Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic potentially life threatening condition resulting from an excessive ovarian stimulation. Its reported incidence varies from one percent to ten percent of in vitro fertilization (IVF) cycles. It seems likely that the release of vasoactive substances, secreted by the ovaries under human chorionic gonadotropin (hCG) stimulation plays a key role in triggering this syndrome. The hallmark of this condition, is a massive shift of fluid from the intra‐vascular compartment to the third space resulting in profound intra‐vascular depletion and haemoconcentration.
Objectives
To evaluate (i) the effectiveness of cryopreservation (embryo freezing) for the prevention of OHSS when compared with human intra‐venous albumin infusion (ii) the effectiveness of the elective cryopreservation (embryo freezing ) of all embryos for the prevention of OHSS when compared with fresh embryo transfer.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Review Group specialised register of controlled trials up to April 2007. In addition, MEDLINE (PUBMED 1985 to March 2007), EMBASE (1985 to April 2007), CINAHL (1985 to March 2007) and the National Research Register (April 2007) were searched.
Selection criteria
Randomised controlled trials (RCTs) in which either human intra‐venous albumin or cryopreservation of all embryos were used as a therapeutic approach to OHSS were included.
Data collection and analysis
The interventions compared were cryopreservation (embryo freezing) versus intra‐venous human albumin administration and elective cryopreservation of all embryos versus fresh embryo transfer. The primary outcomes were: incidence of moderate and severe OHSS versus nil and or mild OHSS, clinical pregnancies and or woman. The secondary outcomes were: number of oocytes retrieved, number of oocytes fertilized, number of embryos transferred, number of embryos frozen, multiple pregnancy rate, live birth rate, number of women admitted to the hospital as inpatient or outpatient and time to the next menstrual period (resolution time). Statistical analysis was performed in accordance with the Cochrane Menstrual Disorders and Subfertility Group guidelines.
Main results
No new studies found for inclusion in the update of this review, the results from the original review published Issue 2 , 2002 (which identified seventeen studies) remain unchanged. It therefore remains that two studies of which met our inclusion criteria one study was included where cryopreservation (embryo freezing) was compared with intra‐venous human albumin administration (Shaker 1996) and one study was included where elective cryopreservation of all embryos was compared with fresh embryo transfer (Ferraretti 1999). When cryopreservation was compared with intra‐venous human albumin administration no difference was found in all the outcomes examined between the two groups. When elective cryopreservation of all embryos was compared with fresh embryo transfer no difference was found in all the outcomes examined between the two groups.
Authors' conclusions
This updated of the review (D'Angelo 2002) has showed that there is insufficient evidence to support routine cryopreservation and insufficient evidence for the relative merits of intra‐venous albumin versus cryopreservation.
Keywords: Female; Humans; Cryopreservation; Embryo, Mammalian; Albumins; Albumins/administration & dosage; Infusions, Intravenous; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Embryo freezing for preventing ovarian hyperstimulation syndrome
More research is needed to determine whether using frozen embryos and or intravenous albumin can reduce the rate of severe ovarian hyperstimulation syndrome in IVF. Ovarian hyperstimulation syndrome (OHSS) is a complication of using hormones to induce ovulation (stimulate the release of eggs) in IVF (in vitro fertilisation). The drugs can sometimes over‐stimulate ovaries. Severe OHSS can be life‐threatening. Fewer hormones are needed if frozen embryos are transferred in a subsequent cycle, although this lowers pregnancy rates. However, this update the review first published in 2002 (D'Angelo 2002) found there is not enough evidence to show whether using frozen embryos and or intravenous albumin infusion (artificial fluid to increase the woman's blood volume) can reduce OHSS in women who are at high risk. More research is needed on effects on pregnancy rates.
Summary of findings
Summary of findings for the main comparison. Cryopreservation compared to fresh embryos transfer for preventing ovarian hyperstimulation syndrome.
| Cryopreservation compared to fresh embryos transfer for preventing ovarian hyperstimulation syndrome | ||||||
| Patient or population: patients with risk of ovarian hyperstimulation syndrome Settings: Intervention: Cryopreservation Comparison: Fresh embryo transfer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fresh embryo transfer | Cryopreservation | |||||
| Moderate and or severe OHSS | 60 per 1000 | 8 per 1000 (1 to 128) | OR 0.12 (0.01 to 2.29) | 125 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Clinical pregnancies | 463 per 1000 | 482 per 1000 (318 to 654) | OR 1.08 (0.54 to 2.19) | 125 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Number of livebirths (livebirth rate) | 373 per 1000 | 380 per 1000 (229 to 558) | OR 1.03 (0.5 to 2.12) | 125 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not enough information given about methods of allocation concealment, and study was not blinded. 2 The summary effect included both the line of no effect and appreciable benefit and harm.
Summary of findings 2. Cryopreservation compared to intra‐venous albumin for preventing ovarian hyperstimulation syndrome.
| Cryopreservation compared to intra‐venous albumin for preventing ovarian hyperstimulation syndrome | ||||||
| Patient or population: patients with risk of ovarian hyperstimulation syndrome Settings: Intervention: Cryopreservation Comparison: Intra‐venous albumin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intra‐venous albumin | Cryopreservation | |||||
| Moderate and or severe OHSS | 77 per 1000 | 308 per 1000 (41 to 824) | OR 5.33 (0.51 to 56.24) | 26 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Nil and or mild OHSS | 538 per 1000 | 307 per 1000 (85 to 689) | OR 0.38 (0.08 to 1.9) | 26 (1 study) | ⊕⊕⊝⊝ low1,3 | |
| Clinical pregnancies | 385 per 1000 | 36 per 1000 (0 to 423) | OR 0.06 (0 to 1.17) | 26 (1 study) | ⊕⊕⊝⊝ low1,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study was not blinded. 2 Very large confidence interval that included the line of no effect. 3 The summary effect included both the line of no effect and apprciable benefit and harm. 4 Very wide confidence interval.
Background
The mechanism of action of albumin in the treatment of women at high risk for OHSS may relate both to increasing the carrier protein capacity and its oncotic properties as albumin is responsible for 75% of the plasma oncotic pressure. Both factors could prevent leakage of fluid from the intra‐vascular space into the peritoneal cavity (Asch 1993). It could be speculated that human albumin binds an undefined factor (ovarian renin‐angiotensin, VEGF) at a specific and critical time of the cycle and thus helps to prevent the development of OHSS (Shoham 1994). Timely administration of albumin, during oocyte recovery or immediately following, may serve to bind and inactivate this factor. However, Doldi 1999 contradicted the above hypothesis and demonstrated that human albumin increases VEGF gene expression in human luteinizing granulosa cells with maximum expression present in cultured granulosa cells when obtained from women with serum oestradiol concentration >2000 pg and or ml on the day of hCG injection.
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic potentially life threatening condition resulting from an excessive ovarian stimulation. Its reported incidence varies from 1% to 10% of In Vitro Fertilization (IVF) cycles (Forman 1990; MacDougall 1992; Wada 1990). The incidence of the severe form of OHSS in women undergoing controlled ovarian hyperstimulation for IVF has been estimated to be approximately 0.5 to 2% (Forman 1990; SART 1992) with a reported positive correlation between younger age, lean appearance and OHSS (Navot 1992). In addition, polycystic ovarian syndrome (PCOS) or ultrasonographic ovarian appearance of polycystic ovaries (presence of multiple, small follicles at the periphery of the ovary with echogenic stroma 'necklace sign' ), establishment of pregnancy during assisted reproduction treatment (ART), human chorionic gonadotrophin (hCG) supplementation of the luteal phase, high serum oestradiol ( less than 2500 pg and or ml) were also reported to be associated with OHSS.
OHSS was originally classified as mild, moderate and severe by Rabau 1967 ; Schenker 1978 and subsequently modified by Golan 1989 to incorporate ultrasonographic measurement of the stimulated ovaries. Briefly, mild OHSS presents clinically as weight gain, thirst and abdominal discomfort; ultrasound examination shows the ovaries to be enlarged (five to ten cm in diameter) with a small amount of fluid in the pelvis. Moderate OHSS is associated with more pronounced symptoms (nausea, vomiting, abdominal distension, pain and dyspnoea), ultrasound examination of the pelvis reveals moderate amounts of ascitic fluid and the ovaries are 10 to 12 cm in diameter. In severe OHSS, all of these symptoms are associated with clinical evidence of excessive third‐space fluid accumulation (ascites, hydrothorax), ovaries larger than 12 cm in diameter and in extreme cases may present with acute respiratory distress, hepato‐renal failure and thromboembolic phenomena (Brinsden 1995)(Table 3). Navot 1992 introduced further modification to the above classification by differentiating between severe and life threatening form of OHSS (Table 4).
1. Golan classification of OHSS (1989).
| Classification | Size ovaries | Grade | Symptoms |
| MILD | 5‐10 cm | grade 1: | abdominal tension and discomfort |
| grade 2: | grade 1 signs plus nausea, vomiting, and/or diarrhoea | ||
| MODERATE | >10 cm | grade 3: | grade 2 signs plus ultrasound evidence of ascites |
| SEVERE | >12 cm | grade 4: | grade 3 signs plus clinical evidence of ascites and/or pleural effusion and dyspnoea |
| grade 5: | grade 4 signs plus haemoconcentration increased blood viscosity, hypovolaemia, decreased renal perfusion, oliguria |
2. Navot classification of severe OHSS (1992).
| Severe OHSS | Critical OHSS |
| Variably enlarged ovary | Variably enlarged ovary |
| Massive ascites +/‐ hydrothorax | Tense ascites +/‐ hydrothorax |
| Hct >45%(30% increment over the baseline value) | Hct >55% |
| WBC >15,000 | WBC >35,000 |
| Oliguria | |
| Creatinine 1.0‐1.5 | Creatinine >1.6 |
| Creatinine clearance >50mL/min | Creatinine clearance <50mL/min |
| Liver dysfunction | Renal failure |
| Anasarca | Tromboembolic phenomena |
| ARDS |
The factors leading to this syndrome have not been completely elucidated. It seems likely that the release of vasoactive substances, e.g. vascular endothelium growth factor (VEGF), secreted by the ovaries under hCG stimulation plays a key role in triggering this syndrome ( Goldsman 1995 ; Tsirigotis 1994). As more follicles are recruited in response to gonadotrophin stimulation, the mass of the granulosa cells increases and at the same time the cells gain functional maturation. These two factors, acting synergistically, cause a concomitant increase in serum oestradiol level and in, as yet poorly defined, vasoactive substances (Agrawal 1998; Al‐Shawaf 2001). The hallmark of this condition, is a massive shift of fluid from the intra‐vascular compartment to the third space resulting in profound intra‐vascular depletion and haemoconcentration (Rabau 1967; Schenker 1978).
Description of the intervention
The crucial event in the development of the OHSS is the administration of hCG. However, some have reported the onset of OHSS after gonadotrophin in stimulation despite withholding hCG ( Allegra 1991 ;Lipitz 1991). Moderate or severe OHSS typically presents in the luteal phase as a consequence of ovulatory hCG or in early gestation phase in which endogenous hCG is produced. When the OHSS develops in the luteal phase and pregnancy does not take place, the syndrome rapidly resolves spontaneously with the onset of the menses, rarely progressing into its severe form. If a pregnancy is established, notable aggravation will be observed and the symptoms can persist for up to 12 weeks of gestation, this is more often associated with multiple pregnancy (Dahl 1994). The elective cryopreservation of all embryos and their subsequent transfer in non gonadotrophin stimulated cycles may be used to avoid the endogenous hCG rise in IVF‐ET programmes (Amso 1990). However, the policy of elective cryopreservation of all embryos in patients at risk would reduce the chances of pregnancy, since frozen‐thawed embryo replacement may be associated with lower pregnancy rate than fresh embryo transfer (Awonuga 1996).
How the intervention might work
In addition, alternative strategies have been proposed for IVF and or ICSI (intra‐cytoplasmic sperm injection) patients at high risk of OHSS: (1) cancellation of the treatment cycle (Forman 1990) (2) gonadotrophins discontinuation prior to hCG triggering injection (coasting) (Sher 1993) (3) early unilateral follicular aspiration (Egbase 1999); (4) avoidance of luteal supplementation with hCG (Araujo 1995); the use of a GnRH agonist instead of hCG to induce the final oocyte maturation prior to retrieval in non GnRH agonist down regulated cycles (Gonen 1990; Segal 1992). Each of these strategies may reduce but not eliminate the risk.
Objectives
To evaluate (i) the effectiveness of cryopreservation (embryo freezing) for the prevention of OHSS when compared with human intra‐venous albumin infusion (ii) the effectiveness of the elective cryopreservation (embryo freezing) of all embryos for the prevention of OHSS when compared with fresh embryo transfer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in which freezing of all embryos was used as a therapeutic approach to OHSS were included. Ovulation induction treatment without IVF and or ICSI was not included in the meta‐analysis. Cross‐over trials were excluded from this meta‐analysis.
Types of participants
Women of reproductive age;
Women down‐regulated by GnRH‐a, undergoing superovulation in IVF and or ICSI cycles.
Types of interventions
Cryopreservation of all embryos versus intra‐venous albumin infusion;
Cryopreservation of all embryos versus fresh embryo transfer.
Types of outcome measures
Primary outcomes
Incidence of moderate and severe OHSS versus nil or mild OHSS, subsequent to oocyte retrieval;
Clinical pregnancy rate and or woman (after fresh or frozen embryo transfer where applicable).
Secondary outcomes
Number of oocytes retrieved
Fertilization rate (number of oocytes fertilised divided by total oocytes inseminated x 100);
Number of embryos transferred;
Number of embryos frozen;
Multiple pregnancy rate;
Livebirth rate;
Number of women admitted to the hospital inpatient versus outpatient;
Number of days to next menstrual period (resolution).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Menstrual Disorders and Subfertility Review Group specialised register of controlled trials up to 22 April 2007.
See Appendix 1
Searching other resources
In addition, the review authors were also involved in hand searching of specialist journals (Human Reproduction Abstract Books 1999 and 2000) retrieving relevant articles from titles and abstracts, checking the reference lists of articles, contacting authors of conference abstracts to obtain details of the subsequent publication, informing the principal journals (Human Reproduction, Fertility and Sterility, British Journal of Obstetrics and Gynaecology and Lancet) asking for new published and unpublished articles, contacting authors of ongoing studies on this topic to obtain study data and update of the not already published paper. The authors of the included published studies were contacted to obtain additional information that was required for the analysis and they kindly replied.
Data collection and analysis
Selection of studies
For the purposes of the update of the review published in 2002 (D'Angelo 2002) two review authors, Mr N.N. Amso (NNA) and Dr A. D'Angelo (ADA) scanned the titles and the abstracts of the reports identified by electronic searching in order to find relevant papers no further trials were identified, In the original review (D'Angelo 2002) Mr N.N. Amso (NNA) and Dr A. D'Angelo (ADA) scanned the titles and the abstracts of the reports identified by electronic searching in order to find relevant papers. One reviewer (ADA) obtained copies of the full text articles and made copies for the other review author (NNA) in which details of authors, institution, results and discussion were removed in order to assess their eligibility for inclusion.
Data extraction and management
Both review authors extracted data independently using forms designed according to Cochrane guidelines. Disagreements were resolved by discussion.
Assessment of risk of bias in included studies
The unit for randomisation was women fulfilling the criteria for inclusion into the study. The quality of allocation concealment was graded as either adequate (A), unclear (B), or inadequate (C), following the detailed descriptions of these categories provided by the Menstrual Disorders and Subfertility Review Group. Additional information on the trial methodology or data were requested by writing to the corresponding authors directly. Included trials data were processed as:
Trial characteristics
Method of randomisation
Number of women randomised, excluded and reasons;
Multicentre or single centre design;
Presence or absence of blinding to treatment allocation;
Whether an intention‐to‐treat analysis was done;
The presence of a power calculation;
Duration, timing, and location of the study;
Sources of any funding
Characteristic of women
Age of the women;
Down‐regulation and superovulation in ART cycles;
Causes of infertility: unexplained subfertility; tubal factor; endometriosis; anovulatory factor; male factor;
Duration of infertility;
Women at risk of developing OHSS based on serum oestradiol level (>1,906 pg and or ml or >7,000 pmol and or L) on the day of human chorionic gonadotrophin (hCG) administration
Interventions used
Treatment:
‐ elective cryopreservation.
Control:
‐ intra‐venous human albumin infusion; ‐ fresh embryo transfer.
Outcomes
Primary
Incidence of moderate and severe OHSS versus nil or mild OHSS;
Clinical pregnancy rate and or woman (after fresh or frozen embryo transfer where applicable).
Secondary
Number of oocytes retrieved;
Fertilization rate;
Number of embryos frozen;
Number of embryos transferred;
Multiple pregnancy rate;
Livebirth rate;
Number of women admitted to the hospital inpatient versus outpatient;
Number of days to the next menstrual period (resolution).
Measures of treatment effect
Statistical analysis was performed in accordance with the guidelines developed by the Menstrual Disorders and Subfertility Group. For a dichotomous data, results for each study were expressed as an odds ratio (OR) with 95% confidence intervals (CI) and combined for meta‐analysis with RevMan software using Peto‐modified Mantel‐Haenszel method. Continuous data were not normally distributed therefore the results for these outcomes have not been combined using WMD and have been reported separately. Because of the small number of studies included no sensitivity analysis was performed.
Unit of analysis issues
None
Dealing with missing data
The data will be analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from the original investigators of Shaker 1996.
Assessment of heterogeneity
Not applicable
Assessment of reporting biases
There were less than ten included studies therefore this was not assessed formally
Data synthesis
Not applicable
Subgroup analysis and investigation of heterogeneity
Not applicable
Sensitivity analysis
Because of the small number of studies included no sensitivity analysis was performed.
Results
Description of studies
There were no new studies identified for inclusion or exclusion in this update.
In the original review published in 2002 (D'Angelo 2002) one randomised controlled study in which cryopreservation of all embryos was compared with intra‐venous albumin infusion and subsequent fresh embryo transfer for the prevention of moderate and severe OHSS was identified and one randomised controlled study in which elective cryopreservation of all embryos was compared with fresh embryo transfer for the prevention of moderate and severe OHSS was identified.
Results of the search
In the original review published in 2002 (D'Angelo 2002) the only study in which cryopreservation of all embryos was compared with intra‐venous albumin infusion and subsequent fresh embryo transfer which met our inclusion criteria was Shaker et al. (Shaker 1996).
Included studies
See Characteristics of included studies Women's age and superovulation protocols are listed in the table of included studies. Women were considered to be at risk of hyperstimulation when: E2 was less than 10,000 pmol and or L and less than 15 oocytes collected or E2 less than 13,000 p mol and or L. A diagnosis of moderate or severe OHSS was made according to the Schenker and Weinstein classification (Schenker 1978). The intervention and control groups were compared in relation to the incidence of moderate or severe versus nil or mild OHSS, the number of clinical pregnancies, number of oocytes retrieved, and the number of embryos transferred and frozen.
The only study in which elective cryopreservation of all embryos was compared with fresh embryo transfer which met our inclusion criteria was Ferraretti 1999. It was a single centre randomised study. Women undergoing superovulation for IVF and or ICSI treatment (GnRH‐a down‐regulation and gonadotrophin stimulation) were included in the study. They were considered to be at risk of hyperstimulation when the E2 level was >1500 pg and or ml ( less than 5500 pmol and or L) and less than 15 oocytes collected. The diagnosis of moderate and severe OHSS was done according to Golan classification (Golan 1989) revised by Navot 1992. The incidence of moderate or severe OHSS, clinical pregnancy rate per woman, number of oocytes retrieved, number of embryos transferred, livebirth rate and number of women admitted as inpatient or outpatient were compared in the intervention and control groups.
Excluded studies
See Characteristics of excluded studies. A total of fifteen studies were excluded in the original review published in 2002 (D'Angelo 2002) . Six were prospective observational studies (Asch 1993; Awonuga 1996; Chen 1997; Queenam 1997; Titinem 1995 ;Wada 1993). Four were randomised controlled studies where intra‐venous albumin was compared with no treatment and or placebo, therefore did not meet our inclusion criteria (Isik 1996; Munoz 2000; Shalev 1995; Shoham 1994), and is the subject of an existing Cochrane review (Aboulghar 1999). Three were retrospective (Pattinson 1994; Ndukwe 1997; Wada 1993 ). One was prospectively randomised but on alternating basis (Panay 1999). One was a cohort study (Ng 1995).
Risk of bias in included studies
See Figure 1 and Figure 2. Women were randomised using drawing cards (Shaker 1996) or not specified (Ferraretti 1999).
1.
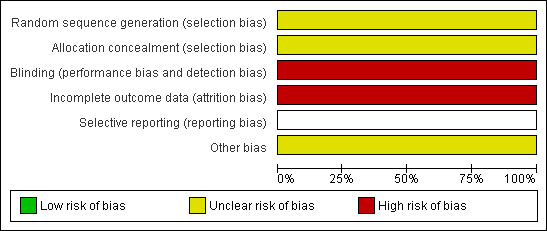
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
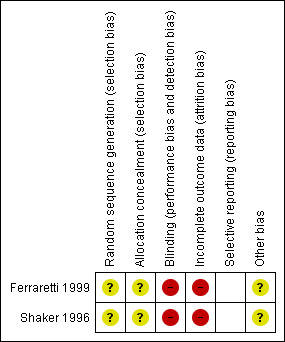
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
None of the studies described allocation concealment adequately.
Blinding
In the original review published in 2002 (D'Angelo 2002) both studies included were single centre unblinded randomised controlled trials.
Incomplete outcome data
Both authors Ferraretti 1999 and Shaker 1996 were contacted by letter to obtain missing data. The number of women included in these studies was small. Three women withdrew from Shaker 1996 requesting to have fresh embryos rather than cryopreservation. No withdrawals or loss of follow up were mentioned in the other study.
Other potential sources of bias
None of the studies described a power of calculation.
Effects of interventions
Because there were no new studies found for inclusion in the update of this review the results from the original review published Issue 2 , 2002 which identified seventeen studies remains unchanged. Two trials met our inclusion criteria in 2002. Comparisons were classified into two categories:
(1) Cryopreservation versus intra‐venous albumin infusion
Incidence of moderate and or severe OHSS: there was no difference between the two groups in the incidence of moderate and or severe OHSS (n=26, OR 5.33, 95% CI 0.51 to 56.24). No cases of severe OHSS were diagnosed in both groups but one case of moderate OHSS were diagnosed in the treatment group (cryopreservation) and four cases in the control group (intra‐venous albumin).
Incidence of nil or mild OHSS: there was no difference between the two groups in the incidence of nil or mild OHSS (n=26, OR 0.38, 95% CI 0.08 to 1.90). Four cases of mild OHSS were diagnosed in the treatment group and seven cases in the control group.
Clinical pregnancy and or woman: there was no difference between the two groups in the number of clinical pregnancies and or women (n=26, OR 0.06, 95% CI 0.00 to 1.17). There were 13 (38%) pregnancies observed in the treatment group and 13 pregnancies in the control group.
There was no mention of multiple pregnancy rate or livebirth rate
Data for number of oocytes retrieved, oocytes fertilized and for embryos transferred were not normally distributed therefore the results for these outcomes have not been combined using WMD and have been reported separately.
Number of oocytes retrieved: the mean (SD) number of oocytes retrieved was 17.15+and or‐ 7.77 in the intra‐venous albumin group and 19.62+and or‐ 5.87 in the cryopreservation group.
Number of oocytes fertilized: the mean (SD) number of oocytes fertilized was 6.0+and or‐3.42 in the intra‐venous albumin group and 7.46+and or‐3.91 in the cryopreservation group.
Number of embryos transferred: the mean (SD) number of embryos transferred was 2.31+and or‐ 0.84 in the intra‐venous albumin group and 1.69+and or‐1.32 in the cryopreservation group.
(2) Cryopreservation versus fresh embryo transfer
Incidence of moderate and or severe OHSS: there was no statistically significant difference between the two groups in the incidence of moderate and or severe OHSS (n=125, OR 0.12, 95% CI 0.01 to 2.29). No cases of moderate and or severe OHSS were diagnosed in the treatment group (cryopreservation of all embryos) versus four cases in the control group (fresh embryo transfer).
Clinical pregnancy and or woman: there was no difference between the two groups (n=125, OR 1.08, 95% CI 0.54 to 2.19). There were 28 and or 58 (48.2%) pregnancies observed in the treatment group and 31 and or 67 (46.3%) in the control group.
Multiple pregnancy rate was overall 30%.
Livebirth rate: there was no difference between the two groups (n=125, OR 1.03, 95% CI 0.50 to 2.12). There were 22 babies born in the treatment group and 25 in the control group.
Number of women admitted as inpatient or outpatient: there was no difference between the two groups (n=125, OR 0.12, 95% CI 0.01 to 2.29). No one was admitted as an inpatient in the treatment group and 4 and or 125 in the control group.
Data for number of oocytes retrieved, embryos transferred and resolution time were not normally distributed therefore the results for these outcomes have not been combined using WMD and have been reported separately.
Number of oocytes retrieved: the mean (SD) number of oocytes retrieved was 20.80+and or‐ 5.50 in the cryopreservation of all embryos group and 19.80 +and or‐ 4.30 in the fresh embryo transfer group.
Number of embryos transferred: the mean (SD) number of embryos transferred was 3.10+and or‐0.80 in the cryopreservation of all embryos group and 3.20+and or‐1.00 in the fresh embryo transfer group.
Resolution time: the mean (SD) time to the next menstrual period was 12.1+and or‐1.0 days in the cryopreservation of all embryos group
Discussion
Summary of main results
This updated systematic review ( and its original published issue 2, 2002) D'Angelo 2002 ,showed that there was no statistically significant difference in the incidence of moderate and or severe OHSS when cryopreservation of all embryos was employed compared to intra‐venous albumin infusion and fresh embryos transfer in women at risk of OHSS.
Overall completeness and applicability of evidence
These results have to be interpreted with caution because of (i) the small number of women in the individual studies and (ii) they were based on an experimental treatment (i.e. Intra‐venous albumin) which has not been validated in large studies. Comparisons of the two different management options for OHSS did not show any significant difference in the incidence of OHSS.
Quality of the evidence
There are a number of methodological concerns which may have affected the results such as the administration of intra‐venous albumin to all women (Ferraretti 1999) and its possible influence on the incidence of severe OHSS. Although there were four cases of severe OHSS in the fresh embryos transfer group and none in the cryopreservation group, this did not reach a statistically significant difference between cryopreservation and fresh embryos transfer group. According to this review and to a previous meta‐analysis done by Aboulghar 1999, which has demonstrated a statistically significant difference in the incidence of severe OHSS between intra‐venous albumin infusion and placebo and or no treatment , there is a need for a larger multi centre RCT of these interventions with sufficient power to show a statistically significant difference in the occurrence of moderate and or severe OHSS.
On the basis of the studies included in both reviews, were carried out power calculations which indicated that to demonstrate a difference of 25% between experimental (intra‐venous albumin infusion) and control (elective cryopreservation of all embryos) groups at a power of 80%, with a statistical significance level of 0.05, we need 185 women in each group (a total of 370 women). To achieve a power of 90%, 235 women are needed in each group (a total of 470 women).
Potential biases in the review process
As far as the clinical pregnancy rate per woman is concerned, none of the studies reached statistical significance. However, in one study (Shaker 1996) there was a trend for higher clinical pregnancy rate in the cryopreservation arm (P = 0.06). It should be noted that this study (i) has a low power because of the small number of women randomised (13 in each arm) and three women withdrawn from the cryopreservation group; (ii) was not blinded and (iii) the authors tried to justify such discrepancy in pregnancy rate with the fact that the second dose of intra‐venous albumin, administered five days after the fresh embryos transfer, might have affected the implantation phase.
Authors' conclusions
Implications for practice.
This updated review has showed that there is insufficient evidence to support routine cryopreservation and insufficient evidence for the relative merits of intra‐venous albumin versus cryopreservation.
Implications for research.
There is (1) a need to have clear definition of women at risk of OHSS, based on endocrinological and or ultrasonographic and or clinical criteria (2) a need for a large RCT looking at (i) severe OHSS for intra‐venous albumin with fresh embryo transfer versus cryopreservation and (ii) pregnancy outcome for intra‐venous albumin with fresh embryo transfer versus cryopreservation. Randomisation should take place when risk is determined (i.e. during the stimulation phase or immediately prior to egg collection) according to serum oestradiol level (less than 1,906 pg and or ml or less than 7,000 pmol and or L) on the day of human chorionic gonadotrophin (hCG) administration.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2012 | Amended | Summary of findings tables added |
| 26 November 2010 | Review declared as stable | It is unlikely that any new studies will be conducted that will influence the findings of this review |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Converted to new review format. |
| 22 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Authors would like to thank the MDSG editorial office staff,for their advice and support throughout the review process.
Appendices
Appendix 1. Search
MEDLINE(R) 1950 to March Week 2 2007
1 cryopreservation/ or tissue preservation/ (19871) 2 Freezing/ (16747) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (5989) 4 thaw$.tw. (10976) 5 freez$.tw. (32035) 6 or/1‐5 (59469) 7 embryo transfer/ or fertilization in vitro/ or sperm injections, intracytoplasmic/ or exp ovulation induction/ (27399) 8 Ovarian Hyperstimulation Syndrome/ (1109) 9 (IVF or ICSI or OHSS).tw. (11422) 10 (in vitro adj5 fertili$).tw. (13045) 11 (intracytoplas$ adj5 sperm$).tw. (2894) 12 (ovar$ adj5 hyperstim$).tw. (2440) 13 (oval$ adj5 induction).tw. (112) 14 or/7‐13 (32507) 15 6 and 14 (3409) 16 randomized controlled trial.pt. (231520) 17 controlled clinical trial.pt. (74401) 18 Randomized Controlled Trials/ (47478) 19 Random allocation/ (57229) 20 Double‐blind method/ (90219) 21 Single‐blind method/ (10694) 22 or/16‐21 (392686) 23 clinical trial.pt. (433538) 24 exp clinical trials/ (187924) 25 (clin$ adj25 trial$).ti,ab,sh. (127269) 26 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. (89503) 27 Placebos/ (25889) 28 placebo$.ti,ab,sh. (113300) 29 random$.ti,ab,sh. (482821) 30 Research design/ (46468) 31 or/23‐30 (854847) 32 animal/ not (human/ and animal/) (3047680) 33 22 or 31 (861864) 34 33 not 32 (790029) 35 15 and 34 (223) 36 (2002$ or 2003$ or 2004$ or 2005$ or 2006$ or 2007$).ed. (3590567) 37 35 and 36 (83) 38 from 37 keep 1‐83 (83)
EBM Reviews ‐ Cochrane Central Register of Controlled Trials 1st Quarter 2007
1 cryopreservation/ or tissue preservation/ (215) 2 Freezing/ (47) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (129) 4 thaw$.tw. (204) 5 freez$.tw. (434) 6 or/1‐5 (783) 7 embryo transfer/ or fertilization in vitro/ or sperm injections, intracytoplasmic/ or exp ovulation induction/ (1439) 8 Ovarian Hyperstimulation Syndrome/ (95) 9 (IVF or ICSI or OHSS).tw. (1695) 10 (in vitro adj5 fertili$).tw. (1130) 11 (intracytoplas$ adj5 sperm$).tw. (302) 12 (ovar$ adj5 hyperstim$).tw. (387) 13 (oval$ adj5 induction).tw. (1) 14 or/7‐13 (2634) 15 6 and 14 (127) 16 from 15 keep 1‐127 (127)
CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature 1982 to March Week 3 2007
1 cryopreservation/ or tissue preservation/ (294) 2 Freezing/ (80) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (61) 4 thaw$.tw. (77) 5 freez$.tw. (329) 6 or/1‐5 (697) 7 embryo transfer/ or fertilization in vitro/ or sperm injections, intracytoplasmic/ or exp ovulation induction/ (766) 8 Ovarian Hyperstimulation Syndrome/ (37) 9 (IVF or ICSI or OHSS).tw. (233) 10 (in vitro adj5 fertili$).tw. (256) 11 (intracytoplas$ adj5 sperm$).tw. (46) 12 (ovar$ adj5 hyperstim$).tw. (43) 13 (oval$ adj5 induction).tw. (1) 14 or/7‐13 (897) 15 6 and 14 (44) 16 exp clinical trials/ (42848) 17 Clinical trial.pt. (20246) 18 (clinic$ adj trial$1).tw. (10023) 19 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw. (6020) 20 Randomi?ed control$ trial$.tw. (8742) 21 Random assignment/ (14868) 22 Random$ allocat$.tw. (1008) 23 Placebo$.tw. (8430) 24 Placebos/ (3415) 25 Quantitative studies/ (3123) 26 Allocat$ random$.tw. (58) 27 or/16‐26 (60065) 28 15 and 27 (7) 29 from 28 keep 1‐7 (7)
EMBASE 1980 to 2007 Week 12
1 cryopreservation/ or tissue preservation/ (12333) 2 Freezing/ (6068) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (4719) 4 thaw$.tw. (8017) 5 freez$.tw. (22290) 6 or/1‐5 (36047) 7 embryo transfer/ or fertilization in vitro/ or sperm injections, intracytoplasmic/ or exp ovulation induction/ (25690) 8 Ovarian Hyperstimulation Syndrome/ (2905) 9 (IVF or ICSI or OHSS).tw. (11537) 10 (in vitro adj5 fertili$).tw. (11595) 11 (intracytoplas$ adj5 sperm$).tw. (2915) 12 (ovar$ adj5 hyperstim$).tw. (2541) 13 (oval$ adj5 induction).tw. (100) 14 or/7‐13 (29590) 15 6 and 14 (2710) 16 Controlled study/ or randomized controlled trial/ (2374884) 17 double blind procedure/ (63195) 18 single blind procedure/ (6449) 19 crossover procedure/ (18389) 20 drug comparison/ (81250) 21 placebo/ (96088) 22 random$.ti,ab,hw,tn,mf. (362109) 23 latin square.ti,ab,hw,tn,mf. (1061) 24 crossover.ti,ab,hw,tn,mf. (32266) 25 cross‐over.ti,ab,hw,tn,mf. (11194) 26 placebo$.ti,ab,hw,tn,mf. (144334) 27 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (105428) 28 (comparative adj5 trial$).ti,ab,hw,tn,mf. (5590) 29 (clinical adj5 trial$).ti,ab,hw,tn,mf. (476127) 30 or/16‐29 (2851591) 31 nonhuman/ (2858114) 32 animal/ not (human/ and animal/) (12843) 33 or/31‐32 (2861716) 34 30 not 33 (1673476) 35 15 and 34 (698) 36 (2002$ or 2003$ or 2004$ or 2005$ or 2006$ or 2007$).em. (2829448) 37 35 and 36 (371) 38 from 37 keep 1‐371 (371)
Data and analyses
Comparison 1. Cryopreservation versus fresh embryos transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Moderate and or severe OHSS | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.29] |
| 2 Clinical pregnancies | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.54, 2.19] |
| 3 Number of livebirths (livebirth rate) | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.12] |
| 4 Number of patients admitted | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.29] |
1.1. Analysis.
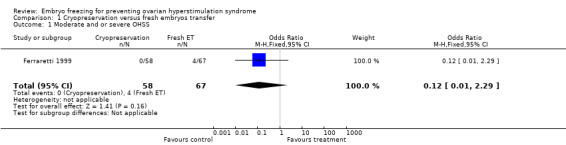
Comparison 1 Cryopreservation versus fresh embryos transfer, Outcome 1 Moderate and or severe OHSS.
1.2. Analysis.
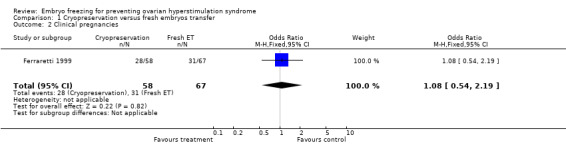
Comparison 1 Cryopreservation versus fresh embryos transfer, Outcome 2 Clinical pregnancies.
1.3. Analysis.
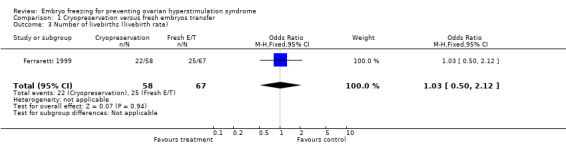
Comparison 1 Cryopreservation versus fresh embryos transfer, Outcome 3 Number of livebirths (livebirth rate).
1.4. Analysis.
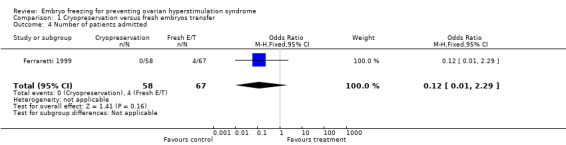
Comparison 1 Cryopreservation versus fresh embryos transfer, Outcome 4 Number of patients admitted.
Comparison 2. Cryopreservation versus intra‐venous albumin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Moderate and or severe OHSS | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.33 [0.51, 56.24] |
| 2 Nil and or mild OHSS | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.90] |
| 3 Clinical pregnancies | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.17] |
2.1. Analysis.
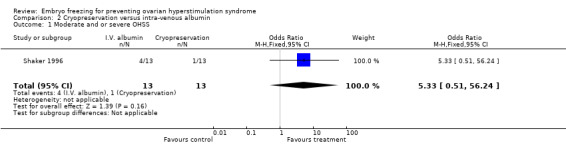
Comparison 2 Cryopreservation versus intra‐venous albumin, Outcome 1 Moderate and or severe OHSS.
2.2. Analysis.
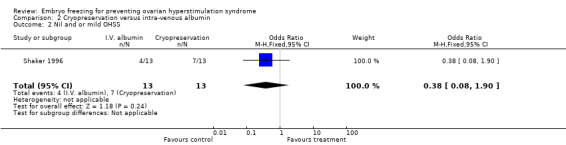
Comparison 2 Cryopreservation versus intra‐venous albumin, Outcome 2 Nil and or mild OHSS.
2.3. Analysis.
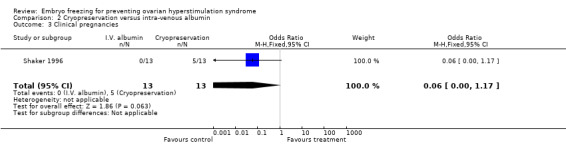
Comparison 2 Cryopreservation versus intra‐venous albumin, Outcome 3 Clinical pregnancies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ferraretti 1999.
| Methods | Randomised study; parallel prospective design; single centre; power calculation: not stated; method of randomisation: not specified. | |
| Participants | 125 infertile women considered at risk of OHSS (58/125 had cryopreservation;67/125 had fresh embryos transfer); E2 (major risk factor for OHSS) was >1500 pg/ml; Age (31.6 versus 31.4 years); Duration of infertility (3.9 versus 4.1 years); Causes of infertility (%): tubal factor (27 versus 30), male factor (28 versus 33), PCO (8 versus 7), others (3 versus 4) BMI (<30) | |
| Interventions | Study group: cryopreservation of all embryos immediately (zygotes); control group: fresh embryo transfer after 48 hours of culture. Both groups received 20 gr. of human albumin intravenously on the day of oocytes recovery. | |
| Outcomes | Method of diagnosing different grades of OHSS: Golan (1989) and Navot criteria, (1992). Severe OHSS (0/58 versus 4/67);
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation concealment not described adequately |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No losses |
| Other bias | Unclear risk | No power calculation. |
Shaker 1996.
| Methods | Randomised study; parallel prospective design; single centre; power calculation: not stated; intention‐ to‐treat analysis done; randomisation done by drawing cards, each contained a number obtained from a table of random numbers. | |
| Participants | 26 infertile women considered at risk of OHSS (13/26 had intra‐venous albumin infusion; 13/26 had cryopreservation of all embryos); E2 (major risk factor for OHSS) was >3,540 pg/ml; Age (33.8 versus 34.0 years); Duration of infertility (4.4 versus. 4.6 years); Causes of infertility: tubal factor (not stated), male factor (not stated), PCO (2 versus 6); BMI (not calculated). | |
| Interventions | Study group: intra‐venous albumin infusion (200 ml of 20% concentration) on the day of eggs collection and repeated 5 days later + fresh embryos transfer; control group: cryopreservation of all embryos at pronucleate stage. | |
| Outcomes | Method of diagnosing different grades of OHSS: Schenker & Weinstein (1978). Severe OHSS (0/13 versus 0/13); moderate OHSS (4/13 versus 1/13); mild OHSS (4/13 versus 7/13);
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation concealment not described adequately |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three women withdrew they requested to have fresh embryos rather than cryopreservation |
| Other bias | Unclear risk | No power calculation. |
OHSS: Ovarian hyperstimulation syndrome E2: oestradiol PCO: Policystic ovaries BMI: Body mass index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asch 1993 | Observational study |
| Awonuga 1996 | Prospective observational study |
| Chen 1997 | Prospective observational study |
| Isik 1996 | Randomised controlled study comparing intra‐venous albumin infusion with no treatment |
| Munoz 2000 | Randomised controlled study comparing intra‐venous albumin infusion with placebo |
| Ndukwe 1997 | Retrospective review and data analysis |
| Ng 1995 | Cohort study not randomised |
| Panay 1999 | Randomisation based on alternating basis |
| Pattinson 1994 | Retrospective review |
| Queenam 1997 | Prospective observational longitudinal study |
| Shalev 1995 | Randomised controlled study comparing intra‐venous albumin infusion with no treatment |
| Shoham 1994 | Randomised controlled study comparing intra‐venous albumin infusion with placebo |
| Titinem 1995 | Observational study |
| Wada 1992 | Prospective observational study |
| Wada 1993 | Retrospective review |
Differences between protocol and review
None
Contributions of authors
Dr A. D'Angelo took the lead in the writing of the update for the review in 2007. The search string was modified and ran no new studies were found. In 2002: Dr A. D'Angelo took the lead in the writing of the review. She scanned the titles and the abstracts of the reports identified by electronic searching in order to find relevant papers she then obtained copies of the full text articles and made copies for the other reviewer (NNA). Extracted data independently using forms designed according to Cochrane guidelines. Disagreements were resolved by discussion.
N.N. Amso scanned the titles and the abstracts of the reports identified by electronic searching in order to find relevant papers. Extracted data independently using forms designed according to Cochrane guidelines. Disagreements were resolved by discussion.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University Hospital of Wales, UK.
External sources
No sources of support supplied
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ferraretti 1999 {published data only}
- Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome:efficiency and safety. Human Reproduction 1999;14(6):1457‐60. [DOI] [PubMed] [Google Scholar]
Shaker 1996 {published data only}
- Shaker AG, Zosmer A, Dean N, Bekir SJ, Jacobs HS, Tan S. Comparison of intravenous albumin and transfer of fresh embryos with cryopreservation of all embryos for subsequent transfer in prevention of ovarian hyperstimulation syndrome. Fertility and Sterility 1996;65(5):992‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asch 1993 {published data only}
- Asch RH, Ivery G, Goldsman M, Frederick JL, Stone SC, Balmaceda JP. The use of intravenous albumin in patients at high risk for severe ovarian hyperstimulation syndrome. Human Reproduction 1993;8(7):1015‐20. [DOI] [PubMed] [Google Scholar]
Awonuga 1996 {published data only}
- Awonuga AD, Pittrof RJ, Zaidi J, Dean N, Jacobs HS, Tan SL. Elective cryopreservation of all embryos in women at risk of developing ovarian hyperstimulation syndrome may not prevent the condition but reduces the live birth rate. Journal of Assisted Reproduction and Genetics 1996;13(5):401‐6. [DOI] [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen C, Wu M, Yang J, Chen S, Ho H, Yang Y. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome. Fertility and Sterility 1997;68(2):287‐91. [DOI] [PubMed] [Google Scholar]
Isik 1996 {published data only}
- Isik AZ, Gokmen O, Zeyneloglu HB, Kara S, Keles G, Gulekli B. Intravenous albumin prevents moderate‐severe ovarian hyperstimulation in in‐vitro fertilization patients: a prospective, randomized and controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;70:179‐83. [DOI] [PubMed] [Google Scholar]
Munoz 2000 {published data only}
- Munoz E, Cuneo S, Ferro J, Vidal C, Pellicer C, et al. Intravenous albumin in the prevention of ovarian hyperstimulation syndrome. Human Reproduction Abstracts from the 16th Annual Meeting of ESHRE. 2000; Vol. 15.
Ndukwe 1997 {published data only}
- Ndukwe G, Thornton S, Fishel S, Dowell K, Aloum M. Severe ovarian hyperstimulation syndrome: is it really preventable by prophylactic intravenous albumin?. Fertility and Sterility 1997;68(5):851‐4. [DOI] [PubMed] [Google Scholar]
Ng 1995 {published data only}
- Ng E, Leader A, Claman P, Domingo M, Spence JEH. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome in an in‐vitro fertilization programme. Human Reproduction 1995;10(4):807‐10. [DOI] [PubMed] [Google Scholar]
Panay 1999 {published data only}
- Panay N, Iammarrone E, Zosmer A, Tozer A, Hussain S, Wilson C, Al Shawaf T, Lower AM, Grudzinskas JG. Does the prophylactic use of intravenous albumin prevent ovarian hyperstimulaton syndrome? A randomized prospective study. Human Reproduction Abstract Book. 1999; Vol. 14, issue 6:105.
Pattinson 1994 {published data only}
- Pattinson HA, Hignett M, Dunphy BC, Fleetham JA. Outcome of thaw embryo transfer after cryopreservation of all embryos in patients at risk of ovarian hyperstimulation syndrome. Fertility and Sterility 1994;62(6):1192‐6. [DOI] [PubMed] [Google Scholar]
Queenam 1997 {published data only}
- Queenam Jr JT, Veeck LL, Toner JP, Oehinger S, Muasher SJ. Cryopreservation of all prezygotes in patients at risk of severe hyperstimulation does not eliminate the syndrome, but the chances of pregnancy are excellent with subsequent frozen‐thaw transfers. Human Reproduction 1997;12(7):1573‐6. [DOI] [PubMed] [Google Scholar]
Shalev 1995 {published data only}
- Shalev E, Giladi Y, Matilsky M, Ben‐Ami M. Decreased incidence of severe ovarian hyperstimulation syndrome in high risk in‐vitro fertilization patients receiving intravenous albumin: a prospective study. Human Reproduction 1995;10(6):1373‐6. [DOI] [PubMed] [Google Scholar]
Shoham 1994 {published data only}
- Shoham Z, Weissman A, Barash A, Borenstein R, Schachter M, Insler V. Intravenous albumin for the prevention of severe ovarian hyperstimulation syndrome in an in vitro fertilization program: a prospective, randomized, placebo‐controlled study. Fertility and Sterility 1994;62(1):137‐42. [DOI] [PubMed] [Google Scholar]
Titinem 1995 {published data only}
- Titinem A, Husa L, Tulppala M, Simberg N, Seppala M. The effect of cryopreservation in prevention of ovarian hyperstimulation syndrome. British Journal of Obstetrics and Gynaecology 1995;10(2):326‐9. [DOI] [PubMed] [Google Scholar]
Wada 1992 {published data only}
- Wada I, Matson PL, Troup SA, Hughes S, Buck P, Lieberman BA. Outcome of treatment subsequent to the elective preservation of all embryos from women at risk of ovarian hyperstimulation syndrome. Human Reproduction 1992;7(7):962‐6. [DOI] [PubMed] [Google Scholar]
Wada 1993 {published data only}
- Wada I, Matson PL, Troup SA, Morroll DR, Hunt L, Lieberman BA. Does elective cryopreservation of all embryos from women at risk of ovarian hyperstimulation syndrome reduce the incidence of the condition?. British Journal of Obstetrics and Gynaecology 1993;100(3):265‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Aboulghar 1999
- Aboulghar M, Evers JH, Al‐Inany H. Intra‐venous albumin for preventing severe ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 1999, Issue 4. [Google Scholar]
Agrawal 1998
- Agrawal R, Conway G, Sladkevicius P, Tan SL, . Engmann L, Payne N, et al. Serum vascular endothelial growth factor and Doppler blood flow velocities in in vitro fertilization: relevance to ovarian hyperstimulation syndrome and policystic ovaries. Fertility and Sterility 1998;70:651‐8. [DOI] [PubMed] [Google Scholar]
Al‐Shawaf 2001
- Al‐Shawaf T, Zosmer A, Hussain S, Panay N, Wilson C, Lower AM, et al. Prevention of severe ovarian hyperstimulation syndrome in IVF with or without ICSI and embryo transfer: a modified "coasting" strategy based on ultrasound identification of high‐risk patients. Human Reproduction 2001;1(16):24‐30. [DOI] [PubMed] [Google Scholar]
Allegra 1991
- Allegra A, Termine N, Raineri L, Corselli F, Traina MC, Giannola C. Ovarian hyperstimulation and twin pregnancy in a patient undergoing GnRH‐a and gonadotropins stimulation protocol without midcycle hCG administration [Iperstimolazione ovarica e gravidanza gemellare in una paziente in trattamento con analogo del GnRH e gonadotropine senza somministrazione di midcycle hCG]. Rivista Ostetrica Ginecologica Perinatologia. 1991;2:209‐11. [Google Scholar]
Amso 1990
- Amso NN, Ahuja KK, Morris N, Shaw RW. The management of predicted ovarian hyperstimulation involving gonadotropin‐releasing hormone analog with elective cryopreservation of all pre‐embryos. Fertility and Sterility 1990;53:1087‐90. [DOI] [PubMed] [Google Scholar]
Araujo 1995
- Araujo E, Bernardini L, Frederick JL, Asch RH, Balmaceda JP. Prospective randomized human chorionic gonadotropin versus intramuscular progesterone for luteal‐phase support in assisted reproduction. Journal of Assisted Reproduction & Genetics 1990;11:74‐8. [DOI] [PubMed] [Google Scholar]
Asch 1993
- Asch RH, Ivery G, Goldsman M, Frederick JL, Stone SC, Balmaceda JP. The use of intravenous albumin in patients at high risk for severe ovarian hyperstimulation syndrome. Human Reproduction 1993;8(7):1015‐20. [DOI] [PubMed] [Google Scholar]
Awonuga 1996
- Awonuga AD, Pittrof RJ, Zaidi J, Dean N, Jacobs HS, Tan SL. Elective cryopreservation of all embryos in women at risk of developing ovarian hyperstimulation syndrome may not prevent the condition but reduces the live birth rate. Journal of Assisted Reproduction & Genetics 1996;13(5):401‐6. [DOI] [PubMed] [Google Scholar]
Brinsden 1995
- Brinsden PR, Wada I, Tan SL, Balen A, Jacobs HS. Diagnosis, prevention and management of OHSS: review. British Journal of Obsterics and Gynaecology 1995;102:767‐72. [DOI] [PubMed] [Google Scholar]
Dahl 1994
- Dahl Lyons CA, Wheeler CA, Frishman GN, Hackett GJ, Seifer DB, Haning RV. Early and late presentation of ovarian hyperstimulation syndrome: two distinct entities with different risk factors. Human Reproduction 1994;9:792‐9. [DOI] [PubMed] [Google Scholar]
Doldi 1999
- Doldi N, Destefani A, Gessi A, Grossi D, Ferrari A. Human albumin enhances expression of vascular endothelial growth factor in cultured human luteinizing granulosa cells: importance in ovarian hyperstimulation syndrome. Human Reproduction 1999;14(5):1157‐9. [DOI] [PubMed] [Google Scholar]
Egbase 1999
- Egbase PE, Al Sharhan M, Grudzinskas JG. Early unilateral follicular aspiration compared with coasting for the prevention of svere ovarian hyperstimulation syndrome: a prospective randomzed study. Human Reproduction 1999;14(6):1421‐5. [DOI] [PubMed] [Google Scholar]
Forman 1990
- Forman RG, Frydman R, Egan D, Ross C, Barlow DH. Severe ovarian hyperstimulation syndrome using agonists of gonadotrophin releasing hormone for in vitro fertilization:a European series and a proposal for prevention. Fertility & Sterility 1990;53:502‐9. [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A, Ron‐El R, Herman A, Soffer Y, Wainraub Z, Caspi E. Ovarian Hyperstimulation Syndrome: an update review. Obstetrics and Gynaecology Survey 1989;44:430‐40. [DOI] [PubMed] [Google Scholar]
Goldsman 1995
- Goldsman MP, Pedram A, Dominiguez CE, Ciuffardi I, Levin E, Asch RH. Increased capillary permeability induced by human follicular fluid: a hypothesis for an ovarian origin of the hyperstimulation syndrome. Fertility & Sterility 1995;63:268‐72. [DOI] [PubMed] [Google Scholar]
Gonen 1990
- Gonen Y, Balakier H, Powell W, Casper RF. Use of GnRH agonist to trigger follicular maturation for in vitro fertilization. Journal of Clinical Endocrinology and Metabolism 1990;71:918‐23. [DOI] [PubMed] [Google Scholar]
Lipitz 1991
- Lipitz S, Zion BR, Bider D, Shalev J, Mashiach S. Quintuplet pregnancy and third degree ovarian hyperstimulation despite withholding human chorionic gonadotropin. Human Reproduction 1991;6:1478‐9. [DOI] [PubMed] [Google Scholar]
MacDougall 1992
- MacDougall MJ, Tan SL, Jacobs HS. In vitro fertilization and the ovarian hyperstimulation syndrome. Human Reproduction 1992;7:579‐600. [DOI] [PubMed] [Google Scholar]
Navot 1992
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies:prevention and treatment. Fertility & Sterility 1992;58:249‐61. [DOI] [PubMed] [Google Scholar]
Rabau 1967
- Rabau E, Serr DM, David A, Maschiac S, Lunenfield B. Human menopausal gonadotropins for anovulation and sterility. American Journal of Obstetrics and Gynaecology 1967;98:92‐8. [DOI] [PubMed] [Google Scholar]
SART 1992
- Medical Research International, Society for Assisted Reproductive Technology (SART), the American Fertility Society. In vitro fertilization‐embryo transfer (IVF‐ET) in the United States: 1990 results from the IVF‐ET registry. Fertility & Sterility 1992;57:15‐24. [PubMed] [Google Scholar]
Schenker 1978
- Schenker JG, Weinstein D. Ovarian hyperstimulation syndrome: a current survey. Fertility & Sterility 1978;30:255‐68. [DOI] [PubMed] [Google Scholar]
Segal 1992
- Segal S, Casper RF. Gonadotropin‐releasing hormone agonist versus human chorionic gonadotropin for triggering follicular maturation in in vitro fertilization. Fertility & Sterility 1992;57:1254‐8. [PubMed] [Google Scholar]
Sher 1993
- Sher G, Zouves C, Feinman M, Dodge S, Zouves C, Knutzen V. Eliminating the risk of life‐endagering complications following overstimulation with menotropin fertility agents: a report on women undergoing in vitro fertilization and embryo transfer. Obstetric and Gynaecology 1993;81:1009‐11. [PubMed] [Google Scholar]
Shoham 1994
- Shoham Z, Weissman A, Barash A, Borenstein R, Schachter M, Insler V. Intravenous albumin for the prevention of severe ovarian hyperstimulation syndrome in an in vitro fertilization program: a prospective, randomised, placebo‐controlled study. Fertility & Sterility 1994;62(1):137‐42. [DOI] [PubMed] [Google Scholar]
Tsirigotis 1994
- Tsirigotis M, Craft I. Ovarian Hyperstimuation Syndrome (OHSS): how much do we really know about it?. European Journal of Obstetrics and Gynaecology 1994;55:151‐5. [DOI] [PubMed] [Google Scholar]
Wada 1990
- Wada I, Matson PL, Troup SA, Erard P, Wisanto A, Steirteghem AC. Ovarian hyperstimulation syndrome in GnRH‐a/hMG cycles for IVF and GIFT. Journal of Obstetrics and Gynaecology 1990;11:88‐9. [Google Scholar]
References to other published versions of this review
D'Angelo 2002
- D'Angelo A, Amso N. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/1465185] [DOI] [PubMed] [Google Scholar]


