Abstract
Key Clinical Massage
Although tumor necrosis factor (TNF) inhibitors are used to prevent autoimmune disease, but they can cause adverse effects. Multiple sclerosis syndrome is one of the rare complications following treatment with TNF inhibitor. We reported a case of rheumatoid arthritis with unfavorable outcome post Etanerecept therapy.
Abstract
Tumor necrosis factor (TNF) inhibitors are prescribed to treat various autoimmune diseases such as rheumatoid arthritis (RA). Etanercept, a human anti‐TNF monoclonal antibody, is a therapeutic option in autoimmune and inflammatory diseases. One of the rare adverse effects of Etanercept is developing central nervous system and peripheral nervous system demyelination however, there is controversy in the cause of it. In this case, we presented a 35‐year‐old woman with multiple sclerosis (MS)‐like symptoms due to taking Etanercept. She had a history of RA since the age of 18 years and was referred to the emergency department with signs and symptoms of para paresthesia, progressive paraparesis of both lower limbs, and urinary retention a year after Etanercept treatment. We discontinued the Etanercept and started Methylprednisolone and Rituximab. Rapid clinical improvement was noted and in the approximately 6‐month follow‐up, she did not exhibit any neurological worsening. So it is probable that the initiation of Etanercept triggered MS‐like syndrome in these patients and healthcare practitioners should take heed of this adverse effect.
Keywords: case report, Etanerecept, MS‐like symptoms, multiple sclerosis, rheumatoid arthritis
1. INTRODUCTION
Tumor necrosis factor‐α (TNF‐α) is a potent pro‐inflammatory cytokine that plays a crucial role in many autoimmune and inflammatory diseases such as ulcerative colitis, rheumatoid arthritis (RA), psoriasis, ankylosing spondylitis, and Crohn's disease. 1 The prescription of TNF‐α inhibitors has greatly ameliorated the treatment of chronic inflammatory and autoimmune diseases. Today, various biological medications such as Etanercept, Adalimumab, and Infliximab act as antagonists and prevent TNF‐α from performing its function. These biological medications' therapeutic effects on reducing inflammation in several autoimmune diseases have been proven. 1 , 2 In recent years, the increased use of TNF‐α inhibitors due to the advantage and potential to prevent the development of structural damage caused various adverse effects such as serious infections, urticaria, psoriasis, diabetes mellitus (DM), mononeuritis multiplex, malignant lymphoma, congestive heart failure, lupus‐like syndrome, and demyelinating disorders. 3 , 4 One of the rare side effects of these medications is demyelination in the central nervous system (CNS) and peripheral nervous system (PNS) which can cause multiple sclerosis (MS)‐like syndrome, transverse myelitis, optic neuritis, and Guillain Barre syndrome. Although the mechanism underlying these adverse effects has not yet been clarified, several studies reported TNF‐ α inhibitors usage adverse effects. 5 , 6 , 7
TNF‐α inhibitors have proven to be more effective than disease‐modifying antirheumatic drugs. 8 , 9 On the other hand, patients with rheumatological disease may develop neurological manifestations often due to cervical spine involvement and CNS disease caused by vasculitis. Therefore, distinguishing these demyelination lesions from MS and treating them as soon as possible is important and can help physicians handle patients during treatment with TNF‐α inhibitors and prevent unwanted events. 10 , 11 Here, we encountered a case of MS‐like symptoms with a history of RA who was under treatment with Etanercept.
2. CASE HISTORY/EXAMINATION
A 35‐year‐old female with a past medical history of RA presented to the emergency department (ED) of Rajaei Hospital of Karaj, Iran with the chief complaint of para paresthesia and progressive paraparesis of both lower limbs with slow progression started 10 days before her admission. The patient was a known case of RA from 18 years ago and had been under the supervision of her rheumatologist. She had no history of hypertension, DM, seizure, dyslipidemia, heart disease, stroke, or other significant diseases. She had been treated with Prednisolone 10 mg daily and azathioprine 50 mg every 12 h. At the age of 32, due to the patient's plan to conceive, her previous medication was discontinued. After pregnancy, she was started on Methotrexate at an initial dose of 10 mg weekly, which was gradually increased to 20 mg weekly, along with Prednisolone 5 mg daily. A month after delivery due to RA flare‐up and increased Disease Activity Score 28 (DAS28) for our patient from low disease activity (DAS28 2.6–3.2) to high disease activity (DAS28 > 5.1), Etanercept 50 mg weekly, a TNF‐α inhibitor, was initiated during the treatment process. One year after initiating Etanercept, during which the patient's arthritis symptoms and joint pain were well‐controlled and she was in remission, she began experiencing paraesthesia and progressive paraparesis in both lower limbs. These symptoms developed gradually over the 10 days preceding her visit to the ED. At the time of admission, the physical examination revealed an awake patient with a 15/15 score on the Glasgow coma scale, who was afebrile (T:36.1°C) and the blood pressure was 130/85 mmHg, RR:15 breaths/min and other vital signs were normal. The patient presented with a sensory level at T5 and urinary retention indicative of sphincter impairment. The mental status and cranial nerve examinations were within normal limits. Upper limb assessment revealed normal sensory and motor function, with muscle strength rated at 5/5 and reflexes at 2+ bilaterally. Lower limb examination showed knee and Achilles reflexes at 3+ on both sides. Proximal and distal muscle strength in the lower limbs was 4/5, with an upward plantar reflex noted. In the sensory examination, the superficial sensation was normal and the deep sensation of both limbs was decreased. The cerebellar examination showed no evidence of tremor and dysmetria. The finger‐to‐nose and heel‐to‐shin tests were performed accurately and smoothly, although the gait exhibited paraparesis with spasticity.
3. Diagnosis Assessment
Laboratory data showed: hemoglobin:13.8 g/dL, white blood cell: 6.7 × 103 cells/mm3, platelet: 215 × 103 cells/mm3, prothrombin time: 14.7 s, partial thromboplastin time: 28 s, international normalized ratio: 0.9, alanine transaminase: 38 U/L, aspartate aminotransferase: 30 U/L, alkaline phosphatase: 99 U/L, creatinine: 0.86 mg/dL, lactate dehydrogenase: 237 U/L, cobalamin: 347 pg/mL, folic acid: 10.5 ng/mL, and Vitamin D: 43 ng/mL and C‐reactive Protein and erythrocyte Sedimentation Rate were normal (Table 1). Extensive serological testing was performed to exclude differential diagnoses. Results for Brucella, Borrelia, HIV, EBV, CMV, Toxoplasma, and syphilis serologies were negative (Table 2). Neuromyelitis optica antibodies, antinuclear antibody, anti‐double stranded deoxyribonucleic acid antibodies (anti‐dsDNA), anti‐neutrophil cytoplasmic autoantibody, and perinuclear anti‐neutrophil cytoplasmic antibodies were negative and protein S, protein C, and anti‐thrombin 3 were normal. Also, the patient's electrocardiography was normal, and the ejection fraction was 55%. Lumbar puncture was not performed due to the patient's unwillingness. The diagnosis of MS‐like syndrome was confirmed based on the presence of multiple lesions observed on brain MRI (conducted with a Siemens 1.5 Tesla unit and IV contrast), specifically in the left periventricular, subcortical, and juxtacortical regions (Figure 1). The result showed a moderate load of left periventricular active‐enhancing plaque and multiple periventricular, subcortical, and Juxtacortical plaque hyperintensities on T2‐weighted images without active enhancement (Figure 1). In the Fluid‐attenuated inversion recovery cut of cervical MRI, there is a spinal cord lesion in the posterior column in the C1‐C2 region, which has been enhanced (Figure 2). In the thoracic, a spinal cord injury is seen in T5‐T6 with spinal encroachment (Figure 3). Normal anatomy of deep and superficial cerebral veins and superficial sagittal, straight, inferior sagittal, transverse sagittal, and sigmoid dural venous sinuses were seen in the magnetic resonance venography of the brain.
TABLE 1.
The complete blood count and laboratory result.
| Test | Result | Unit | Normal range |
|---|---|---|---|
| WBC | 6.7 | 103 cells/mm3 | 4.0–10.0 |
| RBC | 4.9 | 103/μL | 4.2–5.4 |
| Hgb | 13.8 | g/dL | 11.5–15.0 |
| Plt | 215 | 103 cells/mm3 | 14.0–440 |
| Neutrophils | 63.5 | % | 50–70 |
| Lymphocytes | 27.1 | % | 20–40 |
| PT | 14.7 | Second | 10–14 |
| PTT | 28 | Second | 25–35 |
| INR | 0.9 | ‐ | Up to 1.1 |
| ALT | 38 | U/L | Up to 45 |
| AST | 30 | U/L | Up to 35 |
| ALT | 99 | U/L | 80–306 |
| Creatinine | 0.86 | mg/dL | 0.7–1.4 |
| LDH | 237 | U/L | 225–500 |
| Cobalamin | 347 | pg/mL | 160–950 |
| Folic acid | 10.5 | ng/mL | 2.7–7 |
| Vitamin D | 43 | ng/mL | 20–50 |
| CRP | 2 | mg/L | Up to 6 |
| ESR (1 hour) | 1 | mm/h | Up to 15 |
Abbreviations: ALP, alkaline phosphates; ALT, alanine transaminase; AST, aspartate aminotransferase; CRP, C‐ reactive protein; ESR, erythrocyte sedimentation rate; Hgb, hemoglobin; INR, international normalized ratio; LDH, lactate dehydrogenase; Plt, platelet; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell.
TABLE 2.
Serological test result.
| Test | Result |
|---|---|
| Toxoplasma IgM | Negative |
| Toxoplasma IgG | Negative |
| Brucella IgM titer | Negative |
| Brucella IgG titer | Negative |
| Borrelia IgM | Negative |
| HIV Ag and Ab | Negative |
| EBV | Negative |
| CMV PCR | Negative |
| VDRL | Negative |
FIGURE 1.
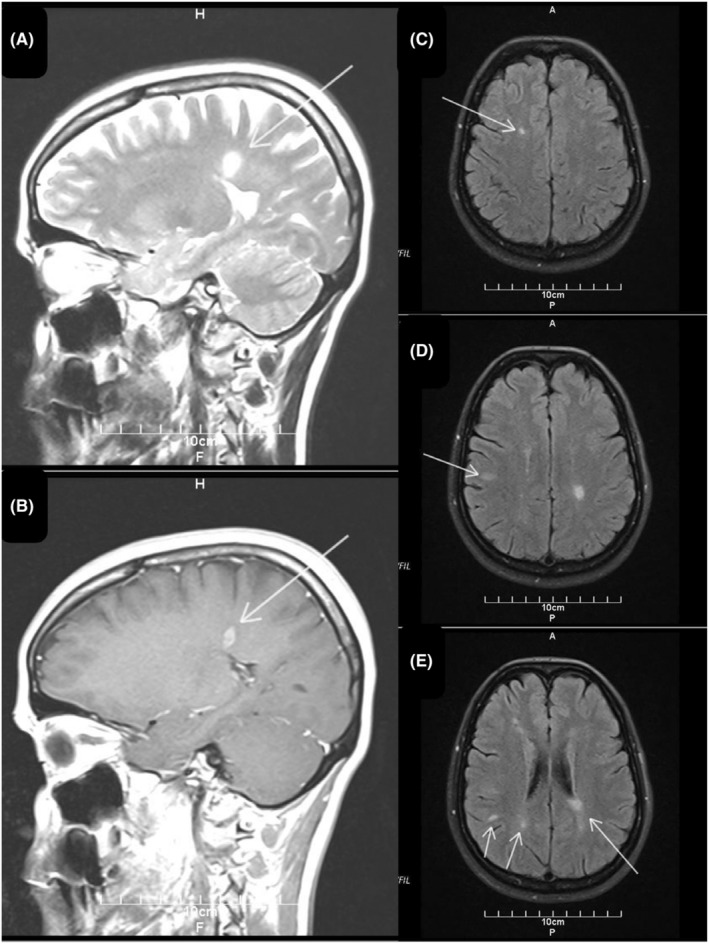
Brain magnetic resonance imaging was performed at the onset of symptoms in 2023. The sagittal fluid‐attenuated inversion recovery (FLAIR) image shows a periventricular (A, arrow) lesion and gadolinium enhancement in periventricular (B, arrow) suggesting multiple sclerosis. Transverse fluid‐attenuated inversion recovery images (FLAIR) show left subcortical (C, arrow), juxtacortical (D, arrow), and periventricular, subcortical, juxtacortical (E, arrows) hyperintense round lesions consistent with demyelination areas.
FIGURE 2.
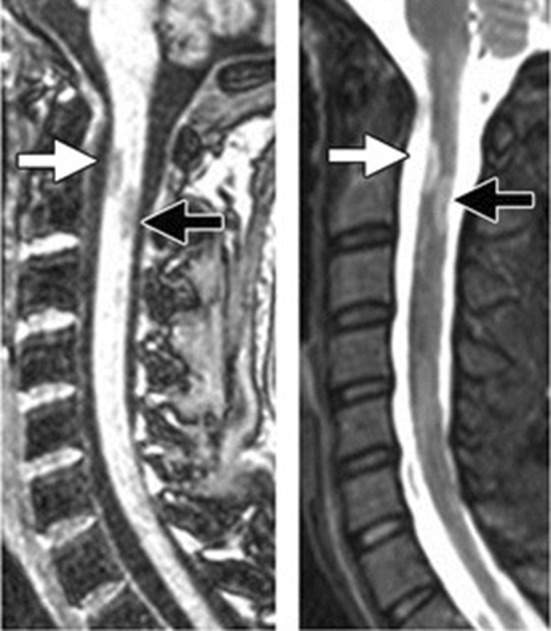
The sagittal T2‐weighted (W) image discloses a focal T2‐hyperintense cervical lesion.
FIGURE 3.
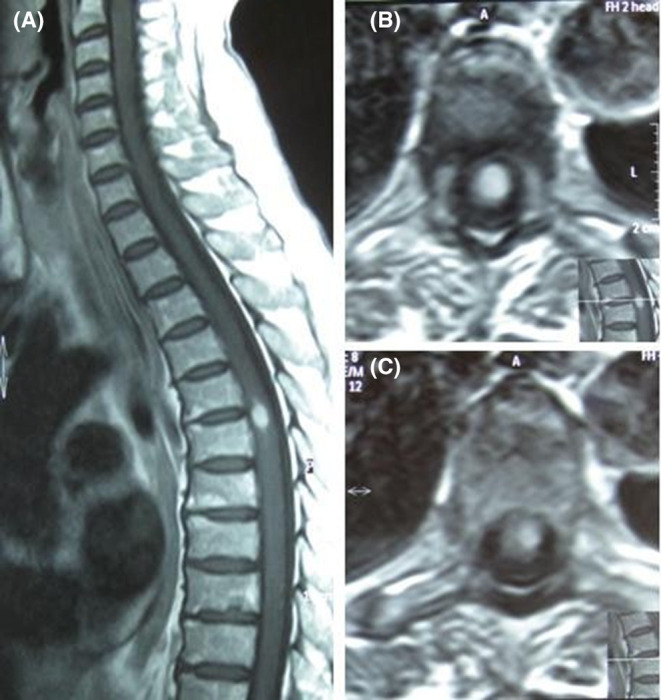
The sagittal (A) and axial (B and C) T2‐weighted (W) image discloses a focal T2‐hyperintense T5‐T6 thoracic lesion.
4. THERAPEUTIC INTERVENTION
In this case, the potential link between Etanercept and CNS demyelination prompted a reevaluation of the patient's treatment. Consequently, Etanercept was discontinued, and the patient was treated with intravenous boluses of Methylprednisolone (1000 mg daily for 5 days) and Rituximab (1 g). Rapid clinical improvement in demyelination symptoms was observed. At the 6‐month follow‐up, the patient remained free of neurological symptoms, normal laboratory results, a normal neurological examination, and no flare of RA. The patient has since been lost to follow‐up.
5. DISCUSSION
In the presented case, we suspected MS‐like syndrome because of the emergence of neurologic symptoms and demyelination lesions found consistent in brain and spinal cord MRI. As noted in our case report and other literature discontinuation of TNF‐α inhibitors ended in remission of the signs and symptoms. 10 , 12 Several studies demonstrated that TNF‐α, which is secreted by microglia and macrophages in the CNS, plays a key role in the etiology and pathogenesis of demyelination. 13 They found elevated TNF‐α levels in serum and CSF of patients with progressive MS and demonstrated that TNF‐α levels correlated with disease severity. 14 Therefore, TNF‐α inhibitor therapy has been assessed as a treatment for autoimmune diseases. Despite the clinical effectiveness of TNF‐α inhibitors, well‐known adverse effects including injection site reactions, congestive heart failure, risk of infections, and hemocytopenia, 15 some literature has reported neurological adverse events such as new onset MS‐like syndrome, lupus‐like syndromes and vasculitis, psoriasis, DM, interstitial lung diseases, autoimmune hepatitis, myositis, uveitis, and MS due to TNF‐α inhibitor therapy. 4 , 16 , 17
A prospective study was conducted by Kaltsonoudis et al. 10 on rheumatologic patients who were eligible for anti‐TNF‐α therapy. They observed that 3 out of 75 (4%) patients developed neurological adverse events such as paresis of the left facial nerve, optic neuritis, tingling, and numbness of the lower extremities. Another cohort study was conducted by Ali Theibich et al. 18 in Copenhagen with around 550 rheumatologic outpatients who underwent treatment with TNF‐α inhibitors every year from January 2008 to December 2011. They observed six patients with signs of demyelinated neurological adverse events: four cases of MS, one MS‐like symptom, and one multifocal motor neuropathy. Likewise, a study by Mohan et al. 17 reported demyelinating processes in 20 inflammatory arthritis patients during the use of biological medications (18 following Etanercept administration and 2 following Infliximab). The most common presenting clinical symptoms were paresthesias (13 out of 20) and visual disturbances secondary to optic neuritis (8 out of 20). Confusion was an atypical feature that occurred in 25% of patients which is uncommon in MS. The average time between the initiation of the TNF‐α inhibitor and the onset of the neurologic adverse effect was 5 months (ranging between 1 week and 15 months). They reported that discontinuation of the medication ended in the remission of neurologic symptoms either partially or completely in most patients. In a case series by Andreadou et al. 19 they reported 4 cases who had various rheumatologic diseases and were treated by Etanrecept. They showed various adverse neurologic events such as dysesthesias up to the T4 level, right unilateral tinnitus, difficulty in speech, and ptosis of the right corner of the mouth. In all cases, lesions observed in brain MRI that suggestive of demyelination.
The underlying mechanisms that cause demyelination in patients treated with TNF‐α inhibitors are undecipherable but several mechanisms have been hypothesized. “Lack of entry” is one of the theories that describe demyelination concerning treatment with TNF‐α inhibitor. This theory says that TNF‐α inhibitors are unable to permeate from the intact brain blood barrier to withdrawal demyelination but they can enhance demyelination by increased entry of peripheral autoreactive T cells into the CNS. In this theory, we can understand that due to the failure of TNF‐α inhibitors in decreasing demyelination, adverse events can be susceptible. In another theory called the sponge effect, it is assumed that TNF‐α is blocked systematically by TNF‐α inhibitors, but not within the CNS because TNF‐α inhibitors can not penetrate within the brain blood barrier which leads to the accumulation of TNF‐α in the CNS. It seems TNF‐α inhibitors had a potential role in the induction of demyelination so it is interesting to find that the TNF‐α inhibitor therapy causes or prevents the demyelinating process. 12 , 20
Different treatments and approaches have been reported to control MS‐like symptoms caused by TNF‐α inhibitors. In some cases, symptoms improved just by cessation of TNF‐α inhibitors but the symptoms re‐emerged by re‐administration. 10 In other cases besides cessation of the TNF‐α inhibitor, administration of glucocorticoids such as intravenous boluses of methyl‐prednisone (1 g/day for 5 days) showed significant clinical improvement. Other therapeutic approaches such as gabapentin, interferon β, plasmapheresis, and intravenous immunoglobulin (IVIG) have been reported besides TNF‐α inhibitor cessation. 10 , 17 , 18 Thus Clinicians should consider preventing the administration of TNF‐α inhibitors in patients who are at increased risk of developing immune‐mediated demyelination, preexisting diagnosis of MS or strong familial history of MS, or other demyelinating diseases.
Generally, besides the proven therapeutic effect of TNF‐α inhibitors on MS, an interesting relation between TNF‐α inhibitors and the new onset of MS‐like syndromes has been confirmed and these studies indicated that TNF‐α inhibitors may initiate an underlying demyelinating disease. 20 As mentioned, many various adverse neurological events have been reported concerning TNF‐α inhibitors such as Guillain‐Barre syndrome, Miller‐Fisher syndrome, CNS demyelination, polyneuropathies, and leukoencephalopathy. 21 MRI findings in many studies disclosed large areas of increased signal intensity on T2‐weighted images all through the periventricular and subcortical without abnormal contrast enhancement. 16 , 17 Therefore in the face of developing new neurologic signs or symptoms suggestive of demyelination in a patient who is under treatment of TNF‐α inhibitors immediate discontinuation of TNF‐α inhibitors, neurologic examination, assessment by MRI, CSF analysis, and therapies used in MS, such as glucocorticoids, IFN‐β, or IVIG, should be considered.
Our case study contributes to the expanding pool of data that substantiates a potential connection between TNF‐α inhibitor agents and demyelination. Healthcare practitioners should take heed of this information. In the event of any neurological adverse effects, promptly discontinuing the use of TNF‐α blockers and promptly requesting an MRI scan to confirm the diagnosis are crucial actions to prioritize.
AUTHOR CONTRIBUTIONS
Ali Hosseinpour: Investigation; methodology; validation; writing – review and editing. Arsh Haj Mohamad Ebrahim Ketabforoush: Investigation; writing – review and editing. Elnaz Daneshzad: Investigation; writing – original draft. Ali Babanezhad Gajouti: Writing – original draft. Samaneh Hajimollarabi: Supervision; validation.
FUNDING INFORMATION
The authors received no funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The authors would like to thank the Clinical Research Development Unit (CRDU) of Shahid Rajaei Hospital, Alborz University of Medical Sciences, Karaj, Iran for their support, cooperation, and assistance throughout the study.
Hosseinpour A, Haj Mohamad Ebrahim Ketabforoush A, Daneshzad E, Babanezhad Gajouti A, Hajimollarabi S. Unexpected multiple sclerosis‐like symptoms in a rheumatoid arthritis patient treated with Etanercept: A case report. Clin Case Rep. 2024;12:e9486. doi: 10.1002/ccr3.9486
DATA AVAILABILITY STATEMENT
The original contributions presented in this study are included in the article and further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Chen W, Ding Y, Lu J, Shi Y, Gao Y, Peng C. Efficacy and survival of infliximab in psoriasis patients: a single‐center experience in China. Dermatol Ther. 2020;33(6):e14227. [DOI] [PubMed] [Google Scholar]
- 2. Sabaei M, Rahimian S, Haj Mohamad Ebrahim Ketabforoush A, et al. Salivary levels of disease‐related biomarkers in the early stages of Parkinson's and Alzheimer's disease: a cross‐sectional study. IBRO Neurosci Reports. 2023;14:285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haj Mohamad Ebrahim Ketabforoush A, Molaverdi G, Nirouei M, Abbasi Khoshsirat N. Cerebral venous sinus thrombosis following intracerebral hemorrhage after COVID‐19 AstraZeneca vaccination: a case report. Clin Case Reports. 2022;10(11):e6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramos‐Casals M, Brito‐Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF‐targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86(4):242‐251. [DOI] [PubMed] [Google Scholar]
- 5. Sökmen O, Göçmen R, Tuncer A. Multiple sclerosis‐like demyelinating lesions during Adalimumab treatment in a case with Crohn's disease. Noropsikiyatri Ars. 2022;59(4):342‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsumoto T, Nakamura I, Miura A, Momoyama G, Ito K. New‐onset multiple sclerosis associated with adalimumab treatment in rheumatoid arthritis: a case report and literature review. Clin Rheumatol. 2013;32:271‐275. [DOI] [PubMed] [Google Scholar]
- 7. Mohamad Ebrahim Ketabforoush AH, Ahmadian Z, Ariaei A, Hosseinpour A, Khoshsirat NA. Spontaneous intracranial hypotension and bilateral subdural hematoma in the spectrum of antiphospholipid syndrome and systemic lupus erythematosus: a challenging clinical encounter. Future Neurol. 2024;19(1):2337450. [Google Scholar]
- 8. Hosseinpour A, Daneshzad E, Dezfouli RA, Zamani S, Qorbani M. The association between antioxidants and COVID‐19 outcomes: a systematic review on observational studies. Biol Trace Elem Res. 2023;201(11):5098‐5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiganer EH, Lessa CF, Di Pace JL, et al. Transverse myelitis in systemic lupus erythematosus: clinical features and prognostic factors in a large cohort of Latin American patients. J Clin Rheumatol. 2021;27(6 S):S204‐S211. [DOI] [PubMed] [Google Scholar]
- 10. Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA. Neurological adverse events in patients receiving anti‐TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res Ther. 2014;16(3):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashami L, Haj Mohamad Ebrahim Ketabforoush A, Nirouei M. Giant cell arteritis with rare manifestations of stroke and internal carotid artery dissection: a case study. Clin Case Rep. 2022;10(3):e05597. doi: 10.1002/ccr3.5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gharib MH, AlKahlout MA, Garcia Canibano B, Theophiel Deleu D, Malallah AlEssa H, AlEmadi S. Demyelinating neurological adverse events following the use of anti‐TNF‐α agents: a double‐edged sword. Case Rep Neurol Med. 2022;2022:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eder P, Korybalska K, Łykowska‐Szuber L, et al. An increase in serum tumour necrosis factor‐α during anti‐tumour necrosis factor‐α therapy for Crohn's disease – a paradox or a predictive index? Dig Liver Dis. 2016;48(10):1168‐1171. [DOI] [PubMed] [Google Scholar]
- 14. Wong M, Ziring D, Korin Y, et al. TNFα blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126(2):121‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon TA, Suissa S, Boers M, et al. Malignancy outcomes in patients with rheumatoid arthritis treated with abatacept and other disease‐modifying antirheumatic drugs: results from a 10‐year international post‐marketing study. Semin Arthritis Rheum. 2024;64:152240. doi: 10.1016/j.semarthrit.2023.152240 [DOI] [PubMed] [Google Scholar]
- 16. Thomas CW Jr, Weinshenker BG, Sandborn WJ. Demyelination during anti‐tumor necrosis factor α therapy with infliximab for Crohn's disease. Inflamm Bowel Dis. 2004;10:28‐31. [DOI] [PubMed] [Google Scholar]
- 17. Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti–tumor necrosis factor α therapy for inflammatory arthritides. Arthrit Rheumat. 2001;44(12):2862‐2869. [DOI] [PubMed] [Google Scholar]
- 18. Theibich A, Dreyer L, Magyari M, Locht H. Demyelinizing neurological disease after treatment with tumor necrosis factor alpha‐inhibiting agents in a rheumatological outpatient clinic: description of six cases. Clin Rheumatol. 2014;33:719‐723. [DOI] [PubMed] [Google Scholar]
- 19. Andreadou E, Kemanetzoglou E, Brokalaki C, et al. Demyelinating disease following anti‐TNFa treatment: a causal or coincidental association? Report of four cases and review of the literature. Case Rep Neurol Med. 2013;2013:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kemanetzoglou E, Andreadou E. CNS demyelination with TNF‐α blockers. Curr Neurol Neurosci Rep. 2017;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubcenco E, Ottaway CA, Chen DL, Baker JP. Neurological symptoms suggestive of demyelination in Crohn's disease after infliximab therapy. Eur J Gastroenterol Hepatol. 2006;18(5):565‐566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article and further inquiries can be directed to the corresponding author.


