Abstract
We previously reported that, upon reinoculation into cats, a neutralization-sensitive, tissue culture-adapted strain of feline immunodeficiency virus constantly reverted to the broad neutralization resistance typical of primary virus isolates and identified residue 481 in the V4 region of the surface glycoprotein as a key determinant of the reversion. Here, we found that well-characterized immune sera, obtained from cats in which such reversion had occurred, selected in tissue culture in favor of virus variants that also had a neutralization-resistant phenotype and had amino acid 481 changed, thus indicating that the host's humoral immune response is capable of driving the reversion in the absence of other intervening factors. In contrast, a second group of immune sera, elicited by a virus variant that had already reverted to neutralization resistance in independent cats, induced the emergence of escape mutants lacking broad neutralization resistance and neutralized fewer virus variants. It is proposed that the viral variants used to produce the two sets of sera may have generated different antibody repertoires.
Naturally occurring lentiviruses are only occasionally inhibited by sera of infected subjects in in vitro neutralization assays, and this property is currently the focus of intensive investigation since it is considered instrumental for virus persistence and pathogenicity as well as a formidable obstacle in the development of effective prophylactic vaccines (5, 16). With regard to the latter point, several immunogens have indeed been shown to protect against neutralization-sensitive (NS), tissue culture-adapted (TCA) laboratory lentiviruses but not against isolates recently cultured from infected hosts (11, 25). In spite of considerable recent advances on the structural features that permit wild-type lentiviruses to resist potentially neutralizing antibodies (7, 8, 13, 15, 20, 27), what determines conservation of this important viral attribute of wild-type lentiviruses is poorly understood. In fact, although it seems feasible that continued exposure to antiviral antibody is important (4), this has never been formally demonstrated. In principle, alternative or adjunctive intervening factors include other innate and adaptive immune effectors and the need to conserve the usage of certain receptor-coreceptor systems which determine virus tropism for specific cell types (17).
Feline immunodeficiency virus (FIV) is an important pathogen of domestic cats and, due to extensive similarities with human immunodeficiency virus type 1 (HIV-1), is a valuable model for AIDS studies (10, 18, 24). In particular, the neutralization properties of FIV closely resemble those of HIV-1, including that TCA strains are readily inhibited by immune sera in vitro whereas fresh isolates exhibit a generalized resistance to antibody-mediated neutralization (1, 9). In previous studies, we observed that, upon reinoculation into cats, an exquisitely NS laboratory TCA strain of FIV regained the broadly neutralization-resistant (NR) phenotype typical of wild-type viruses (3, 6). This closely mimicked what also observed with TCA strains of HIV and chimeric simian-HIV following in vivo readaption (2, 7). The NS→NR reversion of FIV—operationally defined as transition from in vitro inhibition by most of a large panel of immune cat sera to inhibition by very few—was observed in all inoculated cats, albeit after variable numbers of months, and was often associated with only a few envelope (Env) amino acid changes. This permitted recognition of variable region 4 (V4) and V5 of the surface glycoprotein (SU) as the major determinants of in vivo reversion. Specifically, sequencing of numerous viral samples obtained at different times of infection, followed by analysis of biological and molecular clones, showed that a 481Lys→Asn or 481Lys→Glu change in V4 was clearly sufficient for early reversion, while a 557Ser→Asn change in V5 appeared to concur with a second less-well-identified change to determine the broad neutralization resistance of long-term revertants reisolated from cats 3 years after infection. Additional mutations, dispersed throughout Env, were associated with the appearance of escape mutants that resisted some sera but lacked broad neutralization resistance (3).
In the above studies, circumstantial evidence had suggested that the host's immune response was implicated in in vivo NS→NR reversion of FIV; however, the precise force(s) that guided the event was not explored. Here, well-characterized immune sera were obtained from cats in which NS→NR reversion of TCA FIV had occurred and, for comparison, sera generated by an NR variant of this virus obtained by repassaging it in vivo were studied for the ability to drive changes in the neutralization phenotype of the same TCA virus in tissue culture. V4 and V5 of emerged virus variants were also sequenced.
Characterization of sera used in in vitro immune selections.
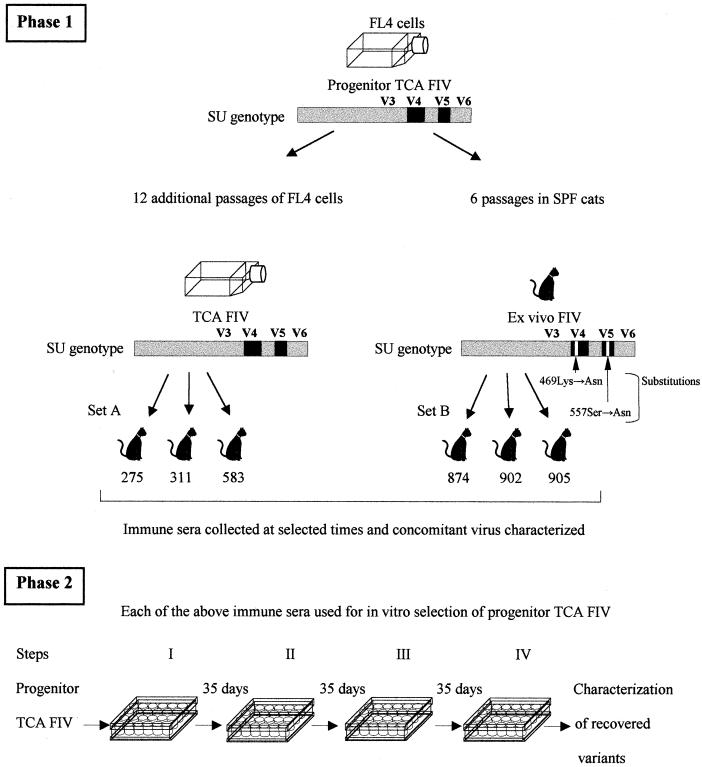
Two sets of immune sera were obtained from specific-pathogen-free female cats (Iffa Credo, L'Arbresle, France) infected intravenously, when 7 to 12 months old, with distinct preparations of the Petaluma strain of FIV (Fig. 1). Four sera (set A) were from cats 275, 311, and 583 described in a previous report (3). These animals had been infected with 1 ml (corresponding to approximately 20 50% cat infectious doses) of supernatant of chronically infected FL4 cells (26; generous gift of Janet K. Yamamoto) on their 193rd passage (TCA FIV) and had an NS phenotype (not shown) and V4 and V5 sequences (Fig. 2) indistinguishable from those of the viral stock designated progenitor TCA FIV which had been harvested 12 passages earlier. Six sera (set B) were from cats infected with a variant of progenitor TCA FIV which, as a consequence of having been readapted to in vivo growth (2 years in cat 3368 of reference 3, followed by five rapid passages in other cats), had already reverted to broad neutralization resistance due to a 557Ser→Asn change that most likely occurred in combination with a 469Lys→Asn change (Fig. 2). In this case, the inoculum consisted of 1 ml of pooled plasma diluted to contain 30 (cats 874 and 905) or 3 (cat 902) 50% cat infectious doses. Individual immune sera also differed with regard to the time of infection they were collected, and this was somewhat though not invariably reflected in the titers of anti-FIV antibodies measured by enzyme-linked immunosorbent assay (ELISA), which ranged between 800 and 3,500 (Table 1). Neutralization tests, performed as previously described (9) against 10 50% tissue culture doses of virus using MBM cells as substrate and quantification of reverse transcriptase (RT) activity as endpoint, showed that all sera neutralized the progenitor TCA FIV at titers varying between 10 and 512, that appeared essentially unrelated to type of inoculum and length of infection (Table 1). Normal cat serum (NCS), pooled from 10 naive animals, was FIV antibody negative in ELISA and neutralization assays. All sera were treated at 56°C for 30 min and checked for the absence of infectious FIV by standard culture.
FIG. 1.
Experimental plan. (Phase 1) While the NS TCA FIV used to infect the donors of set A sera had V4 and V5 amino acid sequences identical to those of progenitor TCA FIV, the NR ex vivo FIV used to infect set B sera donors had a one-amino-acid difference each in V4 and V5. (Phase 2) All the immune sera were independently used for in vitro immune selection of progenitor TCA FIV, and viral variants that emerged were studied for neutralization phenotype and V4 and V5 sequences. SPF, specific pathogen free.
FIG. 2.
Deduced amino acid sequences of the V4 and V5 regions of the SU of FIV variants used to generate sets A and B immune sera and of the viruses reisolated from donor cats at the times the sera were collected. Differences relative to progenitor TCA FIV are shown in capital letters. Numbers on the V regions (14) of progenitor TCA FIV indicate amino acid positions starting with the first methionine of Env, according to the reported sequence of clone 34TF10 of FIV-Petaluma (22). Potential N-linked glycosylation sites are identified by solid bars under the progenitor TCA FIV sequence if common to all variants and by open boxes inside the alignment if present only in some. ∗, conserved cysteines. Progenitor TCA FIV and set A sequences have already been reported (3) but are shown again for the sake of clarity.
TABLE 1.
Sera used in immune selections
| Set | Source of virus used for infection | Source of immune seruma | ELISA titerb | Neutralizing titerc |
|---|---|---|---|---|
| A | FL4 cells | 275-6 | 800 | 35 |
| 275-10 | 3,500 | 64 | ||
| 311-10 | 3,500 | 64 | ||
| 583-20 | 3,500 | 128 | ||
| B | Infected cats | 874-3 | 1,000 | 64 |
| 902-3 | 1,600 | 64 | ||
| 905-3 | 1,200 | 10 | ||
| 874-10 | 1,600 | 64 | ||
| 902-10 | 3,200 | 64 | ||
| 905-10 | 1,600 | 512 |
The figure before the dash identifies the donor cat, and that after the dash is the month postinfection when the serum was obtained.
Titer was determined by ELISA against gradient-purified progenitor TCA FIV (3).
Titers are expressed as the reciprocal of the serum dilution required to reduce by ≥50% the levels of RT activity produced by progenitor TCA FIV in the presence of the corresponding dilution of NCS and were calculated as described by Reed and Müench (19). The experiments were repeated at least twice, with comparable results.
We also sequenced the V4 and V5 regions of the viruses taken from donor cats at the times the immune sera were harvested. The methods used for virus isolation from peripheral blood mononuclear cells, DNA extraction, and env amplification by PCR and sequencing have been previously described (3). Nucleotide sequences were edited and translated by PC/Gene software (IntelliGenetics, Geel, Belgium), potential N-linked glycosylation sites were determined by using PROSITE in the same package, and multiple amino acid alignments were obtained using CLUSTAL W (23). As previously reported (3) and again presented in Fig. 2 for clarity, the viruses reisolated from the donors of the set A sera had an NR phenotype (not shown) and NR genotype (Glu or Asn at position 481), thus showing that such sera had been harvested after input TCA FIV had undergone NS→NR reversion. The viruses reisolated from the donors of set B sera at 3 months of infection had a Ser-for-Asn substitution at position 557, corresponding to an NS phenotype possibly due to improved fitness, and by 10 months had an Asn at position 481, corresponding to an NR phenotype (Fig. 2). Thus, the NR ex vivo FIV used to generate these sera had also evolved in donor cats with regard to neutralization resistance, but the pathways it had followed were at least partially different from the ones followed by the virus that had elicited A sera, possibly due to the different neutralization susceptibilities of the two inocula. Interestingly, an evolution toward increased neutralization sensitivity of the infecting virus reminiscent of the one detected here has also been described for individuals recently infected with HIV (12). The viruses recovered from all cats also showed a few additional amino acid changes in V4 and V5, but based on our previous findings (3), these were considered unimportant for determining broad neutralization resistance.
In vitro immune selections.
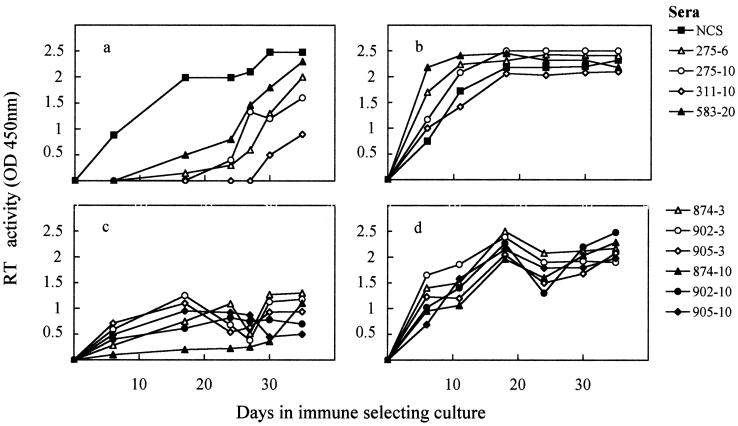
Sets A and B immune sera were studied for the ability to drive changes in the neutralization properties of NS FIV by growing the progenitor TCA FIV in MBM cells in their continuous presence. Virus propagated in the same manner but in the presence of NCS served as a control for possible effects unrelated to antiviral antibodies. One milliliter of progenitor TCA FIV diluted to contain 200 50% tissue culture doses was incubated at 4°C with each serum diluted 1:50 for 1 h, and the mixtures were then inoculated into wells of 24-well flat-bottom plates containing 2 × 106 MBM cells in 1 ml of growth medium. After 24 h, the cells were washed three times and cultured in medium containing 2% of the respective selecting sera. Periodically, the supernatant fluids were harvested, tested for RT activity, and replaced with fresh selecting medium. After 5 weeks, produced viruses were clarified and subjected to three further serial passages exactly as described above. Figure 3 shows the kinetics of virus replication in the first and fourth passages. At the first passage, all the immune sera effectively delayed and/or reduced virus replication compared to NCS, but at the fourth passage, this effect was completely lost, suggestive that all immune selections had led to the emergence of virus variants no longer appreciably susceptible to inhibition by the respective selecting sera.
FIG. 3.
Viral growth curves in the cultures used for immune selection of progenitor TCA FIV. The first (a and c) and last (b and d) passages of selection with A (a and b) and B (c and d) immune sera are shown. Virus growth is expressed as levels of RT activity in the supernatant fluids. OD450, optical density at 450 nm.
Neutralization phenotypes and genotypes of the immune in vitro-selected variants.
Titers of the virus variants that emerged from the in vitro selections described above were determined in MBM cells and then examined for neutralizability by the panel of immune sera (Table 2) exactly as described above. Confirming that the neutralization phenotype of NS FIV is not affected by mere propagation in MBM cells (6), the control virus passaged in the presence of NCS was effectively inhibited by all sera. On the other hand, all the variants passaged in the presence of the immune sera completely or nearly completely resisted neutralization by the respective selecting sera, thus showing that all the selections had indeed been effective. More importantly, the variants selected with the two sets of sera exhibited clearly different sensitivities to inhibition by the other panel sera. The ones selected with set A sera resisted neutralization in nearly all the virus-serum combinations tested, i.e., they exhibited an unequivocal NR phenotype. In contrast, those selected with set B sera were neutralized, often at high titer, by most such sera. Moreover, this checkerboard neutralization study showed that set B sera inhibited considerably fewer virus variants and usually at lower titers than set A sera.
TABLE 2.
Neutralization phenotypes of immune in vitro-selected FIV variants
| Immune serum | Neutralizing antibody titer with virus variants selected in the presence ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCS | Set A sera
|
Set B sera
|
|||||||||
| 275-6 | 275-10 | 311-10 | 583-20 | 874-3 | 902-3 | 905-3 | 874-10 | 902-10 | 905-10 | ||
| Set A | |||||||||||
| 275-6 | 181 | − | − | − | − | >256 | >256 | >256 | 128 | 128 | 128 |
| 275-10 | 181 | − | − | − | − | >256 | >256 | >256 | 256 | 256 | 128 |
| 311-10 | >512 | 45 | 8 | − | − | >128 | >128 | >128 | >128 | >128 | >128 |
| 583-20 | 181 | − | − | − | − | 128 | 128 | 256 | 128 | 128 | 64 |
| Set B | |||||||||||
| 874-3 | 64 | − | − | − | − | − | 8 | 45 | − | 11 | − |
| 902-3 | 64 | − | − | − | − | − | − | 32 | − | − | − |
| 905-3 | 10 | − | − | − | − | − | 8 | − | − | 8 | − |
| 874-10 | 64 | 128 | 8 | − | − | 32 | 512 | >512 | 8 | 11 | 64 |
| 902-10 | 64 | 8 | − | − | − | 45 | 32 | >512 | 13 | − | − |
| 905-10 | 512 | − | − | − | − | 13 | 51 | 13 | − | 32 | 13 |
Titers are expressed as described in Table 1. −, no neutralizing activity detected; mean titers of <8 were not considered significant and were scored as negative; the results of neutralization with the specific immune serum used for selecting each individual variant are in boldface.
In vitro-selected variants were also studied for amino acid changes in V4 and V5 (Fig. 4). All the NR variants selected with set A sera had 481Lys replaced by either Thr (three variants) or Glu (one variant), as well as additional changes of V4 indicative of a preferential pressure exerted by set A sera on this SU region. Although it is possible that other changes contributed to affect the phenotype, the consistency of mutations at residue 481 was remarkable. Instead, none of the FIV variants selected with set B sera had position 481 substituted. Those selected with set B sera harvested 3 months postinfection had completely unmodified V4 and V5, suggesting that escape from the respective selecting sera was due to changes in other Env regions. On the other hand, the variants selected with B sera harvested at 10 months had either the 557Ser→Asn change (two cases) or an adjacent two-amino-acid deletion (one case), thus suggesting that these sera had exerted an intense pressure at or around residue 557 of V5. Two of the latter variants also had a substitution, each at different positions of V4. No amino acid changes were detected in the variants passaged in NCS.
FIG. 4.
Deduced amino acid sequences of the V4 and V5 regions of SU in the FIV variants immune selected in vitro by sets A and B sera. For details, see Fig. 2. The sequences of the control virus propagated as for immune selection but in the presence of NCS were identical to those of progenitor TCA FIV.
Conclusions.
In previous studies (3, 6), the force(s) that had selected in favor of the reversion to broad neutralization resistance which occurred when an NS TCA strain of FIV was readapted to cats had remained undefined. Here, we looked at the issue by exposing the same virus to selection in vitro by four immune sera obtained from three cats in which the NS→NR reversion had recently taken place. This approach had previously proved valuable for identifying the SU substitutions which had rendered a strain of FIV resistant to one immune serum (21) but, at least in FIV, it had never been used to investigate the basis of broad neutralization resistance. The results showed that (i) all four sera selected in favor of mutants which resisted neutralization not only by the specific selecting serum but also by the great majority of the other panel sera, thus behaving as wild-type FIV, (ii) all the NR variants that emerged from the in vitro selections had a substitution at amino acid 481, i.e., at the position previously found to be responsible for NS→NR reversion in the cats that had donated the sera, and (iii) all the in vitro-selected variants had 481Lys replaced by Glu or Thr, that is, by amino acids with identical or with similarly uncharged side groups as the ones that had mediated reversion in vivo (Glu and Asn, respectively). Thus, cats in which a laboratory FIV had recently reverted to wild-type neutralization resistance possessed antibodies capable of driving the same reversion—and with similar structural bases—in the absence of additional or alternative factors that might have played a role in vivo. This clearly points at the host's humoral immune response as the major, and possibly the only, force that had driven NS→NR reversion in the cats that had donated the sera. The observation that in tissue culture the reversion was uniformly complete after 20 weeks whereas in vivo it had generally taken variably longer (3) is most likely attributable to variation in the time needed by individual animals to mount an antibody response capable of driving the reversion. This part of the study hence strongly suggests that antiviral antibodies are the selecting force which sustains generalized neutralization resistance in the FIV strains that circulate in nature.
In parallel, TCA FIV was exposed to in vitro selection by immune sera that had ELISA and neutralizing-antibody titers comparable to the ones discussed above but that had been obtained from cats infected with virus which had already reverted to neutralization resistance in independent animals. This second group of sera effectively led to the emergence of escape mutants resistant to the respective selecting sera and occasional additional sera. Yet, none of the variants they selected possessed a broadly NR phenotype reminiscent of primary FIV isolates and none had V4 and V5 amino acid sequences previously found in NR variants of the same virus. It seems therefore feasible that, although it can be mediated by a single or very few SU amino acid substitutions (3), reversion of TCA FIV to broad neutralization resistance is channeled by a very complex antibody repertoire that might form only when the infecting virus is initially fully vulnerable and needs to undergo extensive changes in order to evolve an Env minimally susceptible to potentially neutralizing antibodies. That the immune sera elicited by NS FIV neutralized a larger spectrum of viral variants than those elicited by NR FIV is in line with this interpretation. We cannot, however, exclude that the different outcomes of the selections also reflected fine differences in the potency of sera. Further studies will be needed to discriminate between these and other possibilities. An improved understanding of the mechanisms controlling neutralization resistance in wild-type lentiviruses may pave the way to new intervention strategies.
Acknowledgments
This work was supported by grants from Ministero della Sanità-Istituto Superiore di Sanità, “Programma per l'AIDS,” and by the Ministero della Università e Ricerca Tecnologica, Rome, Italy. S.G. was the holder of fellowships from ANLAIDS, Rome, Italy.
We are indebted to Janet K. Yamamoto, University of Florida, for the generous gift of FL4 cells.
REFERENCES
- 1.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont T, van Nuenen A, Broersen S, Blattner W A, Lukashov V V, Schuitemaker H. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J Virol. 2001;75:2246–2252. doi: 10.1128/JVI.75.5.2246-2252.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendinelli M, Pistello M, Del Mauro D, Cammarota G, Maggi F, Leonildi A, Giannecchini S, Bergamini C, Matteucci D. During readaptation in vivo, a tissue culture-adapted strain of feline immunodeficiency virus reverts to broad neutralization resistance at different times in individual hosts but through changes at the same position of the surface glycoprotein. J Virol. 2001;75:4584–4593. doi: 10.1128/JVI.75.10.4584-4593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns D P W, Collignon C, Desrosiers R C. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993;67:4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11:S87–S98. [PubMed] [Google Scholar]
- 6.Cammarota G, Matteucci D, Pistello M, Nicoletti E, Giannecchini S, Bendinelli M. Reduced sensitivity to strain-specific neutralization of laboratory-adapted feline immunodeficiency virus after one passage in vivo: association with amino acid substitutions in the V4 region of the surface glycoprotein. AIDS Res Hum Retrovir. 1996;12:173–175. doi: 10.1089/aid.1996.12.173. [DOI] [PubMed] [Google Scholar]
- 7.Cayabyab M, Karlsson G B, Etemad-Moghadam B A, Hofmann W, Steenbeke T, Halloran M, Fanton J W, Axthelm M K, Letvin N L, Sodroski J G. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–984. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Brown A, Harouse J, Luciw P A, Mayer A J. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J Virol. 1999;73:5294–5300. doi: 10.1128/jvi.73.7.5294-5300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Mauro D, Matteucci D, Giannecchini S, Maggi F, Pistello M, Bendinelli M. Autologous and heterologous neutralization analyses of primary feline immunodeficiency virus isolates. J Virol. 1998;72:2199–2207. doi: 10.1128/jvi.72.3.2199-2207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder J H, Phillips T R. Feline immunodeficiency virus as a model for development of molecular approaches to intervention strategies against lentivirus infections. Adv Virus Res. 1995;45:225–247. doi: 10.1016/s0065-3527(08)60062-7. [DOI] [PubMed] [Google Scholar]
- 11.Hosie M J, Dunsford T, Klein D, Willett B J, Cannon C, Osborne R, MacDonald J, Spibey N, Mackay N, Jarrett O, Neil J C. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus. J Virol. 2000;74:9403–9411. doi: 10.1128/jvi.74.20.9403-9411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathey J L, Pratt R D, Spector S A. Appearance of autologous neutralizing antibody correlates with reduction in virus load and phenotype switch during primary infection with human immunodeficiency virus type 1. J Infect Dis. 1997;175:231–232. doi: 10.1093/infdis/175.1.231. [DOI] [PubMed] [Google Scholar]
- 13.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 14.Pancino G, Fossati I, Chappey C, Castelot S, Hurtrel B, Moraillon A, Klatzmann D, Sonigo P. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993;192:659–662. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- 15.Park E J, Gorny M K, Zolla-Pazner S, Quinnan G V., Jr A global neutralization resistance phenotype of human immunodeficiency virus type 1 is determined by distinct mechanisms mediating enhanced infectivity and conformational change of the envelope complex. J Virol. 2000;74:4183–4191. doi: 10.1128/jvi.74.9.4183-4191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13:S137–S162. [PubMed] [Google Scholar]
- 17.Parren P W H I, Wang M, Trkola A, Bilney J M, Purtscher M, Katinger H, Moore J P, Burton D R. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:10270–10274. doi: 10.1128/jvi.72.12.10270-10274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen N C. Feline immunodeficiency virus infection. In: Levy J A, editor. The retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 181–228. [Google Scholar]
- 19.Reed L J, Müench H A. A simple method for estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 20.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;6:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 21.Siebelink K H J, Rimmelzwaan G F, Bosch M L, Meloen R H, Osterhaus A D M E. A single amino acid substitution in hypervariable region 5 of the envelope protein of feline immunodeficiency virus allows escape from virus neutralization. J Virol. 1993;67:2202–2208. doi: 10.1128/jvi.67.4.2202-2208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 25.Wyand M S, Manson K, Montefiori D C, Lifson J D, Johnson R P, Desrosiers R C. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 27.Ye Y, Si Z H, Moore J P, Sodroski J. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J Virol. 2000;74:11955–11962. doi: 10.1128/jvi.74.24.11955-11962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]