Abstract
The intracellular bacterial pathogen Chlamydia trachomatis replicates within a membrane-bound compartment called the inclusion. Upon infection with several chlamydiae, each bacterium creates its own inclusion, resulting in multiple inclusions within each host cell. Ultimately, these inclusions fuse together in a process that requires the chlamydial protein IncA. Here, we show that inclusions form unique contact sites (inclusion contact sites, ICSs) prior to fusion, that serve as fusogenic platforms in which specific lipids and chlamydial proteins concentrate. Fusion depends on IncA clustering within ICSs and is regulated by PI(3,4)P2 and sphingolipids. As IncA concentrates within ICSs, its C-terminus likely interacts in trans with IncA on the apposing membrane, securing a high concentration of IncA at fusion sites. This regulatory mechanism contrasts with eukaryotic or viral fusion systems that are either composed of multiple proteins or use a change in pH to initiate membrane fusion. Thus, our study demonstrates that Chlamydia-mediated membrane fusion is primarily regulated by specific structural domains in IncA and its local organization on the inclusion membrane, which is affected by the host cell lipid composition.
Subject terms: Membrane fusion, Cellular microbiology, Membrane proteins, Phospholipids, Pathogens
The bacterial pathogen Chlamydia trachomatis replicates within a vacuole known as an inclusion. Here, Linton et al. show that in multiply infected cells, apposing inclusions form unique membrane-contact sites that regulate membrane fusion.
Introduction
Membrane fusion occurs when two membranes merge into a continuous bilayer, allowing the contents of both compartments to mix1,2. While fusion driven by eukaryotes and viruses is well described, membrane fusion mediated by bacteria is poorly characterized. One pathogen for which bacteria-driven membrane fusion is critical is Chlamydia trachomatis (Ctr). Ctr is an intracellular bacterium that replicates within a host plasma membrane-derived compartment called the inclusion. Inclusions are essential for Chlamydia’s survival, as they isolate the pathogen from the host cell cytoplasm, thus protecting it from the host defense. Importantly, inclusions also facilitate Chlamydia’s response to environmental and developmental changes3.
Within each host cell, the number of inclusions is tightly regulated. At a multiplicity of infection (MOI) > 1, each bacterium forms its own inclusion in which it begins to replicate, resulting in multiple cytoplasmic inclusions within a cell. Ultimately, these inclusions fuse into a single compartment between 12–16 h post-infection (hpi) through the process of homotypic fusion4–6.
Homotypic fusion requires the chlamydial protein IncA, as IncA knockout (IncAKO) Ctr cannot undergo fusion4–6. Clinical isolates that lack IncA or express mutated IncA on the inclusion are also non-fusogenic7,8. Interestingly, these non-fusogenic isolates are more frequently associated with subclinical infections compared to their fusogenic counterparts7,9. While these isolates can establish infection, infected patients present with lower bacterial burdens, fewer symptoms, and fewer signs of infection, likely resulting in less tissue damage than those infected with fusogenic strains9. In vitro, one of these isolates also grows slower than its fusogenic counterparts10. Notably, only ~1.5% of the clinical isolates successfully cultured are non-fusogenic, suggesting that homotypic fusion may undergo positive selection within the human population. Alternatively, other non-fusogenic strains may elicit asymptomatic infections more frequently. Thus, patients do not seek treatment, and the strains are not detected. These observations suggest that IncA-mediated homotypic fusion supports Ctr pathogenicity7–9.
IncA is expressed on the inclusion surface around 10–12 hpi11. It contains a bilobed transmembrane domain (TMD) flanked by two cytosolic regions, a short N-terminal tail (N-tail), and a large C-terminal domain. Evidence suggests that IncA is a bona fide bacterial fusion protein, but the molecular mechanism of IncA-mediated fusion has yet to be established. This membrane fusion event is unique as (i) IncA is expressed on the entire inclusion surface, while fusion only takes place in specific regions, and (ii) IncA is identical on both membranes, yet there is no known activation step to control fusion. In contrast, the eukaryotic soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) fusion machinery requires a target (t)-SNARE complex on one membrane and a distinct but complementary vesicular (v)-SNARE protein on the other, allowing fusion to occur only when cognate v- and t-SNAREs interact12–14. Viral fusion proteins are also single proteins, but they require activation by a change of pH or binding to a co-receptor to promote fusion15. These observations underscore how unique this bacterial system might be compared to other fusion machinery and raise an intriguing question: how is IncA regulated so that inclusions fuse with the appropriate compartment at the appropriate time?
Previously, we determined the atomic structure of the C-terminal domain of IncA and showed that it forms a non-canonical four-helix bundle encompassing the helices Ha, Hb, Hc, and Hd16. The crystal structure of IncA also revealed intramolecular interactions, which ensure that IncA remains in a folded monomeric conformation on the inclusion surface. In particular, IncA encodes a small clamp, Hclamp, which forms several intramolecular contacts with Hd that are central for controlling its monomeric state and fusogenic activity16. Ultimately, IncA oligomerizes using Hc and Hd, an event that is required for fusion17. Altogether, these data suggest that the activity of IncA requires robust intramolecular regulation to prevent cis oligomerization on one membrane while remaining primed for fusion in trans with the apposing membrane. How IncA switches between its cis monomeric and trans oligomeric forms to mediate fusion is unknown.
Here, we show that inclusions form unique membrane contact sites that we named inclusion contact sites (ICSs). ICSs form before fusion and are enriched in specific chlamydial proteins, including IncA and IPAM (CT223). ICSs also have a unique lipid composition compared to the rest of the inclusion membrane. Interestingly, inclusions form multiple membrane domains that serve distinct functions: microdomains containing phosphorylated forms of the Src kinase (pSrc) family are involved in the trafficking of inclusions to the microtubule-organizing center (MTOC)18, while inclusion-endoplasmic reticulum (ER) contact sites, that are enriched in the lipid transfer protein CERT, mediate lipid transfer to the inclusion19. Here, we establish that ICSs are distinct from pSrc-microdomains and ER-inclusion contact sites, and therefore constitute a novel membrane domain on the surface of the inclusion. In fact, we found that these ICSs form unique fusogenic platforms that control homotypic fusion.
IncA clustering in ICSs is critical for fusion, and preventing the enrichment of IncA significantly delays fusion despite its presence on the rest of the inclusion membrane. IncA clustering is controlled by both its C-terminal domain and the cellular lipid composition, notably PI(3,4)P2 and sphingomyelin. Importantly, IncA must be present on both inclusions to cluster, likely so that IncA on apposing membranes can interact in trans at the level of their C-termini. Such a requirement for IncA topology precludes the inclusion from fusing with the cellular organelles since they do not express IncA on their surface, thus adding yet another regulatory layer to this process. Together, these results indicate that the local membrane environment is critical in controlling IncA-mediated homotypic fusion during Chlamydia infection. This is the first regulatory process identified for this bacterial fusion system.
Results
Inclusions form unique membrane contact sites
A hallmark of eukaryotic cells is the sophisticated endomembrane system that creates physically and functionally distinct organelles. Organelles frequently form local membrane contact sites (CSs) prior to fusion20,21. Specific proteins and lipids cluster within these CSs, thus confining membrane fluidity and curvature changes to a defined region of the membrane. These sites are important for locally regulating fusion20,21. To investigate the mechanism of homotypic fusion during Ctr infection, we first determined whether inclusions form CSs enriched in specific chlamydial proteins and lipids before fusion (Fig. 1a).
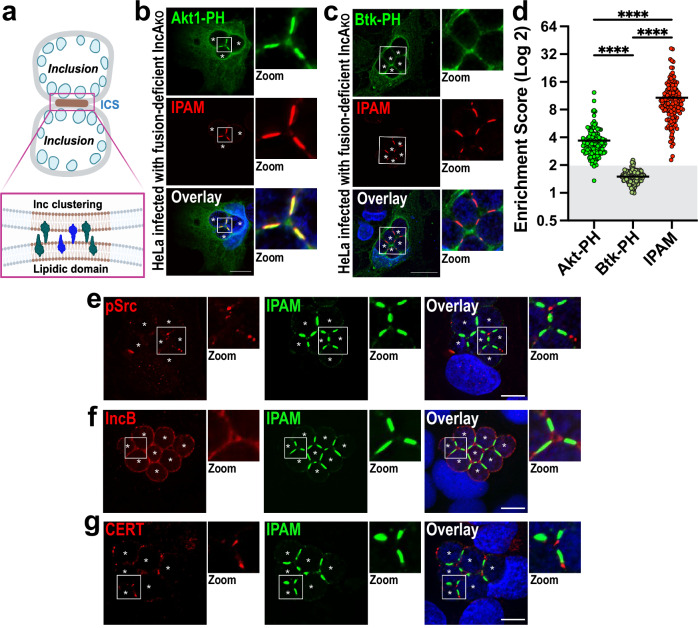
Fig. 1. Inclusions form unique contact sites (ICSs).
a Schematic depicts ICSs as points of contact between inclusion membranes in which specific lipids (brown) and proteins (green and blue) are clustered (see insert). Created in BioRender. Paumet, F. (2023) BioRender.com/s94s603. b–d HeLa cells were infected with IncAKO Ctr (MOI 3). At 2 hpi, cells were transfected with plasmids expressing Akt-PH-GFP (b, green) or Btk-PH-GFP (c, green). Cells were then fixed at 24 hpi and labeled with anti-IPAM antibody (red). DNA was stained with Hoechst (blue). Enlarged ICSs (white box) are shown in the Zoom panel. Scale bar = 20 μm. Images are representative of three independent experiments. d ESs were calculated and plotted for each probe and IPAM as a control. Each dot = individual ES, black horizontal bar = mean. Gray box denotes ESs <2. n = 150 total scores per probe from three independent experiments. ns = not significant, **** denotes p-value < 0.0001 (one-way ANOVA). e–g IncAKO Ctr infected cells were fixed at 24 hpi and labeled with anti-IPAM (green) and anti-pSrc (e), anti-IncB (f), or anti-CERT (g) antibodies (red). DNA was stained with Hoechst (blue). Enlarged ICSs (white box) are shown in the Zoom panel. Scale bar = 10 μm. Asterisks mark the inclusions. Images are representative of three independent experiments.
Phosphoinositides (PIs) are phosphorylated derivatives of the lipid phosphatidylinositol. Despite constituting <1% of cellular lipids, PIs play a disproportionally prominent role in regulating membrane dynamics22. Local PI metabolism allows for the recruitment of specific proteins to intracellular membranes that create functionally distinct domains23. PIs co-segregate with other lipids, such as cholesterol and sphingomyelin, which are essential for lipid raft formation24. Significantly, PIs also influence membrane curvature and interact with the eukaryotic tethering and fusion machinery, ultimately impacting the fusion of cellular membranes25. Here, we tested several fluorescent lipid probes that bind specific PIs. HeLa cells were transfected with the probes following infection with IncAKO Ctr at an MOI of 3 to promote the formation of multiple inclusions. In these conditions, inclusions cannot fuse, and ICSs become enriched and readily detectable. At 24 hpi, IncAKO Ctr–infected cells were stained with an antibody raised against another chlamydial inclusion membrane (Inc) protein, IPAM, to label the inclusion membrane26,27.
Akt1-PH, which binds PI(3,4,5)P3 and PI(3,4)P2, was found to be enriched within ICSs in both fixed (Fig. 1b) and live cells (Fig. S1a), indicating that PI(3,4,5)P3 and/or PI(3,4)P2 clusters within these sites. To discriminate between these two lipids, we used the probe Btk-PH, which targets only PI(3,4,5)P3. Figure 1c and S1b show that the Btk-PH probe is not concentrated in ICSs, suggesting that PI(3,4,5)P3 does not cluster in ICSs. Using anti-PI(3,4)P2 antibodies, we confirmed that PI(3,4)P2 clusters in ICSs (Fig. S1c, d). To assess the degree of PI(3,4)P2 clustering, the enrichment score of Akt1-PH versus Btk-PH in ICSs was quantified by measuring the ratio of their fluorescence intensities at ICSs versus the periphery of the apposing inclusions (See Fig. S2 for the description of the strategy). An enrichment score (ES) of 2 represents the sum of two membranes, an ES > 2 indicates protein enrichment, and an ES < 2 denotes exclusion from ICSs. Using this strategy, ESAkt-PH scores 3.6 on average, indicating that this probe is enriched in ICSs, as opposed to ESBtk-PH, which only scores an average of 1.5, indicating that this probe is depleted from the ICSs (Fig. 1d). Akt1-PH clustering is specific as PLCδ-PH labeling PI(4,5)P2 (Fig. S3a), FAPP1-PH labeling PI4P (Fig. S3b), and EEA1-FYVE labeling PI3P (Fig. S3c) are not enriched in ICSs. Surprisingly, we observed that the chlamydial inclusion protein IPAM also strongly localizes within the ICSs (Fig. 1b, c, red) with an ESIPAM scoring an average of 10.6 (Fig. 1d). IPAM exclusively and consistently labels ICSs, regardless of the cell type infected or the Ctr strain used (Fig. S4a–b). Therefore, we used IPAM as an ICS marker throughout this study.
Inclusions form specific microdomains in which cholesterol concentrates along with active members of the Src kinase family and chlamydial proteins, including IPAM and IncB18. These microdomains serve as nodes for interactions between dynein and the inclusions and allow the inclusions to migrate to the MTOC to complete the developmental cycle18,28. To determine whether ICSs constitute a variant of microdomains or are, in fact, unique, we labeled IncAKO Ctr-infected cells with anti-pSrc and anti-IncB at 24 hpi. In parallel, cells were fed TopFluor-cholesterol at 4 hpi. Neither pSrc (Fig. 1e) nor IncB (Fig. 1f) are localized at the ICSs, and while cholesterol is present, it is not enriched (Fig. S3d), thus indicating that ICSs are distinct from chlamydial microdomains.
Inclusions also form contact sites with the ER within which CERT concentrates to facilitate lipid transfer between the ER and the inclusion19. As shown in Fig. 1g, CERT is excluded from the ICSs, indicating that ICSs also do not overlap with ER-contact sites. Altogether, these data show that the ICS constitutes a novel membrane domain on the inclusion, which likely has a distinct function.
IncA clusters in ICSs before fusion
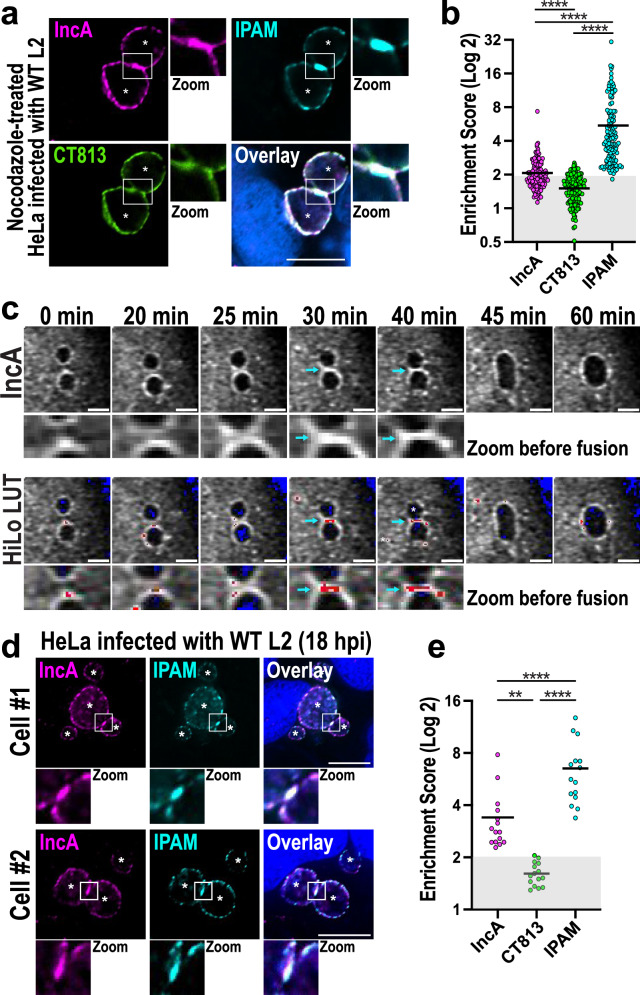
Since IncA is critical for homotypic fusion4–6, we tested whether this chlamydial protein clusters in ICSs prior to fusion. Wild-type (WT) Ctr expresses all the elements necessary for fusion. As such, these inclusions undergo fusion as soon as IncA is expressed ~10–12 hpi, making it a challenge to enrich and visualize ICSs. Thus, to delay fusion and increase the number of ICSs, we used nocodazole, which blocks microtubule polymerization and delays homotypic fusion29. We first infected cells with WT Ctr for 5 h to allow the infection to proceed normally. We then added nocodazole to the infected cells for 16 h, before fixing and labeling them with anti-IncA antibody (Fig. S5). Anti-CT813/InaC (hereafter referred to as CT813) antibody, which labels an Inc protein involved in cytoskeletal remodeling30–32, was used to mark the inclusion membrane, while anti-IPAM antibody was used to mark the ICSs. As expected, 46% of infected cells treated with nocodazole display multiple inclusions at 21 hpi (Fig. S6b, c) compared to 11% in DMSO-treated cells (Fig. S6a, c). In these conditions, endogenous IncA is enriched in ICSs, where it colocalizes with IPAM (Fig. 2a, Fig S6b). CT813, on the other hand, is excluded from these points of contact (Fig. 2a, green). Although the upper 10% values average 2.8 (Fig. 2b), the overall average ESIncA is modest at 2.1, reflecting that only a limited number of IncA-enriched ICSs can be observed in the overall nocodazole-treated cell population. This observation is consistent with ICSs rapidly undergoing fusion once IncA reaches a critical threshold. Furthermore, nocodazole asynchronously delays but does not block homotypic fusion, resulting in significant variation in IncA clustering at each time point, i.e., individual ESIncA scores vary from 1.1 to 7.3. In contrast, CT813, which is involved in cytoskeletal reorganization but has no role in homotypic fusion30–32, displays an ESCT813 consistently ranging from 0.5 to 2.5, with an average of 1.5, indicating that this protein is primarily excluded from ICSs. In contrast, IPAM, which predominantly localizes in the ICS, displays an average ES of 5.7, indicating significant enrichment in ICSs before fusion. Similar to ESIncA, though, ESIPAM also shows a substantial variation from 2 to 32, averaging lower than the usual ESIPAM average of 11.5 (Fig. S4a), further supporting that ICSs asynchronously and transiently form in nocodazole-treated cells.
Fig. 2. The bacterial fusion protein IncA clusters in ICSs.
a HeLa cells were infected with WT Ctr (MOI 3) for 5 h to allow the infection to proceed before being treated with 20 μM nocodazole. Cells were then fixed at 21 hpi and labeled with anti-IncA (magenta), anti-IPAM (cyan), and anti-CT813 (green) antibodies. DNA was labeled with Hoechst (blue). Enlarged ICSs (white box) are shown in the Zoom panel. Asterisks denote inclusions. Scale bar = 10 µm. Images are representative of three independent experiments. b ESs were calculated for each Inc. Each dot = individual ES, black horizontal bar = mean. Gray box denotes ESs <2. N = 130 total scores per Inc from three independent experiments. ns = not significant, **** denotes p-value < 0.0001 (matched-paired, one-way ANOVA). c Anti-FLAG-FrankenbodymScarlet3 HeLa cells were infected with IncAKO Ctr complemented with IncAWT-FLAG (MOI 3). IncAWT-FLAG expression was induced at 5 hpi with 20 ng/mL anhydrotetracycline. z-stacks of infected cells were acquired every 5 min as described in the Methods. Individual frames are shown beginning at 17 hpi (0 min). IncA images show the enrichment of the anti-FLAG FrankenbodymScarlet3 signal at the ICS prior to the fusion of the two inclusions (zoom). The HiLo look-up table (HiLo LUT) was then applied to the IncA images to highlight the grayscale differences (low signal = blue; high signal = red). Scale bar = 5 µm. Blue arrows denote signal enrichment at the ICS. Images are representative of three independent experiments. d HeLa cells were infected with WT Ctr (MOI 3), fixed at 18 hpi, and labeled with anti-IPAM (cyan) and -IncA (magenta) antibodies. DNA was labeled with Hoechst (blue). Enlarged ICSs (white box) are shown in the Zoom panel. Asterisks denote inclusions; Scale bar = 10 μm. Images are representative of three independent experiments. e HeLa cells were infected, fixed, and labeled as in d. ESs for each Inc was measured. N = 15 total scores per Inc from three independent experiments. Gray box denotes ESs <2. ns = not significant, **** denotes p-value < 0.0001 (matched-paired, one-way ANOVA).
To overcome this limitation and further observe IncAWT enrichment in infected cells prior to fusion, we developed a live cell imaging assay. To do so, HeLa cells were transfected with a FLAG-specific intracellular antibody, called a frankenbody, fused to the fluorescent protein mScarlet3. This anti-FLAG frankenbody is a chimeric single-chain variable fragment that binds to FLAG epitopes in living cells33 (Fig. S7a). Frankenbody-transfected cells were then infected with IncAKO Ctr expressing IncAWT-FLAG and GFP16. As IncAWT-FLAG is expressed during infection, the frankenbodies label the FLAG-tagged protein on the inclusion surface in living cells (Fig. S7a). Using this strategy, we monitored the distribution of IncAWT-FLAG on the inclusion membrane over time and observed that IncA concentrates in ICSs until fusion occurs, ~10 min after initial contact (Fig. 2c, Fig. S7b, and Supplementary Movie 1). This clustering was consistently observed in infected cells before inclusion fusion.
Finally, we were able to identify instances of endogenous IncA clustering at early time points during infection (18 hpi) in fixed cells infected with WT Ctr (Fig. 2d). In these conditions, ESIncA is an average of 3.4. As expected, IPAM is also consistently enriched with an average ESIPAM of 6.5, while CT813 is absent with an average ESCT813 of only 1.6. (Fig. 2e). These snapshots of IncA clustering were rare in WT Ctr–infected cells as fusion occurs rapidly after IncA reaches a critical level of enrichment in ICSs. Together, these data from multiple complementary methods demonstrate that IncA clusters in ICSs, further supporting its relevance to the fusion process.
The clustering of IncA in ICSs is necessary for fusion and requires its C-terminus
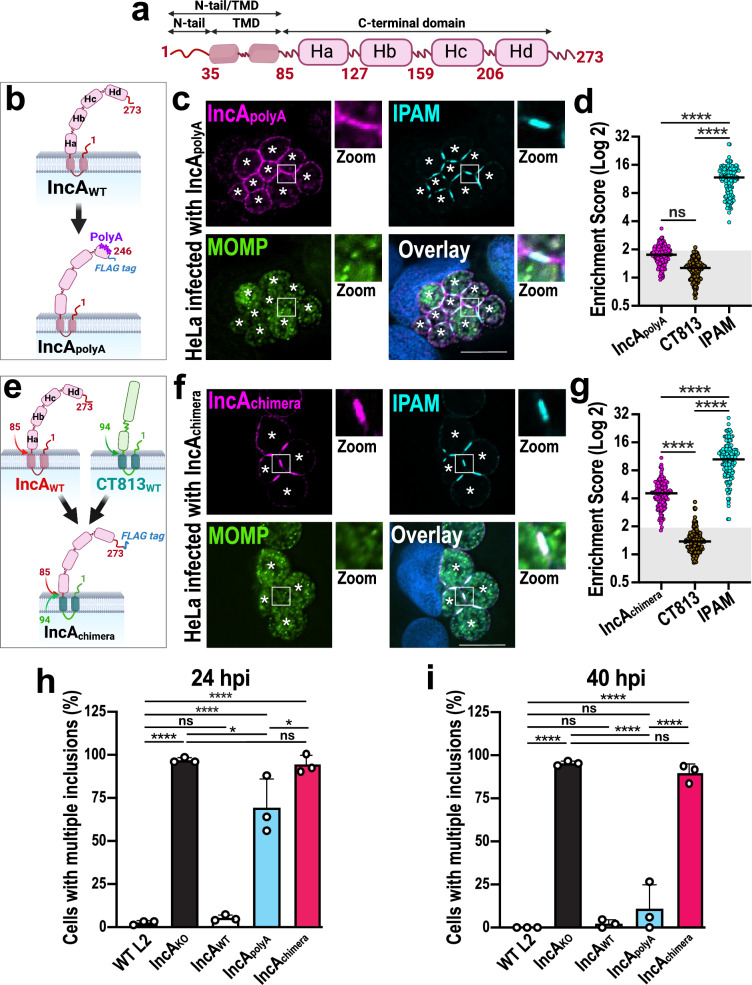
Since inclusions form ICSs in which IncA concentrates, we next assessed whether this clustering is required for homotypic fusion. IncA is an Inc protein that encodes a bilobed transmembrane domain (TMD), a small 35 amino-acid N-terminal tail (N-tail), and a large C-terminus composed of four helices Ha-d (Fig. 3a)16. Interestingly, the C-terminal region of IncA switches between a stable monomeric four-helix bundle and oligomers16,34. In this context, IncA clustering within ICSs could be controlled by one of its protein domains. To test this possibility, we used the Ctr mutant IncApolyA, which expresses an IncA protein in which the helix Hd is mutated, thus disrupting the monomeric four-helix bundle (Fig. 3b)16. As seen in Fig. 3c, IncApolyA does not efficiently cluster within ICSs during infection, with an ES consistently between 1.0 and 3.3 and averaging only 1.8 (Fig. 3d). IPAM and CT813 (Fig. 3c, d and Fig. S8a) remain clustered and excluded from the ICSs, respectively, suggesting that the effect is specific to IncA. The failure of IncApolyA to concentrate at ICSs at 24 hpi correlates with a homotypic fusion defect (Fig. 3h, blue) since 69% of IncApolyA Ctr-infected cells display multiple inclusions at 24 hpi, compared to 3% of WT Ctr-infected cells. Fusion eventually occurs by 40 hpi as 90% of IncApolyA Ctr-infected cells contain fused inclusions- close to that of WT Ctr-infected cells (Fig. 3i, blue). These results suggest that the C-terminus of IncA significantly contributes to its clustering within ICSs, which correlates with the timing of homotypic fusion.
Fig. 3. The C-terminal domain of IncA controls IncA clustering in ICSs and regulates homotypic fusion.
a Schematic of full-length IncA, displaying an N-terminal tail (N-tail) and transmembrane domain (TMD), as well as four C-terminal helices (Ha-d). The N-tail and the C-terminal domain face the host cytoplasm, while the TMD anchors IncA in the inclusion membrane. Created in BioRender. Paumet, F. (2023) BioRender.com/z33h938. b Schematic of IncApolyA-FLAG in which Hd residues were mutated to alanines to disrupt critical intramolecular contacts to create the IncApolyA-FLAG complemented mutant strain16. Created in BioRender. Paumet, F. (2023) BioRender.com/u70l441. c HeLa cells were infected with IncApolyA-FLAG Ctr (MOI 5) for 24 h in the presence of anhydrotetracycline. Cells were fixed and labeled with anti-FLAG (magenta, labels IncApolyA-FLAG), anti-IPAM (cyan), and anti-MOMP (green) antibodies. DNA was stained with Hoechst (blue). Asterisks denote the inclusions; Scale bar = 10 μm. Images are representative of three independent experiments. d ESs for cells infected with IncApolyA-FLAG Ctr at 24 hpi. N = 150 total scores per Inc from three independent experiments. Gray box denotes ESs <2. ns = not significant, **** denotes p-value < 0.0001 (one-way ANOVA). e Schematic of IncAchimera-FLAG in which IncA N-tail and TMD were swapped for those of CT813, to create the IncAchimera-FLAG complemented mutant strain. Created in BioRender. Paumet, F. (2024) BioRender.com/f62h052. f Cells were infected with IncAchimera-FLAG Ctr (MOI 5) for 24 h, fixed, and labeled as in c. Asterisks denote inclusions; Scale bar = 10 μm. Images are representative of three independent experiments. g ESs were calculated as in d. **** denotes p-value < 0.0001 (one-way ANOVA). h, i Homotypic fusion was quantified for the indicated strains in infected cells (MOI 7) at 24 hpi (h) and 40 hpi (i). Graphs depict the average number of cells containing multiple inclusions +/- SD from three independent experiments. ns = not significant, * denotes p-value < 0.05, **** denotes p-value < 0.0001 (one-way ANOVA).
The organization of proteins in membrane domains can also depend on their TMD through interactions with specific lipids or proteins. To exclude the involvement of IncA’s TMD in clustering, we used CT813, which is consistently excluded from ICSs in all the conditions tested (Fig. S4c–d). The N-tail/TMD of IncA was swapped for that of CT813 while keeping the C-terminus of IncA intact, thus creating IncAchimera (Fig. 3e). We then transformed IncAKO Ctr with this construct before infecting HeLa cells with the resulting mutant. As seen in Fig. 3f, IncAchimera is expressed on the inclusion surface, where it clusters in ICSs, with an ES ranging from 1.8 to 10.9 with an average of 4.6 (Fig. 3g). These results indicate that the N-tail/TMD of IncA is not involved in the clustering of IncA in ICSs and further supports the importance of its C-terminus in this process. ICSs in IncAchimera-infected cells appear otherwise unchanged as the phospholipid composition is the same as IncAKO Ctr-infected cells (Fig. S9a–e). Similarly, IPAM remains clustered (Fig. 3f, g and S8b), and cholesterol is present but not enriched (Fig. S9f). Finally, CT813 remains excluded from the ICS (Fig. 3g and S8b), like IncB, pSrc, and CERT (Fig. S10a–c).
Interestingly, even though IncAchimera is clustered in the ICS, homotypic fusion is completely blocked, similar to the IncAKO Ctr, with ~100% of the infected cells still displaying multiple inclusions at 24 hpi and 40 hpi (Fig. 3h, i, red). Note that because fusion is blocked, IncAchimera can accumulate over time in the ICS, resulting in an ESIncAchimera that is significantly higher than ESIncAWT (Fig. 2b), which undergoes fusion as soon as a critical threshold is reached. Altogether, these results demonstrate that (1) the N-term/TMD region of IncA is essential for membrane fusion but does not play a role in the clustering of IncA in ICSs, (2) the C-terminus of IncA is critical for clustering, and (3) a defect in IncA clustering correlates with delayed fusion.
The binding of IncA C-termini in trans is essential for IncA clustering
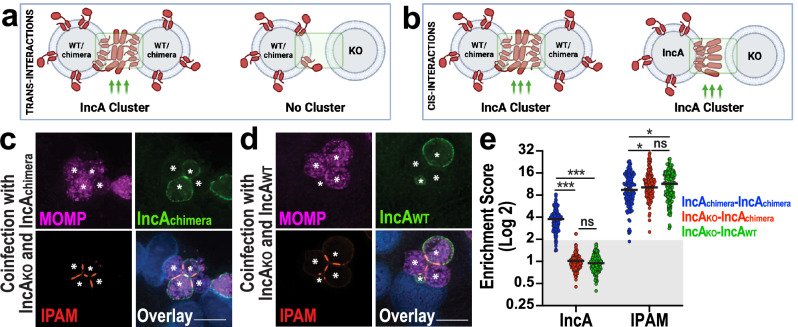
Thus far, we have demonstrated that the C-terminus of IncA is necessary for its clustering in ICSs. However, the mechanism by which this occurs is yet to be identified. One possibility is that the C-terminal domain of IncA promotes clustering by binding specific lipids or proteins enriched in ICSs and drives IncA clustering in cis. Another possibility is that the C-termini of IncA proteins interact with one another in trans to form multimers16,35. Discriminating between IncA oligomerization in trans versus cis using immunoprecipitation is challenging since both types of interactions would result in the same readout16. To circumvent this limitation, we used a co-infection system. If IncA clustering does not occur when IncA is expressed on only one inclusion, then trans-oligomerization is required (Fig. 4a). However, if IncA still clusters in the absence of IncA on the opposite membrane, then cis-oligomerization is involved (Fig. 4b). To assess these possibilities, we co-infected HeLa cells with IncAKO and IncAchimera Ctr. As seen in Fig. 4c and S11a, IncAchimera clusters when both inclusions express it, reaching an ES of 3.8 (Fig. 4e, IncA blue). In contrast, when an IncAchimera inclusion faces an IncAKO inclusion, IncAchimera is unable to cluster, and its ES drops down to 1.0 (Fig. 4e, IncA red). We observe a similar phenomenon with IncAWT (Fig. 4d, S11b), where the ES decreases to 1.0 when the apposing inclusion does not express IncA (Fig. 4e, IncA green), contrasting with the enrichment observed when IncA is expressed on both membranes. These observations also argue against the contribution of another yet-to-be-identified factor to promote IncA clustering since both inclusions would express such a factor on both sides. Together, these results indicate that IncA must be present on both membranes to cluster and fuse.
Fig. 4. IncA clustering in ICSs requires the presence of IncA on both membranes.
a If IncA interacts in trans, then clustering will only be observed when IncA is present on both inclusion membranes. WT = wild-type. KO = IncA knockout. Created in BioRender. Paumet, F. (2024) BioRender.com/w90v943. b If IncA interacts in cis, then IncA clustering will be observed when IncA is on one or both inclusion membranes. Created in BioRender. Paumet, F. (2024) BioRender.com/q49y738. c, d HeLa cells were co-infected with IncAKO Ctr (MOI 2.5) and IncAchimera-FLAG Ctr (MOI 2.5) (c), or with IncAKO Ctr (MOI 2.5) and WT Ctr (MOI 2.5) (d) in the presence of anhydrotetracycline. Cells were fixed at 24 hpi and labeled with anti-MOMP (magenta), which labels Ctr, anti-IncA (green), and anti-IPAM (red) antibodies. DNA was stained with Hoechst (blue). For c, d: Asterisks denote inclusions; Scale bars = 10 μm. Images are representative of three independent experiments. e ES quantification for the co-infections conducted in c and d. Graph depicts the average (black line) and individual ESs (dots). n = 150 total scores per Inc from three independent experiments. Gray box denotes ESs <2. ns = not significant, * denotes p-value < 0.05, *** denotes p-value < 0.001 (two-way ANOVA).
The lipid and protein composition of ICSs controls homotypic fusion
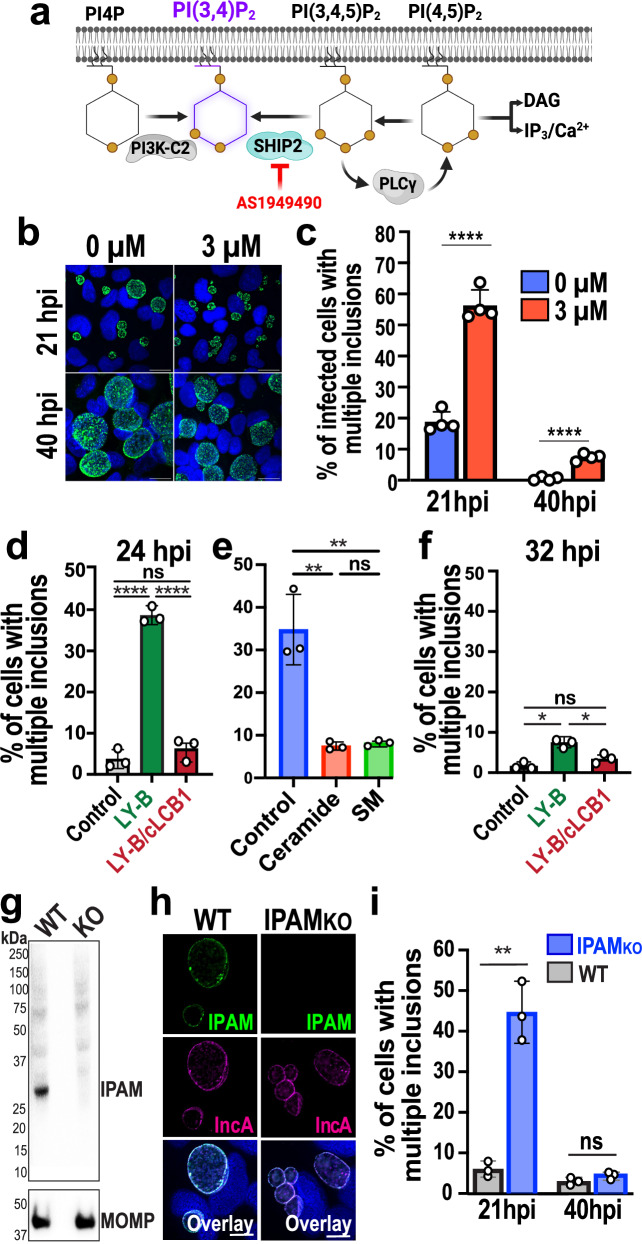
ICSs display specific lipid and protein composition. To test whether the local membrane environment of ICSs plays a role in homotypic fusion, we first altered the lipid composition of the inclusion. PI(3,4)P2 significantly concentrates in the ICS (Fig. 1b and S1a, c, d); thus, we determined whether its depletion would affect homotypic fusion. Two enzymes are critical for the generation of PI(3,4)P2; SH2 domain-containing inositol 5-phosphatase (SHIP2), and class II phosphoinositide 3-kinase A (PI3K-C2). To decrease the concentration of PI(3,4)P2 during infection, we used a potent and selective small-molecule inhibitor of SHIP2, AS194949036 (Fig. 5a). Wild-type Ctr-infected cells were treated with AS1949490 after infection, and the effect of the inhibitor was measured after 21 hpi or 40 hpi (Fig. 5b). When the concentration of PI(3,4)P2 is significantly reduced (Fig. S12a), we observe that 56% of infected cells displayed multiple inclusions at 21 hpi, compared with only 18% in the DMSO-treated (0 µM) control cells (Fig. 5c). Fusion eventually occurs, as 93% of infected cells treated with AS1949490 have undergone fusion at 40 hpi, similar to the DMSO-treated control cells (Fig. 5b, c). Since PIs can be converted into each other, interfering with the concentration of PI(3,4)P2 likely impacts the concentration of the other PIs, including PI(3,4,5)P3, and PI(4,5)P2 (Fig. 5a). However, since neither PI(3,4,5)P3, nor PI(4,5)P2 is present at the ICSs (Fig. 1c, S1b and S3a), it is unlikely that they influence homotypic fusion. Interestingly, IPAM and IncA are no longer enriched in the ICSs in the presence of AS1949490 (Fig. S12b), indicating that PI(3,4)P2 depletion disrupts ICS composition, which in turn likely disrupts the kinetics of fusion.
Fig. 5. The lipid and protein composition of the ICSs regulate homotypic fusion.
a Diagram of PI(3,4)P2 biosynthesis. The inhibitor AS1949490 used to block SHIP2 is indicated in red. Created in BioRender. Paumet, F. (2024) BioRender.com/p53j299. b HeLa cells were infected with WT Ctr (MOI 3) in the presence of 0 µM or 3 µM AS1949490. Cells were fixed at 21 hpi and labeled with anti-MOMP antibody (green) to visualize inclusions. Hoechst was used to label DNA (blue). Scale bar = 20 µm. Images are representative of four independent experiments. c Average percentage of cells treated with 0 µM or 3 µM AS1949490 containing multiple inclusions +/- SD from four independent experiments. 100 cells were counted from multiple fields of view for each experiment and each condition. **** denotes p-value < 0.0001 (two-tailed t-test). d, f CHO (control), LY-B, and LY-B/cLCB1 cells were infected with WT Ctr (MOI 3) and fixed at 24 or 32 (hpi). The average percentage of cells containing multiple inclusions was measured in WT Ctr-infected cells at 24 hpi (d) or 32 hpi (f) +/- SD from three independent experiments. For each replicate, 100 cells for each condition were counted from multiple fields of view. ns = not significant, * denotes p-value < 0.05, ** denotes p-value < 0.01, and **** denotes p-value < 0.0001 (one-way ANOVA). e LY-B cells were infected with WT Ctr (MOI 3) for 2 h followed by the addition of media supplemented with dihydroceramide (ceramide) or sphingomyelin (SM). Cells were then fixed at 24 hpi, and fusion was quantified as in d. g Western blot for the detection of IPAM in HeLa cell lysates infected with WT or IPAMKO Ctr (MOI 1, 24 hpi) shows successful knock out of IPAM (predicted molecular weight 29.4kD) in IPAMKO Ctr. MOMP staining is used as the loading control. Representative of two independent experiments. h HeLa cells were infected with WT or IPAMKO Ctr (MOI 3) and fixed 21 hpi. Cells were labeled with anti-IPAM (green) and anti-IncA (magenta) antibodies. DNA was stained with Hoechst (blue). Scale bar = 10 μm. Images are representative of two independent experiments. i Fusion was quantified as in d at 21 or 40 hpi.
In addition to phosphoinositides, sphingolipids also play a critical role in Chlamydia infection, where they are recruited to the inclusion membrane37–40. In particular, it has been shown that the depletion of sphingolipids impairs homotypic fusion in cells infected with Ctr serovar E41. In light of their critical role during infection and to assess the extent to which these lipids may be involved in controlling homotypic fusion, we determined whether sphingolipids were also involved in homotypic fusion in serovar L2-infected cells. To do so, we used Chinese hamster ovary-derived mutant LY-B cells that cannot synthesize sphingolipids due to a loss of the serine palmitoyltransferase (SPT) cLCB1 subunit42 (Fig. S13a). LY-B cells complemented with cLCB1 were used as a positive control43. As shown in Fig. 5d, homotypic fusion is impaired at 24 hpi in LY-B cells, demonstrating that the absence of sphingolipids interferes with the fusion of Ctr L2 inclusions, as was previously shown for serovar E41.
Among sphingolipids, sphingomyelin is particularly important for Chlamydia development44–46. Sphingomyelin is generated by the mammalian enzymes sphingomyelin synthase 1 and 2 (SMS 1 and 2) as well as by Ctr47 (Fig. S13a). Ceramide is a required precursor for sphingomyelin synthesis, which can be transported to the inclusion by direct interactions with the ceramide transporter CERT19,46,47 (Fig. S13a). Ctr can also use CERT-derived ceramide to de novo synthesize sphingomyelin47. Therefore, we used CERT knockout (ΔCERT), SMS 1 and 2 double knockout (DKO), or CERT, SMS1, and SMS2 triple knockout (TKO) HeLa cell lines47 to assess the extent to which sphingomyelin plays a role in homotypic fusion. Using the TKO cells, we observe that homotypic fusion is also impaired at 24 hpi (Fig. S13b, c, TKO). Both sphingomyelin and ceramide trafficking must be blocked to inhibit fusion, as ΔCERT or DKO inclusions still undergo homotypic fusion, although to varying degrees (Fig. S13b, c, ΔCERT, DKO). The homotypic fusion defect at 24 hpi in the LY-B (Fig. 5e) and TKO (Fig. S13d) cells is reversed when media is complemented with sphingomyelin, validating that the lack of sphingomyelin is responsible for the fusion defect. Furthermore, while the addition of dihydroceramide rescued the fusion defect in LY-B cells, which can use this lipid to synthesize sphingomyelin, dihydroceramide did not rescue the fusion defect in TKO that lack the enzymes needed to convert this precursor into sphingomyelin (Fig. 5e and S13d). These data support that sphingomyelin is the specific sphingolipid involved in regulating homotypic fusion.
Consistent with the fusion defect in LY-B and TKO cells, IncA is excluded from ICSs at 24 hpi (Figs. S14a and S13e), with an average ES of 1.4 and 1.3, respectively (Figs. S14b and S13f). High-resolution 3D reconstruction of inclusions formed in LY-B cells distinctly shows that IncA no longer colocalizes with IPAM but instead, surrounds it at the edge of the ICSs (Fig. S14c). This is not due to a general loss of ICS integrity since Akt-PH (Fig. S14d) and IPAM (Figs. S14a–c and S13e, f) remain concentrated within the ICSs, while CT813 remains excluded (Figs. S14a, b and S13e, f). Due to the toxicity of the fluorescent sphingomyelin probe, Eqt2-SM-oxGFP, we could not determine whether sphingomyelin is clustered within ICSs.
Interestingly, homotypic fusion ultimately occurs in the LY-B cells at ~32hpi, indicating that fusion is only delayed (Fig. 5f). This correlates with delayed IncA clustering as endogenous IncA can be detected within the ICS at around 26 hpi (Fig. S14e). The uptake of exogenous lipids from the cell culture media may facilitate the slow population of the ICS over time. Together, these data indicate that the absence of sphingolipids specifically affects the clustering of IncA within ICSs and delays homotypic fusion.
In conjunction with lipids, proteins present in ICSs could play a major role in homotypic fusion. IPAM has been found almost entirely clustered in ICSs (Fig. S4a, b). To assess whether IPAM regulates fusion, we used an IPAMKO Ctr strain in which IPAM is depleted (Fig. 5g, h). Homotypic fusion was significantly inhibited at 21 hpi in IPAMKO Ctr-infected cells, with 45% of the cells displaying multiple inclusions, compared to only 6% for cells infected with WT Ctr (Fig. 5i). Once again, fusion is only delayed in the absence of IPAM since 95% of infected cells have undergone fusion by 40 hpi for both IPAMKO and WT Ctr. Although the exact role of IPAM in membrane fusion is unknown, these results indicate that inclusions form unique functional platforms at contact sites, in which lipids and proteins directly and/or indirectly control homotypic fusion.
Discussion
Reminiscent of eukaryotic cells in which fusion-competent membranes generate contact sites enriched in specific lipids and proteins20,21, we discovered that chlamydial inclusions also form membrane contact sites where fusion occurs. These contact sites are enriched in PI(3,4)P2 and chlamydial proteins, including the bacterial fusion protein IncA, but are different from previously identified ER-inclusion contact sites or pSrc-containing microdomains. This study establishes that the ICS is a unique molecular platform, the function of which is to regulate homotypic fusion. ICSs carry out this specific task by controlling the clustering of chlamydial proteins, notably IncA. In fact, delaying IncA clustering into these regions significantly delays fusion, indicating that a high concentration of IncA in ICSs is required for homotypic fusion to readily occur. Considering that homotypic fusion is tightly linked to the concentration of IncA in ICSs, we identified elements that control IncA clustering in these sites.
Previously, we established that the C-terminal region of IncA folds into a stable four-helix bundle, thus maintaining the protein as a monomer16. When we disrupt the formation of this four-helix bundle by introducing mutations into one of the helices, as in IncApolyA, this IncA mutant no longer clusters in the ICS. Since IncApolyA cannot form the intramolecular interactions that lock IncA in its monomeric conformation, it can readily self-assemble in cis on the inclusion membrane16. As such, the lack of clustering in the ICSs could be due to the decrease in free non-oligomerized IncApolyA to interact in trans upon inclusion-inclusion contact. Supporting this hypothesis, co-infections of Ctr expressing either WT IncA or IncAchimera with IncAKO Ctr show that when IncA is only expressed on one membrane, it is unable to cluster. Altogether, these data suggest that the clustering of IncA is due to its oligomerization in trans, at the level of the C-terminus, between apposing inclusions. This clustering, in turn, regulates homotypic fusion.
Adding another layer of regulation, we observed that the lipid composition of the ICS plays a significant role in homotypic fusion: the depletion of PI(3,4)P2 or sphingolipids, in particular sphingomyelin, significantly delays fusion. In other systems, sphingolipids, notably sphingomyelin, are critical for maintaining the organization of lipid rafts required for efficient membrane fusion48. It has been proposed that sphingomyelin influences other lipids to drive its organization into lipid rafts49. Interestingly, sphingomyelin has also been shown to act as a critical regulator of the endomembrane distribution of phosphatidylserine, which plays a critical role in membrane fusion50–52. During Chlamydia infection, the precise mechanism by which these lipids control homotypic fusion is unclear. However, we found that their depletion disrupts the composition of ICSs and IncA clustering. Therefore, these lipids may act indirectly by influencing the localization of other lipids and/or proteins, which would then control IncA clustering. This raises the possibility of an indirect impact of sphingolipids on the distribution and organization of other lipids. We have only begun to unravel the composition of ICSs and cannot exclude these possibilities.
IPAM is another chlamydial protein clustered in the ICSs, the deletion of which impairs homotypic fusion. IPAM is thought to regulate the organization of microtubules at the inclusion surface likely directing inclusions to the MTOC53. Although its exact function in membrane fusion is unknown, one possibility is that the high concentration of IPAM in the ICSs ensures that the inclusions are in a tight apposition at the level of the ICSs, thus physically securing their tethering. This is currently under investigation.
Altogether, our results support a model (Fig. 6) in which the concentration of specific proteins and lipids in the ICSs plays a critical role in controlling homotypic fusion through IncA clustering: ❶ IncA is first expressed on the entire inclusion membrane and maintained in a stable monomeric conformation to prevent cis self-assembly on the inclusion membrane. ❷ Once the inclusions are in close proximity, lipids, including sphingomyelin and PI(3,4)P2, facilitate IncA clustering at the ICS prior to fusion. ❸ As IncA concentrates within ICSs, it can then undergo conformational changes and oligomerize in trans with IncA on the apposing membrane, further securing its clustering and ensuring its high local concentration at these points of contact. ❹ Finally, as the trans-oligomerization of IncA increases, it promotes homotypic fusion of inclusions and the mixing of the inclusion contents.
Fig. 6. Regulatory model of IncA-mediated membrane fusion.

❶ IncA (pink) is homogeneously expressed on the inclusion membrane where it is folded as a monomer to prevent cis self-assembly. IPAM (green) is distributed throughout the inclusion membrane. ❷ Lipids (blue), in particular sphingolipids and PI(3,4)P2, trigger IncA clustering within ICSs. PI(3,4)P2 also controls IPAM recruitment into the ICSs. ❸ As IncA concentrates in the ICS, it then oligomerizes in trans, further enhancing its clustering. ❹ At its optimal local concentration, IncA drives the homotypic fusion of inclusions. Ct = Chlamydia trachomatis bacteria. Created in BioRender. Paumet, F. (2023) BioRender.com/c27a297.
In addition to the local regulatory elements, we discovered that the N-tail/TMD of IncA itself is necessary to complete this fusion event, as IncAchimera cannot promote fusion despite its high local concentration. The importance of the membrane anchor for fusion has been previously established for viral and eukaryotic fusion machinery. A fusion protein must be linked to a membrane anchor that spans the bilayer to trigger membrane fusion54–56. The TMDs of IncA and CT813 are both bilobed, have a similar size, span the bilayer, and likely act similarly as membrane anchors. Therefore, the exchange of the TMDs is likely not responsible for the inhibition of fusion seen with the IncAchimera. Another possibility is that the N-tail directly influences the fusogenicity of IncA by modifying its conformation. Note that the N-tail of IncA is significantly enriched in proline, with five prolines present in a seven amino-acid residue stretch. Proline residues can engage aromatic residues within or between peptides in a complex57. As such, the N-tail could interact with the phenylalanine, tyrosine, and histidine residues present in Ha and Hb to influence the folding of IncA, thus priming it for fusion. Since the N-tail of CT813 is not as proline-rich, it may not prime IncA for fusion. As a result, IncA would not adopt a fusogenic conformation, leading to the complete inhibition of fusion. Experiments are ongoing to identify the role of each region in IncA-mediated fusion.
In the context of Chlamydia infection, this study opens new avenues of research. Could fine-tuning homotypic fusion via lipids allow Chlamydia to modulate its pathogenicity? In fact, the intracellular environment of infected cells is constantly adapting as the host mounts an immune response and responds to Ctr growth. This ever-changing environment may impact the lipid composition of the inclusion membrane, thus influencing the clustering of IncA, which would then modulate homotypic fusion. As a result, Chlamydia infection could be altered since IncA-mediated homotypic fusion is critical for its pathogenesis. Supporting this possibility, it has been shown that sphingomyelin depletion, which impacts homotypic fusion, also prevents Chlamydia from exiting persistence, a state during which Chlamydia stops replicating and goes dormant until the environment becomes favorable again41. Altogether, deciphering the regulatory mechanisms of IncA-mediated homotypic fusion will help us better understand disease progression.
Overall, these data identify how a bacterial fusion system is regulated at the molecular level. These results could inform the discovery and characterization of new prokaryotic fusion machinery. Ultimately, the mechanisms we identify here may reveal shared or divergent mechanisms of regulating membrane fusion across kingdoms.
Methods
Cell culture
HeLa 229 cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Corning) supplemented with 20 mM HEPES/NaOH (pH 7.5), nonessential amino acids (HyClone), 10% fetal bovine serum (Corning), 10 μg/mL gentamicin (Thermo), and 2 mM L-glutamine (Gemini Bio). CHO-K1, LY-B, and LY-B/cLCB1 cell lines, obtained from RIKEN BioResource Research Center, were cultured in HAM-F12 media (Gibco) supplemented with 2 mM L-glutamine, 10% fetal bovine serum, and 10 μg/mL gentamicin. The parental HeLa cell line (mCAT#8), as well as HeLa knockout cell lines ΔCERT, double-knockout (DKO) for the sphingomyelin synthetases (SMS) 1 and 2, and triple knockout (TKO) for SMS1, SMS2, and CERT, were grown in DMEM supplemented with 20 mM HEPES/NaOH (pH 7.5), nonessential amino acids, 20% fetal bovine serum, 10 μg/mL gentamicin, and 2 mM L-glutamine47.
Antibodies
An anti-IncA antibody was generated by immunization of goats (Thermo) with purified soluble recombinant IncA87-273-His6 protein produced in-house17. The specificity of the antibody was validated by western blot analysis of infected cell lysates (WT and IncAKO Ctr L2), as described below. This analysis revealed a single band at the appropriate size (30.3kD) in the WT L2 Ctr-infected lysate, which is absent in the IncAKO Ctr-infected lysate (Fig. S5a). Using immunofluorescence microscopy, we observed IncA staining on the inclusion membrane in WT-infected cells, while this staining is absent in IncAKO Ctr-infected cells (Fig. S5b), further validating the specificity of the anti-IncA antibody. For western blot, this goat anti-IncA antibody was used at a dilution of 1:12,000 and for immunofluorescence microscopy at 1:4000. The following primary antibodies were also used in immunofluorescence: anti-FLAG (rabbit, 600-401-383; Rockland Immunochemical, 1:1,600 dilution), anti-CT813 (rabbit; T. Hackstadt, NIH, 1:300 dilution), anti-IncB (rabbit; T. Hackstadt, NIH, 1:300 dilution), anti-pSrc (rabbit, ab40660; Abcam, 1:250 dilution), anti-CERT (chicken, GW22128B; Sigma, 1:200 dilution), anti-MOMP (goat, 1621; ViroStat, 1:800 dilution), anti-EB Ctr (rabbit, 1611; Virostat, 1:500 dilution), anti-PI(3,4)P2 (mouse, Z-P034, Echelon, 1:100 dilution), anti-PI(3,4)P2 (mouse, Z-P034B, Echelon, 1:100 dilution), and anti-CT223/IPAM (mouse; D. Rockey, Oregon State University, 1:200 dilution). The following fluorescent secondary antibodies were used in immunofluorescence microscopy: donkey anti-chicken IgY, or donkey anti-mouse, -goat, and -rabbit IgG conjugated to Alexa Fluor −488, −555, or −647 (Thermo, all used at a 1:500 dilution). Hoechst 33342 was used to label DNA. For western blot, HRP-conjugated donkey anti-goat, anti-mouse, and anti-rabbit IgG antibodies were used (Thermo, 1:25,000 dilution).
Gene cloning and mutagenesis
Standard PCR and cloning procedures were used. Primers used are listed in Table 1. DNA sequences were confirmed by Sanger sequencing. All enzymes were purchased from Thermo. CT8131-94IncA85-273-FLAG (IncAchimera) was constructed by Overlap Extension PCR. In the first step, the CT813 portion (Met1 to Leu94) was amplified using FO507/FO1307 and full-length CT813 as the template, while the IncA portion (Gln85 to Ser273) was amplified using FO1306/FO511 and full-length IncA as the template. In the second step, the CT813 and IncA products were allowed to prime each other briefly. The primers FO507/FO511 were then used to amplify the final construct using the second product as the template. The final PCR product was digested with NotI and SalI and ligated into pBOMB3-tet. The pBOMB3-Tet plasmid was constructed by removing the tetracycline promoter from pBOMB4-Tet with XhoI and NotI, followed by ligation into pBOMB35.
Table 1.
Primers
| Name | Sequence (3’−5’) |
|---|---|
| FO507 | TACCACTCCCTATCAGTGATAG |
| FO511 | ACGCAGTCGACCTACTTGTCATCGTCATCCTTGTAGTCGGAGCTTTTTGTAGAGGG |
| FO786 | GGACACTCTAGAATGGACAACACCGAGGACG |
| FO787 | ATATATGGGCCCTCACTGGGAGCCGGAGTGGCG |
| FO1306 | GTATCAGCTTTATGCTTGCAGAAAACCGCTAATCTACATCTATAC |
| FO1307 | GTAGATTAGCGGTTTTCTGCAAGCATAAAGCTGATACCAAGCAG |
| FO1333 | ATTCTCGAGCACCTTGCCGAAAGTGCC |
| FO1334 | CTTGGAGCCGTACTGGAACTG |
| FO1336 | ATTGGTACCGCCACCATGGACTCGGGCCGGGACT |
| FO1337 | ATTCTCGAGCTGAAGGAGCTCAACATCCAG |
| FO1338 | ATTGGTACCGCCACCATGGAGGGGGTGTTGTACAAGTGG |
| FO1339 | ATTCTCGAGTCTTCAGGAAGAGAAGGATGGA |
| FO1344 | ATTGGTACCGCCACCATGGCCGCAGTGATTCTG |
| FO1345 | ATTCTCGAGCGGTTTTAAGCTTCCATTCCTG |
| FO1348 | ATTGGTACCGCCACCATGGTGAAGAAGGAGTGGCAATCTAGT |
| FO1349 | ATTCTCGAGTCCTTGCAAGTCATTGAAACAT |
| FO1759 | GTATACAAGCTTATGGCCGAGGTGAAGCTG |
| FO1762 | GATCCTCTAGATTACTTGTACAGCTCGTCCATCC |
| FO1789 | GTGCTGGGACCAAGCTGG |
| FO1790 | GCGGGTTTAAACGGGCCC |
Anti-FLAG-FrankenbodymScarlet3 was generated as follows. Anti-FLAG-FrankenbodymRuby2 was PCR amplified from pCMV-anti-FLAG-FrankenbodymRuby2 (Addgene #175294) with the primers FD1759 and FO1762. The PCR product was inserted into EF1α-pcDNA3.1 (gift from Mark Tomishima) using the HindIII and XbaI restriction sites to generate EF1α-anti-FLAG-FrankenbodymRuby2. Next, mRuby2 was exchanged for mScarlet3. To do so, mScarlet3 was PCR amplified with the primers FO1789 and FO1790 and inserted into EF1α-anti-FLAG-FrankenbodymRuby2 using the Oli1 and XbaI restriction sites to generate EF1α-anti-FLAG-FrankenbodymScarlet3.
Plasmids encoding Akt-PH-GFP, Btk-PH-GFP, and EEA1-FYVE-GFP were provided by T. Balla (NIH). PLCδ-PH-GFP (#21179) was purchased from Addgene. FAPP1-PH-GFP was provided by P. Wedegaertner (Thomas Jefferson University). Lipid probes with C-terminal mDsRed tags were generated as follows. mDsRed was PCR amplified using primers FO786 and FO787 and cloned into pcDNA3.1+ (Invitrogen) via XbaI and ApaI restriction sites to generate mDsRed pcDNA3.1. Akt-PH, Btk-PH, PLCδ-PH, FAPP1-PH, and EEA1-FYVE were PCR amplified using the primer sets FO1333/1334, FO1344/1345, FO1336/1337, FO1338/1339, FO1348/1349, respectively. The PCR products were then ligated into mDsRed pcDNA3.1 using the restriction sites KpnI and XhoI.
Chlamydia transformation
IncAchimera-FLAG-expressing Ctr was generated by transforming IncAKO Ctr L2 with IncAchimera-FLAG pBomb4S-Tet encoding IncAchimera-FLAG under the control of a tetracycline promoter following the protocol described in ref. 16. GFP IncAKO Ctr L2 was generated by transforming IncAKO Ctr L2 with empty pBomb3-Tet. Individual clones were isolated by limiting dilution and purity was assessed by immunofluorescence microscopy.
Chlamydia strains and infections
Wild-type Ctr serovar L2 (LGV 434/Bu) was provided by T. Hackstadt (NIH). Ctr IncAKO and IncAKO complemented with IncAWT-FLAG or IncApolyA-FLAG Ctr L2 strains were generated previously16. IPAMKO Ctr L2 was provided by R. Bastidas (Duke University). All strains were propagated and density gradient-purified as described16,58. For infections, cells were seeded on glass coverslips in 24-well plates 24 h before infection. HeLa cell lines were seeded at a density of 5 × 104 cells per well, while CHO-derived cell lines were seeded at a density of 2.5 × 104 cells per well. Cells were infected with 500 µL of inoculum in growth media. Anhydrotetracycline (Tet) was added at the time of infection. Plates were then immediately centrifuged at 1000 × g for 1 h at 4 °C. The MOI for each cell line and Ctr strain was determined empirically and is noted in the figure legends. The appropriate concentration of Tet for each IncAmutant-FLAG Ctr strain was determined by immunofluorescence microscopy. The Tet concentrations used to achieve an expression level on the inclusion membrane matching that of IncA on the inclusion membrane of WT Ctr L2 were IncAWT-FLAG (5 ng/mL), IncAchimera-FLAG (0.4 ng/mL) and IncApolyA-FLAG (5 ng/mL). If cells were infected for >24 h, the growth media was replaced at 24 hpi with media containing fresh Tet at the appropriate concentration.
Recombinant protein expression and purification for IncA antibody production
IncA85-273-His6 was expressed and purified as described16.
Lipid probe transfection
HeLa and CHO cell lines were transfected at 2 hpi with 25-50 ng of purified biosensor plasmid DNA per 2.5-5×104 cells. This was mixed with an empty vector pcDNA3.1 to achieve a total of 250 ng DNA per transfection. Transfection was performed using the Continuum transfection reagent (Gemini Bio) according to the manufacturer’s instructions. For HeLa and CHO cell lines, a 1:1 or 1:1.5 DNA to Continuum ratio was used, respectively.
Live-cell imaging of lipid probes and fluorescent cholesterol
HeLa cells were infected with the indicated strains at an MOI of 3 in glass bottom plates. After centrifugation, 5 µM TopFluor or TopFluor-TMR cholesterol (Avanti) was added to the cell culture media and maintained throughout the infection. Lipid probes were transfected as described above. At 22 hpi, the cells were washed twice with imaging media (FluorBrite DMEM supplemented with 20 mM HEPES/NaOH [pH 7.5], nonessential amino acids, 10% fetal bovine serum, 10 μg/mL gentamicin, and 2 mM L-glutamine). To label nuclei, cells were incubated with 10 µg/mL Hoechst in imaging media for 15 min at 37 °C followed by several washes with imaging media. The media was then replaced with imaging media containing a 1:50 dilution of ProLong Live antifade reagent (Invitrogen), and the cells were incubated at 37 °C for 1 h before imaging. Cells were imaged on a Nikon TiE inverted fluorescence microscope with a 60x oil immersion lens and NIS-Elements software (Nikon). Images were then deconvolved using Legacy deconvolution (NIS-elements) and processed using ImageJ (NIH).
Immunofluorescence microscopy
Cells transfected with lipid biosensors, and those labeled with anti-pSrc and -CERT antibodies were fixed at the indicated time points, with 4% paraformaldehyde (PFA) in HBSS containing calcium and magnesium for 15 min at room temperature (RT). For all the other experiments, infected cells were fixed with ice-cold methanol for 10 min at RT. Fixed cells were then washed with IF-G buffer (25 mM HEPES/NaOH [pH 7.5], 150 mM NaCl, 900 nM CaCl2, 500 nM MgCl2, 100 mM glycine) for PFA fixation, or IF buffer (25 mM HEPES/NaOH [pH 7.5], 150 mM NaCl, 900 nM CaCl2, 500 nM MgCl2) for methanol fixation. PFA-fixed cells were permeabilized with 0.1% Triton X-100 in IF buffer for 15 min before blocking. Fixed and permeabilized cells were blocked in IF buffer containing 10% donkey serum, 0.1% bovine serum albumin, and 0.05% sodium azide for 1 h at RT. After blocking, cells were incubated with the primary antibodies diluted in labeling buffer (10% donkey serum, 0.1% bovine serum albumin, 0.05% sodium azide, and 0.1% Triton X-100 in IF buffer) for 1 h at RT. Next, cells were washed with 0.1% Triton X-100 in IF buffer and then incubated with Alexa Fluor-conjugated secondary antibodies (1:500 dilution) and Hoechst diluted in labeling buffer for 1 h at RT. Cells were washed with 0.1% Triton X-100 in IF buffer, followed by washes with IF buffer, and finally, briefly washed with purified water immediately before mounting. Coverslips were mounted with ProLong Glass antifade mountant (Invitrogen) and allowed to cure overnight before imaging. Cells were imaged on a Nikon TiE inverted fluorescence microscope with a 60x oil immersion lens and NIS-Elements software (Nikon). Images were processed using ImageJ (NIH). To analyze the detailed localization of IncA in LY-B-infected cells, z-stacks were acquired on a Nikon A1 Confocal Laser Scanning Microscope at 60x oil immersion in 0.085 µm sections and deconvolved using the Landweber method. 3D reconstruction was performed using the NIS-Elements software.
PI(3,4)P2 labeling with antibody
Infected cells were fixed with 4% PFA in HBSS containing calcium and magnesium at RT for 20 min, followed by three washes with TBS. Cells were permeabilized with 0.2% Triton X-100 in TBS for 15 min, then washed thrice with TBS. Samples were blocked for 1 h at RT in a blocking buffer (10% goat serum in TBS). Antibodies (Z-P034 and Z-P034B) were diluted 1:100 in blocking buffer and incubated with the samples for 2 h at RT. Following several washes in TBS, cells were incubated with secondary antibodies diluted 1:500 in a blocking buffer for 45 min at RT. Cells were washed three times with TBS, mounted with ProLong Glass antifade mountant, and imaged as described above.
Generation of stable anti-FLAG-FrankenbodymScarlet3 HeLa cell line
HeLa 229 cells (5 × 105) were transfected with EF1α-anti-FLAG-FrankenbodymScarlet3 DNA (2 µg) by nucleofection using kit R and program I-013 (Amaxa). Antibiotic selection was initiated 24 h after transfection with 0.7 mg/mL active G-418. After 14 days of selection, individual clones were isolated by limiting dilution. Purity was assessed by fluorescence microscopy.
Live-cell time-lapse imaging
Anti-FLAG-FrankenbodymScarlet3 HeLa cells (4×104) were seeded in glass bottom plates 24 h before infection with IncAKO Ctr L2 complemented with IncAWT-FLAG at MOI 3. At 5 hpi, the monolayers were washed, and the media was replaced with imaging media containing ProLong Live antifade reagent (1:50 dilution) and 20 ng/mL anhydrotetracycline. Images were acquired on a Nikon A1 Confocal Laser Scanning Microscope outfitted with a Tokai-Hit temperature and CO2-controlled chamber and a Plan Fluor 40x/1.3 NA oil immersion objective. Nine-micron z-stacks with 0.5 µm slices were acquired using the resonant scanner every 5 min beginning at 8 hpi until 24 hpi. Images were deconvolved using the Richardson-Lucy method and processed with Nikon NIS-Elements software.
SHIP2 inhibitor, nocodazole, and lipid complementation
To inhibit SHIP2, 3 µM of AS1949490 (Echelon) or DMSO (control) was added to the cell culture medium after infection and centrifugation, and maintained throughout the infection. To complement LY-B and TKO cells with lipids, the cells were first infected for 2 h followed by a brief wash in HBSS with calcium and magnesium. Cell culture media containing 5 µM C6 dihydroceramide (Enzo), TopFluor- Sphingomyelin (Avanti), or ethanol (control) was added to cells and maintained throughout infection. For nocodazole treatment, cells were first infected for 5 h, then washed briefly in HBSS with calcium and magnesium. 20 μM nocodazole or DMSO (control) in cell culture media was then added to cells and maintained throughout infection.
ICS enrichment score quantification
IPAM labeling was used as a proxy to mark the ICSs (Fig. S4a, b). Using NIS-Elements software (Nikon), a fluorescence intensity profile line was drawn across touching inclusions that formed IPAM-positive ICSs. Using the associated fluorescence intensity profile trace, three peaks were measured for each ICS reading: two for the two outer membranes of touching inclusions, called outer periphery (Op), and one for the ICS (Ics). Peak intensity measurements were obtained using the measure horizontal line tool drawn from baseline to maximum peak intensity to provide a numerical intensity for each peak. The enrichment score was calculated by dividing the fluorescence intensity peak of the ICS by the average of the two outer periphery fluorescence intensity peaks. A total of 150 ICS enrichment scores (50 scores from 3 independent experiments) were calculated and plotted for each inclusion membrane protein (see Fig. S2 for a schematic of this method).
Homotypic fusion quantification
Homotypic fusion was measured as previously described16. Briefly, cells were infected at the indicated MOIs, fixed, and labeled at the indicated time. For each of the three independent experiments, 100 cells from each sample were classified as containing one or multiple inclusions. The percentage of total infected cells with a single inclusion (having undergone homotypic fusion) or multiple inclusions (impaired fusion) was quantified.
Western blotting
For Fig. 5g and Fig. S5a, protein lysates were generated from monolayers of HeLa 229 cells infected at an MOI of 1 with WT Ctr, IPAMKO Ctr (Fig. 5g) or IncAKO Ctr (Fig. S5a). To prepare cell lysates, monolayers were washed briefly with cold HBSS without calcium or magnesium and lysed at 24 hpi with cold lysis buffer (1x NuPAGE LDS Sample Buffer [Novex] containing protease and phosphatase inhibitor cocktails [Apex Bio], and 250 units/mL of turbonuclease [Accelagen]) for 10 min. β-Mercaptoethanol was added to a final concentration of 360 mM. Samples were incubated at 95 °C for 10 min followed by centrifugation at 20,800 × g at 4 °C for 10 min. The supernatant was collected, and the total protein concentration was measured using a 660 nm protein assay reagent kit with the ionic detergent compatibility additive (Pierce). Absorbance measurements were obtained at 660 nm on a SpectraMax M2 plate reader (Molecular Devices). Lysates were diluted in LDS-Sample buffer for gel loading and separated on 4–12% Bis-Tris SDS-PAGE gels (Invitrogen). The amount for each sample was normalized to MOMP prior to loading. Samples were transferred to polyvinylidene difluoride membranes for 1 h at 90 volts, at 4 °C, in transfer buffer (25 mM Tris/HCl, 192 mM glycine, 10% methanol). Membranes were blocked for 1 h at RT in blocking buffer (3% bovine serum albumin and 0.05% sodium azide in TBST [25 mM Tris/HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20]). After blocking, membranes were incubated in primary antibody overnight at 4 °C in the blocking buffer. Goat anti-IncA was used at a 1:12,000 dilution, mouse anti-IPAM was used at a 1:1000 dilution, and goat anti-MOMP was used at a 1:20,000 dilution. Following primary antibody incubation, membranes were washed with TBST and then incubated with HRP-conjugated secondary antibodies (Thermo, 1:25,000 dilution) for 1 h at RT in 5% milk diluted in TBST. Membranes were then washed with TBST, followed by several washes with TBS. HRP-positive bands were detected using SuperSignal West Dura extended duration substrate (Thermo). Blots were imaged on a FluorChem R system (ProteinSimple).
Statistical analysis
All data was analyzed in GraphPad Prism v.10. For ICS enrichment score analyses, the same ICS was analyzed for all three Incs (IncA, CT813, and IPAM), and a one-way ANOVA using a matched-paired design was used. In experiments where Inc or lipid probe enrichment was measured across different ICSs, Grubb’s test was applied to detect single outliers followed by a non-matched/non-paired one-way ANOVA analysis. For homotypic fusion quantification, analysis was done with non-matched/non-paired one-way ANOVA. Significance was assumed at a p-value < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We would like to thank Drs. Hackstadt (NIH) for the anti-IncB and anti-CT813 antibodies, Bastidas (Duke University) for the IPAMKO Ctr strain, Ballas (NIH) and Wedergaertner (Thomas Jefferson University) for the lipid probes, and Rockey (Oregon State University), for the anti-IPAM antibody. We would like to thank Drs. Giraudo, Ramage, and Snyder for their critical feedback on the manuscript. We thank the members of the Paumet lab for their valuable discussions during this study. We also thank Yolanda Covarrubias and Jason Hill of the Sidney Kimmel Cancer Center Bioimaging Shared Resource (NCI 5 P30 CA-56036) for their technical assistance. Schematics were created with BioRender.com. All figures were generated in Adobe Illustrator. This work was supported by R01 AI116983 and R01 AI181264 (to F. P).
Author contributions
C.L., J.W., A.L., and F.P. contributed to the experimental design, data acquisition, and manuscript writing and editing. T.Y. and K.H. generated the HeLa mutant cell lines and contributed to the editing of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that all relevant data supporting the findings of this study are included in this published article and its Supplementary Information files, or from the corresponding authors upon request. Source data are provided in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jordan Wesolowski, Email: Jordan.wesolowski@jefferson.edu.
Fabienne Paumet, Email: Fabienne.paumet@jefferson.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53443-7.
References
- 1.Mellman, I. & Warren, G. The road taken: past and future foundations of membrane traffic. Cell100, 99–112 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal, R., Clague, M. J., Durell, S. R. & Epand, R. M. Membrane fusion. Chem. Rev.103, 53–69 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Fischer, A. & Rudel, T. Safe haven under constant attack-the Chlamydia-containing vacuole. Cell Microbiol.20, e12940 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Hackstadt, T., Scidmore-Carlson, M. A., Shaw, E. I. & Fischer, E. R. The Chlamydia trachomatis IncA protein is required for homotypic vesicle fusion. Cell. Microbiol.1, 119–130 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Weber, M. et al. A functional core of IncA is required for Chlamydia trachomatis inclusion fusion. J. Bacteriol.198, 1347–1355 (2016). [DOI] [PMC free article] [PubMed]
- 6.Johnson, C. & Fisher, D. Site-specific, insertional inactivation of IncA in Chlamydia trachomatis using a group II intron. PLoS One8, e83989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannekoek, Y. et al. Interrelationship between polymorphisms of incA, fusogenic properties of Chlamydia trachomatis strains, and clinical manifestations in patients in the Netherlands. J. Clin. Microbiol.43, 2441–2443 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suchland, R. J., Rockey, D. D., Bannantine, J. P. & Stamm, W. E. Isolates of Chlamydia trachomatis that occupy nonfusogenic inclusions lack IncA, a protein localized to the inclusion membrane. Infect. Immun.68, 360–367 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler, W., Suchland, R., Rockey, D. & Stamm, W. Epidemiology and clinical manifestations of unique Chlamydia trachomatis isolates that occupy nonfusogenic inclusions. J. Inf. Dis.184, 879–884 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Xia, M. et al. Chlamydia trachomatis variant with nonfusing inclusions: Growth dynamic and host-cell transcriptional response. J. Inf. Dis.192, 1229–1236 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Scidmore-Carlson, M., Shaw, E., Dooley, C., Fischer, E. & Hackstadt, T. Identification and characterization of a Chlamydia trachomatis early operon encoding four novel inclusion membrane proteins. Mol. Microbiol.33, 753–765 (1999). [DOI] [PubMed] [Google Scholar]
- 12.McNew, J. A. et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature407, 153–159 (2000). [DOI] [PubMed]
- 13.Nickel, W. et al. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc. Natl Acad. Sci. USA96, 12571–12576 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parlati, F. et al. Topological restriction of SNARE-dependent membrane fusion. Nature407, 194–198 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Stegmann, T. & Helenius, A. Viral Fusion Mechanisms. (CRC Press, 1993).
- 16.Cingolani, G. et al. Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA. Nat. Commun.10, 2747 (2019). [DOI] [PMC free article] [PubMed]
- 17.Ronzone, E. & Paumet, F. Two coiled-coil domains of Chlamydia trachomatis IncA affect membrane fusion events during infection. PLoS One8, e69769 (2013). [DOI] [PMC free article] [PubMed]
- 18.Mital, J., Miller, N., Fischer, E. & Hackstadt, T. Specific chlamydial inclusion membrane proteins associate with active Src family kinases in microdomains that interact with the host microtubule network. Cell Microbiol.12, 1235–1249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derré, I., Swiss, R. & Agaisse, H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog.7, e1002092 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gui, L., Ebner, J., Mileant, A., Williams, J. & Lee, K. Visualization and sequencing of membrane remodeling leading to influenza virus fusion. J. Virol.90, 6948–6962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernomordik, L. V. & Kozlov, M. M. Protein-lipid interplay in fusion and fission of biological membranes. Ann. Rev. Biochem. 72, 175–207 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Balla, T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev.93, 1019–1137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posor, Y., Jang, W. & Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol.23, 797–816 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myeong, J., Park, C.-G., Suh, B.-C. & Hille, B. Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquid-ordered/raft domains. Proc. Natl Acad. Sci. USA118, e2025343118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, H. & Wickner, W. Phosphoinositides function asymmetrically for membrane fusion, promoting tethering and 3Q-SNARE subcomplex assembly. J. Biol. Chem.285, 39359–39365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzhanov, D., Weeks, S., Burnett, J. & Rockey, D. Cytokinesis is blocked in mammalian cells transfected with Chlamydia trachomatis gene CT223. BMC Microbiol. 9, 1–10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockey, D. D., Viratyosin, W., Bannantine, J. P., Suchland, R. J. & Stamm, W. E. Diversity within inc genes of clinical Chlamydia trachomatis variant isolates that occupy non-fusogenic inclusions. Microbiology148, 2497–2505 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Mital, J. & Hackstadt, T. Diverse requirements for SRC-family tyrosine kinases distinguish chlamydial species. mBio2, e00031–00011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grieshaber, S., Grieshaber, N. & Hackstadt, T. Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci.116, 3793–3802 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Haines, A., Wesolowski, J., Ryan, N., Monteiro-Bras, T. & Paumet, F. Cross talk between ARF1 and RhoA coordinates the formation of cytoskeletal scaffolds during Chlamydia Infection. MBio12, e0239721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haines, A., Wesolowski, J. & Paumet, F. Chlamydia trachomatis subverts alpha-actinins to stabilize its inclusion. Microbiol. Spectr.11, e0261422 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokes, M. et al. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe17, 716–725 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, Y. et al. Visualizing looping of two endogenous genomic loci using synthetic zinc-finger proteins with anti-FLAG and anti-HA frankenbodies in living cells. Genes Cells26, 905–926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronzone, E. et al. An α-helical core encodes the dual functions of the chlamydial protein IncA. J. Biol. Chem.289, 33469–33480 (2014). [DOI] [PMC free article] [PubMed]
- 35.Delevoye, C., Nilges, M., Dautry-Varsat, A. & Subtil, A. Conservation of the biochemical properties of IncA from Chlamydia trachomatis and C. caviae: oligomerization of IncA mediates interaction between facing membranes. J. Biol. Chem.279, 46896–46906 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Suwa, A. et al. Discovery and functional characterization of a novel small molecule inhibitor of the intracellular phosphatase, SHIP2. Br. J. Pharm.158, 879–887 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackstadt, T., Scidmore, M. A. & Rockey, D. D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl Acad. Sci. USA92, 4877–4881 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scidmore, M., Fisher, E. & Hackstadt, T. Sphingolipids and Glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J. Cell Biol.134, 363–374 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Ooij, C. et al. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol.2, 627–637 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Capmany, A. & Damiani, M. Chlamydia trachomatis intercepts Golgi-derived Sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS One5, e14084 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson, D., Gu, L., Rowe, R. & Beatty, W. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog.5, e1000664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanada, K. et al. Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. J. Biol. Chem.273, 33787–33794 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Monasterio, B. et al. CHO/LY-B cell growth under limiting sphingolipid supply: Correlation between lipid composition and biophysical properties of sphingolipid-restricted cell membranes. FASEB J.35, e21657 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Hackstadt, T., Rockey, D., Heinzen, R. & Scidmore, M. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J.15, 964–977 (1996). [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, E., Fischer, E., Mead, D. & Hackstadt, T. The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic9, 2130–2140 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elwell, C. et al. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog.7, e1002198 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tachida, Y. et al. Chlamydia trachomatis-infected human cells convert ceramide to sphingomyelin without sphingomyelin synthases 1 and 2. FEBS Lett.594, 519–529 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Rogasevskaia, T. & Coorssen, J. Sphingomyelin-enriched microdomains define the efficiency of native Ca2+-triggered membrane fusion. J. Cell Sci.119, 2688–2694 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Skotland, T. & Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun.10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinkla, S. et al. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis.3, e410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maekawa, M., Lee, M., Wei, K., Ridgway, N. & Fairn, G. Staurosporines decrease ORMDL proteins and enhance sphingomyelin synthesis resulting in depletion of plasmalemmal phosphatidylserine. Sci. Rep.6, 35762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitlock, J. & Chernomordik, L. Flagging fusion: phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem.296, 100411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumoux, M., Menny, A., Delacour, D. & Hayward, R. A Chlamydia effector recruits CEP170 to reprogram host microtubule organization. J. Cell Sci.128, 3420–3434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNew, J. A. et al. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol.150, 105–117 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemble, G., Danieli, T. & White, J. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell76, 383–391 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Melikyan, G., White, J. & Cohen, F. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J. Cell Biol.131, 679–691 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharyya, R. & Chakrabarti, P. Stereospecific interactions of proline residues in protein structures and complexes. J. Mol. Biol.331, 925–940 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Caldwell, H., Kromhout, J. & Schachter, J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun.31, 1161–1176 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that all relevant data supporting the findings of this study are included in this published article and its Supplementary Information files, or from the corresponding authors upon request. Source data are provided in this paper.