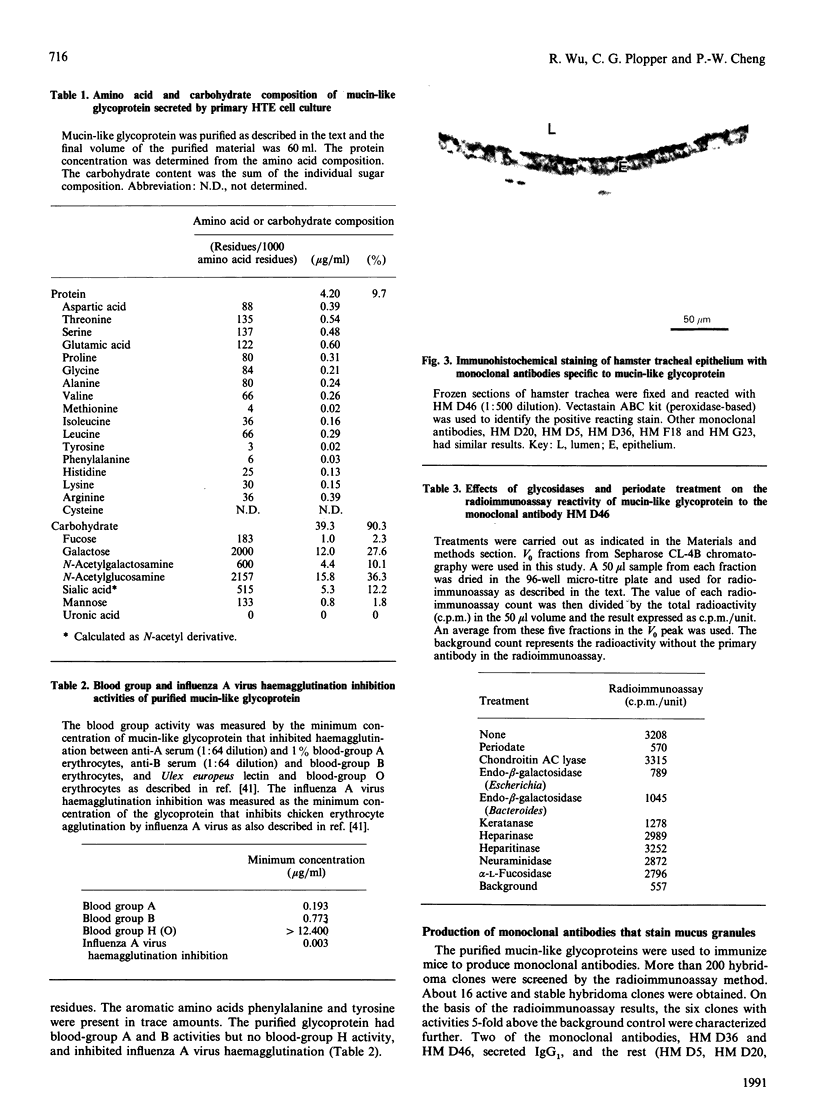
We isolated mucin-like glycoproteins from the conditioned medium of primary hamster tracheal epithelial (HTE) cell culture and characterized them biochemically and immunologically. These glycoproteins were purified on Sepharose CL-4B after Streptomyces hyaluronidase treatment and then by CsCl-density-gradient centrifugation in the presence of 4 M-guanidinium chloride. The purified glycoproteins were resistant to digestion by chondroitin AC lyase, heparinase, heparitinase and endo-N-acetylglucosaminidases A, D and H, but susceptible to endo-beta-galactosidase and keratanase. SDS/PAGE demonstrated no contamination by low-molecular-mass proteins. The purified glycoproteins showed a peak buoyant density of 1.56 g/ml in CsCl-density-gradient centrifugation, and contained 10% peptide and 90% carbohydrate by weight. Carbohydrates in these glycoproteins contained N-acetylglucosamine, N-acetylgalactosamine, galactose, fucose, sialic acid and a trace amount of mannose, but no uronic acid. Serine and threonine together accounted for 27% of the total amino acid residues. In addition, the mucin-like glycoproteins exhibited blood-group A and B activities, and very strong inhibitory activity for influenza A virus haemagglutination. With the use of the purified glycoprotein as an antigen, six monoclonal antibodies that stained mucus granules in hamster tracheal epithelium were obtained. We characterized the antibody produced by one of the clones, HM D46. We conclude that HTE cells cultured in the serum-free medium secrete a glycoprotein with physicochemical properties similar to those known in various airways mucins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Iyer R. N., Carlson D. M., Polony I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1976 Nov;177(1):95–104. doi: 10.1016/0003-9861(76)90419-7. [DOI] [PubMed] [Google Scholar]
- Bottenstein J., Hayashi I., Hutchings S., Masui H., Mather J., McClure D. B., Ohasa S., Rizzino A., Sato G., Serrero G. The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 1979;58:94–109. doi: 10.1016/s0076-6879(79)58127-0. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P. W. High-performance liquid chromatographic analysis of galactosamine, glucosamine, glucosaminitol, and galactosaminitol. Anal Biochem. 1987 Dec;167(2):265–269. doi: 10.1016/0003-2697(87)90162-x. [DOI] [PubMed] [Google Scholar]
- Cheng P. W., Sherman J. M., Boat T. F., Bruce M. Quantitation of radiolabeled mucous glycoproteins secreted by tracheal explants. Anal Biochem. 1981 Nov 1;117(2):301–306. doi: 10.1016/0003-2697(81)90782-x. [DOI] [PubMed] [Google Scholar]
- Clark J. N., Marchok A. C. Characterization of mucin isolated from rat tracheal transplants. Biochim Biophys Acta. 1979 Dec 11;588(3):357–367. doi: 10.1016/0304-4165(79)90344-1. [DOI] [PubMed] [Google Scholar]
- Finkbeiner W. E., Nadel J. A., Basbaum C. B. Establishment and characterization of a cell line derived from bovine tracheal glands. In Vitro Cell Dev Biol. 1986 Oct;22(10):561–567. doi: 10.1007/BF02623514. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W. Structure and metabolism of glycoproteins and glycosaminoglycans secreted by organ cultures of rabbit trachea. Biochem J. 1975 May;148(2):187–196. doi: 10.1042/bj1480187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Hansson G. C., Sheehan J. K., Carlstedt I. Only trace amounts of fatty acids are found in pure mucus glycoproteins. Arch Biochem Biophys. 1988 Oct;266(1):197–200. doi: 10.1016/0003-9861(88)90250-0. [DOI] [PubMed] [Google Scholar]
- Houdret N., Lamblin G., Scharfman A., Humbert P., Roussel P. Activation of bronchial mucin proteolysis by 4-aminophenylmercuric acetate and disulphide bond reducing agents. Biochim Biophys Acta. 1983 Jul 5;758(1):24–29. doi: 10.1016/0304-4165(83)90005-3. [DOI] [PubMed] [Google Scholar]
- Houdret N., Ramphal R., Scharfman A., Perini J. M., Filliat M., Lamblin G., Roussel P. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta. 1989 Jul 21;992(1):96–105. doi: 10.1016/0304-4165(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Kaizu T., Lyons S. A., Cross C. E., Jennings M. D., Last J. A. Composition of glycoproteins secreted by tracheal explants from various animal species. Comp Biochem Physiol B. 1979;62(3):195–200. doi: 10.1016/0305-0491(79)90199-8. [DOI] [PubMed] [Google Scholar]
- Kim K. C., Rearick J. I., Nettesheim P., Jetten A. M. Biochemical characterization of mucous glycoproteins synthesized and secreted by hamster tracheal epithelial cells in primary culture. J Biol Chem. 1985 Apr 10;260(7):4021–4027. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Wu R., Brody A. R., Barrett J. C., Nettesheim P. Growth and differentiation of hamster tracheal epithelial cells in culture. Exp Lung Res. 1984;6(1):27–45. doi: 10.3109/01902148409087893. [DOI] [PubMed] [Google Scholar]
- Leigh M. W., Cheng P. W., Boat T. F. Developmental changes of ferret tracheal mucin composition and biosynthesis. Biochemistry. 1989 Nov 28;28(24):9440–9446. doi: 10.1021/bi00450a029. [DOI] [PubMed] [Google Scholar]
- Leprat R., Michel-Briand Y. Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):209–222. [PubMed] [Google Scholar]
- Liao T. H., Blumenfeld O. O., Park S. S. Isolation and characterization of glycoproteins from canine tracheal pouch secretions. Biochim Biophys Acta. 1979 Apr 25;577(2):442–453. doi: 10.1016/0005-2795(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Lafitte J. J., Houdret N., Pruvot F. R., Lamblin G., Slayter H. S., Roussel P. Peptides of human bronchial mucus glycoproteins. Size determination by electron microscopy and by biosynthetic experiments. Biochem J. 1987 Nov 15;248(1):189–195. doi: 10.1042/bj2480189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L., Bhaskar K., Coles S. Control and modulation of airway epithelial cells and their secretions. Exp Lung Res. 1983 Feb;4(2):157–170. doi: 10.3109/01902148309055011. [DOI] [PubMed] [Google Scholar]
- Ringler N. J., Selvakumar R., Woodward H. D., Simet I. M., Bhavanandan V. P., Davidson E. A. Structure of canine tracheobronchial mucin glycoprotein. Biochemistry. 1987 Aug 25;26(17):5322–5328. doi: 10.1021/bi00391a016. [DOI] [PubMed] [Google Scholar]
- Rizzino A., Rizzino H., Sato G. Defined media and the determination of nutritional and hormonal requirements of mammalian cells in culture. Nutr Rev. 1979 Dec;37(12):369–378. doi: 10.1111/j.1753-4887.1979.tb06646.x. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Pritchett T. J., Lane J. L., Paulson J. C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983 Dec;131(2):394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Brown C. F., Jacoby J. Z., 3rd, Lynn W. S., Kaufman B. Biochemical properties of tracheobronchial mucins from cystic fibrosis and non-cystic fibrosis individuals. Pediatr Res. 1987 Nov;22(5):545–551. doi: 10.1203/00006450-198711000-00015. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Lynn W. S., Kaufman B. Resolution of the major components of human lung mucosal gel and their capabilities for reaggregation and gel formation. Biochemistry. 1979 Sep 4;18(18):4030–4037. doi: 10.1021/bi00585a029. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Brown C. F., Kaufman B. Structural features of human tracheobronchial mucus glycoprotein. Biochem J. 1984 Sep 1;222(2):371–377. doi: 10.1042/bj2220371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev G. P., Fox O. F., Wen G., Schroeder T., Elkins R. C., Carubelli R. Isolation and characterization of glycoproteins from canine tracheal mucus. Biochim Biophys Acta. 1978 Sep 26;536(1):184–196. doi: 10.1016/0005-2795(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell J. R., Jr, Chakrin L. W., Payne B. J. The canine tracheal pouch. A model for use in respiratory mucus research. Am Rev Respir Dis. 1970 May;101(5):741–754. doi: 10.1164/arrd.1970.101.5.741. [DOI] [PubMed] [Google Scholar]
- Wu R., Martin W. R., Robinson C. B., St George J. A., Plopper C. G., Kurland G., Last J. A., Cross C. E., McDonald R. J., Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol. 1990 Nov;3(5):467–478. doi: 10.1165/ajrcmb/3.5.467. [DOI] [PubMed] [Google Scholar]
- Wu R., Nolan E., Turner C. Expression of tracheal differentiated functions in serum-free hormone-supplemented medium. J Cell Physiol. 1985 Nov;125(2):167–181. doi: 10.1002/jcp.1041250202. [DOI] [PubMed] [Google Scholar]