Abstract
To investigate the correlation of cervical central lymph node metastasis (CLNM) in stage T1a unifocal papillary thyroid carcinoma (PTC) with the clinicopathological characteristics, ultrasonography features and the number of lymph node dissection, and to analyze the risk factors of CLNM. Data from 493 unifocal PTC patients (T1a) who underwent partial or total thyroidectomy and pCLND at the Guizhou Provincial People’s Hospital were collected and retrospectively analyzed. They were divided into two groups in accordance with cervical CLNM or not. Their information, including clinical characteristics, ultrasound (US) features, pathological results, and other characteristics of the groups, was analyzed and compared using univariate and multivariate logistic regression analyses. A total of 493 patients were eligible in this study. Among them, 33.7% (166/493) of PTC patients had cervical CLNM, and 66.3% (327/493) did not. The two groups were compared using a univariate analyses, and there were no significant differences between the two groups in age, maximum tumor size, tumor location, aspect ratio, boundary, morphology, echogenicity, BRAFV600E and HT (P > 0.05), and there were significant differences between gender, capsule contact, microcalcifications, rich vascularity, and number of lymph node dissection (P < 0.05). A multivariate logistic regression analyses was performed to further clarify the correlation of these indices. However, only male (OR = 1.770, P = 0.009), microcalcifications (OR = 1.791, P = 0.004), capsule contact (OR = 1.857, P = 0.01), and number of lymph node dissection (OR = 2.274, P < 0.001) were independent predictors of cervical CLNM. In conclusion, four independent predictors of cervical CLNM, including male, microcalcifications, capsule contact, and number of lymph node dissection, were screened out. Therefore, a comprehensive assessment of these risk factors should be conducted when designing individualized treatment regimens for PTC patients.
Keywords: Papillary thyroid carcinoma, Central lymph node metastasis, BRAFV600E mutation, Prophylactic central lymph node dissection
Subject terms: Cancer, Diseases, Health care, Medical research
Introduction
Thyroid nodules are a prevalent condition among Chinese adults, with a prevalence rate of 20.43% when nodules larger than 0.5 cm in diameter are detected through ultrasound examination. Among these nodules, 8–16% are identified as malignant tumors1. Differentiated thyroid cancer (DTC), which includes papillary thyroid carcinoma (PTC) and follicular carcinoma, is the most prevalent malignant tumor affecting the thyroid gland. There has been a significant increase in the incidence of thyroid cancer in recent years. Despite its relatively low malignancy, DTC still poses a threat to patients’ lives, health, and overall well-being. Furthermore, standardized diagnosis, treatment, and follow-up are crucial due to the low mortality rate and long survival period associated with DTC.
The main route of spread for PTC is lymph node metastasis in the neck, with the central compartment lymph nodes being a frequently affected site. According to literature reports, the rate of central lymph node metastasis (CLNM) in papillary thyroid microcarcinoma (PTMC) ranges from 24.1–64.1%2. Clinicians can use analysis of clinical pathology and ultrasound imaging features to determine the presence of CLNM3,4.
Postoperative complications, such as hypoparathyroidism and recurrent laryngeal nerve injury, are the main concerns associated with central compartment lymph node dissection. Enhanced training, improved surgical skills, and increased experience have been shown to reduce these complications5. Additionally, following thyroid surgery, inevitable issues such as tissue adhesion and surgical scarring can complicate future surgeries, making the complete and thorough removal of the lesion and the minimization of postoperative complications, particularly in terms of preserving the function of the recurrent laryngeal nerve and parathyroid glands, more challenging. Furthermore, tumor recurrence has a significant impact on the lives and psychological well-being of patients6,7. The objective of this study is to analyze the clinical and pathological characteristics, along with ultrasound features, of stage T1a solitary PTC patients. Additionally, it aims to identify high-risk factors for central lymph node metastasis and investigate the relationship between the number of lymph nodes removed and CLNM, a perspective that has received limited scholarly attention.
Materials and methods
Patient selection
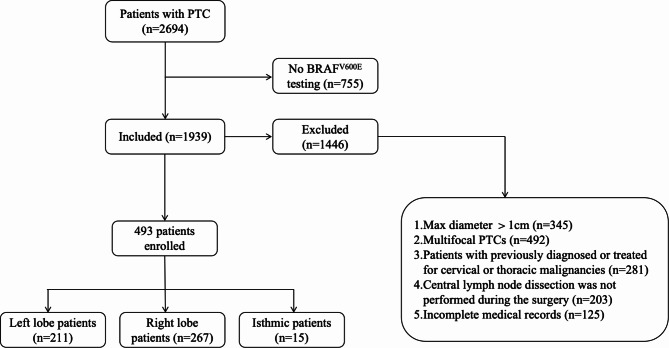
This retrospective study received approval from the Institutional Review Board of Guizhou Provincial People’s Hospital, and the requirement for informed consent was waived due to its retrospective nature. The study were performed in accordance with the relevant guidelines and regulations. The study involved reviewing clinical medical data of patients diagnosed with pathologically confirmed PTC between October 2021 and June 2023. Inclusion criteria were as follows: (1) Preoperative ultrasound indicating a suspected malignant tumor; (2) Intraoperative frozen pathology confirming T1a stage PTC with concurrent prophylactic central lymph node dissection (pCLND); (3) Postoperative pathology confirming stage T1a solitary PTC; (4) Clinically negative central compartment lymph node (cN0); (5) Absence of previous thyroid surgery or radioactive iodine therapy. Exclusion criteria were as follows: (1) Concurrent presence of other types of thyroid cancer; (2) Multifocal thyroid cancer; (3) Incomplete medical records; (4) Simultaneous presence of other malignant tumors. According to the guidelines of the Chinese Anti-Cancer Association for Thyroid Cancer (2022 edition), the upper boundary of pCLND extends to the thyroid cartilage, the lower boundary is the suprasternal notch, and the lateral boundary is the medial side of the carotid sheath, encompassing prelaryngeal, pretracheal, and paratracheal lymph nodes as well as adipose tissue. The clinical data included a total of 493 surgically treated cases of stage T1a solitary PTC, which were categorized into CLNM-positive and CLNM-negative groups based on the presence of CLNM (Fig. 1).
Fig. 1.
Flow chart of study participants.
Conventional and color doppler ultrasound
The ultrasound examination was conducted using SuperSonic Imagine Aixplorer Sxc6-1 (4–15 MHz) and SonoScape 12 L-A (3-17 MHz) line array probes. Two senior ultrasound physicians reanalyzed and assessed the ultrasound images for each patient, documenting the following information: (1) Long diameter of the tumor; (2) clarity of tumor boundaries; (3) regularity of tumor morphology; (4) status of thyroid capsule invasion, determined by the presence of contact between the capsule and the tumor without any glandular tissue in between, irrespective of the contact area’s size; (5) tumor aspect ratio greater than 1; (6) presence of tumor calcification; (7) abundance of tumor blood flow; (8) presence of coexisting Hashimoto’s thyroiditis. According to the classification of Adler Grades of Blood Flow8, the images were further categorized as follows: (1) Grade-0, no blood flow signal; (2) Grade-I, a small amount of blood flow with 1 or 2 dot-like or short rod blood flow signals; (3) Grade-II, moderate blood flow with 3 or 4 dot-like or 1 longer blood vessel in the lesion, with a length and diameter approaching or exceeding the radius of the lesion; 3) Grade-III, rich blood flow with ≥ 5 visible dot-like or 2 long blood vessels. In this study, grades 0-I were classified as absence/not vascularity, while grades II-III were classified as rich vascularity.
BRAFV600E mutation analysis
BRAFV600E mutation analysis was performed at the pathology department of our hospital9. Briefly, DNA was extracted from the specimens and real-time polymerase chain reaction (PCR) was performed using the Stratagene Mx3000P real-time QPCR system (Agilent, LA Jolla, California, USA). The gene encoding BRAFV600E was amplified with specific primers under the following thermal cycling conditions: an initial denaturation of 1 cycle for 5 min at 95 °C, followed by 15 cycles of 95 °C for 25 s, 64 °C for 20 s, and 72 °C for 20 s with a final step of annealing and elongation of 31cycles at 93 °C for 25 s, 60 °C for 35 s and 72 °C for 20 s. The BRAFV600E mutation status of each primary PTC was determined using the Human BRAF Gene V600E Mutation Fluorescence PCR Diagnostic Kit (Amoy Aide Diagnostics). The FAM signals of the mutation detection system indicate the mutation status of the sample. The HEX/VIC signals were used to assess the internal control status. The FAM Ct value was evaluated for each sample: samples with a FAM Ct value ≥ 27 were classified as negative or undetected, while samples with a FAM Ct value < 27 were classified as positive for the mutation. The run files were interpreted according to the manufacturer’s instructions.
Thyroid surgery and pathological analysis
Different therapeutic strategies were used for different situations of PTC. According to the Chinese CACA Guidelines for Thyroid Cancer, for isolated stage T1a patients without any high-risk factors, such as no history of head and neck radiation in adolescence or family history of thyroid cancer, unilateral lobe with isthmus resection and ipsilateral central compartment lymph node dissection are recommended. However, for patients with thyroid extrathyroidal extension, clinically suspicious metastatic lymph nodes, or intraoperative frozen section indicating central compartment lymph node metastasis, total or near-total thyroidectomy with bilateral central compartment lymph node dissection should be performed. The pCLND boundary was established by the carotid arteries laterally, hyoid bone superiorly, and suprasternal notch inferiorly, including pre- and paratracheal nodes, relaryngeal lymph nodes, perithyroidal nodes, and lymph nodes along recurrent laryngeal nerves. During the procedure, the medical team ensured the protection of the parathyroid gland and recurrent laryngeal nerve. Pathological type, presence or absence of BRAFV600E mutation, number of lymph node dissections, and number of metastases were confirmed by two pathologists independently in a blinded fashion. Case in which findings were inconsistent were discussed with a third pathologist.
Statistical analysis
The statistical analysis was conducted using SPSS statistical software, version 25.0 (IBM, Armonk, NY, USA). Data were presented as mean ± standard deviation. Comparison of continuous and categorical variables were analyzed by Pearson chi-square test or Fisher’s exact test, respectively. Univariate analysis was used to examine the relation between predicting elements and cervical CLNM. Additionally, multivariate logistic regression was performed to assess independent risk factors for cervical CLNM, using the factors screened by univariate analysis. All P values were two-sided, and a value of less than 0.05 was considered statistically significant.
Results
Patients characteristics
In total, this retrospective analysis incorporated data from 493 patients (134 male, 359 female), all of whom were pathologically diagnosed with stage T1a unifocal PTC. Of all patients, 166 (33.7%) had cervical CLNM, while 327 (66.3%) did not. The median age of 41.71 years (range 19–78 years) were included for this research. Briefly, 422 (85.6%) were less than 55 years, and 71 (14.4%) were 55 years and older. No distant metastases were found in our research. In 230 (46.7%) patients, the tumor size was ≤ 0.5 cm, and 263 (53.3%) patients had nodules between 0.5 and 1.0 centimetre. Of the PTCs, 211 (42.8%) were in the left lobe, 267 (54.2%) were in the right lobe, and 15 (3%) were in the isthmus. Typically, BRAFV600E mutation was observed in 449 (91.1%) PTC patients, 74 (15.0%) participants were concomitant Hashimoto’s thyroiditis (HT), and 419 (85.0%) patients were without HT (Table 1). Suspicious US features with respect to taller than wide shape, capsule contact, microcalcifications, burrs or small lobes margin, unclear boundary, irregular morphology, and rich vascularity were presented in 274 (55.6%), 96 (19.5%), 193 (39.1%), 237 (48.1%), 232 (47.1%), 293 (59.4%) and 150 (30.4%) of PTC samples, respectively (Table 2; Figs. 2, 3 and 4).
Table 1.
Basic clinicopathologic characteristics in stage T1a solitary papillary thyroid carcinoma.
| Characteristics | Case n = 493(%) |
Cervical CLNM (%) | x2 | p value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Gender | 5.432 | 0.020 | |||
| Female | 359 (72.8) | 249 (69.4) | 110 (30.6) | ||
| Male | 134 (27.2) | 78 (58.2) | 56 (41.8) | ||
| Age, mean ± SD (years) | 41.71 ± 11.21 | 2.570 | 0.109 | ||
| <55 | 422 (85.6) | 274 (64.9) | 148 (35.1) | ||
| ≥55 | 71 (14.4) | 53 (74.6) | 18 (25.4) | ||
| Maximum tumor size (cm) | 2.022 | 0.155 | |||
| ≤0.5 | 230 (46.7) | 160 (69.6) | 70 (30.4) | ||
| >0.5, ≤1.0 | 263 (53.3) | 167 (63.5) | 96 (36.5) | ||
| Tumor location | 1.481 | 0.477 | |||
| Left lobe | 211 (42.8) | 146 (69.2) | 65 (30.80) | ||
| Right lobe | 267 (54.2) | 172 (64.4) | 95 (35.6) | ||
| Isthmus | 15 (3.0) | 9 (60.0) | 6 (40.0) | ||
| Number of lymph node dissection | 15.415 | <0.001 | |||
| <6 | 317 (64.3) | 230 (72.6) | 87 (27.4) | ||
| ≥6 | 176 (35.7) | 97 (55.1) | 79 (44.9) | ||
| BRAFV600E mutation | 0.368 | 0.544 | |||
| Negative | 44 (8.9) | 31 (70.5) | 13 (29.5) | ||
| Positive | 449 (91.1) | 296 (65.9) | 153 (34.1) | ||
| HT | 1.721 | 0.190 | |||
| Non-concomitant | 419 (85.0) | 273 (65.2) | 146 (34.8) | ||
| Concomitant | 74 (15.0) | 54 (73.0) | 20 (27.0) | ||
Table 2.
Basic ultrasonography features in stage T1a solitary papillary thyroid carcinoma.
| Characteristics | Case n = 493(%) |
Cervical CLNM (%) | x2 | p value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Echogenicity | 1.010 | 0.603 | |||
| Hypoechoic | 454 (92.1) | 301 (66.3) | 153 (33.7) | ||
| Markedly hypoechoic | 28 (5.7) | 20 (71.4) | 8 (28.6) | ||
| Equal or high echo | 11 (2.2) | 6 (54.5) | 5 (45.5) | ||
| Echo texture | 0.980 | 0.322 | |||
| Homogeneous | 67 (13.6) | 48 (71.6) | 19 (28.4) | ||
| Heterogeneous | 426 (86.4) | 279 (65.5) | 147 (34.5) | ||
| Component | 0.699 | 0.403 | |||
| Solid | 482 (97.8) | 321 (66.6) | 161 (33.4) | ||
| Cystic-solid | 11 (2.2) | 6 (54.5) | 5 (45.5) | ||
| Aspect ratio | 1.018 | 0.313 | |||
| <1 | 219 (44.4) | 140 (63.9) | 79 (36.1) | ||
| ≥1 | 274 (55.6) | 187 (68.2) | 87 (31.8) | ||
| Capsule contact | 9.306 | 0.002 | |||
| No | 397 (80.5) | 276 (69.5) | 121 (30.5) | ||
| Yes | 96 (19.5) | 51 (53.1) | 45 (46.9) | ||
| Microcalcifications | 6.457 | 0.011 | |||
| No | 300 (60.9) | 212 (70.7) | 88 (29.3) | ||
| Yes | 193 (39.1) | 115 (59.6) | 78 (40.4) | ||
| Margin | 0.372 | 0.542 | |||
| Slippery | 256 (51.9) | 173 (67.6) | 83 (32.4) | ||
| Burrs or small lobes | 237 (48.1) | 154 (65.0) | 83 (35.0) | ||
| Boundary | 0.028 | 0.866 | |||
| Clear | 261 (52.9) | 174 (66.7) | 87 (33.3) | ||
| Unclear | 232 (47.1) | 153 (65.9) | 79 (34.1) | ||
| Morphology | 0.068 | 0.794 | |||
| Regular | 200 (40.6) | 134 (67.0) | 66 (33.0) | ||
| Irregular | 293 (59.4) | 193 () | 100 () | ||
| CDFI | 4.724 | 0.030 | |||
| Absence/not rich vascularity | 343 (69.6) | 238 (69.4) | 105 (30.6) | ||
| Rich vascularity | 150 (30.4) | 89 (59.3) | 61 (40.7) | ||
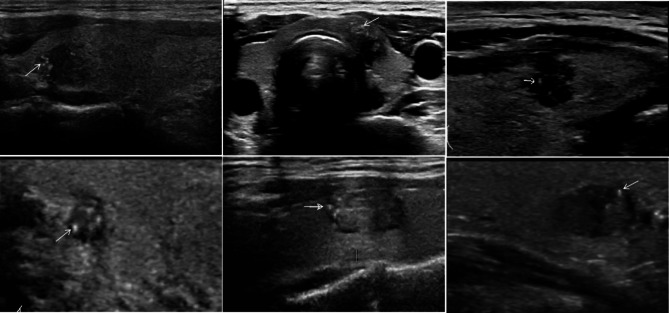
Fig. 2.
The ultrasound images of thyroid nodule with microcalcification, and all patients were accompanied by cervical CLNM. The intranodal microcalcifications manifest as punctate or sediment-like strong echoes, with no obvious posterior acoustic shadow.
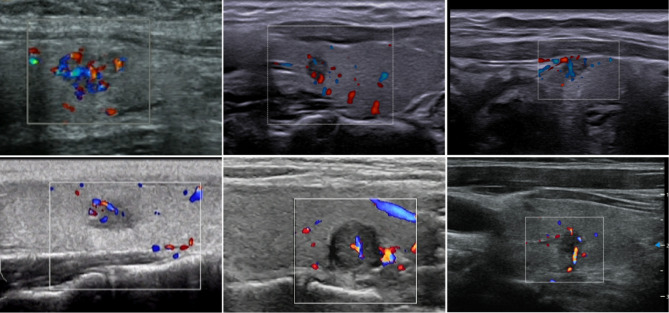
Fig. 3.
The ultrasound images of thyroid nodule with grades II-III vascularity, and all patients were accompanied by cervical CLNM. Short rod-like and stripe-like blood flow signals can be observed within the nodules, classifying them as nodules with abundant blood flow signals.
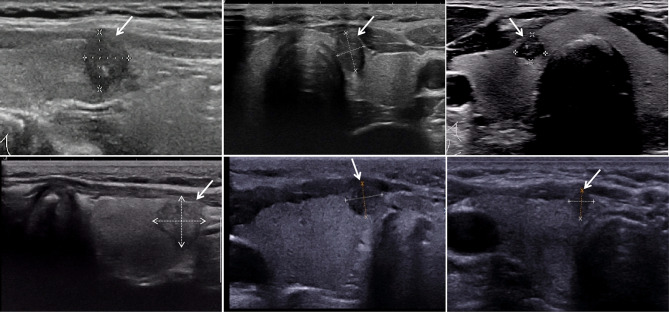
Fig. 4.
The ultrasound images of thyroid nodule with capsule contact or invasion, and all patients were accompanied by cervical CLNM. The nodule demonstrates a discontinuity in the adjacent capsule, an indistinct boundary between the tumor and the surrounding glandular tissue, and the absence of normal glandular tissue between the tumor and the capsule.
Univariate and multivariate logistic regression analysis
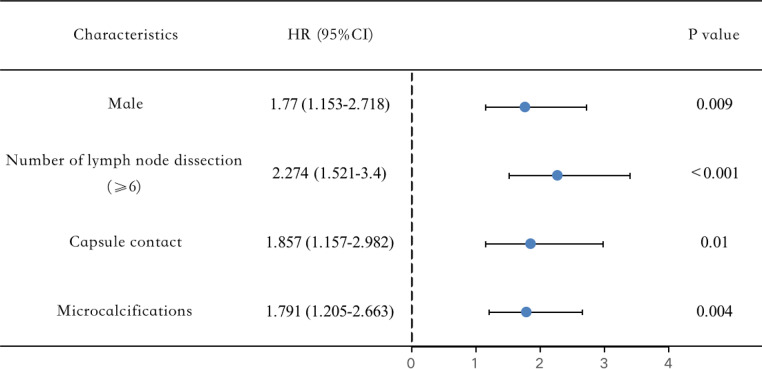
The relationships between medical information and cervical CLNM are presented in Tables 1 and 2. There were significant differences in gender (male, P = 0.02), number of lymph node dissection (≥ 6, P<0.001), capsule contact (P = 0.002), microcalcifications (P = 0.011), and rich vascularity (P = 0.03) between the cervical CLNM and the non-CLNM. Meanwhile, age, maximum tumor size, tumor location, BRAFV600E, HT, echogenicity, echo texture, component, aspect ratio, margin, boundary, and morphology were not related to cervical CLNM in T1a unifocal PTC (all P value > 0.05). Moreover, independent risk factors were determined after multivariate analysis (Table 3). In our research, predictive risk factors, including male (OR = 1.770, P = 0.009), number of lymph node dissection (OR = 2.274, P<0.001), capsule contact (OR = 1.857, P = 0.01), and microcalcifications (OR = 1.791, P = 0.004) turned out to be risk predictors for cervical CLNM in participants with T1a unifocal PTC (Fig. 5).
Table 3.
Multivariate logistic regression analysis of the relationship between predictors of T1a solitary PTC (significant by univariate analysis) and cervical CLNM.
| Independent variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.009 | |||
| Female | Reference | Reference | ||
| Male | 1.770 | 1.153 | 2.718 | |
| Number of lymph node dissection | <0.001 | |||
| <6 | Reference | Reference | ||
| ≥6 | 2.274 | 1.521 | 3.400 | |
| Capsule contact | 0.010 | |||
| No | Reference | Reference | ||
| Yes | 1.857 | 1.157 | 2.982 | |
| Microcalcifications | 0.004 | |||
| No | Reference | Reference | ||
| Yes | 1.791 | 1.205 | 2.663 | |
| CDFI | 0.089 | |||
| Absence/not rich vascularity | Reference | Reference | ||
| Rich vascularity | 1.435 | 0.946 | 2.178 | |
Fig. 5.
Forest plot of the risk factors of the cervical CLNM in T1a solitary PTC patients.
Discussion
Although T1a stage PTC has a favorable prognosis, active surgical treatment is recommended when high-risk factors such as CLNM occur10,11. However, it has been observed that 30–60% of T1a stage PTC patients do not have detectable lymph node metastasis before surgery and it is only after CLND that the presence of lymph node metastasis is discovered12. In this study, the rate of CLNM was found to be 33.7%, which is lower than the rates reported by Liu et al.13. (47.5%) and Chen et al.14. (40.9%). The reason for this difference could be attributed to the fact that all patients in our study had stage T1a unifocal PTC, whereas the aforementioned studies included patients with stage T1a multifocal PTC, which is associated with higher invasiveness and is an independent risk factor for CLNM13–15.
Male patients in this study were found to have a higher incidence of CLNM compared to female patients (41.8% vs. 30.6%, p = 0.02), and is an independent risk factor for CLNM. This finding is consistent with the results of most studies that have shown a higher incidence of CLNM in male patients16,17. There was no statistically significant difference in the incidence of CLNM among patients of different age groups in our study. The association between age and CLNM is inconsistent across various studies, with some studies suggesting that younger patients are more prone to CLNM18–20.
The risk of CLNM and tumor recurrence and mortality are increased in T1a stage PTC patients with thyroid capsule invasion21,22. However, there is no consensus on the preoperative ultrasound criteria for determining tumor invasion of the thyroid capsule. Therefore, in this study, the criteria for preoperative capsule invasion were defined as follows: in addition to continuous interruption of the capsule and unclear boundary between the tumor and extrathyroidal tissue, any absence of normal glandular tissue between the tumor and the capsule, regardless of the contact area, was defined as tumor invasion of the thyroid capsule. This study found that tumor invasion of the thyroid capsule is an independent factor influencing CLNM, consistent with the results of Chen et al.23. and Xue et al.24. The reason for this association is that tumor cells are more likely to enter the lymphatic network after invading the thyroid capsule, leading to lymph node metastasis.
This study found that the occurrence of microcalcification within thyroid nodules is an independent risk factor for CLNM in T1a stage PTC patients. It provides a new predictive factor for preoperative diagnosis of lymph node metastasis, improves the accuracy of preoperative diagnosis of lymph node metastasis, and provides a basis for the formulation of clinical treatment strategies. This finding is consistent with the results of most studies14,23,25. There is still controversy regarding the relationship between tumor blood flow and CLNM. Some studies suggesting a correlation argue that the incomplete basement membrane of tumor neovasculature and higher vascular permeability increase the likelihood of tumor cell detachment and metastasis26. However, some researchers who hold a negative view argue that the possibility of CLNM does not increase with the increase in tumor blood flow27. The multifactorial analysis in this study showed no correlation between tumor blood flow and CLNM.
Studies have found a correlation between the occurrence of CLNM and the number of lymph node dissections. The optimal number of lymph nodes to be dissected for pCLND in PTC patients requires further research. However, it is consensus that obtaining an adequate number of lymph node dissections helps reduce the risk of residual metastatic lymph nodes28,29. Robinson et al.28 and Lee et al.30. recommend dissecting at least 6 lymph nodes in PTC patients to minimize residual lymph nodes. Chung et al.31. also found a higher rate of lymph node metastasis in PTC patients with > 7 lymph node dissections compared to those with ≤ 7 dissections. This study also found that patients with < 6 lymph node dissections had a CLNM occurrence rate of 27.4% (87/166), while patients with ≥ 6 dissections had a CLNM occurrence rate of 44.9% (79/166), with a statistically significant difference. This supports the view that a higher number of CLNDs leads to the detection of more metastatic lymph nodes. The number of lymph node dissections is influenced by various factors, including differences in technical expertise. Ji et al.29. found that teaching hospitals obtained a greater number of lymph nodes than community hospitals in a study of 23,131 PTC patients. The number of lymph nodes in the central neck region also varies among different patients. Ofo et al.32. found a range of 1 to 16 lymph nodes with a median of 4 in a study of 28 cadavers, suggesting that individual differences can affect the number of lymph node dissections in PTC patients. Coexisting Hashimoto’s thyroiditis can also affect the number of CLNDs, as PTC patients with coexisting Hashimoto’s thyroiditis tend to have more enlarged lymph nodes33. The purpose of dissection, whether therapeutic or preventive, as well as the thoroughness of pathological examination can also affect the number of lymph node dissections. Therefore, to obtain accurate CLNM, precise and thorough surgical procedures and pathological examinations are necessary to obtain an adequate number of lymph node dissections.
The limitations of this study are as follows: First, this research is a single-center retrospective study with a limited number of cases, which introduces potential selection bias. Future studies should consider expanding the sample size to provide a more comprehensive understanding of the impact of lymph node clearance on metastasis. Second, the evaluation of ultrasound characteristics may be subjective, which introduces a degree of variability. Third, our study focused exclusively on papillary thyroid carcinoma, thereby neglecting other types of thyroid malignancies. Future research should aim to include a broader range of thyroid malignancies to enhance our understanding. Furthermore, to improve the quality of future studies, it will be essential to increase the sample size and explore the potential of new technologies, such as contrast-enhanced ultrasound, elastography, and artificial intelligence.
In summary, male patients, tumor invasion of the thyroid capsule, and nodules with microcalcifications are more prone to CLNM in stage T1a unifocal PTC patients. Preventive CLND should be performed in patients with high-risk factors, and efforts should be made to obtain an adequate number of lymph node dissections to achieve a more accurate CLNM occurrence rate.
Author contributions
All authors contributed to the study conception and design. Material preparation was performed by Hai-ying Tian, Zhao-yan Yu and Ting Dong. Data collection and analyses were performed by Qing Xie, Yi Mu, Wei Liao and Ning Ma. The first draft of the manuscript was written by Hai-ying Tian and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Guizhou Provincial Science and Technology Projects (ZK[2022]257) and Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwjkj2024-477).
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li, Y. et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid. 30(4), 568–579 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Zheng, X. et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1,587 patients. Cancer Biol. Med. 16 (1), 121–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun, R. Predictive role of intraoperative clinicopathological features of the central compartment in estimating lymph nodes metastasis status. Ann. Transl Med. 7 (18), 471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu, S. Clinical characteristics and ultrasonographic features for predicting central lymph node metastasis in clinically node-negative papillary thyroid carcinoma without capsule invasion. Head Neck. 41 (11), 3984–3991 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Sun, R. et al. Relationship between the extent of central node dissection and parathyroid function preservation in thyroid cancer surgery. Gland Surg. 10 (3), 1093–1103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ondik, M. Secondary central compartment surgery for thyroid cancer. Laryngoscope. 119 (10), 1947–1950 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Lefevre, J. Reoperative surgery for thyroid disease. Langenbecks Arch. Surg. 392 (6), 685–691 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Peng, C. et al. Differential diagnosis of non-diffuse primary thyroid lymphoma and papillary thyroid carcinoma by ultrasound combined with computed tomography. BMC Cancer. 22 (1), 938 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma, N. et al. Integrating US-guided FNAB, BRAFV600E mutation, and clinicopathologic characteristics to predict cervical central lymph-node metastasis in preoperative patients with cN0 papillary thyroid carcinoma. Eur. Arch. Otorhinolaryngol. 4 (2023). [DOI] [PMC free article] [PubMed]
- 10.Liu, Z. et al. Ultrasound lymphatic imaging for the diagnosis of metastatic central lymph nodes in papillary thyroid cancer. Eur. Radiol. 31 (11), 8458–8467 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Zou, Y. et al. Extreme gradient boosting model to assess risk of central cervical lymph node metastasis in patients with papillary thyroid carcinoma: individual prediction using SHapley Additive exPlanations. Comput. Methods Programs Biomed. 225, 107038 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Laird, A., Gauger, M., Miller, P. G. & Doherty, B. S. Evaluation of postoperative radioactive iodine scans in patients who underwent prophylactic central lymph node dissection. World J. Surg. 36 (6), 1268–1273 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Liu, S. et al. A prediction model incorporating the BRAFV600E protein status for determining the risk of cervical lateral lymph node metastasis in papillary thyroid cancer patients with central lymph node metastasis. Eur. J. Surg. Oncol. 47 (11), 2774–2780 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Chen, J. et al. Ultrasound validation of predictive model for central cervical lymph node metastasis in papillary thyroid cancer on BRAF. Future Oncol. 16 (22), 1607–1618 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Wang, Z. et al. A clinical predictive model of central lymph node metastases in papillary thyroid carcinoma. Front. Endocrinol. (Lausanne). 13, 856278 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. Role of BRAF V600E mutation as an indicator of the extent of thyroidectomy and lymph node dissection in conventional papillary thyroid carcinoma. Surgery. 158 (6), 1500–1511 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Tang, T. et al. Risk factors of central lymph node metastasis in papillary thyroid carcinoma: a retrospective cohort study. Int. J. Surg. 54(Pt A), 129–132 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Dai, Q. et al. Nomograms based on preoperative multimodal ultrasound of papillary thyroid carcinoma for predicting central lymph node metastasis. Eur. Radiol. 32 (7), 4596–4608 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Feng, Y. et al. Construction and validation of a nomogram for predicting cervical lymph node metastasis in classic papillary thyroid carcinoma. J. Endocrinol. Invest. 44 (10), 2203–2211 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Feng, J. Nomograms based on sonographic and clinicopathological characteristics to predict lateral lymph node metastasis in classic papillary thyroid carcinoma. J. Endocrinol. Invest. 45 (11), 2043–2057 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Jin, W. Prediction of central lymph node metastasis in papillary thyroid microcarcinoma according to clinicopathologic factors and thyroid nodule sonographic features: a case-control study. Cancer Manag Res. 10, 3237–3243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamaya, A. et al. Sonographic Detection of Extracapsular extension in papillary thyroid Cancer. J. Ultrasound Med. 34 (12), 2225–2230 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Chen, J. et al. Conventional ultrasound, immunohistochemical factors and BRAFV600E mutation in predicting central cervical lymph node metastasis of papillary thyroid carcinoma. Ultrasound Med. Biol. 44 (11), 2296–2306 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Zhang, K. et al. Preoperative prediction of central cervical lymph node metastasis in fine-needle aspiration reporting suspicious papillary thyroid cancer or papillary thyroid cancer without lateral neck metastasis. Front. Oncol. 12, 712723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao, X. et al. Predictors and a prediction model for central cervical lymph node metastasis in papillary thyroid carcinoma (cN0). Front. Endocrinol. 12, 789310 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan, W. Differences in sonographic features of papillary thyroid carcinoma between neck lymph node metastatic and nonmetastatic groups. J. Ultrasound Med. 31 (6), 915–920 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Huang, X. Relationship between expression of vascular endothelial growth factor and cervical lymph node metastasis in papillary thyroid cancer: a meta-analysis. J. Huazhong Univ. Sci. Technolog Med. Sci. 37 (5), 661–666 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Robinson, T. How many lymph nodes are Enough? Assessing the adequacy of Lymph Node yield for papillary thyroid Cancer. J. Clin. Oncol. 34 (28), 3434–3439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji, K. et al. Adequacy of lymph node yield for papillary thyroid Cancer: an analysis of 23,131 patients. J. Surg. Res. 244, 566–573 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Lee, J. et al. Clinical value of lymph node ratio integration with the 8th edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid Cancer. Sci. Rep. 9 (1), 13361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung, E. The impact of the number of harvested central lymph nodes on the lymph node ratio. Auris Nasus Larynx. 46 (2), 267–271 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Ofo, E. et al. Quantification of lymph nodes in the central compartment of the neck: a cadaveric study. Eur. Arch. Otorhinolaryngol. 273 (9), 2773–2778 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Hu, J. The extent of lymph node yield in central neck dissection can be affected by preoperative and intraoperative assessment and alter the prognosis of papillary thyroid carcinoma. Cancer Med. 9 (3), 1017–1024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.