Abstract
Jaagsiekte sheep retrovirus (JSRV) replicates in the lungs of sheep and causes the secretion of copious lung fluid containing the virus. Adaptation of JSRV to infection and replication in the lung and its apparent resistance to the denaturing activity of lung fluid suggest that vectors based on JSRV would be useful for gene therapy targeted to the lung. We show here that a retrovirus vector bearing the JSRV Env is stable during treatment with lung surfactant while an otherwise identical vector bearing an amphotropic Env is inactivated. Furthermore, the JSRV vector was stable during centrifugation, allowing facile vector concentration, and showed no loss of activity after six freeze-thaw cycles. However, the JSRV vector was inactivated by standard disinfectants, indicating that JSRV vectors pose no unusual safety risk related to their improved stability under other conditions.
Much effort has been devoted to the development of gene therapy for the treatment of lung disease (1). A major target is cystic fibrosis, which affects 1 in 3,000 Caucasian births and is caused by a defect in the cystic fibrosis transmembrane regulator chloride ion channel (3, 17). Early attempts to treat cystic fibrosis involved vectors derived from adenovirus, but the utility of these vectors was limited by immune responses against viral proteins encoded by these vectors, the lack of vector integration, and short-lived transgene expression (8). Vectors based on various serotypes of adeno-associated virus can mediate relatively high gene transfer rates in the lung and can integrate into the genome of target cells to promote long-term gene expression (2, 7), but these vectors have a capacity of only ∼4.6 kb, and it has proven difficult to accommodate the 4.44-kb cystic fibrosis transmembrane regulator coding region with suitable promoter, enhancer, and transcription termination signals in an adeno-associated virus vector. Vectors based on simple retroviruses have also been considered for transfer of genes to the airway, but transfer rates in intact airways of animals have been low and may be related to the sensitivity of these vectors to surfactants and proteases present in lung fluid (18). Alternatively, a lentiviral vector provided higher rates of gene transduction but required disruption of airway epithelial cells to allow vector transduction through the basal aspect of these cells (6). Lipid-mediated transfer of DNA has been used to treat cystic fibrosis. However, this technique is limited by low transduction rates and transient gene expression (1), and gene transfer can be inhibited by lung surfactant (5).
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of a contagious lung cancer of sheep called ovine pulmonary carcinoma or sheep pulmonary adenomatosis (12). Late stages of the disease are accompanied by the secretion of copious lung fluid containing the virus, and while this pathological lung fluid may have somewhat different properties than normal lung fluid, this finding indicates that the virus is resistant to inactivation by the surfactants and proteases in normal lung fluid that have been shown to inactivate other retroviruses (18). These observations suggest that retrovirus vectors made by using components from JSRV, especially the envelope protein (Env), might prove useful for gene transfer to the lung. As a first step in the development of gene therapy vectors based on JSRV, a retrovirus packaging line that expressed the Gag and Pol proteins from Moloney murine leukemia virus (MoMLV) and the Env protein of JSRV was developed, and it was shown that MoMLV-based vectors could be produced from this line at relatively high titers (13). Importantly, from the standpoint of gene therapy vector development, the host range of these vectors included human cells (13).
JSRV is a simple retrovirus with typical gag, pol, and env genes. In newborn sheep, purified virus induces multifocal tumors in as little as 10 days (15), suggesting the role of a viral oncogene rather than insertional activation of cellular oncogenes, and it has recently been shown that expression of the Env protein alone can induce transformation in cultured rodent cell lines (9, 14). This raises a concern regarding the development of vectors for gene therapy and the safety of recombinant viruses made with JSRV Env in the laboratory. While vectors bearing JSRV Env protein should not be oncogenic since the vector genome does not contain or express the env gene, recombination events might generate viruses that express the JSRV env gene and that might be oncogenic in humans.
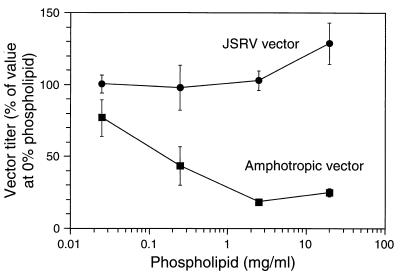
Here we have examined the properties of JSRV vectors in relation to possible use for gene therapy and from the standpoint of safety. We first tested whether JSRV vectors were resistant to treatment with Survanta, a clinical-grade bovine-derived lung surfactant which contains ∼25 mg of phospholipid per ml (including 13 mg of dipalmitoylphosphatidylcholine per ml), 1.2 mg of triglycerides per ml, 2.5 mg of free fatty acid per ml, and 0.6 mg of protein (surfactant proteins B and C) per ml in 0.9% sodium chloride. This preparation is used as a lung surfactant replacement to aid breathing in premature human infants who do not yet secrete surfactant. Vector virions contained a vector that encodes human placental alkaline phosphatase and neomycin phosphotransferase (LAPSN) (11), the Gag and Pol proteins from MoMLV, and the Env protein from either JSRV or amphotropic retrovirus 4070A. These vectors were made using PJ4 (JSRV pseudotype) (13) or PA317 (amphotropic pseudotype) (10) retrovirus packaging cell lines. Vector stocks were incubated with various amounts of Survanta for 30 min at room temperature, and the vector titers were determined using primary sheep skin fibroblasts (SSF-123; gift from William Osborne, University of Washington, Seattle) and HT-1080 human fibrosarcoma cells (American Type Culture Collection cell line CCL-121) as targets for infection (Fig. 1). If anything, the titer of the JSRV vector increased after incubation at the highest concentration of Survanta (20 mg of phospholipid per ml; the normal level in the lung is estimated to be 25 mg/ml), whereas the titer of the amphotropic vector decreased by ∼80% compared with the value found after incubation without Survanta. Furthermore, the titer of the JSRV vector did not change during a 30-min incubation at room temperature without Survanta, while that of the amphotropic vector decreased by ∼30% (data not shown). Thus, the presence of the JSRV Env protein confers resistance to inactivation by lung surfactant and also stabilizes the vector against inactivation during incubation at room temperature.
FIG. 1.
JSRV pseudotype vector transduction rate is not decreased by prior incubation with Survanta. SSF and HT-1080 cells were plated at 105 cells per 3.5-cm-diameter well of six-well plates. Both cell types were grown in Dulbecco's modified Eagle medium with 10% fetal bovine serum in a 10% CO2–air atmosphere. One day later, LAPSN vectors produced from PJ4 (JSRV pseudotype) or PA317 (amphotropic pseudotype) packaging cells were incubated with various concentrations of Survanta for 30 min at room temperature. The target cells were fed 2 ml of medium containing 5 μg of Polybrene per ml, and portions of the vector-Survanta mixtures were added. SSF cells were used as targets for JSRV vector transduction, and HT-1080 cells were used for the amphotropic vector. At the dilutions used, Survanta had no apparent effect on the health or growth rate of the cells. Three days after vector exposure, the cells were fixed and stained for alkaline phosphatase-positive foci. Means and standard deviations are shown from four experiments for the JSRV vector and from two experiments for the amphotropic vector.
Measurement of the vector titer described above was performed in the presence of Polybrene (hexadimethrine bromide), as is typically done to improve infection by many retroviruses. Polybrene is polycationic and is thought to act by neutralizing negative charges on the surfaces of cells and virions to promote virus attachment and entry (16). We measured the effects of Polybrene on transduction by the JSRV vector and explored whether Survanta at a similar concentration might affect transduction rates (Table 1). Polybrene increased the apparent titer of the JSRV vector 38-fold and that of an amphotropic vector 9-fold. In contrast, Survanta at a concentration of 10 μg/ml had little effect on vector titer measured in the presence or absence of Polybrene.
TABLE 1.
JSRV vector transduction rate is increased by Polybrene but not by Survantaa
| Vector transduction conditions | JSRV vector titer (FFU/ml) | Fold increase | Amphotropic vector titer (FFU/ml) | Fold increase |
|---|---|---|---|---|
| No additions | 8.1 × 103 | 1 | 1.6 × 104 | 1 |
| Polybrene | 3.1 × 105 | 38 | 1.4 × 105 | 9 |
| Survanta | 9.7 × 103 | 1.2 | 1.0 × 104 | 0.6 |
| Polybrene + Survanta | 3.5 × 105 | 43 | 7.9 × 104 | 5 |
SSF and HT-1080 cells were plated at 105 cells per 3.5-cm-diameter well of six-well plates. The following day the culture medium was removed and replaced with either medium alone or medium containing 5 μg of Polybrene per ml, 10 μg of Survanta per ml, or both 5 μg of Polybrene and 10 μg of Survanta per ml. JSRV [LAPSN(PJ4)] or amphotropic [LAPSN(PA317)] vectors were then added to the SSF and HT-1080 cells, respectively, and the cells were fixed and stained for alkaline phosphatase-positive foci 3 days later. Results are means from two to three experiments. Titer determinations in separate experiments varied by less than twofold.
JSRV packaging cells can produce JSRV pseudotype vectors at up to 106 transducing units per ml measured on sheep skin fibroblasts, but titers are ∼10-fold lower when measured on human cells (13). Given the stability imparted by JSRV Env to inactivation by lung surfactant, we tested whether JSRV vectors might be stable during concentration by centrifugation to increase vector titer. We collected the LAPSN vector from the JSRV packaging cells, centrifuged the virus at 700 × g for 7 min to remove cell debris, pelleted the virus by centrifugation at 70,000 × g (using a Beckman SW-28 rotor at 23,000 rpm) for 1.5 h at 4°C, and resuspended the virus in a small amount of medium. We were able to achieve a 43-fold increase in vector titer with a 61% yield, showing that the vector could be effectively concentrated by using a simple centrifugation step. This finding parallels the observation that vesicular stomatitis virus G protein can stabilize a retrovirus vector during centrifugation, while a similar vector with an amphotropic Env was unstable (4).
As a final test of the stability of the JSRV vector, we subjected the JSRV pseudotype LAPSN vector to repeated cycles of freezing to −80°C and thawing to 37°C. The titer of the vector remained the same between one and six freeze-thaw cycles (data not shown), further establishing the stability of the vector. In parallel experiments, the titer of an amphotropic pseudotype LAPSN vector decreased by 40% after six freeze-thaw cycles (data not shown).
There is presently no evidence for disease induction by JSRV in humans exposed to infected sheep. However, the ability of vectors bearing the JSRV Env protein to infect human cells and the ability of the viral Env protein to directly transform rodent fibroblasts raise a concern regarding safe handling of JSRV. In addition, while we have not detected replication-competent virus in vector stocks prepared from JSRV pseudotype retrovirus packaging cells, this possibility exists and again raises concerns about the safety of these vectors. In particular, given the relative stability of JSRV vectors during treatment with lung surfactant, during concentration, and during multiple freeze-thaw cycles, we wondered if these vectors might be resistant to commonly used disinfectants as well. To address this issue, we measured inactivation of a JSRV vector and an otherwise identical vector with an amphotropic Env protein using ethanol or a laboratory detergent, ES 7X cleaning solution (ICN Biomedicals). ES 7X is composed of water plus phosphonate, diethylene glycol monobutyl ether, surfactants, and dioctyl sodium sulfosuccinate. Both the JSRV and amphotropic vectors were inactivated by treatment with ethanol at similar concentrations for 10 s or 1 min (Table 2). Both vectors were completely inactivated by 10 s of treatment with ethanol at concentrations of at least 50%. Similarly, both vectors were inactivated by treatment with ES 7X detergent at similar concentrations for 10 s or 1 min (Fig. 2). Both vectors were completely inactivated by 10 s of treatment with ES 7X at concentrations of at least 0.5%. Thus, the JSRV vector was as sensitive to inactivation by common disinfectants as the amphotropic vector was. Given the potential for evaporation of ethanol during disinfection, treatment with ES 7X or similar detergents at concentrations of 1 to 5% for at least 10 s appears to provide adequate disinfection under typical laboratory conditions.
TABLE 2.
JSRV and amphotropic vectors show similar levels of sensitivity to ethanol inactivationa
| Ethanol concn (%) | JSRV vector inactivation (%) after incubation with ethanol for:
|
Amphotropic vector inactivation (%) after incubation with ethanol for:
|
||
|---|---|---|---|---|
| 10 s | 1 min | 10 s | 1 min | |
| 0 | 0 | 0 | 0 | 0 |
| 10 | 38 ± 14 | 56 ± 11 | 39 ± 39 | 62 ± 16 |
| 30 | 99.1 ± 0.8 | >99.8 | 99.3 ± 0.9 | >99.9 |
| 50 | >99.7 | >99.8 | >99.9 | >99.9 |
| 70 | >99.7 | >99.7 | >99.9 | >99.9 |
HT-1080 cells were plated at 7.5 × 104 cells per 3.5-cm-diameter well in six-well plates on day 1. On day 2, the medium was replaced with 2 ml of medium containing 4 μg of Polybrene per ml. JSRV [LAPSN(PJ4)] and amphotropic [LAPSN(PA317)] vectors were treated with ethanol at the indicated concentrations, and 1-, 5-, and 10-μl samples of the treated vectors were then added to the cells either 10 s or 1 min after ethanol addition. At this dilution, the ethanol had no apparent effect on the health or growth rate of the cells. Two days after exposure to the vectors, the cells were fixed and stained for alkaline-phosphatase-positive foci. Means and standard deviations from three to four experiments are shown.
FIG. 2.
JSRV and amphotropic vectors show similar levels of sensitivity to inactivation by ES 7X cleaning solution. HT-1080 cells were plated at 7.5 × 104 cells per 3.5-cm-diameter well of six-well plates. One day later, the cells were fed 2 ml of medium containing 4 μg of Polybrene per ml. LAPSN(PJ4) (A) and LAPSN(PA317) (B) vectors were treated with ES 7X at the indicated concentrations for 10 s or 1 min, and 1-, 5-, and 10-μl samples of the treated vectors were then added to the cells. At these dilutions, the ES 7X had no apparent effect on the health or growth rate of the cells. Two days after exposure to the vectors, the cells were fixed and stained for alkaline phosphatase-positive foci. Means and standard deviations from three to four experiments are shown. Data points on the x axes represent values below the limit of detection: <0.4% for the JSRV vector and <0.14% for the amphotropic vector.
In conclusion, we have found that incorporation of the JSRV Env protein into virions containing an MoMLV-based retrovirus vector and Gag and Pol components from MoMLV confers resistance to lung surfactant, centrifugation, and freeze-thaw cycling in comparison to an otherwise identical vector bearing the amphotropic murine leukemia virus Env. These are useful properties for the application of JSRV vectors to gene therapy in the lung. JSRV vectors do not transduce cells from mice, rats, or hamsters; thus, gene transfer to the lung cannot be tested in these accessible animal models. We are generating transgenic mice expressing HYAL2, the human cell surface receptor for JSRV (14), to allow such testing. Given the oncogenic properties of the JSRV env gene, it is important that vectors bearing the JSRV Env protein are as sensitive to inactivation by standard disinfectants, ethanol and detergent, as are vectors bearing the amphotropic Env protein. Thus, JSRV vectors do not pose an unusual biohazard in this regard. In addition, preliminary results indicate that the JSRV Env gene can be modified to eliminate its transforming activity while preserving its ability to facilitate gene transfer (S.-L. Liu and A. D. Miller, unpublished results), which would further improve the suitability of JSRV vectors for human use.
Acknowledgments
D. A. Coil and J. H. Strickler contributed equally to this work.
This work was supported by grants DK47754 and HL54881 from the National Institutes of Health.
REFERENCES
- 1.Albelda S M, Wiewrodt R, Zuckerman J B. Gene therapy for lung disease: hype or hope. Ann Intern Med. 2000;132:649–660. doi: 10.7326/0003-4819-132-8-200004180-00008. [DOI] [PubMed] [Google Scholar]
- 2.Allen J M, Halbert C L, Miller A D. Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6. Mol Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- 3.Boucher R C. Status of gene therapy for cystic fibrosis lung disease. J Clin Investig. 1999;103:441–445. doi: 10.1172/JCI6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan J E, Whitsett J A, Horowitz A D. Pulmonary surfactant inhibits cationic liposome-mediated gene delivery to respiratory epithelial cells in vitro. Hum Gene Ther. 1997;8:431–438. doi: 10.1089/hum.1997.8.4-431. [DOI] [PubMed] [Google Scholar]
- 6.Guoshun W, Slepushkin V, Zabner J, Keshavjee S, Johnston J C, Sauter S L, Jolly D J, Dubensky T W, Jr, Davidson B L, McCray P B., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Investig. 1999;104:R55–R62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey B-G, Leopold P L, Hackett N R, Grasso T M, Williams P M, Tucker A L, Kaner R J, Ferris B, Gonda I, Sweeney T D, Ramalingam R, Kovesdi I, Shak S, Crystal R G. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Investig. 1999;104:1245–1255. doi: 10.1172/JCI7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci USA. 2001;98:4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmarini M, Sharp J M, De las Heras M, Fan H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai S K, DeMartini J C, Miller A D. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J Virol. 2000;74:4698–4704. doi: 10.1128/jvi.74.10.4698-4704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai S K, Duh F-M, Vigdorovich V, Danilkovitch-Miagkova A, Lerman M I, Miller A D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp J M, Angus K W, Gray E W, Scott F M. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Arch Virol. 1983;78:89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima K, Vogt P K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969;38:414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 17.Welsh M J. Gene transfer for cystic fibrosis. J Clin Investig. 1999;104:1165–1166. doi: 10.1172/JCI8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zsengeller Z K, Halbert C, Miller A D, Wert S E, Whitsett J A, Bachurski C J. Keratinocyte growth factor stimulates transduction of the respiratory epithelium by retroviral vectors. Hum Gene Ther. 1999;10:341–353. doi: 10.1089/10430349950018797. [DOI] [PubMed] [Google Scholar]