Abstract
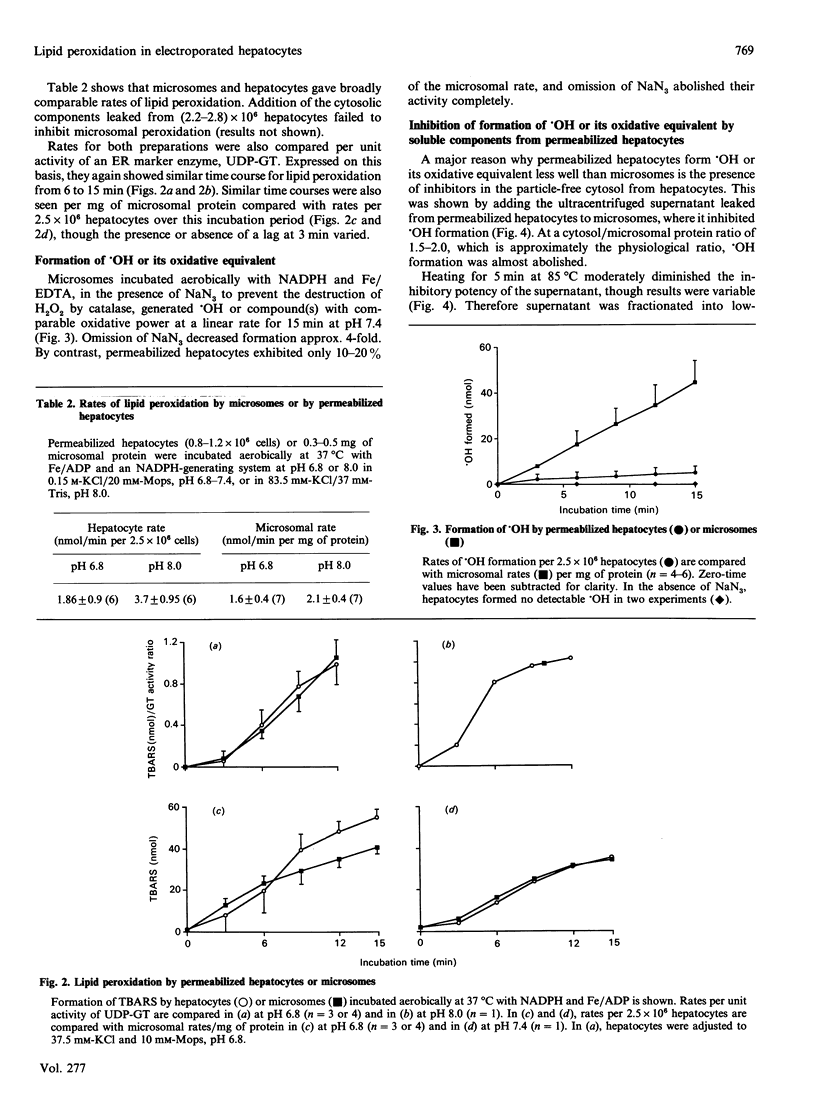
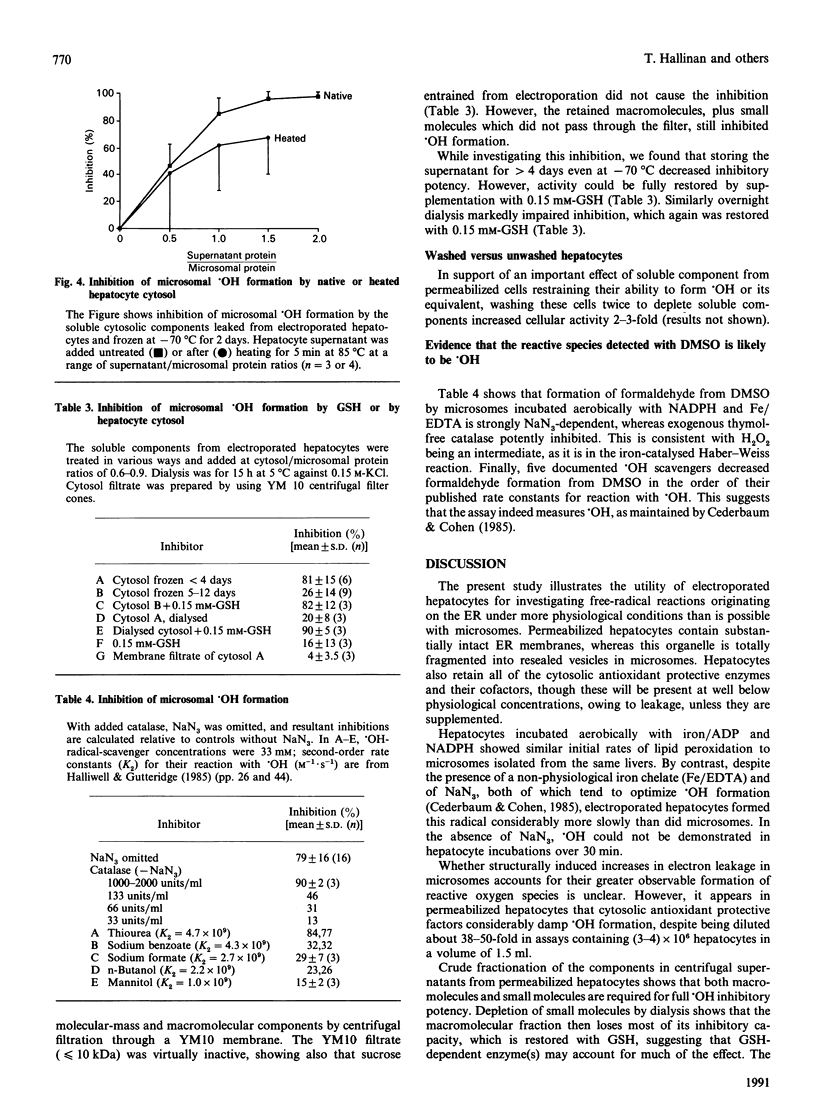
1. Rat hepatocytes suspended in 0.25 M-sucrose were electropermeabilized. This completely disrupted their plasma-membrane permeability barrier. 2. The endoplasmic reticulum in electroporated hepatocytes appeared morphologically preserved and maintained its permeability barrier as evidenced by electron-microscopic examination and latency measurements on luminal reticular enzymes. 3. Upon aerobic incubation with an NADPH-generating system and iron/ADP, porated hepatocytes peroxidized their membrane lipids at rates similar to those of matched microsomal preparations. 4. When hepatocytes were incubated with iron/EDTA and azide, radical formation detectable with dimethyl sulphoxide (DMSO) was only 10-20% that shown by microsomes. Omitting azide abolished hepatocyte reactivity with DMSO completely. Effects of hydroxyl-radical (.OH) scavengers and of added catalase suggest that the radical detected by DMSO is .OH. 5. Cytosolic inhibitor(s) from hepatocytes seemed to be a major factor limiting .OH formation. These were macromolecular, but showed a degree of heat-stability. Dialysis largely abolished inhibition, but this could be restored again by adding GSH. 6. Since .OH formation in hepatocytes seems to be much more stringently prevented than lipid peroxidation, free-radical damage originating from intracellular redox systems seems more likely to take the form of lipid peroxidation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J. Measurement of intactness of rat liver endoplasmic reticulum. Methods Enzymol. 1989;174:58–67. doi: 10.1016/0076-6879(89)74010-6. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- Bock K. W., van Ackeren G., Lorch F., Birke F. W. Metabolism of naphthalene to naphthalene dihydrodiol glucuronide in isolated hepatocytes and in liver microsomes. Biochem Pharmacol. 1976 Nov 1;25(21):2351–2356. doi: 10.1016/0006-2952(76)90027-7. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Debeer L. J., Mannaerts G. P. Role of glycerol 3-phosphate and glycerophosphate acyltransferase in the nutritional control of hepatic triacylglycerol synthesis. Biochem J. 1982 Apr 15;204(1):247–256. doi: 10.1042/bj2040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. B., Seglen P. O. Autophagic sequestration of [14C]sucrose, introduced into rat hepatocytes by reversible electro-permeabilization. Exp Cell Res. 1982 Nov;142(1):1–14. doi: 10.1016/0014-4827(82)90402-5. [DOI] [PubMed] [Google Scholar]
- Högberg J., Orrenius S., Larson R. E. Lipid peroxidation in isolated hepatocytes. Eur J Biochem. 1975 Jan 15;50(3):595–602. doi: 10.1111/j.1432-1033.1975.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Thor H., Andersson B., Orrenius S. Detoxification reactions in isolated hepatocytes. Role of glutathione peroxidase, catalase, and formaldehyde dehydrogenase in reactions relating to N-demethylation by the cytochrome P-450 system. J Biol Chem. 1978 Sep 10;253(17):6031–6037. [PubMed] [Google Scholar]
- Kamath S. A., Rubin E. Interaction of calcium with microsomes: a modified method for the rapid isolation of rat liver microsomes. Biochem Biophys Res Commun. 1972 Oct 6;49(1):52–59. doi: 10.1016/0006-291x(72)90008-3. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Elwell J. H., Oberley L. W. A simultaneous visualization of the antioxidant enzymes glutathione peroxidase and catalase on polyacrylamide gels. Free Radic Res Commun. 1988;5(2):67–75. doi: 10.3109/10715768809066913. [DOI] [PubMed] [Google Scholar]
- Wibo M., Amar-Costesec A., Berthet J., Beaufay H. Electron microscope examination of subcellular fractions. 3. Quantitative analysis of the microsomal fraction isolated from rat liver. J Cell Biol. 1971 Oct;51(1):52–71. doi: 10.1083/jcb.51.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]



