Abstract
Background
A subarachnoid hemorrhage (SAH) is a serious and potentially life‐threatening condition where blood leaks out of blood vessels over the surface of the brain. Delayed ischemic neurological deficit (DIND) and the related feature of vasospasm, where patients experience a delayed deterioration, have long been recognized as the leading potentially treatable cause of death and disability in patients with SAH. Endothelin is a potent, long‐lasting endogenous vasoconstrictor that has been implicated in the pathogenesis of DIND. Therefore, endothelin receptor antagonists (ETAs) have emerged as a promising therapeutic option for SAH‐induced cerebral vasospasm.
Objectives
To assess the efficacy and tolerability of ETAs for SAH.
Search methods
We searched the Cochrane Stroke Group Trials Register (December 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 11), MEDLINE (1950 to December 2011), EMBASE (1946 to December 2011) and the Chinese Biomedical Database (1978 to December 2011). In an effort to identify further published, unpublished and ongoing trials we searched additional Chinese databases, ongoing trials registers, Google Scholar and Medical Matrix, handsearched journals, scanned reference lists, and contacted researchers and pharmaceutical companies.
Selection criteria
We only included randomized controlled trials (RCTs) that compared an ETA with placebo for SAH in adult (18 years of age or older) patients who met the diagnostic criteria for SAH based on clinical symptoms, with confirmation on computerized tomography scan results or angiography. Two review authors independently selected RCTs according to the inclusion criteria. We resolved disagreements by discussion with a third review author.
Data collection and analysis
Two review authors independently selected relevant articles and assessed their eligibility according to the inclusion and exclusion criteria. We resolved disagreements by discussion with a third review author. We used the random‐effects model and expressed the results as risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes with 95% confidence intervals (CI).
Main results
We included four RCTs with 2024 participants that compared ETAs with placebo for SAH. All RCTs were multicenter, double‐blind studies with a low risk of bias. ETAs reduced the incidence of DIND (RR 0.80; 95% CI 0.67 to 0.95) and angiographic vasospasm (RR 0.62; 95% CI 0.52 to 0.72) but did not reduce the incidence of unfavorable outcomes (RR 0.87; 95% CI 0.74 to 1.02) or mortality (RR 1.05; 95% CI 0.77 to 1.45). ETAs increased the incidence of hypotension (RR 2.53; 95% CI 1.77 to 3.62) and pneumonia (RR 1.56; 95% CI 1.23 to 1.97).
Authors' conclusions
ETAs appear to reduce DIND and angiographic vasospasm but there were adverse events and the impact on clinical outcome is unclear. Additional well‐designed RCTs are needed.
Keywords: Humans; Endothelin Receptor Antagonists; Brain Ischemia; Brain Ischemia/prevention & control; Conflict of Interest; Dioxanes; Dioxanes/adverse effects; Dioxanes/therapeutic use; Hypotension; Hypotension/chemically induced; Pneumonia; Pneumonia/chemically induced; Pyridines; Pyridines/adverse effects; Pyridines/therapeutic use; Pyrimidines; Pyrimidines/adverse effects; Pyrimidines/therapeutic use; Randomized Controlled Trials as Topic; Research Support as Topic; Subarachnoid Hemorrhage; Subarachnoid Hemorrhage/complications; Subarachnoid Hemorrhage/drug therapy; Sulfonamides; Sulfonamides/adverse effects; Sulfonamides/therapeutic use; Tetrazoles; Tetrazoles/adverse effects; Tetrazoles/therapeutic use; Vasospasm, Intracranial; Vasospasm, Intracranial/prevention & control
Plain language summary
Endothelin receptor antagonists for subarachnoid hemorrhage
Subarachnoid hemorrhage is an uncommon cause of stroke that often occurs at a young age, producing a relatively large burden of premature mortality. Delayed ischemic neurological deficit (DIND), a condition where the patient's condition deteriorates, has long been recognized as the leading potentially treatable cause of death and disability in patients with subarachnoid hemorrhage. Endothelin is a long‐lasting agent that causes blood vessel constriction, which has been implicated in the cause of DIND. Drugs that reverse this effect (endothelin receptor antagonists, ETAs) have emerged as a promising treatment for subarachnoid hemorrhage. This review of four trials, involving 2024 participants, showed that ETAs reduced the risk of DIND but did not improve clinical outcomes and had potentially serious side effects, such as low blood pressure and chest infection. There is not enough evidence to conclude that ETAs are beneficial in SAH.
Background
Description of the condition
Subarachnoid hemorrhage (SAH) is a serious, potentially life‐threatening condition where blood leaks out of blood vessels over the surface of the brain (NHS 2012). The incidence of SAH is about 10 per 100,000 population per year in Western Europe and the US, and is higher in Japan and Finland (about 15 per 100,000 population per year) in 2008 (Traill 2008). Case fatality after aneurysmal hemorrhage is 50%; one in eight patients with SAH dies outside hospital (Van Gijn 2007). Approximately 85% of patients bleed from intracranial arterial aneurysms, 10% from a non‐aneurysmal peri‐mesencephalic hemorrhage, and 5% from other vascular abnormalities including arteriovenous malformations (Van Gijn 2001). Age‐adjusted mortality rates for SAH are 62% greater in females than in males, and 57% greater in black people than in white people. Although SAH is an uncommon cause of stroke mortality it occurs at a young age, producing a relatively large burden of premature mortality (Johnston 1998).
Description of the intervention
Delayed ischemic neurological deficit (DIND), which is often associated with cerebral vasospasm, has long been recognized as the leading potentially treatable cause of death and disability in patients with aneurysmal SAH (Zimmermann 2004). It usually occurs between four and 10 days after the initial bleeding, has a gradual onset and is multi‐focal (Van den Bergh 2004). At present, the dihydropyridine L‐type calcium channel antagonist, nimodipine, is used to treatment patients with SAH, starting within days of the ictus (Weyer 2006). An ideal drug would prevent DIND and vasospasm, would not alter blood pressure and would have cerebrovascular selectivity. This is not achieved by the current standard treatment with nimodipine (Uhlmann 2006). Endothelin is a potent, long‐lasting endogenous vasoconstrictor that has been implicated in the pathogenesis of vasospasm (Zimmermann 2004) and endothelin receptor antagonists (ETAs) have been developed to reverse this effect.
How the intervention might work
Endothelin‐1 (ET‐1) is the only endothelin produced by endothelium cells and released on the abluminal side. It acts in a paracrine manner on neighboring smooth muscle tissue, producing vasoconstriction (Wagner 1992). ET‐1 mediates functions by its interaction with two endothelin receptor subtypes: ETA and ETB. The ETA receptor has the highest binding affinity toward ET‐1 and ET‐2 (Masaki 2000). Levels of ET‐1 are increased in the cerebrospinal fluid and plasma of patients with SAH in close correlation with the development of vasospasm (Fassbender 2000) and have been verified by many experimental and clinical studies. Thus, ETAs have emerged as a promising therapeutic option for aneurysmal SAH‐induced cerebral vasospasm (Chow 2002).
Why it is important to do this review
Clinical trials have demonstrated marked prevention of vasospasm with the ETA, clazosentan. However, patient outcome was not improved (Macdonald 2008). There are more recent trials of ETAs, including trials in progress. Moreover, there are concerns about safety hazards such as hypotension and pulmonary complications. Therefore, it is necessary to perform a systematic review on the effectiveness and safety of ETAs in SAH. The aim of this review is to integrate all available randomized trials to provide more reliable evidence for clinicians.
Objectives
To assess the efficacy and tolerability of ETAs for SAH.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomized controlled trials (RCTs) that compared ETAs with placebo for SAH regardless of language and publication status.
Types of participants
Participants were adults (18 years of age or older), both men and women, who met the diagnostic criteria for SAH based on clinical symptoms, with confirmation on computerized tomography scan results or angiography.
Types of interventions
We included studies performed with ETAs given regularly with the aim of preventing the occurrence of DIND in patients with SAH, regardless of dose or drug.
Types of outcome measures
Primary outcomes
Primary outcome was the development of DIND. This is usually defined as delayed neurological worsening with or without locally defined cerebral vasospasm on digital subtraction catheter angiography or transcranial Doppler ultrasound. Death at scheduled follow‐up was the primary clinical outcome.
Secondary outcomes
Secondary outcomes included angiographic vasospasm, drug‐related adverse events, and unfavorable outcome. Unfavorable outcome was defined as a Glasgow Outcome Scale Extended (GOSE) categorization of "death, persistent vegetative state or severe disability". We planned to include rebleeding within six months but data were not available.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of papers published in languages other than English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (December 2011), MEDLINE (1950 to December 2011) (Appendix 1), EMBASE (1974 to December 2011) (Appendix 2), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2011, Issue 11) (Appendix 3) and the Chinese Biomedical Database (1978 to December 2011). We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and adapted it for the other databases.
Searching other resources
In an effort to identify further published, unpublished and ongoing trials we:
searched the China National Knowledge Infrastructure (CNKI) (www.cnki.net) (1978 to December 2011) and Wanfang Data (www.wanfangdata.com) (1997 to December 2011);
-
we searched the following relevant trials and research registers (December 2011):
ClinicalTrials.gov (www.clinicaltrials.gov);
Current Controlled Trials (www.controlled‐trials.com);
Stroke Trials Registry (www.strokecenter.org/trials);
handsearched the Chinese Journal of Stroke, Stroke and Nervous Diseases, Chinese Journal of Neuromedicine, Journal of Apoplexy and Nervous Diseases, Stroke Neurosurgery and Neurocritical Care in the library of Lanzhou University or the Second Hospital of Lanzhou University (2000 to November 2011);
used the search engines Google Scholar (scholar.google.co.uk/) and Medical Matrix (www.medmatrix.org/) to identify relevant studies on the Internet (December 2011);
searched the reference lists of identified studies, key textbooks, review articles and relevant studies;
contacted authors, researchers and trial investigators in the field of study;
contacted Patheon Inc. by searching the website.
Data collection and analysis
Selection of studies
We used the search strategies described above to obtain titles and abstracts of studies that were potentially relevant to this review. Two review authors (JG, LJ) independently selected RCTs of ETAs for SAH by screening titles and abstracts against the predetermined eligibility criteria. If we could not decide whether the articles satisfied the inclusion criteria, we obtained the full texts of the trials. If there were two or more papers relating to one trial, either the publication with the most complete data or the pooled data from all the papers was included. We resolved disagreements by discussion with a third review author (ZS).
Data extraction and management
The same two review authors (JG, LJ) carried out data extraction independently and the results were checked for accuracy by a third review author (JT). We resolved disagreements by discussion. We designed a data extraction form and used it to record the following characteristics.
Study details: title, author, year of publication, number of people randomized and analyzed per arm.
Participants: age, case, sex, characteristic of patients (including whether angiography was performed before randomization), time of therapy.
Type of intervention: dosage of use, route and timing of the drug administration, type and timing of aneurysm treatment, duration of follow‐up.
Outcomes: DIND, angiographic vasospasm, death, unfavorable outcome, hypotension and pneumonia.
Assessment of risk of bias in included studies
Two review authors (JG, LJ) assessed the quality of eligible studies independently based on the information available in the full‐text article. If we could not determine from the full‐text article that all of these criteria were met, we contacted the study authors. If it remained unclear, we discussed with the third review author (KY) whether the study should be excluded. If it was not excluded, we performed a sensitivity analysis.
We assessed the following items using The Cochrane Collaboration's tool for assessing the risk of bias (Higgins 2011) (see Appendix 4).
Was there adequate sequence generation?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we defined measures of treatment effects as follows: for dichotomous outcomes, we planned to express results as risk ratio (RR) with 95% confidence interval (CI). We used a fixed‐effect model unless there was significant heterogeneity in which case we used a random‐effects model. If there were continuous scales of measurement to assess the effects of treatment, we planned to use the mean difference (MD), or the standardized mean difference (SMD) if different scales were used. We planned to analyze heterogeneity using an I2 statistic on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance (Higgins 2011).
Unit of analysis issues
Individual participants were the unit of analysis because we included only individually RCTs with a parallel design.
Dealing with missing data
We attempted to contact all the authors of the original studies for missing data. If the authors of the study did not respond within four weeks, we extracted all the available data from the publication. If data were missing because participants dropped out or were lost to follow‐up, we planned to conduct a primary analysis based on complete data and a sensitivity analysis with missing data imputed based on the worst‐case and best‐case scenarios.
Assessment of heterogeneity
We planned to examine heterogeneity among trials using the I2 statistic. We planned to regard an I2 statistic estimate greater than 50% as showing substantial or considerable heterogeneity and we planned to investigate its causes by performing subgroup analyses or sensitivity analyses by excluding studies thought to cause the heterogeneity.
Assessment of reporting biases
If possible, we planned to use funnel plots to detect potential publication bias in this review.
Data synthesis
We used the Review Manager software, RevMan 5.1, for statistical analysis (RevMan 2011). According to the level of heterogeneity between trials, we planned to use either a fixed‐effect or random‐effects model, where appropriate. We planned to report the results qualitatively if we found significant heterogeneity and we could not find the reasons for the heterogeneity.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to explore possible sources of heterogeneity, for example drug dose, route and timing of drug administration, type and timing of aneurysm, duration of follow‐up, etc.
Sensitivity analysis
We conducted sensitivity analyses based on the methodological quality of the studies and pending results of the assessment of heterogeneity. In addition, we subjected included trials to a sensitivity analysis based on quality to assess the effect of these studies on their reported outcomes.
Results
Description of studies
See Characteristics of included studies
Results of the search
After comprehensive searches, we found 714 citations identified via electronic databases (MEDLINE 308 citations; CENTRAL 23 citations; EMBASE 426 citations; Chinese Database 0 citations). We identified an additional 10 citations through handsearching. After removing duplicates, we identified 698 potential citations for screening. Of these, we excluded 235 citations that were not RCTs, 179 citations that were not ETAs, 108 citations that were not SAH and 156 citations for other reasons. Of the 20 remaining articles, we excluded four citations that used the same data that came from one trial, seven citations that were not RCTs, four citations that were not SAH and one citation was for an ongoing trial. We ultimately included four trials (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011) (see Figure 1).
1.
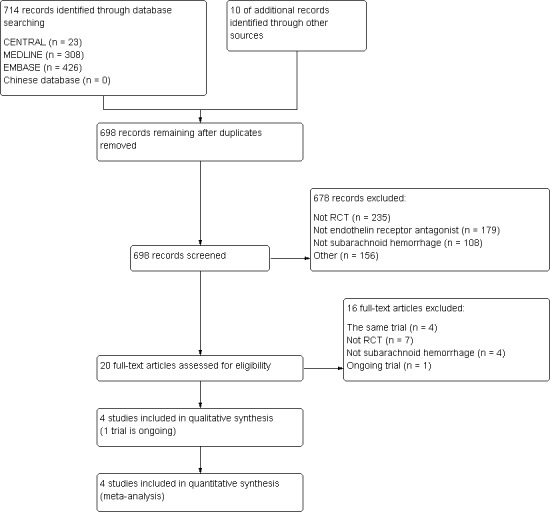
Study flow diagram
Included studies
We included four RCTs (2024 participants) (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011) that fulfilled our inclusion criteria. All four RCTs were multicenter, randomized, double‐blind, placebo‐controlled studies of clazosentan or TAK‐044. Baseline characteristics of patients were similar between the intervention and placebo groups. The characteristics of the included trials are presented in the Characteristics of included studies table.
Excluded studies
We excluded one excluded study (Nogueira 2007), which is described in the Characteristics of excluded studies table.
Ongoing studies
One randomized, double‐blind, placebo‐controlled Phase III study is underway (CONSCIOUS 3 2010).
Risk of bias in included studies
The 'Risk of bias' assessment for the included studies is presented in the Characteristics of included studies table, Figure 2 and Figure 3. Based on our criteria for assessing the risk of bias, these studies had a low risk of bias.
2.
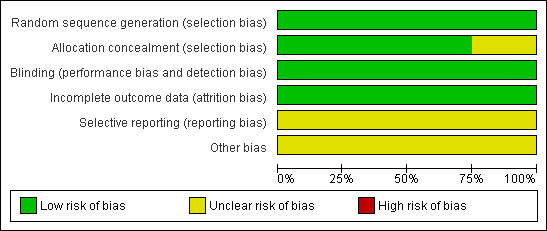
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies
3.
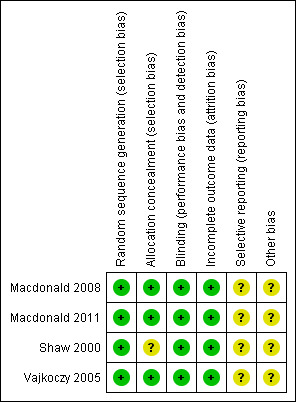
Methodological quality summary: review authors' judgments about each methodological quality item for each included study
Allocation
One trial (Vajkoczy 2005) reported that patients were randomized to receive clazosentan or placebo but did not report the methods of sequence generation and allocation. One trial (Macdonald 2008) reported that patients were stratified by site and procedure, allocation concealment was robust because both the drug and placebo were clear and colorless with no obvious acute effects that would compromise blinding. One trial (Shaw 2000) used a computer‐generated randomization schedule but did not report the methods of allocation. One trial (Macdonald 2011) used an interactive web response system to perform sequence generation and allocation.
Blinding
Double blinding was reported in all four trials (Shaw 2000; Vajkoczy 2005; Macdonald 2008: Macdonald 2011). Macdonald 2008 reported that all investigators, patients and individuals responsible for the conduct, monitoring and analysis of the study were blinded to treatment, Macdonald 2011 reported that clinical and imaging data were assessed by a masked assessor, Vajkoczy 2005 reported that the investigator or other staff member was not allowed to witness the preparation of study medication by the pharmacist and Shaw 2000 was reported to be double‐blind but did not provide details.
Incomplete outcome data
All four trials (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011) reported the number and reason for drop‐outs and losses to follow‐up.
Selective reporting
We did not acquire the protocols for the four trials (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011) so we were unsure about the risk of selective reporting.
Other potential sources of bias
One trial (Vajkoczy 2005) was supported by Axovan Ltd and Actelion Ltd, two trials (Macdonald 2008; Macdonald 2011) were funded by Actelion Pharmaceuticals and one trial (Shaw 2000) was funded in its entirety by Takeda Euro Research and Development Centre GmbH. There may be potential conflicts of interest.
Effects of interventions
Primary outcomes
Delayed ischemic neurological deficit (DIND)
Three RCTs were included in the analysis (Shaw 2000; Macdonald 2008; Macdonald 2011). DIND was significantly less common with ETA than with placebo (RR 0.80; 95% CI 0.67 to 0.95) and there was no heterogeneity of the studies (P = 0.73, I2 = 0%) (Analysis 1.1).
1.1. Analysis.
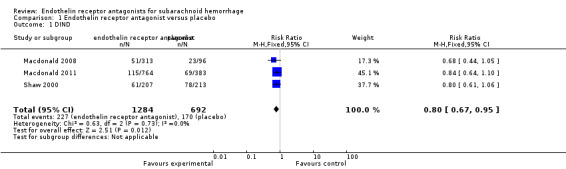
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 1 DIND.
Mortality
Four RCTs reported mortality (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011). The meta‐analysis illustrated no statistically significant differences for mortality (RR 1.05; 95% CI 0.77 to 1.45) between ETA and placebo and there was no heterogeneity of the studies (P = 0.90, I2 = 0%) (Analysis 1.2).
1.2. Analysis.
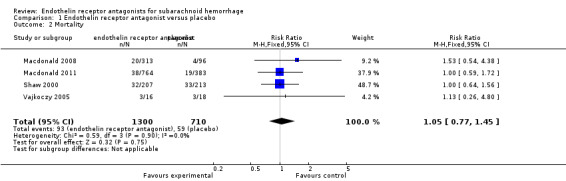
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 2 Mortality.
Secondary outcomes
Angiographic vasospasm
Three RCTs were included in the analysis (Vajkoczy 2005; Macdonald 2008; Macdonald 2011). Compared with placebo, angiographic vasospasm was significantly less common with ETA (RR 0.62; 95% CI 0.52 to 0.72) but there was heterogeneity between the studies (P = 0.47, I2 = 0%) (Analysis 1.3).
1.3. Analysis.
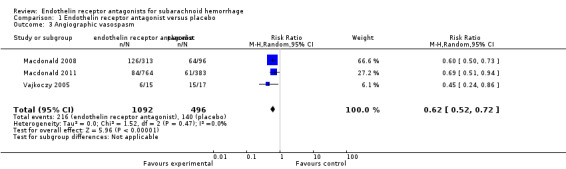
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 3 Angiographic vasospasm.
Unfavorable outcome
Meta‐analysis of three included RCTs (Shaw 2000; Macdonald 2008; Macdonald 2011) showed no statistically significant differences for unfavorable outcome of death, persistent vegetative state or severe disability (RR 0.87; 95% CI 0.74 to 1.02) and there was no heterogeneity of the studies (P = 0.86, I2 = 0%) (Analysis 1.4).
1.4. Analysis.
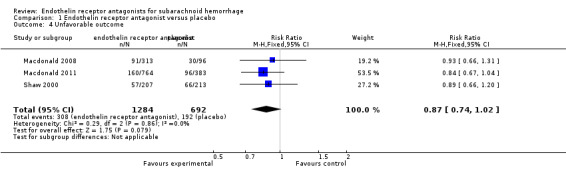
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 4 Unfavorable outcome.
Adverse events incidence: hypotension
Four RCTs were included in the analysis (Shaw 2000; Vajkoczy 2005; Macdonald 2008; Macdonald 2011). Compared with placebo, hypotension was significantly higher with ETA (RR 2.53; 95% CI 1.77 to 3.62) and there was no heterogeneity of the studies (P = 0.55, I2 = 0%) (Analysis 1.5).
1.5. Analysis.
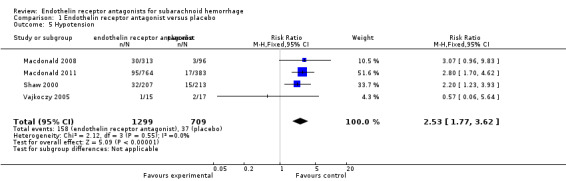
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 5 Hypotension.
Adverse events incidence: pneumonia
Three RCTs were included in the analysis (Shaw 2000; Macdonald 2008; Macdonald 2011). Compared with placebo, pneumonia was significantly more common with ETA (RR 1.56; 95% CI 1.23 to 1.97) and there was no heterogeneity of the studies (P = 0.45, I2 = 0%) (Analysis 1.6).
1.6. Analysis.
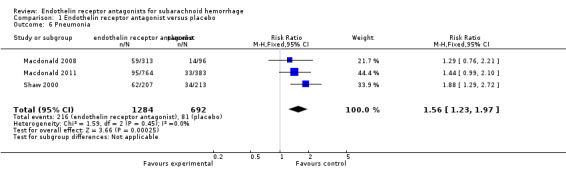
Comparison 1 Endothelin receptor antagonist versus placebo, Outcome 6 Pneumonia.
We did not perform subgroup analyses.
Discussion
Summary of main results
This review demonstrated that ETAs could reduce the risk of DIND and angiographically confirmed vasospasm, but had potentially serious adverse effects such as hypotension and pneumonia. There were no statistically significant differences in mortality and improving clinical outcomes. There is a suggestion that trials using higher dosages of ETAs might be more effective in preventing DIND and angiographic vasospasm. Analysis of hypotension showed no apparent dose‐dependent effect. These results all need testing in a larger trial.
Overall completeness and applicability of evidence
We planned to include all patients who were adults and met the clinical and radiological diagnostic criteria, regardless of gender or severity. However, only moderate to severe patients (World Federation of Neurological Surgeons (WFNS) Grade II, III or IV, or Grade I with a neurological deficit or severe meningism; or Grade Hunt and Hess Grade III or IV) were enrolled. Therefore, these findings might not apply to patients classified as Grade I or V and who have other concomitant conditions or diseases. The included studies in this review were from the English literature: we found no non‐English language studies, which might limit the review's applicability. We were unable to address all of the objectives of this review because the results were based on only four studies (too small a sample to detect relevant differences between effects of treatment). Not all the studies reported our outcomes of interest (mortality, DIND and unfavorable outcome); for example, none of the studies mentioned rebleeding within six months of SAH. Therefore, any further studies conducted in this area must be well‐designed RCTs that ideally assess these outcomes.
Quality of the evidence
We only included RCTs in this review in an attempt to minimize bias. To provide the best available evidence, this review was based on only four RCTs. These studies compared two types of ETAs with placebo over 10 to 14 days. The diagnostic criteria of the four studies were clear and the age, gender, severity and other baseline features of the patients were comparable. All of them had a well‐balanced distribution of prognostic factors for outcome between treatment and control groups, which adds to the validity of the results. They were all multicenter, double‐blind, randomized trials. However, only Shaw 2000 described computer generated sequence allocation; the other studies did not provide the specific method.
Potential biases in the review process
We searched for all relevant studies using sensitive and validated search strategies in several major medical electronic databases and other sources. However, it was possible that not all the relevant studies were identified from computerized searching. The studies used in our review were from the published literature: there were no studies from the 'gray literature' such as special reports, unpublished information, government reports and other traditional or non‐traditional literature. Based on the limited number of RCTs comparing ETAs with placebo for patients with SAH, it was difficult to assess the benefits of the intervention. Our results were based on original studies and were therefore subject to the potential biases inherent in such studies. Limitations in this review included the use of various doses of two different drugs, one of which was a non‐selective ETA. The four studies did not provide sample size estimates and the numbers were too small to classify according to different drugs. There were losses to follow‐up in all four studies. Although the reasons for the losses were described in detail and intention‐to‐treat (ITT) analysis was provided, there still might have been attrition bias. Further studies should assess clinical end point outcome measures, such as mortality and improved clinical outcome.
Agreements and disagreements with other studies or reviews
Few meta‐analyses have been published in this area. Kramer 2009 indicated that ETAs prevent both radiographic vasospasm and DINDs, but did not indicate that they improve clinical outcomes. The results of our review support this. The evolving concept is that DIND (and vasospasm) is multifactorial, with other multiple associated processes that cause poor outcome and vasospasm. Microcirculatory dysfunction, thromboembolism and cortical‐spreading depression have also been described, mostly after experimental SAH and probably contribute to poor outcome after SAH (Macdonald 2008). The lack of impact of ETAs on such factors could, in part, explain the discrepancy between their impressive effects on large‐vessel vasospasm compared with the lack of clinical outcome benefit (Kramer 2009). Similar results have also been observed with other drugs, including tirilazad and nicardipine (Haley 1993; Jang 2009). However, our results also show how the adverse effects of ETAs might offset the reduction in DIND and vasospasm. Hypotension can be extremely detrimental for cerebral perfusion as it may aggravate cerebral ischemia and affect clinical outcome. Similarly, pneumonia, pulmonary edema and acute lung injury (ALT) may further aggravate cerebral hypoxia, which have been associated with worse outcomes after SAH (Kramer 2009). We suggest that future studies should incorporate well‐planned strategies to address the benefit and adverse effects of ETAs.
Authors' conclusions
Implications for practice.
ETAs have not been shown to reduce death or unfavorable clinical outcomes and the reduction in DIND and vasospasm was offset by significant increases in hypotension and pneumonia. At present there is insufficient evidence to support the routine use of ETAs.
Implications for research.
More well‐designed RCTs are needed and future RCTs should ensure concealed treatment allocation, blinded outcome assessment and long‐term follow‐up in order to evaluate the effect of ETAs on the preservation of nervous function, and concern on cost‐effectiveness of ETAs in treatment of SAH. Researchers should ensure that they use pragmatic clinical outcomes, such as mortality, disability, quality of life, and standardized DIND and GOSE categories. The trials should be reported in full and preferably conform to the Consolidated Standards of Reporting Trials (CONSORT) statement.
Acknowledgements
We would like to thank Hazel Fraser and Brenda Thomas for their editorial advice during the preparation of this review. Also thanks to the referees for their editorial advice.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ or exp meninges/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. Vasospasm, Intracranial/ 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. Receptors, Endothelin/ai or Receptor, Endothelin A/ai or Receptor, Endothelin B/ai 15. endothelins/ai or endothelin‐1/ai or endothelin‐2/ai or endothelin‐3/ai 16. ((endothel$ or ET‐1 or ET$) adj5 antag$).tw. 17. (atrasentan or ambrisentan or Letairis or Volibris or bosentan or bozentan or Tracleer or clazosentan or darusentan or edonentan or enrasentan or sitaxsentan or Thelis or "TAK 044" or tezosentan or Valetri or Veletri).tw. 18. (atrasentan or ambrisentan or Letairis or Volibris or bosentan or bozentan or Tracleer or clazosentan or darusentan or edonentan or enrasentan or sitaxsentan or Thelis or "TAK 044" or tezosentan or Valetri or Veletri).nm. 19. 14 or 15 or 16 or 17 or 18 20. 13 and 19 21. limit 20 to humans
Appendix 2. EMBASE search strategy
1. 'subarachnoid hemorrhag'/exp 2. 'brain hemorrhage'/exp 3. 'intracranial aneurysm'/exp 4. 'uterus rupture'/exp 5. 3 and 4 6. 'aneurysm ruptured'/exp 7. 'brain'/exp 8. 'meninx'exp 9. 6 and (7 or 8) 10. ((subarachnoid or arachnoid) and (haemorrhage* or hemorrhage* or bleed* or blood*)):ti,ab,kw 11. 'brain vasospasm '/exp 12. ((cerebral or intracranial or cerebrovascular) and (vasospasm or spasm)):ti,ab,kw 13. sah:ti,ab,kw 14. 1 or 2 or 5 or 9 or 10 or 11 or 12 or 13 15. 'endothelin receptor '/exp 16.'endothelin A receptor '/exp 17.'endothelin B receptor '/exp 18.'endothelin'/exp 19. ((endothel* or ET‐1 or ET*) and antag*):ti,ab,kw 20. (atrasentan or ambrisentan or Letairis or Volibris or bosentan or bozentan or Tracleer or clazosentan or darusentan or edonentan or enrasentan or sitaxsentan or Thelis or "TAK 044" or tezosentan or Valetri or Veletri):ti,ab,kw 21. 15 or 16 or 17 or 18 or 19 or 20 22. 14 and 21
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
1. MeSH descriptor Subarachnoid Hemorrhage explode all trees 2. MeSH descriptor Intracranial hemorrhages explode all trees 3. MeSH descriptor Cerebral hemorrhage explode all trees 4. MeSH descriptor Intracranial Aneurysm explode all trees 5. MeSH descriptor Rupture, Spontaneous explode all trees 6. 4 and 5 7. MeSH descriptor Aneurysm, Ruptured explode all trees 8. MeSH descriptor Brain explode all trees 9. MeSH descriptor Meninges explode all trees 10. 7 and (8 or 9) 11. ((subarachnoid or arachnoid) and (haemorrhage* or hemorrhage* or bleed* or blood*)):ti,ab,kw 12. MeSH descriptor Vasospasm, Intracranial explode all trees 13. ((cerebral or intracranial or cerebrovascular) and (vasospasm or spasm)):ti,ab,kw 14. sah:ti,ab,kw 15. 1 or 2 or 3 or 6 or 10 or 11 or 12 or 13 or 14 16. MeSH descriptor Receptors, Endothelin explode all tree 17. MeSH descriptor Receptors, Endothelin A explode all tree 18. MeSH descriptor Receptors, Endothelin B explode all tree 19. MeSH descriptor Endothelin 1 explode all tree 20. MeSH descriptor Endothelin 2 explode all tree 21. MeSH descriptor Endothelin 3 explode all tree 22. ((endothel* or ET‐1 or ET*) and antag*):ti,ab,kw 23. (atrasentan or ambrisentan or Letairis or Volibris or bosentan or bozentan or Tracleer or clazosentan or darusentan or edonentan or enrasentan or sitaxsentan or Thelis or "TAK 044" or tezosentan or Valetri or Veletri):ti,ab,kw 24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 15 and 24
Appendix 4. Quality assessment
Allocation concealment
Yes (low risk of bias): randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study.
Unclear (uncertain risk of bias): randomization stated but no information on method used is available.
No (high risk of bias): method of randomization used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group.
Blinding
Blinding of investigators: yes/no/not stated.
Blinding of participants: yes/no/not stated.
Blinding of outcome assessor: yes/no/not stated.
Blinding of data analysis: yes/no/not stated.
The above are considered not blinded if the treatment group can be identified in more than 20% of participants because of the side effects of treatment.
Incomplete outcome data
Yes (low risk of bias): if there were no postrandomization drop‐outs or withdrawals or if the postrandomization drop‐outs were balanced in both groups and the reasons for missing data were unlikely to be related to true outcome.
Unclear (uncertain risk of bias): if it is not clear whether there are any drop‐outs or withdrawals or if the reasons for these drop‐outs are not clear.
No (high risk of bias): if the reasons for missing data likely to be related to true outcomes, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcomes, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that as‐signed at randomization; potentially in appropriate application of simple imputation.
Selective outcome reporting
Yes (low risk of bias): if all the important outcomes (primary outcomes stated in the review) were reported or if the trial protocol was available and all of the trial's prespecified (primary and secondary) outcomes that are of interest in their view have been reported.
Unclear (uncertain risk of bias): if there is insufficient information to assess whether the risk of selective outcome reporting is present.
No (high risk of bias): if not all the prespecified outcomes were reported, the primary outcomes were changed, or if some of the important outcomes were incompletely reported.
Other biases
Baseline imbalance
Yes (low risk of bias): if there was no baseline imbalance in important characteristics.
Unclear (uncertain risk of bias): if the baseline characteristics were not reported.
No (high risk of bias): if there was a baseline imbalance due to chance or owing to imbalanced exclusion after randomization.
Early stopping
Yes (low risk of bias): if sample size calculation was reported and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect owing to chance was low.
Unclear (uncertain risk of bias): if sample size calculations were not reported and it is not clear whether the trial was not stopped early.
No (high risk of bias): if the trial was stopped early by informal stopping rules.
Data and analyses
Comparison 1. Endothelin receptor antagonist versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DIND | 3 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.95] |
| 2 Mortality | 4 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.77, 1.45] |
| 3 Angiographic vasospasm | 3 | 1588 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.52, 0.72] |
| 4 Unfavorable outcome | 3 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 5 Hypotension | 4 | 2008 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.77, 3.62] |
| 6 Pneumonia | 3 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.23, 1.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Macdonald 2008.
| Methods | Study type: interventional
Study design: Phase IIb randomized double‐blind, placebo‐controlled, dose‐finding study
Sequence generation: patients were stratified by site and procedure (coiling or clipping) Allocation: drug and placebo were clear and colorless with no obvious acute effects that would compromise blinding Blinding: with the exception of the pharmacist and an independent pharmacy monitor who was not involved in other study tasks, all investigators, patients and individuals responsible for conduct, monitoring and analysis of the study were blinded to treatment Incomplete outcome data addressed: reported the number and reason Free of selective outcome reporting: unclear Free of other bias: this trials was supported by Actelion Pharmaceuticals Duration of study: 12 weeks |
|
| Participants | Ages eligible for study: 18 to 70 years Genders eligible for study: both Total number of participants: 413 Location: 52 entered in 11 countries Inclusion criteria: eligible patients were aSAH due to a ruptured saccular aneurysm confirmed by DSA. SAH had to be diffuse (long axis 20 mm) or localized (long axis 20 mm) thick (short axis 4 mm) subarachnoid clot on CT scan within 48 hours of SAH. Patients had a WFNS Grade I to IV on admission or were Grade V patients who had improved to Grade IV or less after resuscitation and ventriculostomy Exclusion criteria: SAH from a lesion other than a ruptured saccular aneurysm; intraventricular or intracerebral blood in the absence of localized thick or diffuse SAH; no or localized thin SAH on CT; cerebral vasospasm on admission DSA; hypotension (systolic blood pressure 90 mm Hg) refractory to fluid therapy; neurogenic pulmonary edema or cardiac failure requiring inotropic support; severe or unstable concomitant condition or disease or chronic condition, which, in the opinion of the investigator, could affect assessment of the safety or efficacy of the study drug; kidney (plasma creatinine 177 mol/L) or liver disease (total bilirubin 51.3 mol/L), or both; and prior cerebral damage on CT scan such as stroke (2 cm maximum diameter), traumatic brain injury, previously treated cerebral aneurysm, arterial venous malformation or pre‐existing cerebrovascular disorder that would affect diagnosis and evaluation of SAH. Women of childbearing potential had negative pretreatment serum pregnancy tests | |
| Interventions | Treatment group: 1, 5, or 15 mg/hour clazosentan Control group: placebo |
|
| Outcomes | Primary outcome measures: the primary end point was moderate or severe vasospasm within 14 days of SAH Secondary outcome measures: the secondary end point was morbidity and mortality within 6 weeks of SAH; DIND; death, vegetative or severe disability defined as a GOSE; adverse events included mortality | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were stratified by site and procedure (coiling or clipping) |
| Allocation concealment (selection bias) | Low risk | Drug and placebo were clear and colorless with no obvious acute effects that would compromise blinding |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The pharmacist and an independent pharmacy monitor who was not involved in other study tasks, all investigators, patients and individuals responsible for conduct, monitoring and analysis of the study were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported the number and reason |
| Selective reporting (reporting bias) | Unclear risk | There was not enough information to permit judgment of the other potential sources of bias |
| Other bias | Unclear risk | Supported by Actelion Pharmaceuticals |
Macdonald 2011.
| Methods | Study type: interventional
Study design: randomized, double‐blind, placebo‐controlled, Phase III study
Sequence generation: an interactive web response system Allocation: an interactive web response system Blinding: clinical and imaging data were assessed by a masked assessor Incomplete outcome data addressed: reported the number and reason Free of selective outcome reporting: unclear Free of other bias: this trials was funded by Actelion Pharmaceuticals Duration of study: 12 weeks |
|
| Participants | Ages eligible for study: 18 to 75 years Genders eligible for study: both Total number of participants: 1157 Location: 102 sites in 27 countries Inclusion criteria: patients had SAH due to ruptured saccular aneurysm secured by surgical clipping. Eligible patients had a diffuse clot (long axis ≥ 20 mm or present in both hemispheres) on admission CT scan and a WFNS grade I to IV SAH before the securing procedure Exclusion criteria: individuals with SAH due to non‐aneurysmal causes, intraventricular or intracerebral hemorrhage without subarachnoid blood, angiographic vasospasm on admission angiography or major complications during the securing procedure were excluded | |
| Interventions | Treatment group: 5 mg/hour clazosentan Control group: placebo |
|
| Outcomes | Primary outcome measures: all‐cause mortality and vasospasm‐related morbidity within 6 weeks of aSAH, as defined by at least 1 of the following events: death; vasospasm‐related cerebral infarction; DIND due to vasospasm or neurological signs or symptoms, in the presence of a positive angiogram, leading to rescue therapy Secondary outcome measures: the GOSE score dichotomized as good or poor outcome at week 12 was the main secondary end point. The other secondary end points were the occurrence of the individual components of the composite primary end point and total volume of new or worsened cerebral infarcts of all causes at week 6 after aSAH | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive web response system |
| Allocation concealment (selection bias) | Low risk | An interactive web response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinical and imaging data were assessed by a masked assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported the number and reason |
| Selective reporting (reporting bias) | Unclear risk | There was not enough information to permit judgment of the other potential sources of bias |
| Other bias | Unclear risk | Funded by Actelion Pharmaceuticals |
Shaw 2000.
| Methods | Study type: interventional
Study design: multicenter, double‐blind, randomized, placebo‐controlled, parallel‐group investigation
Sequence generation: computer‐generated randomization schedule Allocation: method of allocation not reported but was multicenter study Blinding: double‐blind Incomplete outcome data addressed: reported the number and reason Free of selective outcome reporting: unclear Free of other bias: this study was funded in its entirety by Takeda Euro Research and Development Centre GmbH Duration of study: 3 months |
|
| Participants | Ages eligible for study: 18 years of age or older Genders eligible for study: both Total number of participants: 420 Location: 20 neurosurgical units in the UK, 7 in the Netherlands, and 1 in the Republic of Ireland Inclusion criteria: the patient was included if an angiogram confirmed that the patient harbored an aneurysm. Patients included also had to have an SAH classified as WFNS 14 Grades II, III, or IV, or Grade I with a neurological deficit or severe meningism (the coexistence of at least 2 of the following: severe headache, photophobia or neck stiffness) Exclusion criteria:
|
|
| Interventions | Treatment group: TAK‐044 (≤ 50 mg given 3 times per day) Control group: placebo |
|
| Outcomes | Primary outcome measures: the primary end point of the study was the incidence of delayed neurological deterioration caused by ischemia within 3 months Secondary outcome measures: the secondary end points included delayed neurological deterioration within 10 days; visual infraction; unfavorable outcome based on the GOS 4 score; the incidence of adverse events and abnormal laboratory indices | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported, but was multicenter study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐bind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported the number and reason |
| Selective reporting (reporting bias) | Unclear risk | There was not enough information to permit judgment of the other potential sources of bias |
| Other bias | Unclear risk | Funded in its entirety by Takeda Euro Research and Development Centre GmbH |
Vajkoczy 2005.
| Methods | Study type: interventional
Study design: randomized, double‐blind, placebo‐controlled, multicenter Phase IIa study
Sequence generation: randomization of eligible patients to the treatment groups followed a 1:1 ratio Allocation: method of allocation not reported but was multicenter study Blinding: the investigator or other staff member was not allowed to witness the preparation of study medication by the pharmacist Incomplete outcome data addressed: reported the number and reason Free of selective outcome reporting: unclear Free of other bias: was funded by Axovan Ltd and Actelion Ltd Duration of study: 14 days |
|
| Participants | Ages eligible for study: 18 to 65 years Genders eligible for study: both Total number of participants: 34 Location: 5 neurosurgical centers in Germany Inclusion criteria: patients with severe aSAH (Grade III or IV according to the Hunt and Hess classification and Grade ≥ III according to the Fisher scale) and diagnosed by CT scanning and a saccular aneurysm confirmed by digital subtraction angiography Exclusion criteria:
|
|
| Interventions | Treatment group: clazosentan (0.2 mg/kg/hour) Control group: placebo |
|
| Outcomes | Primary outcome measures: the primary end points of the study were to assess the safety and tolerability of clazosentan in patients with aSAH and to evaluate whether its administration following surgical aneurysm clipping can reduce the incidence or severity, or both of angiographic vasospasm Secondary outcome measures: secondary end points included the effect of clazosentan on ischemic deficits as documented by CT scanning, and its peripheral and cardiopulmonary hemodynamic effects. An exploratory tertiary objective was to assess the effect of clazosentan on the reversal of angiographically confirmed vasospasm | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Method of allocation not reported but was multicenter study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐bind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported the number and reason |
| Selective reporting (reporting bias) | Unclear risk | There was not enough information to permit judgment of the other potential sources of bias |
| Other bias | Unclear risk | Funded by Axovan Ltd and Actelion Ltd |
aSAH: aneurysmal subarachnoid hemorrhage CT: computerized tomography DIND: delayed ischemic neurological deficit DSA: digital subtraction catheter angiography GOS: Glasgow Outcome Scale GOSE: Glasgow Outcome Scale Extended SAH: subarachnoid hemorrhage WFNS: World Federation of Neurological Surgeons
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Nogueira 2007 | An open‐label feasibility study of high dosages of the endothelin receptor antagonist bosentan (ETA/B) in SAH patients at high‐vasospasm risk |
SAH: subarachnoid hemorrhage
Characteristics of ongoing studies [ordered by study ID]
CONSCIOUS 3 2010.
| Trial name or title | Clazosentan in aneurysmal subarachnoid hemorrhage |
| Methods | Phase III clinical study |
| Participants | Inclusion criteria: 1. Males and females aged 18 to 75 years (inclusive). 2. Patients with a ruptured saccular aneurysm, confirmed by angiography, and which has been successfully secured by endovascular coiling. 3. Women of childbearing potential must have a negative serum pregnancy test and must use a reliable method of contraception during the 12 weeks following study drug discontinuation. 4. Written informed consent Exclusion Criteria: 1. Patients with SAH due to causes other than a saccular aneurysm (e.g. trauma or rupture of fusiform or mycotic aneurysms). 2. Patients who experienced a major complication during the endovascular coiling procedure, such as massive intracranial bleeding, intracranial thromboembolism, coil migration, aneurysm perforation or rupture, arterial dissection, major arterial occlusion, a large territorial cerebral infarct defined as involving > 1/3 of a vascular territory, or a new major neurological deficit postprocedure. 3. Patients who have had their current ruptured aneurysm previously secured (successfully or not) by clipping. 4. Patients coiled with coiling material, which has not been approved by local health authorities. 5. Use of liquid embolism aneurysmal treatment or flow diverting device. 6. Patients with several aneurysms among which the ruptured one cannot be identified with certainty and which are not all secured during the coiling procedure. 7. Patients with no DSA at end of procedure. 8. Patients planned to have another securing procedure for any aneurysm after randomization and prior week 12 post aSAH. 9. Patients for whom study drug cannot be started within 56 hours after the aneurysm rupture. 10. Patients for whom it is known, at the time of screening, that certain follow‐up, or protocol‐mandated imaging assessments will not be feasible. 11. Patients with hypotension (systolic blood pressure ≤ 90 mmHg) that is refractory to treatment. 12. Patients with aspiration pneumonia. 13. Patients with pulmonary edema or severe cardiac failure requiring inotropic support at the time of randomization. 14. Any severe or unstable concomitant condition or disease (e.g. known significant neurological deficit, cancer, hematological, or coronary disease), or chronic condition (e.g. psychiatric disorder), which, in the opinion of the investigator, would affect the assessment of the safety or efficacy of the study drug. 15. Significant kidney or liver disease, or both. 16. Patients receiving iv nimodipine or iv nicardipine must have these drugs discontinued at least 4 hours prior to initiation of the study treatment. 17. Patients who have received iv fasudil within the 24‐hour period immediately preceding the planned start of study drug initiation. 18. Patients starting statins less than 2 weeks prior to admission must have them discontinued prior to study drug initiation. 19. Patients receiving cyclosporine A or other calcineurin inhibitors (e.g. tacrolimus), or patients for whom it is known at the time of randomization that these medications will be started during the study drug infusion period. 20. Patients who have received an investigational product including investigational coil material within 28 days prior to randomization or those who have already participated in the current study. 21. Patients unlikely to comply with the protocol (e.g. unable to return for follow‐up visits). 22. Known hypersensitivity to other endothelin receptor antagonists. 23. Patients with current alcohol or drug abuse or dependence |
| Interventions | Clazosentan (5 mg/hour) versus clazosentan (15 mg/hour) versus placebo |
| Outcomes | Cerebral vasospasm‐related morbidity and mortality of all‐causes; Glasgow Outcome Scale Extended at week 12 post‐aSAH, dichotomized into good (score > 4) and poor (score ≤ 4) outcome |
| Starting date | July 2009 |
| Contact information | Alexa Richie (richie.alexa@mayo.edu) |
| Notes | ‐ |
aSAH: aneurysmal subarachnoid hemorrhage DSA: digital subtraction catheter angiography iv: intravenous SAH: subarachnoid hemorrhage
Differences between protocol and review
There are no significant differences between the protocol and the review, except that we did not have data for the secondary outcome of 'rebleeding within six months of SAH'.
Contributions of authors
Writing of review: Zhenghong Shi, KeHu Yang, Jia Guo, Jinhui Tian, Lei Jiang Screening of titles and abstracts: Jia Guo, KeHu Yang Assessment for inclusion: Zhenghong Shi, Jinhui Tian Quality assessment: Zhenghong Shi, Jia Guo Data extraction: Zhenghong Shi, Jinhui Tian, Lei Jiang Data entry into RevMan: Jiang L Data analysis: Zhenghong Shi, Jia Guo, Jinhui Tian Disagreement resolution: KeHu Yang, Jinhui Tian
Declarations of interest
None known.
New
References
References to studies included in this review
Macdonald 2008 {published data only}
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemias and infarction occurring after subarachnoid hemorrhage (CONSCIOUS‐1): randomized, double‐blind, placebo‐controlled phase 2 dose‐finding trial. Stroke 2008;39(11):3015‐21. [DOI] [PubMed] [Google Scholar]
Macdonald 2011 {published data only}
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double‐blind, placebo‐controlled phase 3 trial (CONSCIOUS‐2). Lancet Neurology 2011;10(7):618‐25. [DOI] [PubMed] [Google Scholar]
Shaw 2000 {published data only}
- Shaw MD, Vermeulen M, Murray GD, Pickard JD, Bell BA, Teasdale GM. Efficacy and safety of the endothelin A/B receptor antagonist TAK‐044 in treating subarachnoid hemorrhage: a report by the Steering Committee on behalf of the UK/Netherlands/Eire TAK‐044 Subarachnoid Haemorrhage Study Group. Journal of Neurosurgery 2000;93(6):992–7. [DOI] [PubMed] [Google Scholar]
Vajkoczy 2005 {published data only}
- Vajkoczy P, Meyer B, Weidauer S, Raabe A, Thome C, Ringel F, et al. Clazosentan (AXV‐034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double‐blind, placebo‐controlled, multicenter Phase IIa study. Journal of Neurosurgery 2005;103(1):9–17. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Nogueira 2007 {published data only}
- Nogueira RG, Bodock MJ, Koroshetz WJ. High‐dose bosentan in the prevention and treatment of subarachnoid hemorrhage‐induced cerebral vasospasm: an open‐label feasibility study. Neurocritical Care 2007;7:194–202. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CONSCIOUS 3 2010 {unpublished data only}
- Clazosentan in aneurysmal subarachnoid hemorrhage. Ongoing study July 2009.
Additional references
Chow 2002
- Chow M, Dumont AS, Kassell NF. Endothelin receptor antagonists and cerebral vasospasm: an update. Neurosurgery 2002;51:1333‐41. [PubMed] [Google Scholar]
Fassbender 2000
- Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, et al. Endothelin 1 in subarachnoid hemorrhage: an acute‐phase reactant produced by cerebrospinal fluid leukocytes. Stroke 2000;31:2971‐5. [DOI] [PubMed] [Google Scholar]
Haley 1993
- Haley EC, Kassell FN, Torner JC. A randomized trial of nicardipine in subarachnoid hemorrhage: angiographic and transcranial doppler ultrasound results. A report of the Cooperative Aneurysm Study. Journal of Neurosurgery 1993;78:548–53. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jang 2009
- Jang YG, Ilodigwe D, Macdonald RL. Metaanalysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Neurocritical Care 2009;10:141–7. [DOI] [PubMed] [Google Scholar]
Johnston 1998
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998;50:1413‐8. [DOI] [PubMed] [Google Scholar]
Kramer 2009
- Kramer A, Fletcher J. Do endothelin‐receptor antagonists prevent delayed neurological deficits and poor outcomes after aneurysmal subarachnoid hemorrhage? A meta‐analysis. Stroke 2009;40:3403‐6. [DOI] [PubMed] [Google Scholar]
Masaki 2000
- Masaki T. The endothelin family: an overview. Cardiovascular Pharmacology 2000;35 Suppl 2:S3‐5. [DOI] [PubMed] [Google Scholar]
NHS 2012
- National Health Service (NHS). Subarachnoid haemorrhage. April 2012. http://www.nhs.uk/conditions/subarachnoid‐haemorrhage/Pages/Introduction.aspx (accessed 28 June 2012).
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Traill 2008
- Epidemiology and pathophysiology of cerebral aneurysms. http://www.anzca.edu.au/events/asm/asm2008/abstracts/epidemiology‐and‐pathophysiology‐of‐cerebral‐aneurysms.html (updated on 4 July 2008).
Uhlmann 2006
- Uhlmann D. Current opinion in investigational drugs. Clazosentan Actelion 2006;7:272‐81. [PubMed] [Google Scholar]
Van den Bergh 2004
- Bergh WM, Dijkhuizen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnesium Research 2004;17:301‐13. [PubMed] [Google Scholar]
Van Gijn 2001
- Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249‐78. [DOI] [PubMed] [Google Scholar]
Van Gijn 2007
- Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306‐18. [DOI] [PubMed] [Google Scholar]
Wagner 1992
- Wagner OF, Christ G, Wotja J, Vierhapper H, Parzer S, Nowotny PJ, et al. Polar secretion of endothelin‐1 by cultured endothelial cells. Journal of Biological Chemistry 1992;267:16066‐8. [PubMed] [Google Scholar]
Weyer 2006
- Weyer GW, Jahromi BS, Aihara Y, Agbaje‐Williams M, Nikitina E, Zhang ZD, et al. Expression and function of inwardly rectifying potassium channels after experimental subarachnoid hemorrhage. Journal of Cerebral Blood Flow and Metabolism 2006;26:382‐91. [DOI] [PubMed] [Google Scholar]
Zimmermann 2004
- Zimmermann M, Seifert V. Endothelin receptor antagonists and cerebral vasospasm. Clinical Autonomic Research 2004;14:143‐5. [DOI] [PubMed] [Google Scholar]


