Abstract
Two heptad repeat regions in the ectodomain of the human immunodeficiency virus type 1 (HIV-1) transmembrane subunit (gp41) self-assemble into a six-helix bundle structure that is critical for virus entry. Immunizations with peptides corresponding to these regions generated antibodies specific to the receptor-activated conformations of gp41.
The envelope glycoprotein (Env) of the human immunodeficiency virus type 1 (HIV-1) mediates virus entry by fusing viral and cellular membranes, resulting in delivery of the viral nucleocapsid to the cell cytoplasm. Binding of the gp120 surface subunit of Env to cellular receptors triggers in the oligomeric Env complex conformational changes that turn on the membrane fusion activity of the transmembrane subunit (gp41). Two heptad repeat regions in the gp41 ectodomain (Fig. 1A) self-assemble into a six-helix bundle structure, which has been proposed to form after receptor binding and to be critical for membrane fusion (1, 11, 13). This structure consists of a triple-stranded coiled-coil internal layer (N heptad) and an external layer with three helices (C heptad) that pack in the grooves of the coiled coil in an antiparallel manner (Fig. 1B). Peptides corresponding to these heptad repeats are potent inhibitors of HIV infection (7, 15, 16). They appear to block virus entry by mimicking helices of the six-helix bundle and forming a peptide-gp41 complex (4), which interferes with the formation of the gp41 six-helix bundle in a dominant negative manner (reviewed in reference 2).
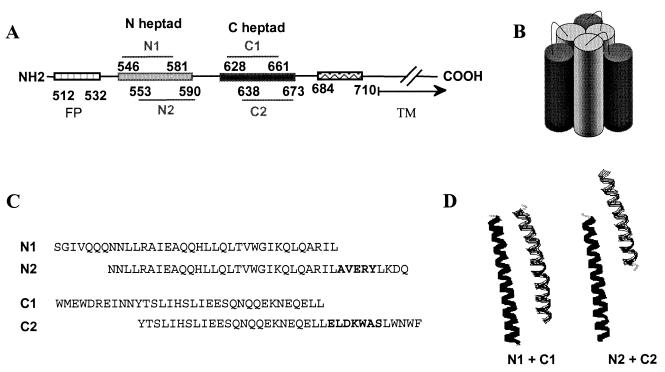
FIG. 1.
(A) Linear representation of domains in gp41. (B) Schematic diagram of the six-helix bundle structure generated by self-assembly of the N and C heptad repeat domains. (C) Sequence of the N and C heptad repeat peptides. (D) Modeling of the interactions between the N and C heptad repeat peptides. Images showing gp41 ribbon diagrams of heptad repeat regions from monomers were generated with RasMol V2.6, using atomic coordinates from Weissenhorn et al. (13)
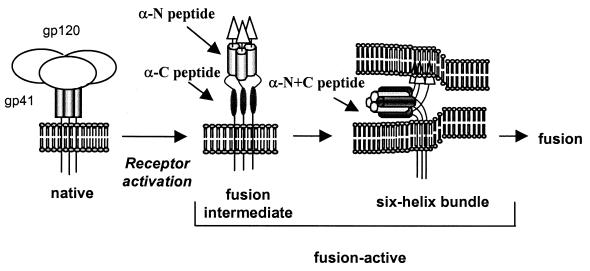
To generate antibodies targeting the highly conserved, heptad repeat domains of gp41 for studies of Env-mediated entry and its inhibition, we immunized New Zealand White rabbits with two versions of peptides corresponding to the N and C heptad repeat domains (Fig. 1C). The N1 peptide corresponds to the sequences of the coiled coil in the protease-resistant six-helix bundle (1), and the N2 peptide corresponds to the DP-107 peptide inhibitor (15). Both peptides potently inhibit HIV infection and form helices in solution (1, 15), but the N2 peptide contains the immunodominant AVERY epitope at the C terminus (12). The C1 peptide corresponds to the external helices in the protease-resistant six-helix bundle (1), while the C2 peptide corresponds to the DP-178 peptide inhibitor (16). Although neither C peptide has a stable solution structure, both potently inhibit HIV infection (8; C. Wild, T. Greenwell, and T. Matthews, Letter, AIDS Res. Hum. Retrovir. 9:1051–1053, 1993). The C2 peptide contains the epitope for the 2F5 gp41 neutralizing monoclonal antibody (10). Immunizations with the individual peptides might generate antibodies that preferentially target the heptad repeat domains in the receptor-activated (fusion-active) conformations of Env, particularly the fusion intermediate, when these domains could be most exposed (Fig. 2).
FIG. 2.
Postulated HIV entry mechanism involving gp41 conformational changes and potential targets for antipeptide antibodies.
In an attempt to create antibodies to the six-helix bundle structure, rabbits were also immunized with mixtures of the N1 and C1 or the N2 and C2 peptides. A recombinant gp41 (rgp41) (18), which contains the immunodominant loop connecting the N and C heptad regions, was also used for comparison. The N1-C1 peptide mixture (N1+C1) has previously been shown to form a thermostable six-helix bundle (8). The N2 and C2 peptides have previously been shown to interact with each other (Wild et al., letter), but the precise structure is not known. All animals were primed subcutaneously with a total of 200 μg of total peptide (equimolar for mixtures) in complete Freund's adjuvant and boosted twice with 100 μg of total peptide in incomplete Freund's adjuvant at approximately 4-week intervals.
All peptide immunogens proved to be immunogenic, even in the absence of carrier protein. In enzyme-linked immunosorbent assays (data not shown), geometric mean endpoint titers were 2.5 × 104 against the N peptides and 1.6 × 103 for the C peptides, without significant difference between the two versions of the peptides. Geometric mean titers were 1.6 × 104 and 1.6 × 103 for the N1+C1 and N2+C2 immunogens, respectively. It is unclear to what extent the peptides adopted a coiled-coil or six-helix bundle structure when immobilized on plastic.
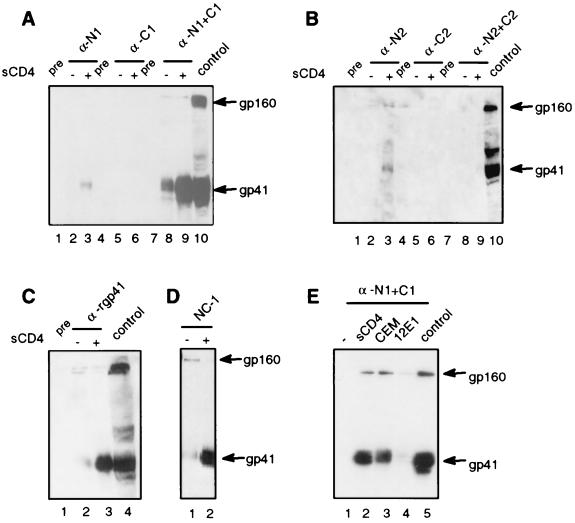
To assess whether the antibodies could bind native or receptor-activated Env, we performed immunoprecipitation assays using intact Env-expressing cells that were incubated with sera in the presence or absence of the CD4 receptor (Fig. 3). Briefly, approximately 107 293T cells were transfected with an Env expression vector (HXB2 strain) using FUGENE 6 according to the manufacturer's protocol and incubated with sera in the presence or absence of 3 × 106 target cells or 8 μg of soluble CD4 (sCD4; kindly provided by Ray Sweet, SmithKline Beecham) per ml at 37°C for 1 h. Cells were then washed twice with phosphate-buffered saline to remove antibodies prior to lysis and immunoprecipitation as previously described (14). Antisera against the N1 peptide (Fig. 3A, lane 3), the N2 peptide (Fig. 3B, lane 3), the N1+C1 peptide mixture (Fig. 3A, lanes 8 and 9), rgp41 (Fig. 3C, lane 3), and a monoclonal antibody specific for the six-helix bundle (NC-1) (Fig. 3D, lane 2) immunoprecipitated gp41 from the cell surface. All C peptide and N2+C2 peptide antisera failed to immunoprecipitate cell surface Env (Fig. 3A, lanes 5 and 6, and Fig. 3B, lanes 5 and 6 and lanes 8 and 9).
FIG. 3.
Immunoprecipitation of gp41 expressed on cell surface in the presence or absence of sCD4 or target cells. (A) Sera from animals immunized with N1, C1, or N1+C1 peptides. (B) Sera from animals immunized with N2, C2, or N2+C2 peptides. (C) Serum from an animal immunized with rgp41. (D) Monoclonal antibody (NC-1) specific for the six-helix bundle. (E) Immunoprecipitation in the presence of sCD4 or CD4+CXCR4+ cells (CEM) or CD4-CXCR4+ cells (12E1) with sera from N1+C1 animals. One representative immunoprecipitation is shown for each immunogen. All sera were used at 1:50 dilutions, except the N1+C1 and rgp41 antisera, which were used at 1:250, and the NC-1 hybridoma supernatant was used at 1:10. Env-expressing cell lysate was run as a positive control. Pre, preimmune sera.
The weak gp41 bands seen in surface immunoprecipitations with the N peptide antisera could be due to inefficient binding to a transient fusion intermediate that exposes the N helix, binding to a limited number of N-helix epitopes in the six-helix bundle, or to an immunogen that does not efficiently mimic the trimeric coiled coil. Importantly, sera from all three animals immunized with the N1+C1 peptide mixture were qualitatively different from sera from animals immunized with a single peptide (Fig. 3A and B and data not shown). All N1+C1 peptide antisera immunoprecipitated much more gp41 than did the antisera raised against individual peptides. These results indicate that the N1+C1 peptides interacted to form a new structure distinct from the individual peptides, most likely a six-helix bundle. Sera against the N2+C2 peptide mixture did not immunoprecipitate gp41 in the cell surface assay. This finding is consistent with molecular modeling of the peptides (Fig. 1D), which shows that compared to the N1+C1 peptides, the N2+C2 peptides overlap less well to form a six-helix bundle and may consequently be less stable.
All sera that could bind cell surface Env showed strong enhancement of immunoprecipitation in the presence of sCD4 (Fig. 3A through D). The N1+C1 peptide antisera also showed enhancement with CD4+ CEM human lymphoblasts (Fig. 3E, lane 3), but not with mutant CEM cells that lack CD4 expression (12E1 cells; Fig. 3E, lane 4). Thus, the CD4 receptor is both sufficient and necessary for triggering the conformational changes that allow the antibodies to bind. While increased antibody binding after receptor activation might only reflect increased accessibility of gp41 epitopes due to displacement of gp120, this explanation seems unlikely to completely account for the differences in antibody binding. The relatively weak binding of the N peptide antisera compared to that of the N1+C1 peptide antisera and the lack of binding of the N2+C2 peptide antisera, which all bind well to Env in cell lysates (see below), suggest that gp41 on the cell surface is undergoing conformational changes that lead to differential exposure of gp41 epitopes. The enhanced immunoprecipitation by the anti-N1+C1 sera in the presence of cellular receptors is consistent with the hypothesis that the six-helix bundle forms after receptor-induced conformational changes and further indicates that CD4 is sufficient for triggering changes that lead to the formation of the six-helix bundle. Similar results were reported in flow cytometry experiments with a monoclonal antibody specific for the six-helix bundle (6) and were seen in the cell surface immunoprecipitation assays using this monoclonal (Fig. 3D, lanes 1 and 2). Experiments using the envelopes from the SF162 and JR-FL strains, which are relatively resistant to shedding, gave results identical to those observed with the HXB2 strain (data not shown).
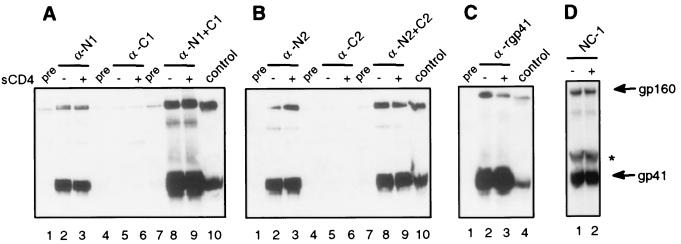
Sera were also assessed for Env binding in immunoprecipitation assays using cell lysates (Fig. 4). Briefly, approximately 4 × 106 Env-expressing cells per serum sample were lysed with 1% IGEPAL (CA-630; Sigma) in 150 mM NaCl–50 mM Tris (pH 8), clarified, and stored at −20°C prior to immunoprecipitation and immunoblotting as described above. All sera that immunoprecipitated gp41 in the cell surface assays also immunoprecipitated gp41 from cell lysates. In contrast to the surface immunoprecipitation, the N2+C2 antisera also immunoprecipitated gp41 from cell lysates, though the intensity of the gp41 bands was much less than for the N1+C1 antisera. Overall the gp41 bands from the cell lysate immunoprecipitations were much stronger than the bands in the cell surface assay, despite the fact that fewer cells were used in the lysates. Likewise, pooled sera from HIV-positive individuals showed much stronger immunoprecipitations in the cell lysate assay than in the cell surface assay (data not shown), indicating that most of the cellular Env is intracellular, as previously reported (17). In contrast to the cell surface immunoprecipitations, addition of receptor did not enhance immunoprecipitation of gp41 from cell lysates (Fig. 4A through C). These results suggest that a significant fraction of Env in cell lysates may be in a nonnative and/or receptor-activated conformation due to lysis conditions and/or to being in an immature or misfolded state. The latter possibility might explain why the majority of the total cellular Env does not get transported to the cell surface.
FIG. 4.
Immunoprecipitation of gp41 in cell lysates in the presence or absence of sCD4. (A) Sera from animals immunized with the N1, C1, or N1+C1 peptides. (B) Sera from animals immunized with the N2, C2, or N2+C2 peptides. (C) Serum from an animal immunized with rgp41. (D) Monoclonal antibody specific for the six-helix bundle (NC-1). Sera shown are the same as those of Fig. 3 at the indicated dilutions. Sera that immunoprecipitated gp41 were two of two for the N1 peptide antisera, one of two for the N2 peptide antisera, zero of two for the C1 peptide antisera, zero of two for the C2 peptide antisera, three of three for the N1+C1 peptide antisera, one of two for the N2+C2 peptide antisera, and one of one for the rgp41 antisera. Env-expressing cell lysate was run as a positive control. Pre, preimmune sera. *, mouse immunoglobulin band.
All sera were further assessed for the ability to inhibit virus entry, using a single-round infectivity assay (3). For this assay, 80 ng of p24-containing HIV pseudovirion stock (HXB2 Env strain) was mixed with serum and immediately added to U87-CD4+CXCR4+ target cells at final serum concentrations of 1:20 and 1:40 and then incubated overnight at 37°C before being washed and harvested 48 h after infection, as previously described (14). Although the 2F5 anti-gp41 monoclonal antibody showed potent neutralization in this assay, none of the gp41 peptide or rgp41 antisera showed significant inhibition (data not shown). The lack of inhibition of virus entry with all anti-N1+C1 sera, which efficiently bind gp41 in all assays and appear specific for the six-helix bundle, indicates that it may be difficult to block infection with antibodies against this structure. A likely explanation for this result is that the six-helix bundle structure is sterically inaccessible to antibodies at the time that fusion occurs or that the six-helix bundle forms coincidently with membrane fusion (9). Immunoprecipitation of gp41 in the presence of target cells (Fig. 3D) is probably due to antibody binding the six-helix bundle after fusion occurs, but we cannot rule out the possibility that the antibodies bind the six-helix bundle during fusion without inhibiting the process. Our results using the high-titered polyclonal sera are in agreement with other studies showing that monoclonal antibodies specific for the six-helix bundle are not neutralizing (5, 6). Efforts are currently under way to modify virus neutralization assays to optimize binding to receptor-activated structures and to assess neutralization activity against additional virus isolates in different assay formats.
Sera against the isolated N or C peptides did not show strong binding to Env under physiologic conditions that permit membrane fusion (Fig. 3). Therefore, it is difficult to draw conclusions about blocking virus entry with antibodies targeting the heptad repeat regions of the fusion-intermediate. The weak binding of the N peptide antisera to gp41 in the surface immunoprecipitations could be due to difficulties binding a transient fusion intermediate or to peptide immunogens that do not efficiently present stable structures. It was previously reported that the N peptides aggregate in solution (8). More structured immunogens that better mimic the heptad repeat domains in the fusion-intermediate, such as the coiled-coil trimer for the N heptad repeat, are needed to evaluate the potential of antibodies to these regions to block virus entry. Nevertheless, it is noteworthy that sera against the N peptides immunoprecipitated CD4-triggered Env at the cell surface, albeit weakly, despite strong binding to gp41 from cell lysates. These results contrast with those for the N1+C1 peptide antisera, which show strong binding to receptor-activated gp41 at the cell surface and in cell lysates. The different binding patterns for these sera may possibly be explained by one serum binding to the fusion intermediate and the other binding to the six-helix bundle. The findings with the N peptide antisera suggest that it may be possible to bind the fusion-intermediate with antibodies, though it is conceivable that these antibodies are binding to epitopes in the N heptad coiled coil, which may be exposed in the six-helix bundle.
In summary, we have shown that gp41 peptides can efficiently raise antibodies to receptor-activated conformations of gp41. The N1+C1 peptide mixture generated high-titered sera against the six-helix bundle structure, though these antibodies did not block HIV infection. The N heptad repeat peptides elicited antibodies that could bind to Env in a CD4-dependent manner, but these sera bound Env less well than the N1+C1 peptide antisera. The C heptad repeat peptides did not elicit antibodies that could bind Env in our assays. These studies provide information for generating conformation-specific antibodies and designing novel immunogens that target receptor-activated conformations of Env.
Acknowledgments
We thank Ira Berkower and Hana Golding (Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Bethesda, Md.) for critical reading of the manuscript. We also thank Shibo Jiang (Lindsley F. Kimball Research Institute, New York Blood Center, New York) for providing the NC-1 monoclonal antibody, Dan R. Littman (New York University, New York) for providing U87 cells and plasmids for the single-round infectivity assay, and Hermann Katinger (Polymun Scientific Inc., Vienna, Austria) for providing the 2F5 gp41 antibody.
REFERENCES
- 1.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 2.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen B K, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta R A, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 5.Gorny M K, Zolla-Pazner S. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J Virol. 2000;74:6186–6192. doi: 10.1128/jvi.74.13.6186-6192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang S, Lin K, Lu M. A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1998;72:10213–10217. doi: 10.1128/jvi.72.12.10213-10217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang S, Lin K, Strick N, Neurath A R. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 9.Melikyan G B, Markosyan R M, Hemmati H, Delmedico M K, Lambert D M, Cohen F S. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–424. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J J, Steel S, Wisniewolski R, Wang C Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci USA. 1986;83:6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 14.Weng Y, Weiss C D. Mutational analysis of residues in the coiled-coil domain of the human immunodeficiency virus type 1 gp41. J Virol. 1998;72:9676–9682. doi: 10.1128/jvi.72.12.9676-9682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willey R L, Bonifacino J S, Potts B J, Martin M A, Klausner R D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci USA. 1988;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingfield P T, Stahl S J, Kaufman J, Zlotnick A, Hyde C C, Gronenborn A M, Clore G M. The extracellular domain of immunodeficiency virus gp41 protein: expression in Escherichia coli, purification, and crystallization. Protein Sci. 1997;6:1653–1660. doi: 10.1002/pro.5560060806. [DOI] [PMC free article] [PubMed] [Google Scholar]