Abstract
Maternal smoking during pregnancy (MSDP), driven by nicotine crossing the placenta, causes lifelong decreases in offspring pulmonary function and vitamin C supplementation during pregnancy prevents some of those changes. We have also shown in animal models of prenatal nicotine exposure that vitamin C supplementation during pregnancy improves placental function. In this study we examined whether vitamin C supplementation mitigates the effects of MSDP on placental structure, function, and gene expression in pregnant human smokers. Doppler ultrasound was performed in a subset of 55 pregnant smokers participating in the “Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function” (VCSIP) randomized clinical trial (NCT01723696) and in 33 pregnant nonsmokers. Doppler ultrasound measurements showed decreased umbilical vein Doppler velocity (Vmax) in placebo-treated smokers that was significantly improved in smokers randomized to vitamin C, restoring to levels comparable to nonsmokers. RNA-sequencing demonstrated that vitamin C supplementation to pregnant smokers was associated with changes in mRNA expression in genes highly relevant to vascular and cardiac development, suggesting a potential mechanism for vitamin C supplementation in pregnant smokers to improve some aspects of offspring health.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73005-7.
Subject terms: Nutritional supplements, Paediatrics, Neonatology, Risk factors, Translational research, Outcomes research
Introduction
Despite strong smoking cessation efforts, over 50% of smokers who become pregnant will continue to smoke during pregnancy1,2. Maternal smoking during pregnancy (MSDP) affects the development of the placenta and multiple fetal organ systems including lung, brain, kidney and vasculature3–6. Offspring of women who smoked during pregnancy have decreased lung function caused by narrowing and increased branching of airways7,8. The vasculature of offspring of smokers is affected by increased intimal and medial thickness and they show modest increases in blood pressure and decreased renal glomerular filtration rates9–12.
Not surprisingly, MSDP also effects placental structure and function. Both animal studies and histologic analysis of placentas from pregnant human smokers show that nicotine accumulates in amniotic fluid and freely crosses the placental barrier13 where it can bind to nicotinic acetylcholine receptors expressed in multiple placental cell types14 to potentially alter placental development. MSDP is associated with increased connective tissue in the placenta and thickening of the trophoblastic basement membrane15. Doppler ultrasound (US) measurements in pregnant smokers show increased umbilical artery pulsatility index, suggesting increased placental vascular resistance, and decreased umbilical venous volume blood flow, consistent with reduced blood supply to the fetus3,16,17. Along with the decrease in placental function there is an increase in relative weight of placentas after correction for the intrauterine growth restriction (IUGR) associated with MSDP, such that the ratio of fetal weight to placental weight (F/P ratio) is decreased in offspring of smokers; suggesting a potential compensatory mechanism for a less robust placental unit that is growing larger to meet the oxygen demands of the fetus18,19.
In nonhuman primates (NHP), we have demonstrated that the negative effects of nicotine on offspring lung function are primarily caused by nicotine crossing the placenta to interact with nicotinic receptors that are highly expressed in fetal lung20. NHP exposed to nicotine in utero show decreases in pulmonary function similar to human infants exposed to tobacco smoke in utero, thus supporting the central role of nicotine21. This finding has been replicated in multiple other animal models as well22,23. In NHPs exposed to nicotine during pregnancy, we have also demonstrated that prenatal vitamin C supplementation mitigated the nicotine-induced changes in offspring pulmonary function24,25. This study led to two human double-blind, randomized controlled trials (RCTs) demonstrating significantly improved pulmonary function and decreased wheeze in the offspring of pregnant smokers randomized to vitamin C supplementation in pregnancy26,27.
We next examined the impact of prenatal nicotine exposure on placental function in NHP, as well as the potential protective effect of vitamin C supplementation. As we have previously reported25, prenatal nicotine exposure in NHP resulted in decreased placental blood flow that correlated with placental histological findings and this effect was partially prevented by vitamin C supplementation. Based on our NHP studies, we hypothesized that placental hemodynamics would be similarly improved in human smokers who were given supplemental vitamin C during pregnancy.
To test this hypothesis, we conducted an ancillary study in a subset of participants in the “Vitamin C to Decrease Effects of Smoking in Pregnancy on Infant Lung Function” (VCSIP; NCT#01723696) RCT, using Doppler-US to quantify the effect of vitamin C supplementation on measures of placental hemodynamics. Informed by our prior NHP studies, we chose to assess the umbilical artery pulsatility index (UAPI) as an indicator of vascular impedance, umbilical vein doppler velocity (Vmax) and the calculated quantification of umbilical vein blood volume (cQuv) as a comprehensive measure that accounts for both blood velocity and vessel lumen size. In addition, to better understand the underlying mechanisms for the protective effect of supplemental vitamin C on placental function in MSDP, we also performed placental transcriptome profiling to identify changes in gene expression associated with vitamin C supplementation.
Results
Figure 1 shows the overall design of the study and Table 1 presents the maternal and infant demographic and birth statistics per group (nonsmoker, placebo supplemented-smoker, vitamin C supplemented-smoker) for the 88 subjects who underwent ultrasound assessment. As expected, fasting plasma ascorbic acid (AA) levels were increased in vitamin C-supplemented smokers versus placebo at mid- and late-gestation. Maternal body mass index (BMI) at enrollment was also higher in the placebo group (p = 0.02). Tobacco exposure as measured by self-report and maternal cotinine were similar between the randomized-smoking groups across pregnancy. The demographics for this subpopulation did not differ significantly from the previously published demographics of the entire study population28.
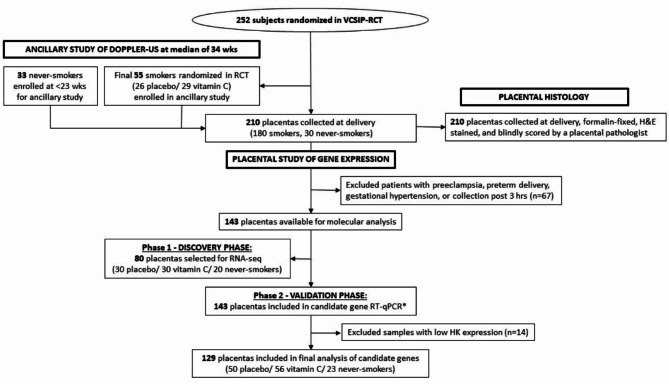
Fig. 1.
Consort diagram and study design. Pregnant smokers were randomized to vitamin C or placebo at less than 23 weeks of gestation as part of a clinical trial to evaluate impact on offspring lung function. Non-smoking subjects were recruited as part of an ancillary study to perform doppler ultrasound. Placentas were collected at delivery from 210 subjects for histology and gene expression analysis. Samples with pregnancy-related complications and poor-quality control metrics were excluded from molecular analysis.
Table 1.
Population characteristics of mothers and infants with completed doppler ultrasound.
| Nonsmokers (n=33) | Placebo-treated smokers (n=26) | Vitamin C-treated smokers (n=29) | p-value vitamin C vs. placebo | |
|---|---|---|---|---|
| Maternal characteristics at randomization | ||||
| Age at enrollment, median (IQR), years | 32 (29–33) | 24 (22.25–32) | 28 (21–32) | 0.953 |
| Race, n white (%) | 30 (90.9) | 21 (80.8) | 27 (93.1) | 0.308 |
| Gravida, median (IQR) | 2 (1–3) | 2.5 (1–4.8) | 3 (1–3) | 0.753 |
| Body mass index at enrollment, mean (SE) kg/m2 | 27.83 (1.01) | 30.78 (1.37) | 26.71 (0.79) | 0.020 |
| History of asthma, n (%) | 5 (15.2) | 5 (19.2) | 10 (34.5) | 0.335 |
| Private health insurance, n (%) | 31 (93.9) | 5 (19.2) | 4 (13.8) | 0.471 |
| Gestational age at randomization, median (IQR), weeks | NA | 17.75 (14.8–19.53) | 17.9 (14.7–19.4) | 0.627 |
| Maternal cigarettes/day during pregnancy, median (IQR) | NA | 8 (4.25–10) | 8 (5–10) | 0.898 |
| Urine cotinine at randomization, median (IQR), ng/mL | Not detectable | 5197 (1885–8302) | 5418 (1872–7278) | 0.959 |
| Plasma ascorbic acid at randomization, mean (SE), µmol/L | Not measured | 41.9 (3.1) | 46.2 (3.3) | 0.416 |
| Plasma ascorbic acid at mid-gestation mean (SE), µmol/L | Not measured | 32.2 (2.8) | 57.9 (4.3) | <0.001 |
| Plasma ascorbic acid at late-gestation mean (SE), µmol/L | Not measured | 33.47 (3.1) | 45.7 (3.9) | 0.016 |
| Patient characteristics at Doppler ultrasound | ||||
| Time from last cigarette, median (IQR), h | NA | 1.3 (0.58–1.41) | 1.11 (0.53–1.46) | 0.454 |
| Gestational age at ultrasound, median (IQR), weeks | 35 (33.4–36.2) | 32.8 (32.3–34.3) | 34.2 (32.3–35.2) | 0.423 |
| Birth characteristics of infants | ||||
| Birth weight, mean (SE), g | 3571.8 (76.3) | 3175.9 (97.1) | 3142.4 (105.2) | 0.958 |
| Placental weight, mean (SE), g | Not measured | 584.45 (142.75) | 612.19 (119.88) | 0.551 |
| Gestational age at birth, median (IQR), weeks | 39.6 (39.1-40.0) | 39.1 (38.3-39.6) | 38.6 (37.3-39.6) | 0.140 |
| Female, n (%) | 19 (57.6) | 12 (46.2) | 11 (37.9) | 0.731 |
| Preterm, <37 weeks gestation, n (%) | 0 (0) | 0 (0) | 5 (17.23) | 0.080 |
Significant values are in bold.
Normally distributed variables are expressed as mean and standard error and compared for group differences using an unadjusted F-test. Non-normally distributed variables are expressed as median and interquartile range (25th–75th percentile) and the Wilcoxon rank sum test was used to compare groups. Chi-square test was used to compare categorical variables.
Third trimester doppler ultrasound (Doppler-US)
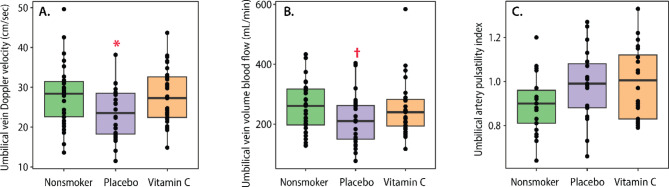
Mean umbilical vein Doppler velocity was significantly decreased in placebo-treated smokers compared to nonsmokers and vitamin C during pregnancy restored levels to that of nonsmokers (Fig. 2A; Table 2). Mean umbilical vein volume blood flow (cQUV) trended downward in placebo-treated smokers compared to nonsmokers (209.4 ± 14.6 vs. 254.9) and was partially restored in smokers randomized to vitamin C compared to nonsmokers (249.2 ± 16.0 vs. 254.9 ± 14.2 mL/min), (Fig. 2B; Table 2). Mean umbilical artery pulsatility index (PI) trended upward in both placebo- supplemented and vitamin C-supplemented smokers compared to nonsmokers and was not decreased by vitamin C supplementation (Fig. 2C; Table 2). There were no significant differences between groups in terms of gestational age at ultrasound, intraabdominal umbilical vein diameter, intraabdominal umbilical vein cross sectional area, or fetal heart rate (Tables 1 and 2).
Fig. 2.
Comparison of Doppler ultrasound measures. Boxplots show effects by treatment group on (A) Umbilical vein Doppler velocity; (B) Umbilical vein volume blood flow; (C) Umbilical artery pulsatility index. Plots show median and interquartile ranges. * p < 0.05 compared to nonsmokers and vitamin-C treated groups, † p < 0.09 compared to vitamin-C treated group by F-test using mixed linear model per measurements adjusted for gestational age at ultrasound.
Table 2.
Doppler ultrasound measurements of fetal and placental hemodynamics.
| Nonsmokers (n=33) | Placebo-treated smokers (n=26) | Vitamin C-treated smokers (n=29) | p-value nonsmoker vs. placebo | p-value nonsmoker vs. vitaminC | p-value vitamin C vs. placebo | |
|---|---|---|---|---|---|---|
| Umbilical vein Doppler velocity (Vmax), cm/s | 28.00 ± 1.31 | 23.49 ± 1.10 | 27.88 ± 1.21 | 0.05 | 0.92 | 0.04 |
| Umbilical vein volume blood flow (cQUV), mL/min | 254.9 ± 14.2 | 209.4 ± 14.6 | 249.2 ± 16.0 | 0.17 | 0.71 | 0.09 |
| Umbilical artery pulsatility index (UAPI) | 0.90 ± 0.02 | 0.99 ± 0.03 | 0.99 ± 0.03 | 0.07 | 0.05 | 0.95 |
| IAUV—intraabdominal umbilical vein diameter (IAUV), cm | 0.62 ± 0.02 | 0.61 ± 0.02 | 0.61 ± 0.01 | 0.85 | 0.78 | 0.93 |
| Cross sectional area of the umbilical vein (CSA), cm2 | 0.31 ± 0.02 | 0.30 ± 0.02 | 0.30 ± 0.01 | 0.83 | 0.75 | 0.94 |
| Fetal heart rate (FHR), beats/min | 143.0 ± 1.6 | 137.3 ± 1.9 | 141.3 ± 2.0 | 0.02 | 0.59 | 0.06 |
Data shown as mean ± SE. CSA calculated as π(UV diameter (cm)/2)2; cQUV calculated as 0.5 × Vmax × CSA × 60. P-values for F-test using mixed linear model per measurement adjusted for gestational age at ultrasound and BMI. Logarithmic transformation were performed for the following measurements to reduce skewness of residuals: IAUV, CSA, cQUV, and FHR.
Placental histology
The standard “Amsterdam criteria”29 was used to evaluate and score for a comprehensive range of potential placental pathology including chorangiosis (placental villous capillary growth). In general, the placentas from the two randomized-smoking groups had more histologic abnormalities than the placentas from the non-smoking group (Supplementary Table S1). However, the histologic analysis did not show any meaningful differences between the placebo-supplemented and vitamin C-supplemented subjects other than 2 cases of acute chorioamnionitis in the vitamin C-supplemented group (Supplementary Table S1). Differences were not detected between area and intensity of trichrome staining in placental villi or blood vessels between vitamin-C treated subjects and smokers, though the area of trichrome staining surrounding blood vessels was increased in the non-smoker group (data not shown).
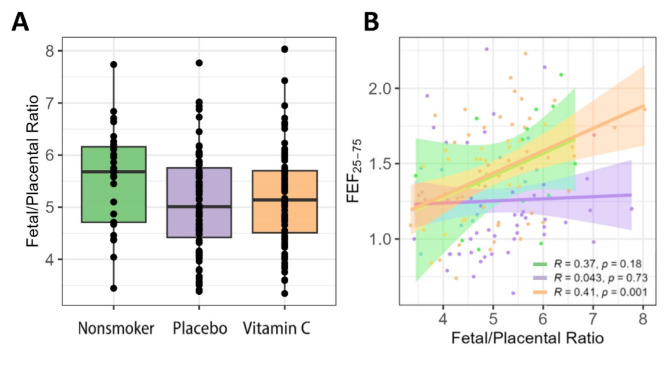
While there was only a slight increase in the fetal/placental weight ratio (F/P ratio) in smokers randomized to vitamin-C treatment compared to placebo, there was a significant correlation between offspring pulmonary function at 5 years of age and the F/P ratio in the nonsmoker and vitamin-C treated groups that was absent in the placebo-treated group (Fig. 3).
Fig. 3.
Fetal/placental weight ratio by group and correlation with pulmonary function at 5 years of age. (A) Boxplot shows the distribution by treatment group. (B) Scatterplot shows the Pearson correlation between fetal/placental weight ratio and FEF25 − 75 measured at 5-year follow-up visit for each treatment group. The correlation was significant in the vitamin C treated smokers (orange) with a similar trend in nonsmokers (green), however this relationship was not observed in placebo treated smokers (purple).
RNA-seq differential gene expression
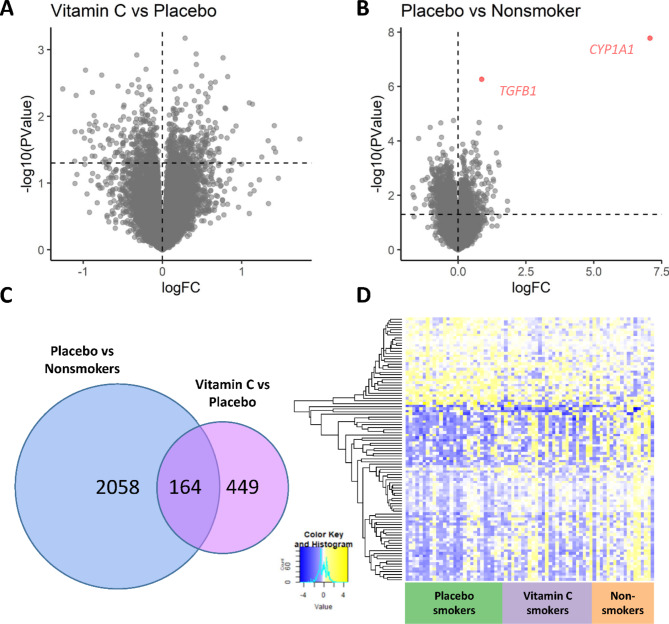
Eighty placental samples were selected for RNA-sequencing using a blocked randomization design described in the methods. Seven samples were removed from analysis as outliers based on correlation of PCs with seqQC metrics, suggesting technical problems with the libraries (3 placebo, 2 vitamin C, and 2 nonsmokers). The primary analysis compared placental expression in pregnant smokers randomized to placebo (n = 27) versus vitamin C-supplemented pregnant smokers (n = 28), adjusted for batch, infant sex, and cell type PC1 (Supplemental Materials). Out of 19,927 genes included in analysis, 613 were nominally significant between randomization groups (Fig. 4A; p < 0.05), of which 313 were up-regulated and 300 down-regulated in the placebo-smokers versus the vitamin C-smokers. Two genes reached FDR significance in the comparison of placebo supplemented-smokers to nonsmokers: CYP1A1 and TGFB1 (both upregulated), and 2222 genes were nominally significant (Fig. 4B; p < 0.05; 838 up-regulated; 1384 down-regulated in placebo group). The overlap of nominally significant genes (n = 164) between the two comparisons (placebo vs. vitamin C-supplemented smokers; placebo-supplemented smokers vs. nonsmokers) is illustrated in Fig. 4C. Out of 164 genes overlapping between the two comparisons, 160 were normalized with vitamin C supplementation by at least 40% toward the level of nonsmokers (Fig. 4D). The complete table of differential expression is shown in Supplementary Table S2.
Fig. 4.
RNA-sequencing results of placentas. Volcano plots show difference in log fold change (log FC) versus –log10 p-value in vitamin C vs. placebo (A) and placebo vs. nonsmokers (B) Lines at y = 1.3 are equivalent to nominal p = 0.05. (C) Venn-diagram shows overlap of nominally significant changes in expression between groups. (D) Heatmap for overlapping set of 164 genes shown in C with nominally significant differential expression in placentas from smokers randomized to placebo vs. nonsmokers and in placentas from placebo vs. vitamin C-treated smokers. Columns represent individual subjects and rows represent individual genes. The mean direction of effect in 160/164 overlapping genes was reversed with vitamin C.
Functional and pathway enrichment analyses
ConsensusPathDB analysis of the 164 normalized genes identified “circulatory system development “, “blood vessel morphogenesis”, “vascular development”, “cardiovascular system development”, and “cellular response to toxic substance” as the top 5 Gene Ontology (GO) terms (Table 3). Qiagen Ingenuity Pathway Analysis of all nominally significant DEGs between placebo and vitamin C-supplemented groups, predicted “Nerve growth factor (NGF)-stimulated transcription” as the top canonical pathway and we list all 52 canonical pathways with a significant activation score in Supplementary Table S3. A total of 2050 upstream regulators were predicted (Supplementary Table S4), including 99 targets downstream of lipopolysaccharide.
Table 3.
Top 10 enriched GO terms from ConsensusPathDB.
| Gene Ontology (GO) description | q-value |
|---|---|
| Circulatory system development | 0.002 |
| Blood vessel morphogenesis | 0.002 |
| Vasculature development | 0.002 |
| Cardiovascular system development | 0.002 |
| Cellular response to toxic substance | 0.003 |
| MAPK cascade | 0.008 |
| Atrial septum development | 0.012 |
| Drug transport | 0.012 |
| Negative regulation of cellular process | 0.012 |
| Negative regulation of signaling | 0.014 |
Input to analysis included 164 genes nominally significant in comparison of placebo vs. never-smokers and placebo vs. vitamin C; q-value = p-value corrected for multiple testing using the false discovery rate method.
Fluidigm RT-qPCR validation of candidate genes
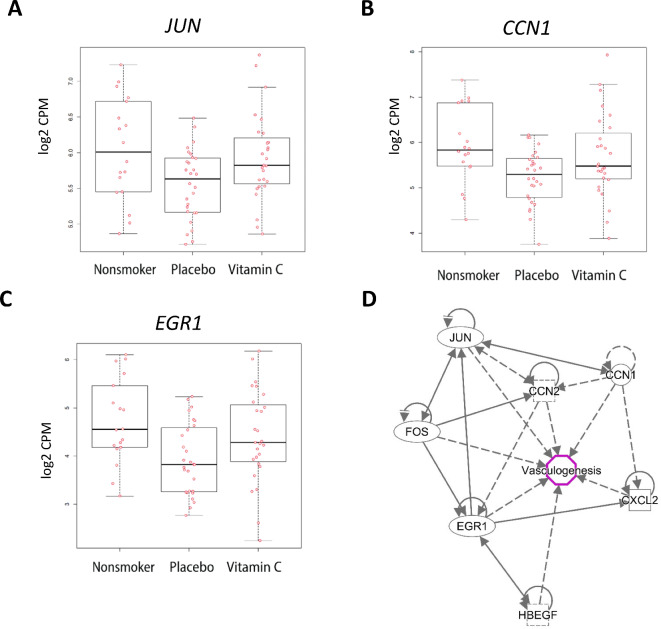
All placentas passing quality control metrics from the parent RCT and from consented nonsmokers (Fig. 1) were included in the validation of candidate genes by RT-qPCR. The characteristics of this subset (n = 129) are provided in Supplementary Table S7, and the primers used for the Fluidigm amplification in Supplementary Table S8. Out of 20 candidate genes measured by RT-qPCR, 8 were validated as differentially expressed (nominal p < 0.05 and consistent direction of effect) between randomized treatment groups (Supplementary Tables S5, S6). Figure 5 shows expression boxplots for 3 candidate genes (JUN, CCN1, and EGR1), as well as the interaction network of validated genes.
Fig. 5.
Top differentially expressed genes validated by qPCR. (A–C) Boxplots for top 3 genes with significant differential expression in placentas from smokers randomized to placebo vs. nonsmokers and in placentas from placebo- or vitamin C-treated smokers validated by qPCR. (D) IPA analysis of nominally significant overlap identified enrichment of canonical signaling related to vasculogenesis. All genes shown were significantly upregulated in vitamin C supplemented smokers versus placebo and in never smokers vs. placebo.
Discussion
This study demonstrates that supplementation with vitamin C (500 mg/day) to pregnant smokers results in umbilical vein Doppler blood flows comparable to nonsmokers. Multiple studies have established that MSDP, as well as prenatal nicotine exposure alone, are associated with decreased placental vascular function as assessed by Doppler-US and abnormal placental histology3,16,30. Our results in this clinical population are consistent with our prior observation in NHPs that vitamin C supplementation mitigated nicotine-induced detrimental changes in placental hemodynamics and correlated with improved offspring lung function24,25. In other studies using animal models, supplemental vitamin C appears to increase the velocity of airway flows by preventing nicotine-induced alterations in airway geometry22,24,31; thus, vitamin C may act similarly in the placenta by preventing alterations in vascular geometry.
Histological studies on placentas of smokers have reported increased thickness of the villous membrane, increased collagen deposition, decreased vascularization of the placental bed, as well as reduced intervillous area and capillary volume15. These changes in the placenta are similar to changes observed in the aorta and other blood vessels in offspring of smokers30,32, suggesting that the mechanism by which vitamin C improves placental blood flow in smokers may resemble the widespread effects of MSDP on pulmonary and cardiovascular development. While we did observe more histologic abnormalities in the placentas from the two randomized groups of smokers, there was little difference observed between the placebo and vitamin C-supplemented groups. Only minor differences in trichrome staining intensity was seen in the placentas from nonsmokers compared to smokers, perhaps representing our limited sample size, or the fact that average smoking intensity has decreased over time33,34. Because of the small differences between the two randomized groups of smokers, further stereometric analysis was not indicated for this study.
Consistent with the findings of decreased vascularization on placental histology and increased umbilical artery PI in both smoking groups combined, by Doppler ultrasound the fetal-side placental blood flow (cQUV) was lower in smokers compared to nonsmokers and smokers randomized to vitamin C supplementation. It is important to note however that we did not see a significant difference in birth weight wit vitamin C supplantation which suggests that while smoking may reduce placental blood flow and oxygen delivery to the fetus, there are likely other contributing factors affecting infant birth weight.
A prospective study of Doppler waveforms at term, reported increased pulsatility index (PI) and resistance in the umbilical artery measurements of pregnant smokers that negatively correlated with infant birth weight35. In this study, we saw no difference in umbilical artery PI between randomized treatment groups. However, umbilical artery PI was increased in both smoking groups (vitamin C-supplemented smokers and placebo-supplemented smokers) combined versus nonsmokers and was negatively correlated with infant birthweight (data not shown). This suggests that the protective effects of vitamin C on placental blood flow may be acting independently of the effects of smoking on the umbilical artery PI. As the umbilical artery PI is clinically used as a measure of placental vascular resistance, this greater effect on placental blood flow than umbilical artery PI mirrors the effects of vitamin C supplementation on lung development and function in which vitamin C significantly increases forced airway expiratory flows, but has less effect on airway resistance11,24.
There is also an interesting potential interaction between vitamin C and fetal-placental weight ratio (F/P) on offspring airway function (Fig. 3). Multiple studies have demonstrated a decreased F/P weight ratio in smokers18,19. While the underlying etiology for the observed increased placental weight associated with smoking is unclear, it may represent an adaptive placental response to the decreased placental functionality. Thus, in placebo-supplemented smokers a larger placenta does not correlate with improved placental function, and we saw no correlation of the F/P weight ratio with offspring pulmonary function in the placebo-supplemented smokers. However, in the vitamin C-supplemented smokers and nonsmokers, there was a clear correlation between F/P ratio and offspring pulmonary function (Fig. 3) suggesting that vitamin C might be restoring an optimal balance between placental function and placental weight. This is consistent with the well-established link between lung development and placental development36,37, though the finding reported here needs further investigation.
Placental expression analysis by RNA-sequencing suggests normalized expression of genes involved in vasculogenesis, endothelial tissue development, and response to growth factors as potential mechanisms. The expression of genes critical to these processes was confirmed by RT-qPCR. Of note, vitamin C supplementation was associated with decreased expression of SLC23A2 (solute carrier family 23 member 2), which encodes one of two vitamin C transporter proteins and is important for ascorbic acid transport across the placenta38. We also observed normalized expression of two members of the CCN family of secreted matricellular proteins, named for the first family members discovered: CYR61 (cysteine-rich protein 61), CTGF (connective tissue growth factor), and NOV (nephroblastoma overexpressed gene). CYR61 and CTGF are immediate early genes, a class of genes that are rapidly inducible in response to growth factors39. CYR61 induces angiogenesis in fibroblasts and endothelial cells through interaction with integrin receptors40 and Cyr61-null mice exhibit placental vascular insufficiency and compromised vessel integrity41. In our study, vitamin C also normalized mean expression of FOS (Fos proto-oncogene) and JUN (Jun proto-oncogene), to the mean level of nonsmokers. In agreement with our results, vitamin C increased mRNA expression of c-jun and c-fos in HL-60 cells induced to differentiation by 1α,25(OH)−dihydroxyvitamin D342. Vitamin C also normalized levels of heparin binding EGF like growth factor (HBEGF) and HB-EGF has been reported to play a role in preventing oxidative stress-mediated uterine decidual damage43.
Comparison of smokers to nonsmokers revealed an increase in placental cytochrome P450 (CYP) enzyme (CYP1A1) expression that was not improved with vitamin C supplementation. Notably, elevated CYP1A1 activity in placentas of smokers has been associated with several adverse health outcomes including preterm delivery, intrauterine growth restriction, and lower infant birth weight44. Placental induction of CYP1A1 in response to cigarette smoke is epigenetically regulated and hypomethylation of the CYP1A1 promoter may serve as an epigenetic record of exposure to maternal smoke that is irreversible45–47. We also observed a small but genome-wide significant increase in transforming growth factor-beta (TGFB1) in smokers versus nonsmokers, in contrast to what has been reported previously48. TGF-β regulates proliferation and differentiation of placental trophoblast cells, in addition to regulating placental steroidogenesis49. The role of TGF-β signaling in vascular development is complex and both pro-angiogenic and anti-angiogenic properties have been described50. Our previous study of placental DNA methylation in this RCT cohort also identified epigenetic dysregulation of TGFB1 and CYP1A1 expression51.
Vitamin C has been shown to reduce blood pressure, especially in patients with hypertension and low plasma levels of vitamin C at baseline. The proposed mechanisms for vitamin C to decrease hypertension include improvement of arterial compliance, redox status and endothelial dysfunction, increased nitric oxide and prostaglandin I2, and reduced leukotrienes and lipids52. In our study, nitric oxide synthase 3 (NOS3) was significantly increased in placentas randomized to placebo-supplementation versus nonsmokers but was not restored by vitamin C supplementation in our wider qPCR analysis. These results agree with our observation that vitamin C supplementation did not normalize umbilical artery PI.
Our study had three major strengths. First, our study population was nested within a double blind, placebo-controlled RCT of vitamin C supplementation (500 mg/day) to pregnant smokers with extensive phenotypic and clinical data that demonstrated significantly increased airway function through 5 years of age. Second, our study used whole-genome transcriptome profiling with RNA-seq to identify changes in placental gene expression, which allows for unbiased discovery of enriched molecular pathways and has not been previously performed. Third, we validated a subset of genes relevant to vascular biology from the discovery phase using qPCR in the larger RCT population.
The major limitation to our study was the relatively small sample size of subjects who underwent Doppler ultrasounds as part of this ancillary clinical study. However, we were still able to demonstrate a significant increase in umbilical vein Doppler velocity in addition to other altered Doppler-US values. Given our sample size, we did not exclude patients from undergoing Doppler-US because of pregnancy complications and ultimately 17% (n = 5) of the vitamin C versus none of the placebo-supplemented smokers delivered preterm, although removing patients who delivered preterm would have further increased the vitamin C effect size on cQUV. Another limitation of the study is that maternal side placental blood flow was not included due to the challenge of achieving rigorous and reproducible doppler measurements in the third trimester, which would have provided additional insight into placental function. We would however expect to have similar findings to a prior study where we performed both maternal and fetal placental doppler measurements to examine the impact of prenatal alcohol53. In that study, we observed that uterine artery doppler blood flow followed a similar trend as the umbilical artery doppler blood flow measurements between alcohol-exposed and control placentas.
In conclusion, our study demonstrates that supplementation of vitamin C to pregnant smokers has the potential to improve placental hemodynamics. The umbilical venous flow directly contributes to the amount of oxygen and nutrients that reach the fetus54, suggesting that vitamin C supplementation to pregnant smokers may lessen the deleterious actions of MSDP by increasing blood supply to the fetus. The potential mechanisms for the observed effects of vitamin C in increasing placental volume blood flow in parallel with changes in offspring pulmonary function may include amelioration of altered vascular geometry associated with nicotine exposure. Pathway analysis of RNA-sequencing results suggest activation of growth factor signaling cascades, blood vessel morphogenesis, and cardiovascular system development. These findings are in line with our previous study of restored placental DNA methylation in this RCT at loci associated with infant lung function51. Future pragmatic clinical studies are needed to confirm these findings in actual obstetrical practice to determine the extent to which vitamin C supplementation for pregnant smokers should be included in standard practice55.
While the clinical impact of the observed placental function changes on fetal development and obstetric outcome is unknown, this finding, combined with the transcriptomic results, suggests that perturbations to fetal hemodynamics by MSDP may be ameliorated by vitamin C supplementation. This supports the findings of our prior studies highlighting the benefits of vitamin C supplementation to mitigate some aspects of offspring morbidity, particularly respiratory morbidity, in pregnancies where abstaining from nicotine products is not feasible. It is important to note however that there is no data to suggest that vitamin C supplementation will affect the majority of effects of smoking on fetal development such as prematurity, neural development, and overall somatic growth.
Materials and methods
Study design
This study was nested within a multi-center, double-blind RCT which demonstrated improved airway function at 3, 12 and 60 months of age in offspring whose mothers were randomized to vitamin C (500 mg/day) versus placebo27,28,56. Recruitment of participants into the parent study began in December 2012 and ended in December 2015. All subjects randomized after August 2014 were consented into an ancillary study to undergo Doppler-US. A group of 33 pregnant nonsmokers were enrolled as a reference group and followed prospectively according to a modified protocol similar to the randomized pregnant smokers (Fig. 1). The study of gene expression was divided into 2 phases: (1) the discovery phase that included total RNA-sequencing of 80 stratified, randomly sampled placental samples and (2) the validation phase of expression of 20 candidate genes by RT-qPCR from all available placental samples meeting inclusion criteria.
Study population
The parent RCT recruited women with singleton pregnancies (≥ 15 years old; between 130/7 and < 23 weeks gestation at randomization) with a history of current smoking and documented refusal/inability to quit. Inclusion criteria included current cigarette smoking of at least 1 cigarette in the last week, collected at randomization. Women were randomized to receive supplemental vitamin C versus placebo in rotations of two and four subjects, and stratified by gestational age at randomization (≤ 18 versus > 18 weeks) and site (Oregon Health & Science University [OHSU], Portland, Oregon; PeaceHealth Southwest Medical Center [SWW], Vancouver, Washington; Indiana University [IU], Indianapolis, Indiana). Both randomized groups received standard prenatal vitamin with minimum daily requirements of vitamin C. A total of 252 pregnant smokers were randomized and 243 infants were available for study at delivery.
The RCT was approved by each site’s Institutional Review Board (OHSU Institutional Review Board and Indiana University Institutional Review Board) and the study itself was monitored by an NIH appointed Data Safety Monitoring Board (Vitamins for Early Lung Health (VITEL)). We obtained written informed consent from all subjects prior to enrollment57. All methods and procedures were performed in accordance with the relevant guidelines and regulations.
Third trimester doppler ultrasound (Doppler-US)
At a median of 34 weeks of gestation, a sonographer blinded to the treatment group performed Doppler-US using image-directed pulsed and color Doppler equipment (Phillips & GE Voluson). The lowest high-pass filter was used and an angle of 30 degrees or less between the Doppler beam was deemed acceptable58. Doppler waveform measurements in 55 randomized smokers and in 33 pregnant nonsmokers were obtained from the straight portion of the intraabdominal umbilical vein (IAUV) as described previously25,59,60. The cross-sectional area (CSA) of the IAUV was calculated as CSA = π (diameter/2)2, and Vmean was calculated as 0.5 × Vmax. The umbilical vein volume blood flow was calculated as Vmean × CSA × 6054,61. The umbilical artery waveform was measured in a free-floating loop of the mid-umbilical cord using machine-specific software to obtain the pulsatility index (PI) and the fetal heart rate62. An obstetrician and a Maternal-Fetal Medicine subspecialist (A.E.F.) blinded to treatment group reviewed all ultrasound images. For each measurement, three consecutive uniform wave-forms were recorded and the mean of three measurements was used for further analysis.
Placental samples
Following delivery, placentas were collected and processed within 3 h of delivery by trained research staff using a standardized protocol57. Fresh placentas were weighed after trimming off the cord and measured (length, width, and average thickness), positioned fetal side up, and fetal membranes folded around the edge. Four quadrants were mapped out visually and cores collected from each quadrant in a radial pattern within 4 cm of the cord insertion site using a 1 cm punch, avoiding major vessels. Each core was rinsed in saline to remove maternal blood, trimmed of maternal decidua and fetal membranes, and cut lengthwise to yield 8 villous samples total. Four samples were formalin-fixed for histological analyses (one per quadrant), 2 were collected into RNAlater to provide RNA/DNA, and 2 were flash frozen. For molecular analyses one RNAlater treated sample per subject was submerged in liquid nitrogen in a pre-chilled mortar and pestle and ground into a fine powder. We simultaneously extracted RNA, miRNA, and DNA from placental powder using the Qiagen AllPrep Universal Kit and the QIAcube for automated nucleic acid extraction (Qiagen, USA). Fixed placental tissue was hematoxylin and eosin (H&E), and Mallory trichrome-stained, and scored by a board-certified placental pathologist (T.K.M.), blinded to treatment group, according to Amsterdam criteria29. All placental samples were reviewed three times to ensure that the placental findings were reproducible.
Cell type deconvolution
Bulk RNA-seq represents a convolution of gene expression from a mixture of cell types. We therefore used a publicly available cell type deconvolution reference signature matrix, developed using single-cell sequencing of healthy term placental samples, to estimate proportions of 19 fetal cell types and 8 maternal cells63. The signature matrix and our mixture file were input to CIBERSORTx with default settings and 1000 permutations to evaluate imputation goodness-of-fit. We then performed principal component analysis of estimated cell proportions, and determined that principal component 1 (PC1) represented 98.39% of variance63.
RNA-seq differential expression analysis
For molecular analyses, we excluded subjects with gestational hypertension, preeclampsia, and preterm delivery (< 37 weeks), and placentas sampled greater than 3 h after delivery (Fig. 1). We used a blocked randomization design to randomly select initial placental RNA samples for sequencing with 10 blocks of 8 (3 placebo, 3 vitamin C, 2 nonsmokers). We constructed a model representing the study design (~ group + batch + sex + cell PC1) and used glmQLFit64 to fit the negative binomial generalized linear model for each gene. Genes with nominally significant differential expression were included in functional and pathway enrichment analyses using ConsensusPathDB65 and Ingenuity Pathway Analysis (Qiagen Inc., MD, USA)66.
Fluidigm biomark gene expression by RT-qPCR
We performed candidate gene validation of RNA-seq results by RT-qPCR using the Fluidigm Biomark system and 96.96 Dynamic Array IFCs (Fluidigm, CA, USA) according to the Fluidigm Delta Gene Assay protocol. We calculated delta Ct (∆Ct) for each sample and assay (target): ∆Ct = mean Ct of target − mean Ct of housekeeping genes (combined mean Ct from RPL19, TOP1).
Statistical analysis
For comparison of Doppler measurements between groups, we followed a pre-defined statistical design to give 80% power at level 0.05 to detect a difference of 0.12 in umbilical artery PI between pairs of groups. To achieve this we projected to enroll 33 subjects for each of the three groups (nonsmokers, smokers randomized to placebo, and smokers randomized to vitamin C). Assuming a drop-out rate of 2.4% from randomization until the 34 week ultrasound, this would yield 32 subjects per group and give 80% power at level 0.05 to detect a difference of 0.12 in umbilical artery PI between pairs of groups based on SD’s of 0.09 and 0.2267 We constructed linear regression models for each measurement adjusted for gestational age at ultrasound (GAUS) and maternal BMI as continuous values. Normality was assessed for each measurement using the Shapiro–Wilks test and logarithmic transformations performed as appropriate.
For demographic variables presented in Table 1, normally distributed variables were expressed as mean and standard error and compared for group differences using an unadjusted F-test. Non-normally distributed variables were expressed as median and interquartile range (25th–75th percentile), and the Wilcoxon rank sum test was used to compare groups. Chi-square test was used to compare categorical variables. Significance was defined as p < 0.05. We performed all statistical analyses using “EdgeR,” “stats”, “car”, and “emmeans” packages in R version 3.6.1. Due to our limited sample size, we did not adjust for multiple testing and considered a nominal p < 0.05 as significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the women and their infants who participated in the VCSIP RCT to make this study possible. We also thank all VCSIP team members who contributed to clinical data and sample collection.
Author contributions
Conception and design: L.E.S., C.T.M., R.S.T., T.K.M., V.H.R., J.O.L., A.E.F., D.M.H., B.P., L.G., A.V., C.D.M., E.R.S. Acquisition, analysis or interpretation of data: all authors. Drafting of manuscript: L.E.S., C.T.M., R.S.T., T.K.M., V.H.R., J.O.L., A.E.F., D.M.H., B.P., L.G., A.V., C.D.M., E.R.S.
Funding
Supported by the NHLBI (R01 HL105447 and R01 HL 105460) with co-funding from the Office of Dietary Supplements (ODS) and by P51 OD0110925 and NIH grant UH3 OD023288 from the Environmental influences on Child Health Outcomes Program. Additional support from the Oregon Clinical Translational Research Institute funded by the National Center for Advancing Translational Sciences (UL1TR000128).
Data availability
The raw and processed RNAseq data is publicly accessible through the NCBI Gene Expression Omnibus (GEO) via accession series GSE253158.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tong, V. T. et al. Trends in smoking before, during, and after pregnancy—pregnancy risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 62, 1–19 (2013). [PubMed] [Google Scholar]
- 2.Lange, S., Probst, C., Rehm, J. & Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 6, e769–e776 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Rizzo, G., Capponi, A., Pietrolucci, M. E. & Arduini, D. Effects of maternal cigarette smoking on placental volume and vascularization measured by 3-dimensional power Doppler ultrasonography at 11 + 0 to 13 + 6 weeks of gestation. Am. J. Obstet. Gynecol. 200, 415e1–415e5 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Block, D. B., Mesquita, F. F., de Lima, I. P., Boer, P. A. & Gontijo, J. A. Fetal kidney programming by maternal smoking exposure: effects on kidney structure, blood pressure and urinary sodium excretion in adult offspring. Nephron. 129, 283–292 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Roza, S. J. et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur. J. Neurosci. 25, 611–617 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Zdravkovic, T., Genbacev, O., McMaster, M. T. & Fisher, S. J. The adverse effects of maternal smoking on the human placenta: a review. Placenta. 26 (Suppl), S81–S86 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Elliot, J., Vullermin, P. & Robinson, P. Maternal cigarette smoking is Associated with increased inner Airway Wall Thickness in Children who die from Sudden Infant Death Syndrome. Am. J. Respir Crit. Care Med. 158, 802–806 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Gilliland, F. D. et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 55, 271–276 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogberg, L. et al. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J. Hypertens. 30, 693–699 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken, E., Huh, S. Y., Taveras, E. M., Rich-Edwards, J. W. & Gillman, M. W. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes. Res. 13, 2021–2028 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy, C. T. & Spindel, E. R. Pulmonary effects of maternal smoking on the Fetus and Child: effects on Lung Development, respiratory morbidities, and Life Long Lung Health. Paediatr. Respir Rev. 21, 27–33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooijman, M. N. et al. Fetal smoke exposure and kidney outcomes in school-aged children. Am. J. Kidney Dis. 66, 412–420 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Pastrakuljic, A. et al. Transplacental transfer and biotransformation studies of nicotine in the human placental cotyledon perfused in vitro. Life Sci. 63, 2333–2342 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Lips, K. S. et al. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 26, 735–746 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Jauniaux, E. & Burton, G. J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 83, 699–706 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Newnham, J. P., Patterson, L., James, I. & Reid, S. E. Effects of maternal cigarette smoking on ultrasonic measurements of fetal growth and on doppler flow velocity waveforms. Early Hum. Dev. 24, 23–36 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Pintican, D., Poienar, A. A., Strilciuc, S. & Mihu, D. Effects of maternal smoking on human placental vascularization: a systematic review. Taiwan. J. Obstet. Gynecol. 58, 454–459 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Jaitner, A. et al. Smoking during pregnancy and its effect on placental weight: a mendelian randomization study. medRxiv (2023). [DOI] [PMC free article] [PubMed]
- 19.Mitsuda, N. et al. Association between maternal active smoking during pregnancy and placental weight: the Japan environment and children’s study. Placenta. 94, 48–53 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Sekhon, H. S. et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J. Clin. Investig. 103, 637–647 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekhon, H. S., Keller, J. A., Benowitz, N. L. & Spindel, E. R. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am. J. Respir Crit. Care Med. 164, 989–994 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Wongtrakool, C., Wang, N., Hyde, D. M., Roman, J. & Spindel, E. R. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. Am. J. Respir Cell. Mol. Biol. 46, 695–702 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehan, V. K., Asotra, K. & Torday, J. S. The effects of smoking on the developing lung: insights from a biologic model for lung development, homeostasis, and repair. Lung. 187, 281–289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proskocil, B. J. et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in Newborn monkeys. Am. J. Respir Crit. Care Med. 171, 1032–1039 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lo, J. O. et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am. J. Obstet. Gynecol. 212, 370–378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEvoy, C. T. et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 311, 2074–2082 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEvoy, C. T. et al. Effect of vitamin C supplementation for pregnant smokers on offspring airway function and wheeze at age 5 years: follow-up of a randomized clinical trial. JAMA Pediatr. 177, 16–24 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEvoy, C. T. et al. Oral vitamin C (500 mg/d) to pregnant smokers improves infant airway function at 3 months (VCSIP). A randomized trial. Am. J. Respir Crit. Care Med. 199, 1139–1147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khong, T. Y. et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab. Med. 140, 698–713 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Geelhoed, J. J. et al. Maternal smoking during pregnancy, fetal arterial resistance adaptations and cardiovascular function in childhood. BJOG. 118, 755–762 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Wongtrakool, C., Roser-Page, S., Rivera, H. N. & Roman, J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L611–L618 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyrklund-Blomberg, N. B., Hu, J. & Gennser, G. Chronic effects of maternal smoking on pulse waves in the fetal aorta. J. Matern Fetal Neonatal Med. 19, 495–501 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Pulvers, K. et al. Light and intermittent smoking among California Black, Hispanic/Latino, and non-hispanic white men and women. Nicotine Tob. Res. 17, 755–759 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Schauer, G. L., Malarcher, A. M. & Mowery, P. National trends in frequency and amount of nondaily smoking, and relation to quit attempts, 2000–2012. Nicotine Tob. Res. 18, 1539–1544 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Alptekin, H. et al. A prospective comparative study to assess the effect of maternal smoking at 37 weeks on doppler flow velocity waveforms as well as foetal birth weight and placental weight. J. Obstet. Gynaecol. 37, 146–150 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Jobe, A. H. Effects of chorioamnionitis on the fetal lung. Clin. Perinatol. 39, 441–457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer, B. W., Kallapur, S., Newnham, J. & Jobe, A. H. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 14, 2–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotiriou, S. et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 8, 514–517 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Holbourn, K. P., Acharya, K. R. & Perbal, B. The CCN family of proteins: structure-function relationships. Trends Biochem. Sci. 33, 461–473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grzeszkiewicz, T. M., Lindner, V., Chen, N., Lam, S. C. & Lau, L. F. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 143, 1441–1450 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Mo, F. E. et al. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol. Cell. Biol. 22, 8709–8720 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Lluch, G. et al. Cellular redox state and activating protein-1 are involved in ascorbate effect on calcitriol-induced differentiation. Protoplasma. 217, 129–136 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Yu, H. F. et al. HB-EGF ameliorates oxidative stress-mediated uterine decidualization damage. Oxid. Med. Cell Longev. 6170936 (2019). (2019). [DOI] [PMC free article] [PubMed]
- 44.Stejskalova, L. & Pavek, P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr. Pharm. Biotechnol. 12, 715–730 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Fa, S. et al. Changes in first trimester fetal CYP1A1 and AHRR DNA methylation and mRNA expression in response to exposure to maternal cigarette smoking. Environ. Toxicol. Pharmacol. 57, 19–27 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Suter, M. A. & Aagaard, K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Epigenomics. 4, 115–118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suter, M. et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 59, 1481–1490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huuskonen, P. et al. The human placental proteome is affected by maternal smoking. Reprod. Toxicol. 63, 22–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, H., Fu, G., Yu, H. & Peng, C. Transforming growth factor-beta inhibits aromatase gene transcription in human trophoblast cells via the Smad2 signaling pathway. Reprod. Biol. Endocrinol. 7, 146 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goumans, M. J. et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21, 1743–1753 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shorey-Kendrick, L. E. et al. Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: association with gene expression and respiratory outcomes. Clin. Epigenet. 13, 177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houston, M. C. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 4, 165–183 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Lo, J. O. et al. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am. J. Obstet. Gynecol. 216, 302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Najafzadeh, A. & Dickinson, J. E. Umbilical venous blood flow and its measurement in the human fetus. J. Clin. Ultrasound. 40, 502–511 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Ford, I. & Norrie, J. Pragmatic trials. N Engl. J. Med. 375, 454–463 (2016). [DOI] [PubMed] [Google Scholar]
- 56.McEvoy, C. T. et al. Vitamin C to pregnant smokers persistently improves infant airway function to 12 months of age: a randomised trial. Eur. Respir J. (2020). [DOI] [PMC free article] [PubMed]
- 57.McEvoy, C. T. et al. Vitamin C to decrease the effects of smoking in pregnancy on infant lung function (VCSIP): Rationale, design, and methods of a randomized, controlled trial of vitamin C supplementation in pregnancy for the primary prevention of effects of in utero tobacco smoke exposure on infant lung function and respiratory health. Contemp. Clin. Trials. 58, 66–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acharya, G., Wilsgaard, T., Berntsen, G. K., Maltau, J. M. & Kiserud, T. Doppler-derived umbilical artery absolute velocities and their relationship to fetoplacental volume blood flow: a longitudinal study. Ultrasound Obstet. Gynecol. 25, 444–453 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Frias, A. E. et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 152, 2456–2464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konje, J. C., Kaufmann, P., Bell, S. C. & Taylor, D. J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 185, 608–613 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Acharya, G., Wilsgaard, T., Rosvold Berntsen, G. K., Maltau, J. M. & Kiserud, T. Reference ranges for umbilical vein blood flow in the second half of pregnancy based on longitudinal data. Prenat Diagn. 25, 99–111 (2005). [DOI] [PubMed] [Google Scholar]
- 62.ISUOG Education Committee recommendations. For basic training in obstetric and gynecological ultrasound. Ultrasound Obstet. Gynecol. 43, 113–116 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Campbell, K. A. et al. Placental cell type deconvolution reveals that cell proportions drive preeclampsia gene expression differences. Commun. Biol. 6, 264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamburov, A., Stelzl, U., Lehrach, H. & Herwig, R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 41, D793–D800 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 30, 523–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalinka, J., Hanke, W. & Sobala, W. Impact of prenatal tobacco smoke exposure, as measured by midgestation serum cotinine levels, on fetal biometry and umbilical flow velocity waveforms. Am. J. Perinatol. 22, 41–47 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and processed RNAseq data is publicly accessible through the NCBI Gene Expression Omnibus (GEO) via accession series GSE253158.