Abstract
Background and Aims
Over the past decades, the number of cardiac patients (e.g., with advanced heart failure or existing cardiovascular comorbidities that expose them to a heightened risk of acute cardiovascular decompensation) requiring noncardiac surgery is rising. For this patient population, potentially curative surgical treatments may be denied due to their prohibitive perioperative risk. Around 30% of patients undergoing general thoracic surgery experience cardiovascular complications of varying severity that may ultimately result in refractory heart failure and/or hemodynamic instability. In both these scenarios, perioperative implantation of temporary mechanical circulatory support (tMCS) may improve patient outcomes by both expanding preoperative surgical eligibility criteria and enabling safer management of unexpected periprocedural complications. This scoping review seeks to summarize the current existing evidence on the role of tMCS for cardiac assistance in thoracic surgery and provide a thorough overview.
Methods
We will perform a scoping review adhering to the Joanna Briggs Institute (JBI) methodology and the extension for Scoping Reviews of the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis checklist (PRISMA). We will carry out a comprehensive search of several online databases to identify studies on the perioperative implantation of tMCS in patients undergoing thoracic surgery to provide cardiac assistance either due to their heightened preoperative cardiac risk (pre‐emptive tMCS) or for acute cardiac failure due to inherent surgical complications (bail‐out tMCS). Standardized forms will be employed to perform data charting and extraction.
Results
Retrieved studies will be presented through a narrative synthesis following initial categorization, supplemented by descriptive statistical analyses of quantitative data if adequate inter‐study homogeneity is observed and further complemented by figures and tables.
Conclusion
The planned scoping review aims to assess the safety and feasibility of perioperative implantation of tMCS in patients undergoing thoracic surgery either to mitigate their heightened cardiovascular risk or as a rescue strategy in the event of life‐threatening surgical complications. It will identify knowledge gaps, offer direction for future research, and improve clinical practices within the field.
Keywords: cardiac assistance, cardiac failure, perioperative anesthesia management, temporary mechanical circulatory support, thoracic surgery
1. Introduction
1.1. Background
With increased life expectancy and advances in disease management, the number of cardiac patients (e.g., with advanced heart failure or existing cardiovascular comorbidities that expose them to a heightened risk of acute cardiovascular decompensation) requiring noncardiac surgery is rising [1, 2]. As a result, many patients with advanced heart failure or existing cardiovascular comorbidities are denied potentially curative surgical treatments due to their prohibitive perioperative risk [3]. For this patient population, perioperative implantation of temporary mechanical circulatory support (tMCS) is a compelling option and has already been associated with improved patient outcomes in several surgical settings [4, 5]. The rationale underlying pre‐emptive tMCS implantation relies upon its ability to support cardiac function by reducing cardiac workload and myocardial oxygen demand while maintaining end‐organ perfusion during the critical surgical phase [6]. However, to date, the use of tMCS in thoracic surgery has been limited to managing refractory respiratory failure (i.e., with veno‐venous extracorporeal membrane oxygenation [VV‐ECMO]) or in lung transplantation surgery [7]. Beyond these scenarios, the clinical utilization of tMCS in this surgical setting is limited, fragmented, and primarily derived from experiences in other noncardiac surgical settings [8, 9, 10].
Nevertheless, a multicenter study found that 1.5% of patients undergoing video‐assisted thoracic surgery (VATS) anatomical lung resections experienced major intraoperative complications, while 30% of patients undergoing general thoracic surgery faced cardiovascular complications of varying severity, contributing to increased morbidity and mortality, prolonged hospital stay, and higher overall healthcare costs [11, 12, 13]. These findings further highlight the underutilization of periprocedural tMCS in thoracic surgery and emphasize the need for improvement.
Therefore, conducting a comprehensive scoping review is imperative to summarize all published evidence on the use of tMCS in thoracic surgery, whether as a bail‐out intervention for managing unexpected perioperative hemodynamic instability or as a pre‐emptive measure for patients with prohibitive cardiovascular risk.
In these contexts, tMCS may theoretically improve patients' short‐ and long‐term outcomes by expanding preoperative surgical eligibility criteria to include those with heightened cardiac risk who would otherwise be excluded from surgery. Additionally, it allows for safer management of unexpected perioperative complications that may lead to hemodynamic instability and refractory cardiac failure [6, 14].
Such a review has the potential to enhance the utilization of tMCS and reshape perioperative strategies for patients scheduled to undergo thoracic surgery procedures.
1.2. Study Objective
We will conduct a systematically structured scoping review of the published literature—with the results planned for submission to a peer‐reviewed and indexed journal by November 30, 2024—aiming to summarize the available evidence regarding the perioperative implantation of tMCS in thoracic surgery patients, emphasizing its role in enhancing patients' prognosis and outcomes.
We aim to elucidate how accurate preoperative planning—encompassing the potential use of tMCS—may mitigate the heightened cardiovascular risk in patients with existing marginal cardiovascular reserves, potentially facilitating expedited surgery.
Additionally, we will investigate how the implementation of tMCS as a bail‐out strategy offers a viable option for rescuing patients in the event of life‐threatening surgical complications leading to refractory cardiac failure.
2. Materials and Methods
The JBI (formerly known as the “Joanna Briggs Institute”—a global research organization promoting and supporting evidence‐based decisions that improve health and health service delivery by working with universities and hospitals internationally to integrate evidence‐based healthcare within a broader theory‐informed model) and the Preferred Reporting Items for Systematic Review and Meta‐Analyses Protocols (PRISMA‐P) guidelines for Scoping Reviews were employed to formulate this research protocol [15, 16]. The subsequent review process will adhere to the approach outlined in the PRISMA extension for Scoping Reviews (PRISMA‐ScR) checklist [17].
2.1. Registration
In accordance with the guidelines, this scoping review protocol will be registered in the journal Health Science Reports, accessible through https://onlinelibrary.wiley.com/journal/23988835.
2.2. Eligibility Criteria
Under the assumption that tMCS could theoretically improve both short‐ and long‐term outcomes by broadening preoperative surgical eligibility to include high‐risk cardiac patients who would otherwise be excluded, and by enabling safer management of unexpected perioperative complications (e.g., hemodynamic instability and refractory cardiac failure), we will conduct a systematically structured scoping review of the published literature to summarize the available evidence on the perioperative implantation of tMCS in thoracic surgery patients. Our review will focus on its role in improving patient prognosis and outcomes, thus highlighting how accurate preoperative planning—encompassing the potential use of tMCS—may mitigate the elevated cardiovascular risk in patients with limited cardiovascular reserves, potentially facilitating expedited surgery.
Study selection will be performed in accordance with the inclusion and exclusion criteria outlined below.
2.2.1. Inclusion Criteria
Study designs: All types of study designs will be considered.
Population: Only studies involving adult patients undergoing elective or emergent thoracic surgery procedures under general anesthesia or sedation will be eligible.
Interventions: Inclusion will be limited to studies encompassing the perioperative (pre‐, intra‐, or post‐procedural) implantation of tMCS, either as single devices or in combination, due to anticipated heightened cardiac risk or periprocedural occurrence of acute cardiac failure in patients to ensure a safe and uneventful surgical course.
Language: No language restrictions will be applied to the search process. Papers in languages other than English will be retrieved only if clear, adequate, and unambiguous translations can be provided using free online translation tools (e.g., Google Translate, DeepL Translator).
Territories: No geographical restrictions will be applied during the selection of studies.
2.2.2. Exclusion Criteria
Studies concerning adult patients either undergoing lung transplantation or who necessitate perioperative tMCS implantation for refractory respiratory failure, the pediatric population (< 18 years old), pregnant women, preclinical animal models alongside publications not presenting original data (including systematic or narrative reviews, meta‐analyses, commentaries, conference abstracts which have not reached the full publication status, and editorials) will be excluded from this review. No additional limitations on the study design will be applied.
2.3. Information Sources
A systematic search of the following databases will be conducted: PubMed/MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, Scopus, and Google Scholar.
2.4. Search Strategy
The search strategy will encompass search terms that accurately describe the following core research concepts.
Concept 1: tMCS devices.
Concept 2: Thoracic surgery procedures, with the exclusion of lung transplantation.
Concept 3: Adult population.
Given the variability in how some of the research concepts are addressed in the published literature, synonym counterparts for each of the search terms will be included in the search string as well.
Additionally, during the review process, the search terms may be updated to ensure the retrieval of all relevant papers. Any changes made will be clearly outlined in the final paper, along with explanations of why and how they impacted our search.
Boolean operators “OR,” “AND,“ and “NOT” will be used to combine keywords and free terms and enhance search precision.
Database search will be carried out by two experienced and independent investigators (V.T.A. and S.B.) aiming to identify studies pertinent to the research question. An additional final search update will be conducted before submitting the review draft to incorporate any additional relevant information.
2.5. Study Selection Process
Removal of duplicate publications among the literature search findings will be performed using EndNote X9 (Clarivate Analytics) and the resultant citations will then be uploaded to Rayyan for subsequent screening [18]. Rayyan streamlines the process of sorting through studies by providing an intuitive interface that supports the identification, inclusion, and exclusion of articles based on predefined criteria. It simplifies the screening and selection process in systematically structured reviews, particularly, for researchers managing large volumes of scientific literature, while also promoting collaboration and reducing bias in study selection.
Backward and forward snowballing techniques will be employed to scrutinize the references of selected articles, as appropriate.
Every retrieved reference identified through the database search and literature review will undergo an independent assessment by two experienced investigators (V.T.A. and S.B.), at both title and abstract levels.
In cases where concerns or disagreements arose, full‐text articles will be retrieved and consulted, and any disagreements will be resolved through discussion ultimately involving a third, senior investigator (J.D.U.).
The PRISMA flowchart will be used to visually document and summarize the study selection process, detailing the number of records identified, screened, excluded, and included in the final review (Figure 1).
Figure 1.
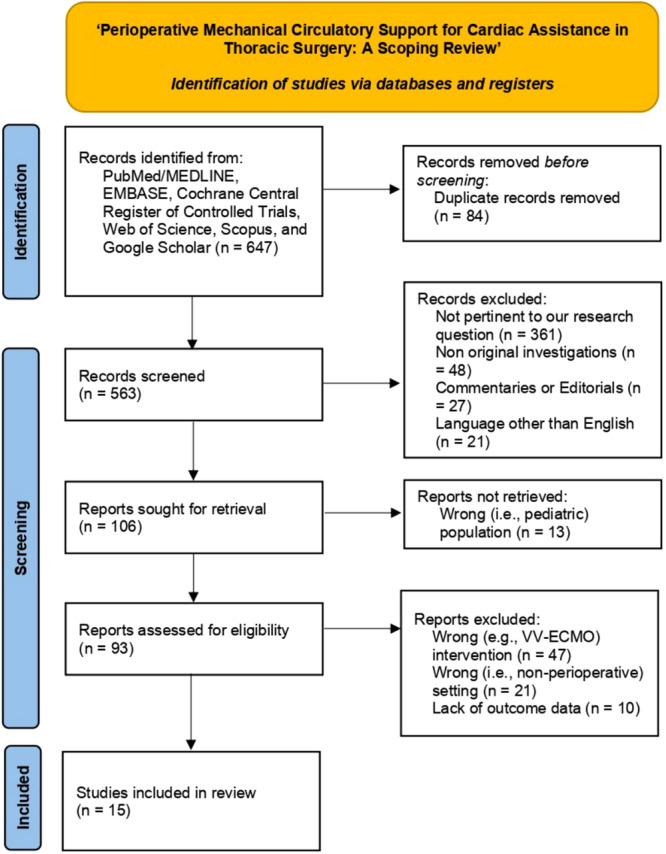
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) 2020 flow diagram for new reviews which included systematic searches of databases and registers only.
2.6. Data Extraction
Standardized forms for data charting and extraction will be employed (Tables 1 and 2). The data charting form may be subject to further reassessment and refinement throughout the review process, with any modifications explained in the final review.
Table 1.
Data extraction: Pre‐emptive tMCS.
| Characteristics of the included studies |
| First author |
| Publication year |
| Study design |
| Number of patients involved |
| Demographic data |
| Age |
| Sex |
| Preoperative concomitant cardiac diasease |
| Preoperative EF |
| Perioperative data |
| Main indication for surgery and surgical procedure performed |
| Surgical approach |
| Intraoperative need for inotropes and vasopressors (with type and dosage, if appropriate) |
| Pre‐emptive tMCS implantation |
| Reason for tMCS implantation |
| Type(s) of tMCS |
| Total duration of tMCS support |
| Outcomes |
| Length of ICU stay (if ICU admission occurred) |
| Length of in‐hospital stay |
| Patient status at latest follow‐up available |
Abbreviations: EF, ejection fraction; ICU, intensive care unit; tMCS, temporary mechanical circulatory support.
Table 2.
Data extraction: bail‐out tMCS.
| Characteristics of the included studies |
| First author |
| Publication year |
| Study design |
| Number of patients involved |
| Demographic data |
| Age |
| Sex |
| Perioperative data |
| Main indication for surgery and surgical procedure performed |
| Surgical approach |
| Intraoperative need for inotropes and vasopressors (with type and dosage, if appropriate) |
| Bail‐out tMCS implantation |
| Reason for tMCS implantation |
| Type(s) of tMCS |
| Total duration of tMCS support |
| Outcomes |
| Length of ICU stay (if ICU admission occurred) |
| Length of in‐hospital stay |
| Patient status at latest follow‐up available |
Abbreviations: EF, ejection fraction; ICU, intensive care unit; tMCS, temporary mechanical circulatory support.
Data from retrieved studies will be manually extracted by two independent investigators (V.T.A. and S.B.) and will further undergo thorough discussion and independent verification by an additional investigator (J.D.U). In cases where data are absent from a study, the corresponding author will be contacted via email for clarification.
A fourth senior investigator (F.M.) will be consulted in instances of discordance to achieve resolution through mutual discussion.
To prevent the duplication of multiple reports on a single study, we will follow the criteria for comparing reports as recommended by Lefebvre et al. in the Cochrane Handbook [19].
2.7. Assessment of the Risk of Bias
The present scoping review will aim to systematically identify, present, and describe the relevant characteristics of the included sources of evidence, rather than combine statistical or qualitative data for synthesized results. As a result, a formal bias assessment of methodological quality or risk of bias of included studies—typically crucial when reporting effect size estimates—employing the Risk Of Bias In Nonrandomised Studies—of Interventions (ROBINS‐I) tool for observational studies (accessible online at: https://www.riskofbias.info) does not constitute a mandatory step and may not occur [20].
However, a comprehensive narrative assessment of the overall quality of the collective evidence will be conducted as outlined within Section 3 of our investigation.
2.8. Data Synthesis
Data will be presented in a narrative fashion to elucidate its relevance with respect to the research objectives of the review itself.
Retrieved articles will be initially categorized into two main groups based on the timeline and rationale for tMCS use as reported by study authors:
“Pre‐emptive tMCS”: This refers to the preplanned implantation of tMCS before the actual start of the surgical procedure;
“Bail‐out tMCS”: This involves the implantation of tMCS in emergency conditions or as a rescue therapy for periprocedural occurrence of refractory cardiac failure.
When applicable and if sufficiently homogeneous, quantitative data will be succinctly summarized utilizing descriptive statistics and visually represented through tables and figures. Results from individual studies will be presented, typically encompassing predictive performance for predefined outcomes. Furthermore, the patient population characteristics will present categorical variables in terms of frequency and percentages, and continuous variables as either means with standard deviation (SD) or median and interquartile range (IQR) based on their distribution.
Nevertheless, in the event that the retrieved papers exhibit heterogeneous data, formal data synthesis or analyses will not be conducted.
2.9. Ethical Considerations and Dissemination
Given the nature of the present investigation, ethical approval is not required, and neither is the consent for publication. Study data information will be made available from the corresponding author upon reasonable request.
We plan to submit the results of the present scoping review for consideration and publication to an indexed and peer‐reviewed journal within November 30, 2024.
3. Discussion
This will be the first systematically structured scoping review seeking to provide a comprehensive overview of the existing published literature concerning the role of perioperative tMCS in supporting cardiac function during thoracic surgery procedures (e.g., video‐assisted thoracoscopic surgery or thoracotomic elective lung resection, pneumonectomy, or emergent thoracic surgery due to trauma), shedding light on knowledge gaps and unanswered research questions.
Our investigation endeavors to summarize all pertinent studies, with particular attention to evaluating patient prognosis and outcomes when tMCS is utilized either as a standalone device or in combination. This is particularly relevant in scenarios involving anticipated patient heightened cardiac risk or unforeseen periprocedural acute cardiac failure, with the overarching goal of ensuring a safe and successful surgical trajectory.
By integrating both quantitative and qualitative data, our primary aim is to refine and potentially broaden existing eligibility criteria for surgical treatment. Through the discourse of our findings, we envision the potential to expand access to a tMCS‐“protected” upfront approach to thoracic surgery, potentially benefitting a larger cohort of patients.
While previous studies in other surgical domains have underscored numerous advantages of tMCS implantation [8, 9, 10], the current literature within the realm of thoracic surgery remains relatively sparse, accentuating the imperative need for a comprehensive review of this topic.
3.1. Strengths and Limitations
Strengths of the proposed scoping review include a predefined protocol, a clear and definite research question, a broad and systematic search of multiple databases relying upon a solid search strategy designed to capture a broad spectrum of literature thereby providing a robust overview of the evidence available on the topic, adherence to JBI methodological guidance and PRISMA checklists, and availability of standardized forms to aid systematic data extraction [15, 16, 17].
However, it is worth noting potential limitations of the proposed investigation, namely the absence of a formal risk of bias assessment for the included studies or the potential retrieval of highly heterogeneous studies, which may impede formal quantitative synthesis. Nonetheless, it is important to acknowledge that the primary objective of a scoping review is to systematically identify and map all available evidence. This aspect still makes this methodology suitable for conducting a comprehensive examination.
4. Conclusion
The perioperative implantation of tMCS, whether as single devices or in combination, could potentially offer a safe and feasible option for patients undergoing thoracic surgery, particularly, those facing heightened preoperative cardiac risk or the possibility of acute cardiac failure due to inherent surgical complications.
By systematically evaluating the breadth of existing research, this scoping review aims to map the current body of evidence, dissect knowledge gaps, and offer valuable insights to help shape future research endeavors.
Author Contributions
Viviana Teresa Agosta: investigation and writing–original draft. Jacopo D'Andria Ursoleo: conceptualization, supervision, investigation, writing–original draft and writing‐review and editing. Samuele Bugo: investigation and writing–original draft. Alice Bottussi: investigation and writing–original draft. Rosario Losiggio: investigation and writing–original draft. Fabrizio Monaco: conceptualization, supervision, and writing–review and editing. All authors of the present work adhere to the authorship guidelines delineated by the International Committee of Medical Journal Editors. All authors have read and approved the final version of the manuscript. Fabrizio Monaco had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Fabrizio Monaco. Fabrizio Monaco affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
The authors have nothing to report. The authors received no specific funding for this work. Open access funding provided by BIBLIOSAN.
Data Availability Statement
Data sharing is not pertinent to this article, as this study will not involve the processing of new data.
REFERENCES
- 1. Burgio G., Martucci G., Panarello G., et al., “Intra‐Aortic Balloon Counterpulsation in High‐Risk Cardiac Patients Undergoing Noncardiac Surgery: A Case Series,” Journal of Cardiothoracic and Vascular Anesthesia 30 (2016): 428–431. [DOI] [PubMed] [Google Scholar]
- 2. Kristensen S. D., Knuuti J., Saraste A., et al., “2014 ESC/ESA Guidelines on Non‐Cardiac Surgery: Cardiovascular Assessment and Management: The Joint Task Force on Non‐Cardiac Surgery: Cardiovascular Assessment and Management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA),” European Journal of Anaesthesiology 31 (2014): 517–573. [DOI] [PubMed] [Google Scholar]
- 3. Novellis P., Monaco F., Landoni G., et al., “Venoarterial Extracorporeal Membrane Oxygenation Support in Lung Cancer Resection,” Annals of Thoracic Surgery 113 (2022): e191–e193. [DOI] [PubMed] [Google Scholar]
- 4. Monaco F., Belletti A., Bove T., Landoni G., and Zangrillo A., “Extracorporeal Membrane Oxygenation: Beyond Cardiac Surgery and Intensive Care Unit: Unconventional Uses and Future Perspectives,” Journal of Cardiothoracic and Vascular Anesthesia 32 (2018): 1955–1970. [DOI] [PubMed] [Google Scholar]
- 5. Monaco F., Ajello S., Calabrò M. G., et al., “Left Ventricular Unloading With an IABP in Patients Undergoing Ventricular Tachycardia Ablation With ECMO Support,” Journal of Cardiothoracic and Vascular Anesthesia 35 (2021): 2686–2693. [DOI] [PubMed] [Google Scholar]
- 6. Rihal C. S., Naidu S. S., Givertz M. M., et al., “2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervention; Affirmation of Value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention,” Journal of the American College of Cardiology 65 (2015): 7. [DOI] [PubMed] [Google Scholar]
- 7. Silva J. S., Cabral D., Calvinho P. A., Olland A., and Falcoz P.‐E., “Extracorporeal Life Support use in Limited Lung Function: A Narrative Review,” Journal of Thoracic Disease 15 (2023): 5239–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siu S. C., Kowalchuk G. J., Welty F. K., Benotti P. N., and Lewis S. M., “Intra‐Aortic Balloon Counterpulsation Support in the High‐Risk Cardiac Patient Undergoing Urgent Noncardiac Surgery,” Chest 99 (1991): 1342–1345. [DOI] [PubMed] [Google Scholar]
- 9. Pinelli F., Romagnoli S., Bevilacqua S., and Macchiarini P., “Extracorporeal Membrane Oxygenation‐Assisted Esophagectomy,” Journal of Cardiothoracic and Vascular Anesthesia 29 (2015): 436–438. [DOI] [PubMed] [Google Scholar]
- 10. Jennings A., Norman A., Whitelock D., Haynes S., and James G., “Elective Peri‐Operative Intra‐Aortic Balloon Counterpulsation During Maxillofacial Free Flap Reconstructive Surgery in a Patient With Severe Cardiomyopathy,” Anaesthesia 65 (2010): 204–206. [DOI] [PubMed] [Google Scholar]
- 11. Decaluwe H., Petersen R. H., Hansen H., et al., “Major Intraoperative Complications During Video‐Assisted Thoracoscopic Anatomical Lung Resections: An Intention‐to‐Treat Analysis,” European Journal of Cardio‐Thoracic Surgery 48 (2015): 588–599 [DOI] [PubMed] [Google Scholar]; discussion: 599.
- 12. Keshava H. B. and Boffa D. J., “Cardiovascular Complications Following Thoracic Surgery,” Thoracic Surgery Clinics 25 (2015): 371–392. [DOI] [PubMed] [Google Scholar]
- 13. Fleisher L. A., Fleischmann K. E., Auerbach A. D., et al., “2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines,” Journal of the American College of Cardiology 64 (2014): e77–e137. [DOI] [PubMed] [Google Scholar]
- 14. Miller M. G. and Hall S. V., “Intra‐Aortic Balloon Counterpulsation in a High‐Risk Cardiac Patient Undergoing Emergency Gastrectomy,” Anesthesiology 42 (1975): 103–105. [DOI] [PubMed] [Google Scholar]
- 15. Peters M. D. J., Marnie C., Tricco A. C., et al., “Updated Methodological Guidance for the Conduct of Scoping Reviews,” JBI Evidence Synthesis 18 (2020): 2119–2126. [DOI] [PubMed] [Google Scholar]
- 16. Moher D., Shamseer L., Clarke M., et al., “Preferred Reporting Items for Systematic Review and Meta‐Analysis Protocols (PRISMA‐P) 2015 Statement,” Systematic Reviews 4 (2015): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tricco A. C., Lillie E., Zarin W., et al., “PRISMA Extension for Scoping Reviews (PRISMA‐ScR): Checklist and Explanation,” Annals of Internal Medicine 169 (2018): 467–473. [DOI] [PubMed] [Google Scholar]
- 18. Ouzzani M., Hammady H., Fedorowicz Z., and Elmagarmid A., “Rayyan—A web and Mobile app for Systematic Reviews,” Systematic Reviews 5 (2016): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lefebvre C., Glanville J., Briscoe S., et al., “Chapter 4: Searching for and Selecting Studies,” In Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, eds. Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., and Welch V. A. (2024), www.training.cochrane.org/handbook.
- 20. Peters M. D. J., Marnie C., Colquhoun H., et al., “Scoping Reviews: Reinforcing and Advancing the Methodology and Application,” Systematic Reviews 10 (2021): 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not pertinent to this article, as this study will not involve the processing of new data.


