Abstract
Objectives:
To investigate the significance of lateral pelvic lymph node dissection (LPLND) in resectable stage IV low rectal cancers, reviewing the treatment outcomes from a single cancer center dedicated to LPLND.
Methods:
Consecutive 56 patients with stage IV low rectal cancers who underwent primary tumor resection (PTR) between 2007 and 2022 were identified. Sixteen patients with non-curative PTR were excluded, and 40 with curative PTR were analyzed.
Results:
The dominant metastatic organ was the liver in 30 (75.0%) patients, followed by the lung in 9 (22.5%). Seven (17.5%) patients had multiple organ metastasis. Five of 40 patients had cT1bN0 or cT2N0 disease, 8 did not receive LPLND for other reasons, and accordingly, 27 (67.5%) finally received LPLND. A total of 15 patients (37.5% of all 40 cases and 55.5% of 27 LPLND cases) had LPLN metastasis. Six (15.0%) patients had bilateral metastasis, and 6 (15.0%) had LD3 metastasis. Eight (20.0%) patients developed local recurrence (LR), and the 5Y-LR rate was 22.3%. Twelve (30.0%) patients underwent preceding chemotherapy before PTR, 26 (65.0%) received chemotherapy after PTR, and 23 (57.5%) achieved complete resection. Twelve (52.2%) of 23 patients developed distant recurrence after complete resection. 5Y-overall survival for all patients was 42.4%.
Conclusions:
A high rate of LPLN metastasis implies the significance of management for LPLN metastasis; meanwhile, an unsatisfactory complete resection rate and overall survival implies that LPLN metastasis in this cohort should be dealt with as a systemic disease.
Keywords: rectal neoplasms, lymph node excision, combined modality therapy
Introduction
Colorectal cancer, the third most common cancer worldwide, currently affects roughly 1.3 million new patients each year globally[1]. Although rectal cancer and colon cancer share many common biological features, the former, which occurs in the narrow pelvis, requires a unique therapeutic approach due to its specific anatomical setting.
The concept of total mesenteric excision (TME) has become broadly accepted in rectal cancer surgery, with a resulting decrease in the rate of local recurrence from 30-50% to <10% over the last decades[2]. However, TME alone cannot sufficiently address the problem occurring in the narrow pelvis. In Western countries, the induction of preoperative chemoradiotherapy (CRT)[2-5], and induction or consolidation chemotherapy around CRT[6-9] improve local control and survival. Meanwhile, Japanese surgeons have taken on the challenge of addressing lateral recurrence with lateral pelvic lymph node dissection (LPLND) to overcome pelvic sidewall recurrence[10-13]. Accordingly, the more advanced tumors are, the more complex treatment management is, including preoperative chemotherapy and/or radiotherapy, LPND, both or neither for locally advanced low rectal cancer.
Furthermore, the treatment management is more complex in the situation of low rectal cancer with resectable distant metastasis. The management principle for resectable advanced rectal cancer includes the radical resection of the visible region and the control for micrometastasis; however, it is impossible to introduce the treatment for local control and distant control at the same time. Thus, we are confronted with a choice to optimize treatment combinations, which enables us to minimize total recurrent risks of low rectal cancer, including the risks for distant, central local, and lateral local recurrence. From this perspective, the actual data for LPLN metastasis in this cohort should be investigated.
We have performed upfront primary tumor resection (PTR) ± metastasectomy of intraabdominal metastasis and two-(three-)stage metastasectomy of the liver and/or lung metastasis for resectable Stage IV low rectal cancers. Also, we have performed LPLND based on the indication as with those without distant metastasis according to the JSCCR guidelines. In the present study, we reviewed the treatment outcomes of our patients to investigate the significance of LPLND in resectable stage IV low rectal cancers.
Methods
Study design
This was a retrospective cohort study using data from a prospectively collected institutional database for patients with colorectal cancer at the Aichi Cancer Center Hospital (ACCH).
Patient identification
Consecutive patients with the American Joint Committee on Cancer (AJCC) stage IV low rectal adenocarcinoma who underwent PTR with curative intent between January 2007 and December 2022 were identified from a prospectively collected database[14]. In the present study, tumors for which the dominant was located below the peritoneal reflection were deemed to be low rectal cancer according to the JSCCR guidelines[15].
Patient characteristics were reviewed and augmented by secondary chart review. Patients were excluded if the patient chart read the palliative intention for their PTR, and only patients who underwent PTR with curative intent were included. Patients were also excluded if they had recurrent rectal cancer, primary colon cancer spreading to the rectum, or those who had undergone urgent surgery without preoperative examination from the present analysis.
Treatment
All rectal cancer patients received a colonoscopy, barium or gastrografin enema, and contrast-enhanced computed tomography (CT) scanning of the chest, abdomen, and pelvis for preoperative staging. Pelvic magnetic resonance imaging (MRI) was also recommended for preoperative staging; however, it was not mandatory during the study period. Liver MRI or positron emission tomography-CT were examined if needed. The primary criteria for diagnosing LPLN metastasis was a short axis of 5 mm or more in CECT or MRI, which ultimately made a general judgment by our radiologist morphologically.
Standard treatment for resectable stage IV low rectal cancer at ACCH was upfront PTR ± metastasectomy of intraabdominal metastasis, e.g., paraaortic lymph node metastasis and peritoneal metastasis. The indication for LPLND was the same as for those without distant metastasis according to the JSCCR guidelines; thus, bilateral LPLND was indicated for all cT3 or deeper tumors. The current JSCCR classification classify the extent of LPLND using the symbol LD0-LD3 as follows; LD0: LPLND is not performed, LD1: LPLND does not satisfy LD2, LD2: Dissection of distal internal iliac, proximal internal iliac, and obturator nodes is performed, LD3: Dissection of all lateral lymph nodes (all distal internal iliac, proximal internal iliac, obturator, external iliac, common iliac, lateral sacral, presacral, and sacral promontory nodes) is performed[16]. In the present study period, LD3 was performed regardless of therapeutic or prophylactic for patients who indicated LPLND.
Liver and/or lung metastasis were considered to perform two-(three-)stage metastasectomy after watchful waiting or induction chemotherapy after PTR. Adjuvant chemotherapy after liver or lung metastasectomy was not mandatory because of the lack of evidence for adjuvant treatment after metastasectomy[17].
Follow-up and survival
After all surgical treatment (and postoperative chemotherapy), patients were recommended to undergo follow-up, included physical examinations, laboratory data including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), and CT scanning of the chest, abdomen, and pelvis every three to four months for the first two years and every six months for the subsequent three years. All follow-up visits, laboratory tests, and radiological examinations were performed at ACCH.
All patients were followed for ten years postoperatively, up to when an event occurred or until October 2023. Overall survival (OS) was calculated as the time to death from any cause, and the cumulative risk for local recurrence (LR) was calculated as the time to LR at any time of their treatment course.
Statistical analysis
Categorical variables were analyzed using Pearson's χ2 test. Continuous variables were presented as medians with ranges and analyzed using the Mann-Whitney's U test. OS and the cumulative risks for LR were estimated by Kaplan-Meier survival analysis. STATA 17.0 (StataCorp LLC, College Station, Texas, US) was used for all statistical analyses.
Ethical approval
The present experimental protocols were approved by the institutional review committee at Aichi Cancer Center Hospital (2020-1-297). Individual patient consent was not required because no identifiable patient data were used.
Results
Patient, surgical, and histopathologic characteristics
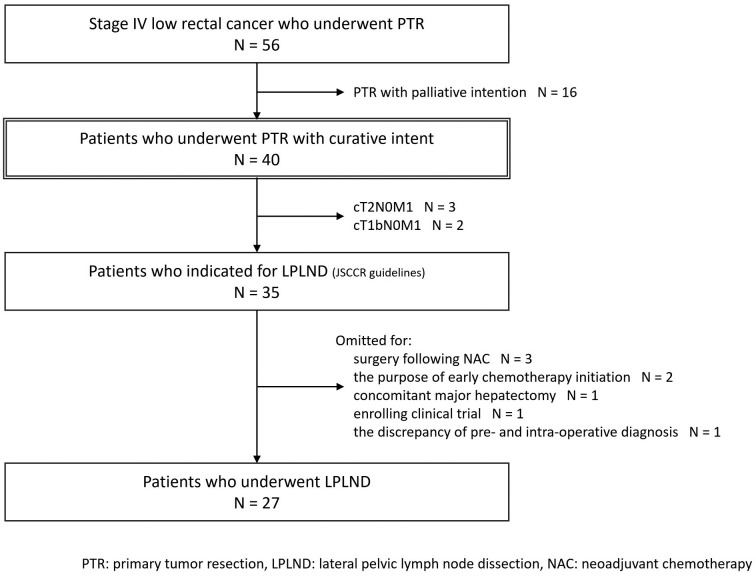
Overall, 56 patients with Stage IV low rectal cancer who underwent PTR at ACCH were identified from a prospectively collected database (Figure 1). Sixteen patients were excluded for PTR with palliative intention, and 40 patients who underwent PTR with curative intent were analyzed in the present study.
Figure 1.
Patient flow diagram.
The clinicocharacteristics of patients are shown in Table 1. Overall, 6 (15.0%) patients had cT4b disease, and 12 (30.0%) patients had cN3 (lateral) disease according to the JSCCR classification, which meant the presence of clinical suspicion of LPLN metastasis. Seven (17.5%) patients had cM1b or cM1c2 disease according to the JSCCR guidelines, which meant the presence of multiorgan metastasis. The dominant metastatic organ was the liver in 30 (75.0%) patients, followed by the lung (22.5%), distant lymph node (15.0%), and peritoneum (7.5%).
Table 1.
Clinicocharacteristics of Patients Who Underwent PTR with Curative Intention (n = 40).
| N = 40 | |||
|---|---|---|---|
| Age, years | 62 | (27-79) | |
| Male gender, n (%) | 31 | (77.5) | |
| BMI, kg/m2 | 22.2 | (15.8-29.1) | |
| cT (JSCCR), n (%) | cT1-4a | 34 | (85.0) |
| cT4b | 6 | (15.0) | |
| invaded to: | urologic | 3 | (7.5) |
| gynecologic | 1 | (2.5) | |
| sacrum | 1 | (2.5) | |
| pelvic side wall | 1 | (2.5) | |
| cN (JSCCR), n (%) | cN0-2 | 28 | (70.0) |
| cN3 (lateral) | 12 | (30.0) | |
| cM (JSCCR), n (%) | cM1a/c1 | 33 | (82.5) |
| cM1b/c2 | 7 | (17.5) | |
| Metastatic organ, n (%) | liver | 30 | (75.0) |
| (duplicate) | lung | 9 | (22.5) |
| distant LN | 6 | (15.0) | |
| peritoneum | 3 | (7.5) | |
| other | 3 | (7.5) | |
Values are median (range).
PTR: primary tumor resection, BMI: body mass index, JSCCR: Japanese Society for Cancer of the Colon and Rectum, LN: lymph node
Surgical details are shown in Table 2. The surgical procedure was low anterior resection, abdominoperineal resection, and total pelvic exenteration in 21 (52.5%), 16 (40.0%), and 3 (7.5%) patients, respectively. The median operating time was 374 min, and the median blood loss was 904 mL. Twenty (50.0%) patients developed any postoperative complications, and the dominant was superficial surgical site infection (SSI) in 5 (12.5%) patients, followed by organ SSI, anastomotic leakage, and ileus.
Table 2.
Surgical Details Patients Who Underwent PTR with Curative Intention (n = 40).
| N = 40 | |||
|---|---|---|---|
| Surgical approach, n (%) | laparoscopy | 4 | (10.0) |
| laparotomy | 36 | (90.0) | |
| Surgical procedure, n (%) | LAR/ISR | 21 | (52.5) |
| APR | 16 | (40.0) | |
| TPE | 3 | (7.5) | |
| Multivisceral resection, n (%) | 6 | (15.0) | |
| LPLND, n (%) | total | 27 | (67.5) |
| laterality: | hemilateral | 2 | (5.0) |
| bilateral | 25 | (62.5) | |
| Operation time, min | 374 | (164-740) | |
| Blood loss, mL | 904 | (0-6100) | |
| Postoperative complication | total | 20 | (50.0) |
| superficial SSI | 5 | (12.5) | |
| organ SSI | 3 | (7.5) | |
| anastomotic leakage | 3 | (7.5) | |
| Ileus | 3 | (7.5) | |
| lymphorrhea | 2 | (5.0) | |
| UTI | 2 | (5.0) | |
| urinary retention | 2 | (5.0) | |
| DVT | 2 | (5.0) | |
| heart failure | 1 | (2.5) | |
Values are median (range).
PTR: primary tumor resection, LPLND: lateral pelvic lymph node dissection, SSI: surgical site infection, UTI: urinary tract infection, DVT: deep vein thrombosis
Histopathologic features are shown in Table 3. Eight (20.0%) had undifferentiated histology, including 1 with pagetoid spread and 1 mixed adenoneuroendocrine carcinoma. Five (12.5%) patients had pT4b disease. Fifteen (37.5%) patients had pN3 disease, which meant the presence of pathologically confirmed LPLN metastasis, and included 6 (15.0%) with bilateral LPLN metastasis and 6 (15.0%) with metastasis spread to LD3. Regarding resection margin, 1 (2.5%) and 6 (15.0%) patients had positive distal and radial margins, respectively.
Table 3.
Histopathologic Features of Patients Who Underwent PTR with Curative Intention (n = 40).
| N = 40 | ||
|---|---|---|
| Histology, n (%) | well-moderate | 32 (80.0) |
| poor-mucinous | 8 (20.0) | |
| poor prognostic histology: | PS | 1 (2.5) |
| MANEC | 1 (2.5) | |
| pT (JSCCR), n (%) | pT1-4a | 35 (87.5) |
| pT4b | 5 (12.5) | |
| invaded to: | urologic | 3 (7.5) |
| gynecologic | 1 (2.5) | |
| pelvic side wall | 1 (2.5) | |
| pN (JSCCR), n (%) | pN0-2 | 25 (62.5) |
| pN3 (lateral) | 15 (37.5) | |
| laterality: | hemilateral | 9 (22.5) |
| bilateral | 6 (15.0) | |
| extent: | localized to LD2 | 9 (22.5) |
| spread to LD3 | 6 (15.0) | |
| DM, n (%) | DM0 | 39 (97.5) |
| DM1 | 1 (2.5) | |
| RM, n (%) | RM0 | 34 (85.0) |
| RM1 | 6 (15.0) |
PTR: primary tumor resection, PS: pagetoid spread, MANEC: mixed adenoneuroendocrine carcinoma, JSCCR: Japanese Society for Cancer of the Colon and Rectum, DM: distal margin, RM: radial margin
Incidence and details of LPLN metastasis in patients who underwent PTR
Five of all 40 patients in the present cohort had cT1bN0 or cT2N0 disease and were not indicated for LPLND. Thus, 35 (87.5%) of 40 patients had diseases that indicated LPLND (Figure 1). Meanwhile, 8 patients did not receive LPLND for other reasons, and 27 (67.5% of all cases and 77.1% of cases indicated for LPLND) patients underwent LPLND with PTR, including 2 hemilateral (1 preoperative decision for concomitant para-aortic lymph node dissection and 1 intraoperative decision for massive bleeding of more than 6,000 mL) and 25 bilateral LPLND (Table 2). Fifteen patients had pathologically confirmed LPLN metastasis in 37.5% of all cases, 42.9% of patients who indicated LPLND, and 55.6% of patients who underwent LPLND, respectively.
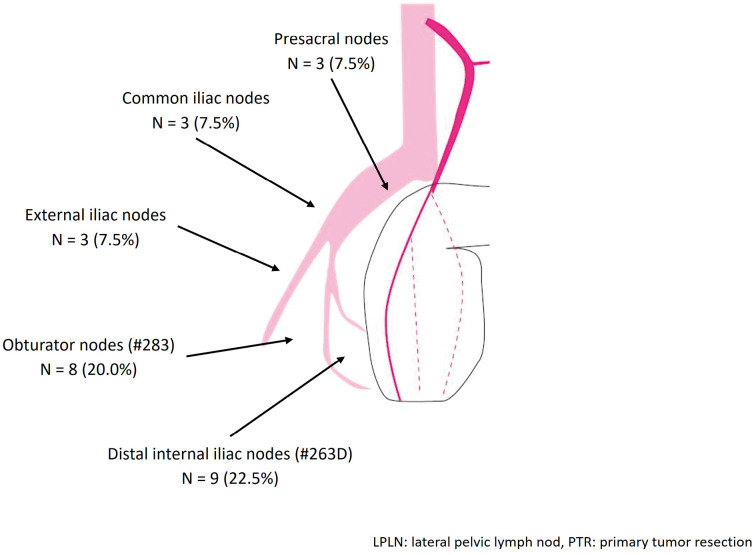
Regarding the location of LPLN metastasis, the most dominant site was distal internal iliac nodes (22.5%), followed by obturator nodes (20.0%), external iliac nodes, common iliac nodes, and presacral nodes (7.5% each) (Figure 2). Six (15.0%) patients had bilateral LPLN metastasis, and 6 (15.0%) had LD3 metastasis, which meant LPLN metastasis beyond distant internal iliac and obturator nodes (Table 2).
Figure 2.
Location of LPLN metastasis in patients who underwent PTR with curative intention (n = 40).
Post-PTR course, local recurrence and survival
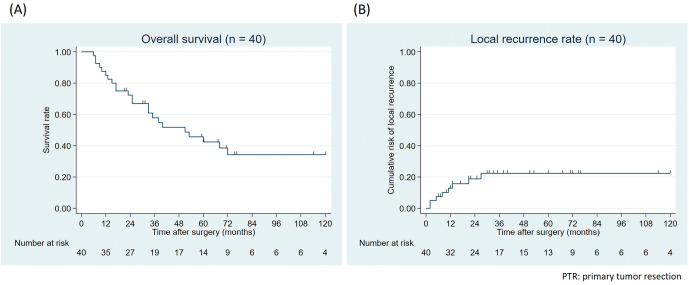
Perioperative treatment and following metastasectomy are shown in Table 4. Twelve (30.0%) patients underwent preceding chemotherapy before PTR, and 26 (65.0%) received chemotherapy after PTR. Metastasectomy was performed for 28 (70.0%) patients, including 8 simultaneous and 20 staged metastasectomy, and completely resected (R0) for 23 (57.5%) patients. Meanwhile, 12 (52.2% of R0 cases) patients developed distant recurrence after PTR and metastasectomy, and 5-year overall survival was 42.4% (Figure 3A).
Table 4.
Perioperative Treatment and Following Metastasectomy in Patients Who Underwent PTR with Curative Intention (n = 40).
| N = 40 | |||
|---|---|---|---|
| Preoperative chemotherapy, n (%) | total | 12 | (30.0) |
| regimen: | triplet+targeted | 1 | (2.5) |
| doublet+targeted | 6 | (15.0) | |
| doublet | 4 | (10.0) | |
| FU mono+targeted | 1 | (2.5) | |
| Post operative chemotherapy, n (%) | total | 26 | (65.0) |
| regimen: | doublet+targeted | 15 | (37.5) |
| doublet | 6 | (15.0) | |
| FU mono+targeted | 1 | (2.5) | |
| FU monotherapy | 3 | (7.5) | |
| unknown | 1 | (2.5) | |
| Metastasectomy, n (%) | total | 28 | (70.0) |
| simultaneous | 8 | (20.0) | |
| procedure (duplicate): | liver | 4 | (10.0) |
| distant LN | 3 | (7.5) | |
| peritoneum | 3 | (7.5) | |
| staged | 20 | (50.0) | |
| procedure (duplicate): | liver | 14 | (35.0) |
| lung | 6 | (15.0) | |
| distant LN | 1 | (2.5) | |
| bone | 1 | (2.5) | |
| not achieved | 12 | (30.0) | |
| Metastasectomy completion rate, n (%) | 23 | (57.5) | |
PTR: primary tumor resection, FU: fluorouracil, LN: lymph node
Figure 3.
(A) Overall survival and (B) local recurrence rate of patients who underwent PTR with curative intention (n = 40).
Eight (20.0%) patients developed LR after PTR. The 5-year cumulative risks for LR was 22.3% (Figure 3B). Details of patients with local recurrence are shown in Table 5. The dominant recurrent site was central pelvis in 5 patients, followed by lateral pelvis in 2 and perineal skin in 1 patient. Of the 2 patients with local recurrence at the lateral pelvis, one had recurrence after only TME, and one had recurrence after bilateral LPLND and recurrence at the left lateral sacral lymph nodes. One of 13 (7.7%) patients without LPLND and 1 of 27 (3.3%) patients with LPLND developed LR in the lateral pelvis.
Table 5.
Details of Patients with Local Recurrence (n = 8) in Patients Who Underwent PTR with Curative Intention (n = 40).
| No. | Age/ gender |
NAC | Procedure | LD | Histology | pT (JSCCR) |
pN (JSCCR) |
cM (JSCCR) |
DM | RM | AC | Time to LR |
Recurrent site |
Details |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56/F | none | LAR+ vagina | LD3 | tub2 | pT3 | pN2 | cM1a (H) | DM0 | RM1 | UFT/UZEL | 11 | central pelvis | vaginal stump |
| 2 | 64/F | none | LAR | LD0 | tub2 | pT3 | pN2 | cM1a (H) | DM0 | RM0 | none | 5 | lateral pelvis | right pelvic wall apart from RM |
| 3 | 74/M | none | APR | LD0 | tub2 | pT2 | pN2 | cM1a (H) | DM0 | RM0 | FOLFOX+ Bmab | 27 | central pelvis | retroperitonium |
| 4 | 50/M | FOLFIRI+ Pmab | APR | LD3 | tub1 | pT3 | pN1 | cM1a (H) | DM0 | RM0 | none | 8 | central pelvis | pelvic floor apart from RM |
| 5 | 59/F | FOLFOX+ Bmab | ISR+ PALN | LD3 | tub2 | pT3 | pN3 | cM1b (PUL, LN) | DM0 | RM0 | none | 2 | central pelvis | pelvic floor apart from RM |
| 6 | 77/F | none | APR+#292 | LD3 | por (PS) | pT3 | pN3 | cM1b (H, LN) | DM1 | RM1 | FOLFOX+ Pmab | 13 | perineal skin | perineal skin |
| 7 | 30/M | FOLFOX+ Pmab | LAR | LD3 | tub2 | pT3 | pN1 | cM1b (H, PUL) | DM0 | RM0 | none | 21 | lateral pelvis | left lateral sacral LN |
| 8 | 50/F | FOLFOX+ Pmab | APR+ uterus | LD0 | tub2 | pT4b (vagina) | pN2 | cM1c2 (H, P, ovary) | DM0 | RM1 | FOLFOX+ Pmab | 2 | central pelvis | retroperitonium |
PTR: primary tumor resection, (N) AC: (neo) adjuvant chemotherapy, LD: lateral dissection, PS: pagetoid spread, JSCCR: Japanese Society for Cancer of the Colon and Rectum, DM: distal margin, RM: radial margin, LR: local recurrence, LN: lymph node
Discussion
In this retrospective cohort study, we showed the actual data for LPLN metastasis in resectable stage IV low rectal cancers by reviewing the treatment outcomes from the single cancer center dedicated to LPLND and extended surgery. Overall, 67.5% of patients underwent LPLND with PTR, 15 had pathologically confirmed LPLN metastasis, 42.9% of patients who indicated LPLND, and 55.6% of patients who underwent LPLND. 57.5% of patients achieved complete resection with simultaneous or staged metastasectomy, and 5-year overall survival was 42.4%. Eight patients developed LR with a 5-year cumulative risk for LR of 22.3% after PTR with our aggressive LPLND strategy, and the dominant recurrent site was the central pelvis in 5 patients, followed by the lateral pelvis in 2 patients.
There are two major concerns with treating low rectal cancer: LR and distant metastasis. LR emerges in two types: lateral recurrence due to LPLN metastasis and central recurrence due to microscopic residual disease in the circumferential resection margin (CRM). The treatment management is more complex amid the resectable distant metastasis[2,4,9,10,12]. NCCN guidelines recommend PTR and simultaneous or staged metastasectomy after initiating any TNT as with locally advanced rectal cancer[18]. Meanwhile, the JSCCR guidelines are skeptical of additional neoadjuvant treatment before PTR and metastasectomy[16]. In addition, there are no clear-cut recommendations regarding LPLN management in any guidelines, including the above. Therefore, no standard management, including the management for LPLN, is established for treating low rectal cancer in this setting.
The high rates of LPLN metastasis, 37.5% of all cases and 55.5% of LPLND cases, were observed in this study based on the indication of LPLND following the JSCCR guidelines. In addition, the lateral LR was observed only in 2 (5.0%) patients, which is relatively low in tumors of such an advanced stage. Tamura et al. also reported a high rate of LPLN metastasis for Stage IV low rectal cancers, even based on selective LPLND with the physician's choice[19]. These results imply the significance of managing LPLN metastasis even in low rectal cancer with resectable distant metastasis. On the other hand, the complete resection rate for distant metastasis remained at 57.5%, and 52.2% of patients developed distant recurrence after PTR and metastasectomy with a 5-year overall survival of 42.4%. Moreover, high rates of widespread lymph node metastasis, 15.0% with bilateral metastasis and 15.0% with LD3 metastasis, were observed in this study. These results also imply that LPLN metastasis in this cohort should be dealt with as a systemically metastasized disease rather than oligometastatic. In that case, when systemic treatment was prioritized in their disease management, the omission of LPLND, which is more invasive and cause delayed initiation of systemic treatment, might be justified for these patients.
The present study has several limitations, some of which are inherently related to its retrospective design with a small number of patients. However, the present study included patients who underwent standardized treatment and follow-up in a single dedicated cancer center. Second, even based on the indication of LPLND following the JSCCR guidelines, 20.0% of patients did not receive LPLND for reasons other than oncologic ones. However, the data used were real-world data directly obtained from our clinical practice. Third, this was a retrospective study, and we could not quantitatively evaluate patients' postoperative urinary and sexual dysfunction from the patient chart. However, even only in a short-term evaluation during admission, the incidence of postoperative urinary retention was low. Fourth, the present results were obtained from a highly experienced, high-volume cancer center that routinely performs LPLND and thus might not be generalizable to all practice settings.
In conclusion, a high rate of LPLN metastasis and low rate of lateral LR was observed in resectable stage IV low rectal cancers, which implies the significance of management for LPLN metastasis even in Stage IV disease. Meanwhile, a high bilateral or LD3 metastasis rate, unsatisfactory complete resection rate, distant recurrence rate, and overall survival implies that LPLN metastasis in this cohort should be dealt with as a systemically metastasized disease rather than oligometastatic, which requires establishing a tailor-made strategy to minimize total recurrent risks, including distant, CME, and beyond TME recurrence.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
AO and CM were responsible for the study concept. However, all authors contributed to the study design. AO, KK, TK, and YuS created the prospectively collected database, and AO and CM collected, assembled, and analyzed the data. AO wrote the initial draft, and all authors approved the final draft of the manuscript.
Approval by Institutional Review Board (IRB)
The present experimental protocols were approved by the institutional review committee at Aichi Cancer Center Hospital (2020-1-297). Individual patient consent was not required because no identifiable patient data were used.
Acknowledgements
We thank Grammarly Inc. (CA, US) and ProEdit Japan Inc. (Kyoto, Japan) for proofreading our draft.
References
- 1.The International Agency for Research on Cancer. World Cancer Report: Cancer Research for Cancer Prevention. Wild CP, Weiderpass E, Stewart BW, editors. 2020. [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001 Aug; 345(9): 638-46. [DOI] [PubMed] [Google Scholar]
- 3.Swedish Rectal Cancer T, Cedermark B, Dahlberg M, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997 Apr; 336(14): 980-7. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004 Oct; 351(17): 1731-40. [DOI] [PubMed] [Google Scholar]
- 5.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009 Mar; 373(9666): 811-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cisel B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019 Aug; 30(8): 1298-303. [DOI] [PubMed] [Google Scholar]
- 7.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Jan; 22(1): 29-42. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Tang Y, Hu C, et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol. 2022 May; 40(15): 1681-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bascoul-Mollevi C, Gourgou S, Borg C, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER PRODIGE 23): Health-related quality of life longitudinal analysis. Eur J Cancer. 2023 Jun; 186: 151-65. [DOI] [PubMed] [Google Scholar]
- 10.Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017 Aug; 266(2): 201-7. [DOI] [PubMed] [Google Scholar]
- 11.Komori K, Fujita S, Mizusawa J, et al. Predictive factors of pathological lateral pelvic lymph node metastasis in patients without clinical lateral pelvic lymph node metastasis (clinical stage II/III): The analysis of data from the clinical trial (JCOG0212). Eur J Surg Oncol. 2019 Mar; 45(3): 336-40. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi A, Komori K, Kinoshita T, et al. Possibilities for and limits of upfront surgical strategy with lateral pelvic node dissection for low rectal cancer. Jpn J Clin Oncol. 2021 Apr; 51(5): 713-21. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi A, Komori K, Kinoshita T, et al. Clinical Relevance of Lateral Pelvic Lymph Node Dissection for Enlarged Lateral Nodes in Locally Advanced Low Rectal Cancer without Preoperative Treatment. J Anus Rectum Colon. 2023 Apr; 7(2): 126-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin MB, Edge, S., Greene, F. et al. AJCC Cancer Staging Manual 8th edition. NY, USA: Springer-Verlag; 2017. [Google Scholar]
- 15.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol Jan. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japanese Classification of Colorectal, Appendiceal, and Anal carcinoma, Third English Edition. Rectum tJSfCotCa, editor. Japan: Kanehara-& Co., Ltd.; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanemitsu Y, Shimizu Y, Mizusawa J, et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J Clin Oncol. 2021 Dec; 39(34): 3789-99. [DOI] [PubMed] [Google Scholar]
- 18.NCCN Guidelines for Treatment of Cancer by Site: Rectal Cancer the United States 2023 [cited 2024 Mar 31]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 19.Tamura H, Shimada Y, Kameyama H, et al. Prophylactic lateral pelvic lymph node dissection in stage IV low rectal cancer. World J Clin Oncol. 2017 Oct; 8(5): 412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]