Abstract
Acute respiratory infections (ARIs) are the most common issue in pediatric clinical practice. They pose a significant public threat, with high morbidity and mortality rates worldwide. Aiming at understanding the epidemiological characteristics of respiratory pathogens and their risk factors among children in eastern Guangdong, China. Samples obtained from 15,993 children hospitalized with ARIs in eastern Guangdong Province were tested for 14 pathogens via multiplex polymerase chain reaction (PCR) from May 2019 to July 2023. The number of hospitalizations for ARIs was correlated with pathogens, age, meteorological parameters, and the pandemic of COVID-19. The data were analyzed by different statistical methods. Among all the samples, the positive rate with ARIs accounted for 68.94% (11,026/15,993) in hospitalized patients. Cytomegalovirus (CMV) (24.49%), Streptococcus pneumoniae (SP) (20.54%), and Respiratory Syncytial Virus (RSV) (14.16%) were the top three pathogens with the greatest infection rates. Among hospitalized patients, there were more single infections in pediatric patients (40.91%, P < 0.001). Compared with bacterial infection and mixed infection, the detection rate of virus infection was higher in pediatric (36.04%, P < 0.001). Age-related increases in Mycoplasma pneumoniae (MP) infection (r = 0.729, P < 0.001) and decreases in RSV infection were observed (r = 0.88, P < 0.001). The virus infection peaked at six months, and the bacterial infection and mixed infection peaked at 1–3 years. Viral pathogens are on the rise in the post-pandemic era. The prevalence of SP infection was more influenced by the Air Quality Index (AQI), RSV infections were more clearly influenced by temperature, and Influenza A virus (IAV) infections were more strongly correlated with both the AQI and relative humidity (P < 0.001). This study highlights the need of keeping an eye on monitoring meteorological factors in assessing hospitalizations for pediatric ARIs in eastern Guangdong, China, especially RSV- and SP-associated hospitalizations.
Keywords: ARIs, Epidemiology, Hospitalization, Meteorological factors
Subject terms: Microbiology, Environmental social sciences
Introduction
Acute respiratory infections (ARIs) are one of the most common pediatric diseases with high morbidity and mortality rates and remain a significant public health issue in both developing and developed countries, posing a global public health threat and economic burden1,2. Although economic development, improved nutrition, and the introduction of vaccines have contributed to a decline in the number of deaths, The World Health Organization in 2019 indicated that respiratory infections continue to rank among the leading causes of mortality, especially in children3. Moreover, it is the leading cause of death from infectious diseases among children under 5 years old4,5.
After the outbreak of the COVID-19 pandemic, ARIs have increasingly received attention as essential public health issues6. Pediatrically hospitalized children accounted for the largest proportion of patients suffering from respiratory diseases, with acute respiratory infections (ARIs) dominating7. Furthermore, ARIs are known to be caused by a variety of respiratory pathogens. Viral infections are the primary cause of ARI morbidity worldwide8,9. Respiratory Syncytial Virus (RSV), Adenovirus (ADV), Influenza A virus (IAV), Influenza B virus (IBV), Parainfluenza virus (PIV), Human bocavirus (HBoV), Human rhinovirus (HRV), Human metapneumovirus (hMPV), and Cytomegalovirus (CMV) are common viruses in ARIs. Additionally, a significant portion of the causes of ARIs are bacterial infections10. The primary respiratory bacteria included Haemophilus influenzae (Hi), Streptococcus pneumoniae (SP), Moraxella catarrhalis (MC), and Bordetella pertussis (BP). In pediatric patients, Mycoplasma pneumoniae (MP) is a prevalent pathogen responsible for ARIs as well. In the aftermath of the COVID-19 pandemic in China, there has been a significant increase in the number of children contracting community-acquired pneumonia caused by MP11, and MP infections need to be treated with antibiotics, therefore, we classified them as bacteria in this study.
Many studies have revealed that the epidemiological characteristics of respiratory pathogens can vary with age, temperature, relative humidity, etc12. Changes in temperature and relative humidity may have an impact on how the pathogen, host, and environment interact, increasing the likelihood of exposure, susceptibility, and infection13,14. Some research has revealed that air pollution has a major impact on the respiratory system15. PM2.5 is an abbreviation for particles with an aerodynamic diameter less than 2.5 μm. Smaller particles are more likely to pass through the blood-gas barrier and become lodged in the alveoli16. It is one of the most widely used and trustworthy markers of air pollution. Numerous epidemiological studies have demonstrated a strong association between particulate air pollution (PM10 and PM2.5) and human illness, including respiratory and cardiovascular disorders17. Different regions, medical conditions, and sanitary circumstances also affect the epidemiological characteristics of respiratory infections in pediatric patients18. We wondered whether there was any relationship between the number of hospitalizations for ARIs and local meteorological factors. Eastern Guangdong is in the eastern part of Guangdong Province and includes five local cities: Shantou, Shanwei, Chaozhou, Jieyang, and Meizhou. with a subtropical climate that is warm and humid. The green line is defined by the Chinese State Environmental Protection Administration (SEPA) as daily average PM2.5 values less than 75 µg/m3, and the air quality in this area is good. It is unknown whether the local air quality influences the transmission of respiratory infections.
Moreover, the current epidemiology statistics and meteorological factors of respiratory pathogens in this area have not been thoroughly examined. A deeper understanding of the actual situation of ARIs in this location can provide information to support the development of appropriate treatment plans and health policies.
Methods
General data
This study enrolled 15,993 pediatric patients with ARIs who were hospitalized at the Children’s Hospital of the Second Affiliated Hospital of Shantou University Medical College, the largest pediatric medical center with the most complete set of specialties and the strongest comprehensive strength in eastern Guangdong. The monthly averages of temperature, relative humidity, PM2.5 concentration and AQI were obtained from the website of the China Air Quality Online Monitoring and Analysis Platform (https://www.aqistudy.cn). These meteorological data were available for the period from May 2019 through July 2023.
From May 2019 to July 2023, 9487 males (59.30%) and 6506 females (40.60%) aged 29 days to 14 years were selected. The diagnostic features of ARIs include cough, fever, cold, sore throat or shortness of breath within the first 7 days. The diagnostic criteria for respiratory infection were cough, sputum production, and one of three conditions: (1) fever, cold, sore throat or shortness of breath; (2) an increase in the total white blood cell count and/or neutrophil proportion; and (3) wet rales appearing in the lungs and/or inflammatory infiltrative lesions in the lungs on X-ray examination. The exclusion criteria were as follows: (1) newborns less than 28 days old; (2) insufficient throat swab samples, for example, too few cells were sampled or nucleic acid extraction; (3) incomplete clinical data, such as lack of medical records or clinical diagnosis; (4) respiratory tract chest radiograph changes caused by pulmonary embolism, heart failure, pulmonary edema, lung cancer, and other noninfectious causes; and (5) except COVID-19 infection. Admission criteria for the Pediatric Intensive Care Unit (PICU) were as follows: (1) poor general condition; (2) refusal to eat or dehydration symptoms; (3) impaired consciousness; (4) a significantly increased respiratory rate (infant RR > 70 beats/min, adult RR > 50 beats/min); (5) respiratory distress (moaning, nasal flutter, convulsions); (6) cyanosis; (7) pulse oximeter ≤ 0.9; (8) pleural effusion; (9) multiple leaf involvement or ≥ 2/3; (10) extrathoracic complications.
The monthly averages of temperature, relative humidity, PM2.5 concentration and AQI were obtained from the website of the China Air Quality Online Monitoring and Analysis Platform (https://www.aqistudy.cn). These meteorological data were available for the period from May 2019 through July 2023.
Multiple PCR
After receiving informed consent from the patients’ parents, nasopharyngeal swabs were collected by a professional physician. In addition, we recorded the patients’ name, age, gender, sampling time, and ward (PICU/general wards). These samples were kept in a refrigerator at 4 °C. DNA/RNA were extracted from the samples by TIANamp virus DNA/RNA fast kit or TIANamp bacteria DNA kit, and respiratory pathogen nucleic acids were detected through multiplex PCR technology. Target bands are massively amplified via temperature modulation. A positive result is indicated when the primers are complementary to these bands. It is fast, sensitive, and specific for the detection of different pathogens19–21. A positive nucleic acid test result for most pathogens can indicate infection in respiratory tract. The above operations were carried out in the clinical testing center of Guangzhou Da’an.
Statistical methods
Statistical analysis was performed with SPSS 25.0. All variables were presented as counts and/or percentages and were tested by the Chi-square test or Fisher’s exact test. The Kruskal-Wallis test was utilized for comparisons between two or more groups, and Pearson or Spearman rank correlations were used for correlation analysis. With a test level ɑ = 0.05 (two-sided), P < 0.05 was considered to indicate statistical significance. Moreover, the data were drawn in Origin 2021 and GraphPad Prism 8.
Results
The distribution features of ARIs in hospitalized children
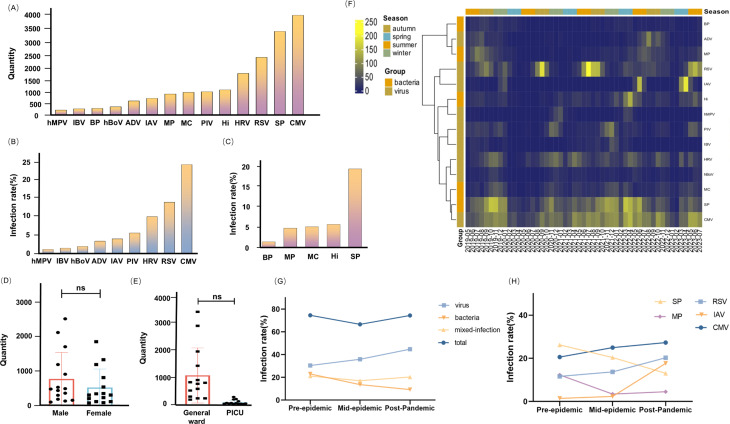
A total of 15,993 pediatric patients (aged 29 days to 14 years) with ARIs in the hospital were identified, 68.94% of whom were positive. In comparison to other pathogens, we discovered that the detection rates of CMV (24.49%), SP (20.54%), RSV (14.16%), HRV (10.2%) and Hi (6.15%) were significantly higher (Fig. 1A). CMV and RSV were the viruses with the highest detection rates. The infection rate of SP was significantly greater than that of the other bacteria (Fig. 1B,C).
Fig. 1.
Distribution of respiratory pathogens. (A) The quantity of different Pathogens among children with ARIs, 2019–2023. (B) The infection rate of viral infection. (C) The infection rate of bacterial infection. (D) The bar chart that described the gender distribution of RIs: “ns” represents P > 0.05. (E) The distribution in general ward and Pediatric Intensive Care Unit (PICU). (F) The heatmap of ARIs in the eastern Guangdong. The deeper the yellow color, the larger the number of infections. (G) The overall infection rate changes of bacteria, viruses, etc. in the pre-, mid-, and post-epidemic. The pre-epidemic was from May 2019 to December 2019, the mid-epidemic was from January 2020 to December 2022, and the post-pandemic was from January 2023 to July 2023. (H) The infection rate changes for SP, MP, RSV, IAV, and CMV at different stages of the pandemic.
There were 6625 (60.10%) males and 4401 (39.90%) females among the positive cases. There was no significant effect of gender on the incidence of respiratory infections in the region (P > 0.05) (Fig. 1D). According to the ward categorization, 10,269 (93.13%) patients were in the general ward, and 757 (6.87%) were in the PICU. There were statistical differences in this distribution in terms of mathematics. However, with clinical analysis showing that the number of children in general wards was significantly greater than that in the PICU, we cannot infer which pathogen causes more serious diseases; combined with these data, we inferred that there was no significant difference in this classification (Fig. 1E).
We analyzed seasonal and monthly changes through heatmaps. Eastern Guangdong has a distinct subtropical monsoon climate, with hot summers and mild winters. The spring lasts from March to May, the summer from June to August, the fall from September to November, and the winter from December to February of the following year. IAV and RSV have distinctive seasonal distribution patterns (Fig. 1F). During the COVID-19 pandemic, the Chinese government implemented a number of measures, including house quarantine, wearing masks, and vaccination, to restrict the spread of COVID-19, which has a substantial influence on other respiratory infections. The pandemic era consists of three stages: pre-epidemic, mid-epidemic, and post-pandemic. Viral pathogens are on the rise in the post-pandemic era (Fig. 1G), especially RSV and IAV. The incidence of MP infections is also experiencing an upward trend (Fig. 1H).
Distribution characteristics at different ages
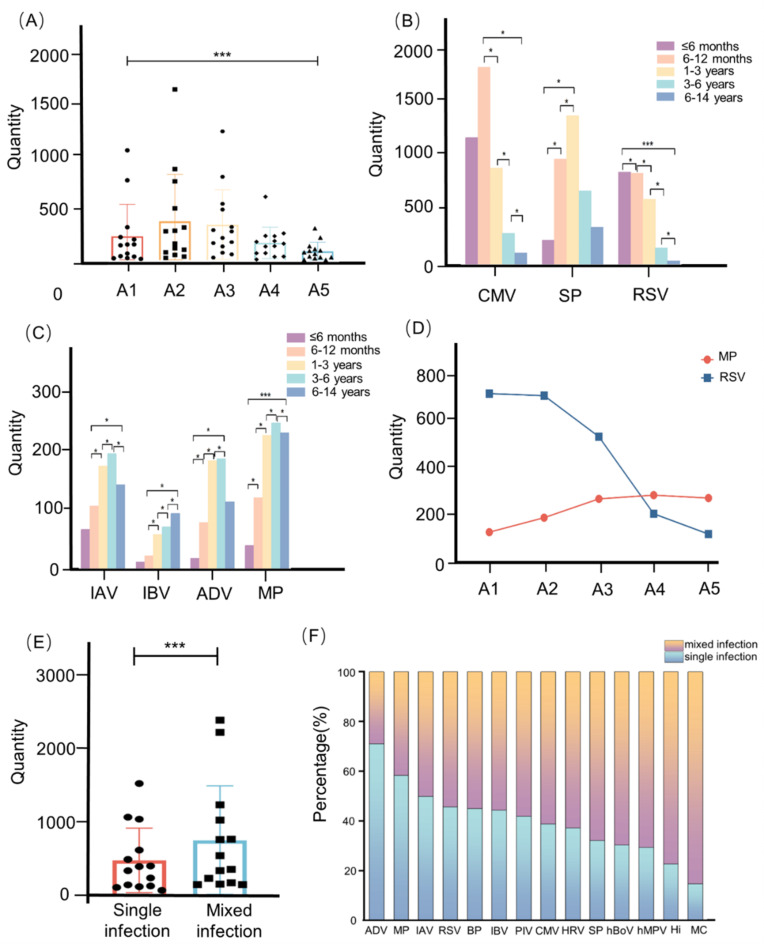
The samples were categorized into five age groups: 29 days to 6 months (A1 in Figs. 2), 6 months to 1 year (A2 in Figs. 2), 1 year to 3 years (A3 in Figs. 2), 3 years to 6 years (A4 in Fig. 2), and 6 years to 14 years (A5 in Fig. 2). Age-related differences were statistically significant (Fig. 2A). Multiple comparisons revealed a significant difference in age among the MP, RSV, IAV, IBV, CMV, ADV and SP (P < 0.05). CMV infection showed a decreasing trend in children after 6 months. The rate of IAV and IBV infection in children older than six months exhibited an increasing trend. The prevalence of SP infection in children under three years old was increasing (Fig. 2B,C). Additionally, we found that MP infection increased with age (r = 0.729, P < 0.001) and that RSV infection decreased with age (r = 0.88, P < 0.001) (Fig. 2D).
Fig. 2.
Distribution characteristics in different ages. (A) The bar chart described the age distribution of RIs: * represents P < 0.05, *** represents P < 0.001. (B) The column diagram described the age distribution of CMV, SP, and RSV. (C) The column diagram described the age distribution of IAV, IBV, ADV, and MP. (D) The linear chart depicted the distribution of MP and RSV at different ages. (E) The error bar chart of single and mixed infections. (F) The distribution of single and mixed infection rates.
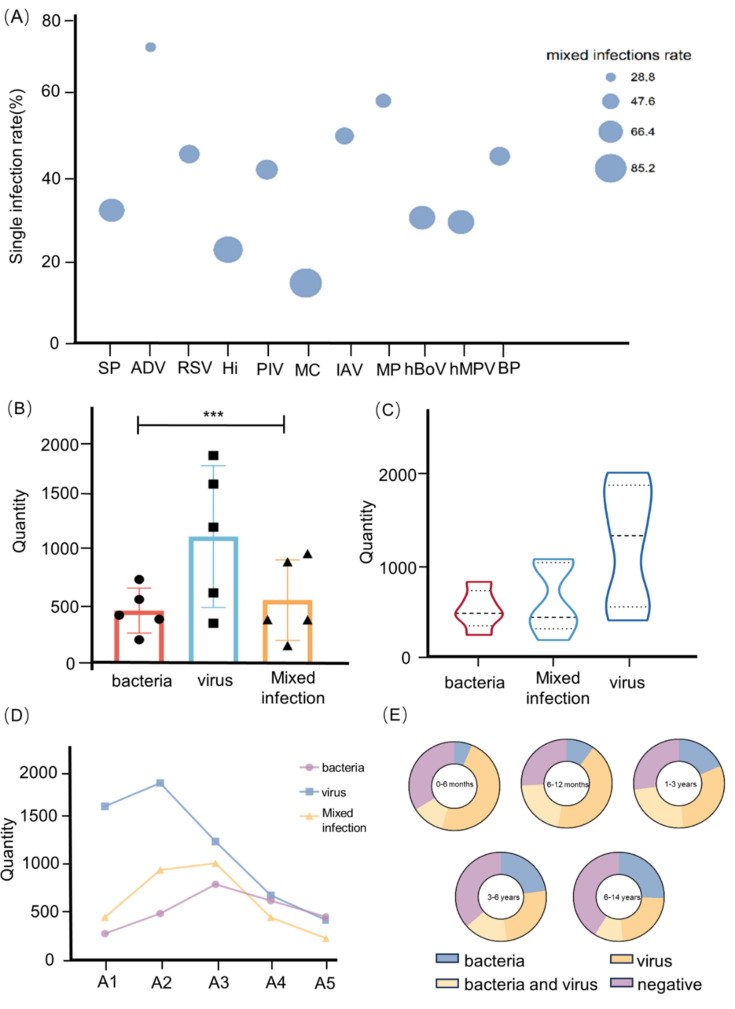
A single infection indicated the presence of only one single pathogen. There were differences in the distributions of single and mixed infections (P < 0.05) (Fig. 2E). Among single infections, ADV (71.14%), MP (59.37%), and IAV (50%) were the top three pathogens. Mixed infections were easily linked to MC (85.08%), Hi (77.11%), and hMPV (70.53%) (Fig. 2F). By analyzing the single infection and mixed infection of each pathogen, we detected differences, except for CMV, HRV, and IBV (the P values for these three pathogens were greater than 0.05) (Fig. 3A). Moreover, we categorized the pathogens into three groups based on their type: viruses, bacteria, and mixed diseases (P < 0.001) (Fig. 3B). To make the statistical analysis easier, we were grouping MP under the bacterial category. Hi, SP, MC, BP, and MP were the members of the bacterial group. The remaining nine pathogens were classified in the virus category. A mixed infection included bacteria and viruses. According to our analysis, viral infections were more common in pediatric patients with ARIs (Fig. 3C). Interestingly, we observed that the age peak for viral infections was between 6 and 12 months, while the age peak for bacterial and mixed infections was between 1 and 3 years, and all infections showed a declining trend with age after the age of three (Fig. 3D,E).
Fig. 3.
Distribution of different infections. (A) The bubble chart of different infections. The mixed infection rate increased with bubble size. (B) The error bar chart of bacteria, viruses and mixed infections. (C) The violin plot showed the distribution of different infections. (D) The linear chart about distribution for different infections: A1 represents 29 days to 6 months, A2 represents 6 months to 1 year, A3 represents 1 year to 3 years, A4 represents 3 years to 6 years, A5 represents 6 years to 14 years. (E) The sector graph of different infections.
Temporal distribution characteristics
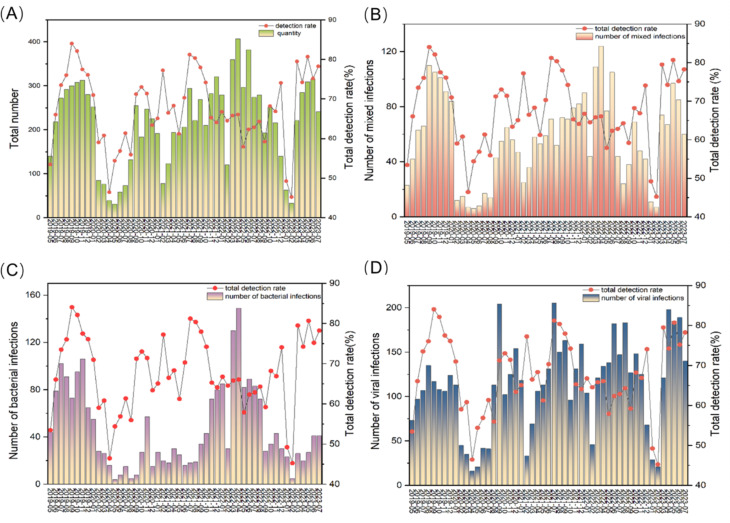
We observed that RSV infection peaked in September 2020 and July 2021, and IAV infection peaked in June 2022 and April 2023. The RSV infection peak generally occurs between April and September. We found two significant decreases: in April 2020 and in February 2023 (Fig. 4A). The peak number of bacteria and mixed infections occurred in April 2022 (Fig. 4B,C). The virus infection peak usually occurs between June and September in this area (Fig. 4D).
Fig. 4.
Temporal distribution characteristics of different infection. (A) The graph of overall quantity of pathogens and total detection rate per month. (B) The graph of mixed infection distribution and total detection rate per month. (C) The graph of bacterial infection distribution and total detection rate per month. (D) The graph of viral infection distribution and total detection rate per month.
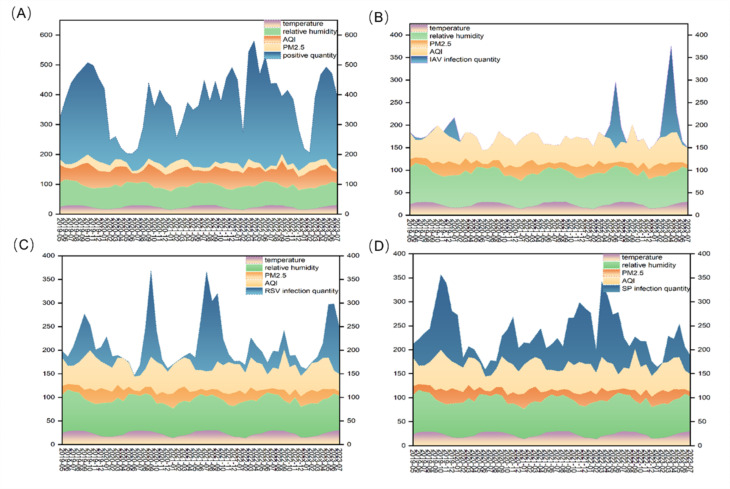
Temperature, relative humidity, PM2.5, and AQI were linked to the number of ARIs in hospitalized children (P < 0.001). From May 2019 to July 2023, the median temperature was 22.0 °C, the median relative humidity was 72%, the median PM2.5 concentration was 21 µg/m3, and the median air quality index (AQI) was 59. Upon analyzing the heatmap, we observed that RSV, CMV, IAV, and SP had significantly higher infection rates than did the other pathogens. As a result, we concentrated on interpreting the relationships between these pathogens and meteorological data. As shown in Fig. 5A, the trends and variations in these meteorological data, along with the quantity of ARIs, were obvious. The distributions of IAV, RSV and SP infections in relation to the meteorological data were shown in Fig. 5B, C&D. Interestingly, we discovered a significant correlation (r = 0.442, P < 0.001) between the AQI and SP infection. Temperature and RSV infection were strongly related (r = 0.607, P < 0.001). There was a weak correlation between IAV infection and the AQI (r = 0.299, P < 0.001), but there was no correlation between IAV infection and temperature (Table 1). Furthermore, outbreaks of these pathogens, which are closely linked to climate change, are predicted using certain temporal prediction models, such as the autoregressive integrated moving average (ARIMA).
Fig. 5.
Monthly distribution of pathogens detection in ARIs and meteorological conditions (temperature, relative humidity, PM2.5 and AQI). (A) Monthly distribution of overall infection and meteorological conditions. (B) Monthly distribution of IAV infection and meteorological conditions. (C) Monthly distribution of RSV infection and meteorological conditions. (D) Monthly distribution of SP infection and meteorological conditions.
Table 1.
Spearman Rank correlation coefficients for the associations between Meteorological Parameters and some pathogens.
| Pathogens | Temperature | Relative humidity | PM2.5 | AQI | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| All pathogens | 0.044 | < 0.001 | 0.038 | < 0.001 | 0.038 | < 0.001 | 0.170 | < 0.001 |
| SP | − 0.196 | < 0.001 | − 0.272 | < 0.001 | 0.377 | < 0.001 | 0.442 | < 0.001 |
| CMV | 0.041 | 0.01 | − 0.107 | < 0.001 | − 0.06 | < 0.001 | 0.112 | < 0.001 |
| RSV | 0.607 | < 0.001 | 0.1 | < 0.001 | − 0.364 | < 0.001 | − 0.157 | < 0.001 |
| IAV | 0.001 | 0.981 | 0.211 | < 0.001 | − 0.102 | 0.009 | 0.299 | < 0.001 |
Discussion
In this study, the positive detection rate of pathogens among hospitalized children with respiratory infection was 68.94%, which was comparable to that in previous reports; for example, in Europe and the United States (60.6%)22, and the rate of virus infection was 52.23%, which was similar to the detection rate of respiratory viruses in a domestic study of 56.6%23. CMV was the pathogen with the highest detection rate in the region. CMV is generally prevalent in the Chinese population. CMV is mainly transmitted through the respiratory tract (saliva, etc.), digestive tract, blood transfusion, and other methods. In vivo, CMV is most likely to infect the lung24, especially alveolar macrophages25. Some studies have reported that the viral shedding rate of nasopharyngeal CMV is 34.4–39.5%26. Moreover, it is believed that most children with CMV infection are in a state of latent infection, and even though CMV replication occurs in vivo, most of them are asymptomatic27. Combined with the high frequency of mixed infections, the high detection rate of CMV in our research was more likely explained by a latent infection or a past infection.
Some studies reported that the detection rate of RSV in patients under 5 years of age was the highest (18.9%)23, which was similar to the results of our study. The proportion of children under 3 years old infected with RSV was the highest in eastern Guangdong, China. In our study, we confirmed that there was a strong correlation between RSV infection and age. RSV infection decreased with increasing age. RSV infection in infants and young children is common because the immune system is still immature and the number of dendritic cells is low28. Moreover, MP infection increased with increasing age. These results are consistent with those of national and international studies29,30. MP is the most common pathogen in children with ARIs31–33. MP infection is more prevalent in the summer and autumn in southern China11. This finding aligns with our observations. The characteristics of MP infection may be due to preschool children’s collective learning in kindergarten. MP spreads easily in densely populated places34,35.
The age peak for virus infections was between 6 and 12 months in our study, and the subsequent infection rate decreased with age. This finding appears to be consistent with a significant decrease in maternal-source antibodies at 6 months of age and the acquisition of specific immunity to an increasingly large array of virus types occurring with age14. The age peak for bacterial and mixed infections was between 1 and 3 years. Children in this age group have achieved a change from standing to walking, and their level of exposure to their surroundings has significantly increased. They might enroll in kindergarten, thereby increasing their collective living and expanding their friendship circle. After the age of three, all infections decreased with age. This may be related to the maturation of the immune system and past infection experience. We discovered that there were two sharp decreases in ARIs in February 2023 and April 2020. This may be related to the strict COVID-19 pandemic control measures, including lockdowns, social distancing, wearing masks, and comprehensive vaccination campaigns. In the post-pandemic era, we discovered that there was a rising incidence of viral infections. In response to this phenomenon, many scholars have proposed hypotheses such as “immune debt,”36 suggesting that due to protective measures during the pandemic, children have had limited exposure to pathogens, which in turn hampers the strengthening of immune stimulation., as well as delays or absence of vaccines37 for children in the mid-epidemic period, has resulted in the susceptibility of children’s immune systems to attacks. Some researchers also put forward the idea that COIVD-19 may have an underlying mechanism for altering immunological function38–40, however this is currently debated. Some researchers have posited that the rise in pediatric viral infection case counts following the emergence of COVID-19 can be partly attributed to an increase in testing41. Overall, it is crucial to detect the prevalence of respiratory pathogens, which can form effective early warning signals and avoid large-scale health threats.
Climate factors certainly have some influence on the spread of respiratory diseases. The peak of IAV infections did not occur in winter as others have reported, possibly related to the regional climate and economic status. The median temperature was 22.0 °C, and the median relative humidity was 72%. In general, the weather in this area was warmer and flatter. The temperature in this area does not seem to have a strong impact on IAV infection outbreaks. However, according to our research, there was a certain correlation between IAV infection and the AQI in this region, however, the underlying reasons remain unknown and more in-depth research is needed. Moreover, we also found that some pathogens are significantly associated with meteorological parameters. The RSV infection rate was 20.53% (2264). Temperature appeared to be the most significant meteorological factor associated with the RSV trends. This result was consistent with some previous research13,42. Our data indicated that the outbreaks of RSV were associated with high temperatures and low PM2.5 concentrations. Although the reasons for this are not clear, it is likely that higher temperature changes may affect the respiratory epithelium, changing its permeability and making the body more vulnerable to infections43, leading to an outbreak of RSV. Generally speaking, the body’s immune and respiratory systems are more likely to be harmed by higher concentrations of PM2.5, which increases the body’s vulnerability to viral infections. However, why would a decrease in PM2.5 lead to an RSV outbreak? The median PM2.5 concentration was 21 µg/m3 in this area. In other words, the area’s air quality has remained good, and lower PM2.5 levels may facilitate virus transmission. Remarkably, we discovered a correlation between the AQI and the frequency of hospitalizations for SP. Poorer air quality is indicated by a higher AQI. The respiratory epithelium may become inflamed and irritated due to these dangerous airborne particles. This causes an outbreak of SP infection.
The high RSV and SP infection prevalence in this area is a major concern, resulting in considerable disease loads and impacts. Based on temporal rhythmicity, the prevalence of RSV and SP infections was predictable within specified bounds according to the temporal prediction models. Predicting the prevalence of RSV infection and SP infection could provide useful guidance to physicians who are promptly coordinating seasonal prophylaxis and initiating early antiviral strategies to minimize RSV and SP transmission and alleviate the region’s acute respiratory infection-related medical resource shortage. Meanwhile, the dissemination of vaccination and health education in the area should be strengthened, especially for children under 3 years old.
Our study had several limitations. Firstly, we detected only 14 common pathogens, and the number of detected pathogen species was limited. Our subsequent studies will research more pathogens. Secondly, a positive nucleic acid test result from multiplex PCR can be interpreted as an infection for the majority of infections. Some pathogens are opportunistic pathogens that can be detected in the respiratory tract of asymptomatic people. With the current technology, we cannot determine whether the pathogens are colonizing or pathogenic. Thirdly, some variables, such as vaccine status, underlying conditions, and household demographics of children, were not recorded, which is significant for pediatric immune system development. These are important influencing factors for respiratory infections. In the future, we’ll go further into these elements by incorporating them in the affecting factors. Lastly, the potential impact of the COVID-19 pandemic on the epidemiology of other respiratory infections due to the implementation of preventive measures like mask-wearing and social distancing.
Conclusion
This study revealed the epidemiological features of important pediatric respiratory infections in eastern Guangdong, China, emphasizing the need for respiratory pathogen surveillance and the importance of vaccination and health education, particularly for children under the age of three.
Acknowledgements
The authors are very grateful to the doctors and nurses who helped collect the pharyngeal samples.
Abbreviations
- ARIs
Acute respiratory infections
- RSV
Respiratory Syncytial Virus
- ADV
Adenovirus
- IAV
Influenza A virus
- IBV
Influenza B virus
- PIV
Parainfluenza virus
- HBoV
Human bocavirus
- HRV
Human rhinovirus
- hMPV
Human metapneumovirus
- CMV
Cytomegalovirus
- Hi
Haemophilus influenzae
- SP
Streptococcus pneumoniae
- MC
Moraxella catarrhalis
- BP
Bordetella pertussis
- MP
Mycoplasma pneumoniae
- AQI
Air Quality Index
Author contributions
CX Lin and Min Lin contributed to the conception and design of the study; TD Yang contributed to the interpretation of the results and the writing of the manuscript; HB Lian contributed to the acquisition, analysis and interpretation of the results; and JY Liao, YM Zeng, and JM Li contributed to the modification and translation of the study. All the authors critically reviewed and provided intellectual input for preparing the manuscript. All the authors have read and approved the final version of the manuscript submitted for publication.
Funding
This study was supported by the Guangdong Science and Technology Special Fund (project approval numbers: 49 and 135) and the Guangdong Medical Research Fund (project number: A2020551).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The informed consent to participate was obtained from the parents of the children, and the study was approved by the Ethics Committee (full name: the Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College) (reference number_2020-31_). All experiments were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tiandan Yang and Haobin Lian.
Contributor Information
Chuangxing Lin, Email: 2179941615@qq.com.
Min Lin, Email: konfutea@hotmail.com.
References
- 1.Cilloniz, C., Martin-Loeches, I., Garcia-Vidal, C., San Jose, A. & Torres, A. Microbial etiology of Pneumonia: Epidemiology, diagnosis and resistance patterns. Int. J. Mol. Sci. 17. 10.3390/ijms17122120 (2016). [DOI] [PMC free article] [PubMed]
- 2.Li, Z. J. et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026. 10.1038/s41467-021-25120-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams, B. G., Gouws, E., Boschi-Pinto, C., Bryce, J. & Dye, C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2, 25–32. 10.1016/s1473-3099(01)00170-0 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Diseases, G. B. D. & Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet (London England). 396, 1204–1222. 10.1016/S0140-6736(20)30925-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ning, G. et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001–2015: a systematic review. Hum. Vaccines Immunotherap. 13, 2742–2750. 10.1080/21645515.2017.1371381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, Z. J. et al. Broad impacts of Coronavirus Disease 2019 (COVID-19) pandemic on acute respiratory infections in China: an observational study. Clin. Infect. Dis. 75, e1054–e1062. 10.1093/cid/ciab942 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niederman, M. S. & Torres, A. Respiratory infections. Eur. Respir. Rev. 31. 10.1183/16000617.0150-2022 (2022). [DOI] [PMC free article] [PubMed]
- 8.Ramaekers, K. et al. Prevalence and seasonality of six respiratory viruses during five consecutive epidemic seasons in Belgium. J. Clin. Virol. 94, 72–78. 10.1016/j.jcv.2017.07.011 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Zhu, G. et al. Epidemiological characteristics of four common respiratory viral infections in children. Virol. J. 18. 10.1186/s12985-020-01475-y (2021). [DOI] [PMC free article] [PubMed]
- 10.Wei, L. et al. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009–2013. Medicine 94, e742. 10.1097/md.0000000000000742 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan, C. et al. Current status of Mycoplasma pneumoniae infection in China. World J. Pediatr.10.1007/s12519-023-00783-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriyama, M., Hugentobler, W. J. & Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 7, 83–101. 10.1146/annurev-virology-012420-022445 (2020). [DOI] [PubMed] [Google Scholar]
- 13.du Prel, J. B. et al. Are meteorological parameters associated with acute respiratory tract infections? Clin. Infect. Dis. 49, 861–868. 10.1086/605435 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Graham, N. M. The epidemiology of acute respiratory infections in children and adults: a global perspective. Epidemiol. Rev. 12, 149–178. 10.1093/oxfordjournals.epirev.a036050 (1990). [DOI] [PubMed] [Google Scholar]
- 15.North, C. M. et al. Air Pollution in the Asia-Pacific Region. A Joint Asian Pacific Society of Respirology/American Thoracic Society Perspective. Am. J. Respir. Crit. Care Med. 199, 693–700. 10.1164/rccm.201804-0673PP (2019). [DOI] [PubMed] [Google Scholar]
- 16.Sun, Q., Hong, X. & Wold, L. E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121, 2755–2765. 10.1161/circulationaha.109.893461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang, Y. et al. PM2.5 in Beijing—temporal pattern and its association with influenza. Environ. Health Glob. Access. Sci. Source 13. 10.1186/1476-069x-13-102 (2014). [DOI] [PMC free article] [PubMed]
- 18.Lin, C. X. et al. Pathogen spectrum changes of respiratory tract infections in children in Chaoshan area under the influence of COVID-19. Epidemiol. Infect. 149. 10.1017/s0950268821001606 (2021).
- 19.Yang, G. et al. Comparison of commercial systems for extraction of nucleic acids from DNA/RNA respiratory pathogens. J. Virol. Methods 171, 195–199. 10.1016/j.jviromet.2010.10.024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, H. S. et al. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin. Microbiol. Infect. 24, 1055–1063. 10.1016/j.cmi.2017.11.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan, Y. et al. A multiplex liquid-chip assay based on Luminex xMAP technology for simultaneous detection of six common respiratory viruses. Oncotarget 8, 96913–96923. 10.18632/oncotarget.18533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aston, S. J. et al. Etiology and risk factors for mortality in an adult community-acquired pneumonia cohort in Malawi. Am. J. Respir. Crit. Care Med. 200, 359–369. 10.1164/rccm.201807-1333OC (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu, Y. et al. A multicenter study of viral aetiology of community-acquired pneumonia in hospitalized children in Chinese mainland. Virol. Sin. 36, 1543–1553. 10.1007/s12250-021-00437-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.li, Jirong, Hongmei, Q. & Huanji, C. Clinical analysis of 49 infants with cytomegalovirus pneumonia. J. Clin. Pediatr. 544–546 (2007).
- 25.Baasch, S. et al. Cytomegalovirus subverts macrophage identity. Cell 184, 3774–3793. 10.1016/j.cell.2021.05.009 (2021). [DOI] [PubMed]
- 26.Miaohui, H. et al. Analysis of 30 cases of respiratory cytomegalovirus infection in children. J. Clin. Pediatr. 114–115 (1997).
- 27.Feng, F. & Yongsui, D. Cytomegalovirus infection and diagnosis of cytomegalovirus infection. Chin. J. Pediatr. 4–6 (1999).
- 28.Ping, L. Advances in pathogenic mechanism of respiratory syncytial virus. Int. J. Pediatr. 48, 459–463 (2021). [Google Scholar]
- 29.Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845. 10.1056/NEJMoa1405870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, H. et al. Surveillance of Mycoplasma pneumoniae infection among children in Beijing from 2007 to 2012. Chin. Med. J. 127, 1244–1248 (2014). [PubMed] [Google Scholar]
- 31.Chun, W. et al. Pathogen spectrum of febrile respiratory syndrome among hospitalized children in Shanghai from 2019 to 2020. Chin. J. Exp. Clin. Virol. 36, 270–275 (2022). [Google Scholar]
- 32.Liu, J. et al. Viral and bacterial coinfection among hospitalized children with respiratory tract infections. Am. J. Infect. Control 48, 1231–1236. 10.1016/j.ajic.2020.01.013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oumei, H. et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J. Med. Virol. 90, 421–428. 10.1002/jmv.24963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foy, H. M., Kenny, G. E., Cooney, M. K. & Allan, I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J. Infect. Dis. 139, 681–687. 10.1093/infdis/139.6.681 (1979). [DOI] [PubMed] [Google Scholar]
- 35.Tong, L., Huang, S., Zheng, C., Zhang, Y. & Chen, Z. Refractory Mycoplasma pneumoniae pneumonia in children: early recognition and management. J. Clin. Med. 1110.3390/jcm11102824 (2022). [DOI] [PMC free article] [PubMed]
- 36.Rubin, R. From immunity debt to immunity theft-how COVID-19 might be tied to recent respiratory disease surges. Jama 331, 378–381. 10.1001/jama.2023.26608 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Cohen, R. et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. 51, 418–423. 10.1016/j.idnow.2021.05.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popescu, I. et al. CD4(+) T-Cell dysfunction in severe COVID-19 disease is tumor necrosis Factor-α/Tumor necrosis factor receptor 1-Dependent. Am. J. Respir. Crit. Care Med. 205, 1403–1418. 10.1164/rccm.202111-2493OC (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.André, S. et al. T cell apoptosis characterizes severe Covid-19 disease. Cell Death Differ. 29, 1486–1499. 10.1038/s41418-022-00936-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roukens, A. H. E. et al. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8(+) T cell responses following COVID-19. Nat. Immunol. 23, 23–32. 10.1038/s41590-021-01095-w (2022). [DOI] [PubMed] [Google Scholar]
- 41.Petros, B. A., Milliren, C. E., Sabeti, P. C. & Ozonoff, A. Increased pediatric RSV case counts following the emergence of SARS-CoV-2 are attributable to increased testing. MedRxiv. 10.1101/2024.02.06.24302387 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirimi, N. et al. Respiratory syncytial virus activity and climate parameters during a 12-year period. J. Med. Virol. 88, 931–937. 10.1002/jmv.24430 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Deal, E. C., McFadden, E. R., Ingram, R. H., Breslin, F. J. & Jaeger, J. J. Airway responsiveness to cold air and hyperpnea in normal subjects and in those with hay fever and asthma. Am. Rev. Respir. Dis. 121, 621–628. 10.1164/arrd.1980.121.4.621 (1980). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.