Abstract
Purpose
There is very little information detailing outcomes and toxicity following reirradiation for ultracentrally located thoracic tumors, and detailed dosimetric data are nonexistent. These data are critical for the safe management of these extremely difficult cases.
Methods and Materials
The records of 15 individuals undergoing 10-fraction hypofractionated stereotactic body radiation therapy for the management of ultracentrally located thoracic tumors between 2009 and 2020 at a single institution were retrospectively reviewed. Treatment outcomes and toxicity were analyzed. A detailed dosimetric analysis of treatment plans and centrally located organs at risk (OARs) from the initial reirradiation and cumulative radiation therapy courses were presented.
Results
At a median follow up of 10 months, the 1- and 3-year overall survival, progression-free survival, and local control were 52% and 28%, 33% and 28%, and 76% and 61%, respectively. Treatment-related adverse events were low, with 5 individuals (33%) developing ≥grade 2 pneumonitis (grade 2 = 4, grade 3 = 1). Dosimetric parameters were not associated with the development of clinically relevant pneumonitis. No adverse events involving central OARs (esophagus, great vessels, and primary bronchial tree) were identified. The median cumulative mean lung dose was 24 Gy equivalent total doses in 2 Gy fractions (EQD2) (range, 10-33 Gy), with a volume receiving 20 G (V20) of 33% (range, 11%-51%). The median esophageal, primary bronchial tree, and great vessel maximum doses (Dmax) were 93.2 Gy (EQD2) (range, 50-148 Gy), 163 Gy (range, 77-204 Gy), and 191 Gy (range, 129-262 Gy), respectively.
Conclusions
The current investigation is the first to provide detailed cumulative dosimetric data from a cohort of patients comprised entirely of ultracentrally located thoracic tumors. Despite unfavorable anatomic tumor location, given an intimate association with critical OARs, delivering an ablative dose with a 10-fraction hypofractionated stereotactic body radiation therapy course can serve as a feasible option for these challenging cases.
Introduction
Lung cancer continues to be the leading cause of cancer-related mortality, with an estimated 23,600 new diagnoses and 13,200 deaths in the US in 2021.1 Among those diagnosed, approximately 50% to 66% are either early-stage or locally advanced diseases rather than de novo metastatic disease.2,3 Despite significant advances in both radiation therapy and systemic therapy options, resulting in dramatically improved outcomes,4,5 a considerable proportion of lung cancer patients will eventually relapse.6,7 Following relapse, cytotoxic chemotherapy has historically been employed but with outcomes less than ideal and chances for survival dramatically diminished,8 and other treatment modalities, including stereotactic body radiation therapy (SBRT), have been explored to control disease.
Even in the upfront radiation therapy setting, early trials exploring the efficacy and safety of SBRT demonstrated an increased risk of severe, and even fatal, treatment-related complications following treatment of centrally located tumors.9 From this, the term “No Fly Zone” was proposed, which refers to tumors located within 2 cm of the proximal bronchial tree. Fortunately, risk-adapted dose fractionation schedules were established, leading to the safe and effective management of centrally located tumors.10,11
More difficult has been the management of ultracentral lung tumors. Though no standardized definition exists, these tumors are described as either direct tumor abutment or planning target volume (PTV) overlapping critical central or mediastinal structures, including the great vessels, proximal bronchial tree/trachea, or esophagus.12, 13, 14 Though outcomes following SBRT for ultracentrally located tumors in the upfront setting exist, it exclusively relies on retrospective single-institution experiences.12, 13, 14, 15, 16 The currently enrolling SUNSET trial is the first to prospectively determine the ideal dose fraction schedule for ultracentrally located lung tumors.17
Despite progress in the upfront SBRT setting for ultracentral tumors, literature characterizing ultracentral reirradiation is exceedingly rare. One recent study has demonstrated favorable rates of tumor control with an acceptable toxicity profile.18 What is lacking, and what is currently unavailable in the literature, is a detailed dosimetric analysis evaluating the cumulative dose delivered to critical, centrally located organs at risk (OARs). The current study includes individuals who have been treated with a 10-fraction hypofractionated SBRT (hfSBRT) course for recurrent ultracentrally located tumors, and all their radiation therapy treatment plans are available for dosimetric analysis. This allows for a detailed report of initial, reirradiation, and cumulative radiation therapy courses and doses received by critical central OARs. This dosimetric data will assist practitioners as they encounter extremely difficult cases of ultracentral reirradiation.
Methods and Materials
Patient population
From 2009 to 2020, a total of 78 consecutive individuals underwent 10-fraction hfSBRT at the Anonymized for Review for the management of ultracentrally located thoracic tumors. Of this cohort, 28 individuals had undergone prior in-field thoracic radiation therapy, necessitating reirradiation. Of the 28 individuals who underwent reirradiation, 15 of them had all treatment plan records available for evaluation and are included in this institutional review board-approved retrospective analysis. Thirteen of these 15 patients had local/regional recurrence without distant metastasis, and 2 of them had dominant progression in hilar/mediastinal disease with limited distant metastasis (Table 1). Reasons for the unavailability of treatment plans include initial treatment at an outside institution (7), corrupt DICOM files (4), or treatment records that were otherwise not available (2).
Table 1.
Patient and tumor characteristics
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 7 (46.6) |
| Female | 8 (53.3) |
| Age (y), median (range) | 74.1 (55.7-85.5) |
| Risk factors | |
| COPD | 8 (53.3) |
| ECOG performance status, median (range) | 2 (0-2) |
| FEV1, median % (range) | 70.0 (36-107) |
| DLCO, median % (range) | 62.0 (32-96) |
| Histology | |
| Adenocarcinoma | 6 (40.0) |
| SCC | 6 (40.0) |
| Other | 3 (20.0) |
| Ultracentral tumor location | |
| Hilum | 6 (40.0) |
| Mediastinum | 4 (26.7) |
| Mediastinum + hilum | 3 (20.0) |
| Parenchyma | 2 (13.3) |
| Prior chemotherapy | |
| Yes | 11 (73.3) |
| No | 4 (26.7) |
| PET maximum SUV prior to treatment, median (range) | 10.9 (4.4-21.8) |
| Patient disease status prior to reirradiation | |
| Local/local-regional recurrence without DM | 13 (86.6) |
| Dominant progression hilar/mediastinal disease with limited DM | 2 (13.3) |
| Initial stage at diagnosis | |
| IA | 1 (6.7) |
| IIA | 1 (6.7) |
| IIB | 1 (6.7) |
| IIIA | 8 (53.3) |
| IIIB | 3 (20.0) |
| IVA | 1 (6.7) |
| Follow-up time (mo) following thoracic reirradiation, median (range) | 10.3 (2.2-79.3) |
Abbreviations: DM = distant metastasis; PET = positron emission tomography; COPD = Chronic obstructive pulmonary disease; ECOG = Eastern Cooperative Oncology Group; FEV1 = Forced expiratory volume in one second; DLCO = Diffusing capacity of lungs for carbon monoxide; SCC = Squamous carcinoma; SUV = Standardized uptake value.
Criteria for receiving reirradiation with hfSBRT include (1) the clinical decision and tumor board recommendation for reirradiation was based on the potentially significant role of local disease control with radiation therapy for local ablation of oligometastatic disease or curative intent treatment of local-regional recurrent disease without metastasis; (2) recurrent tumor in a location meeting criterion for thoracic reirradiation, which was defined as PTV of recurrent treatment plan overlapping with the 50% isodose line of the initial radiation therapy plan; (3) lack of concurrent chemotherapy; and (4) ultracentrally located recurrent or oligometastatic tumor, which was defined as PTV overlap or direct tumor abutment with the great vessels (pulmonary artery, pulmonary veins, and superior and inferior vena cava), esophagus, hilum, central airway, or mediastinum.
Treatment planning
Patients underwent a 4-dimensional simulation computed tomography (CT) scan on a Philips CT simulator using individually shaped body fixation devices and abdominal compression (BlueBAG, BodyFIX system, Medical Intelligence). An internal target volume was delineated on maximum intensity projection image sets and verified with 4-dimensional CT images, with a uniform 5 mm expansion to produce the PTV. OARs, including the bilateral lungs, heart, spinal cord, esophagus, great vessels, and primary bronchus, were contoured on mean intensity projection image sets.
When developing reirradiation plans, prior treatment plans were carefully reviewed, with special attention paid to doses of OARs. The retreatment dose was determined by the treating physicians with the intent to deliver an ablative dose of radiation and respect the tolerant doses on OARs. The dose reduction was permitted in instances of unacceptably high cumulative OAR dose on the spinal cord and esophagus. Institutional guidelines for reirradiation treatment plan OARs were a point maximal dose (Dmax) of 32 Gy to the spinal canal, a Dmax of 48 Gy and a percentage volume receiving at least 40 Gy (V40) < 5 cm3 for the esophagus, and Dmax of 42 Gy for the brachial plexus. There were no specific dose limits for the lungs, great vessels/heart, trachea, and main bronchi, but the intent was to maximally reduce the dose to these OARs. The treatments were delivered daily or every other day.
Treatment plans were generated using either intensity modulated radiation therapy/volumetric modulated arc therapy (Eclipse, Varian) or 3-dimensional (iPlan, Brain lab) conformal techniques. Plans were optimized to achieve 95% or higher of the PTV volume receiving 100% of the prescription dose. Treatment doses ranged from 50 to 70 Gy in 10 fractions, corresponding to a biologically effective dose of 75 to 119 Gy (α/β = 10, biologically effective dose [BED10]) (Table 2).
Table 2.
Treatment details of initial radiation therapy and reirradiation courses
| Characteristic | n (%) |
|---|---|
| Initial treatment | |
| PTV volume (cc), median (range) | 207 (11.3-825.5) |
| Prior thoracic radiation therapy | |
| EBRT | 11 (73.3) |
| SBRT | 4 (26.7) |
| Dose of initial thoracic radiation (Gy), median (range) | |
| EBRT | 61.2 (45-96*) |
| SBRT | 50 (50-70) |
| No. of fractions, median (range) | |
| EBRT | 33 (18-43*) |
| SBRT | 5 (5-7) |
| Fractionation schemes of patients treated with initial SBRT (total dose in Gy/fraction) | |
| 50/5 | 3 (75.0) |
| 70/10 | 1 (25.0) |
| BED10 (in Gy) of initial thoracic radiation, median (range) | |
| EBRT | 72.2 (56.3-118.2*) |
| SBRT | 100 (100-119) |
| Interval between thoracic radiation (mo), median (range) | 16.2 (3.6-60.2) |
| Reirradiation | |
| PTV volume (cm3), median (range) | 49.18 (12.2-149.3) |
| Dose fractionation (total dose in Gy/fraction) | |
| 70/10 | 3 (20.0) |
| 65/10 | 4 (26.7) |
| 63/10 | 1 (6.7) |
| 60/10 | 2 (13.3) |
| 50/10 | 5 (33.3) |
| Reirradiation dose, median (range) | 63.0 (50-70) |
| BED10 (in Gy) of 10-fraction regimen, median (range) | 102.7 (75-119) |
Abbreviations: BED = biologically effective dose; EBRT = external beam radiation therapy; PTV = planning target volume; SBRT = stereotactic body radiation therapy.
A single individual underwent an initial course of conventionally fractionated EBRT to 66 Gy in 33 fractions, with a subsequent course of 30 Gy in 10 fractions at the same site prior to a 10-fraction hypofractionated SBRT course.
Follow-up evaluation
Following treatment completion, patients underwent regularly scheduled follow up, including a CT scan of the thorax every 3 to 6 months to assess treatment response and durability. Treatment failure was primarily radiographically based. If concern for disease progression was identified or if imaging results were equivocal, patients underwent positron emission tomography. A minority of patients required tissue biopsy if both CT and positron emission tomography were equivocal. Toxicity was assessed at each follow-up appointment and was categorized via the Common Terminology Criteria of Adverse Events v5.0.
Dosimetric analysis
A detailed dosimetric analysis of the initial and reirradiation courses, as well as summed cumulative doses to OARs, was performed. OARs were delineated on simulation CT scans corresponding to a radiation therapy course and included the bilateral lungs, esophagus, primary bronchial tree, and great vessels. Lung contours were automatically generated and were defined as lung parenchyma minus PTV. For the construction of the primary bronchial tree, the airway lumen was contoured 2 cm craniocaudally to the PTV. Likewise, the esophagus and great vessels were manually delineated 2 cm craniocaudally to the PTV.
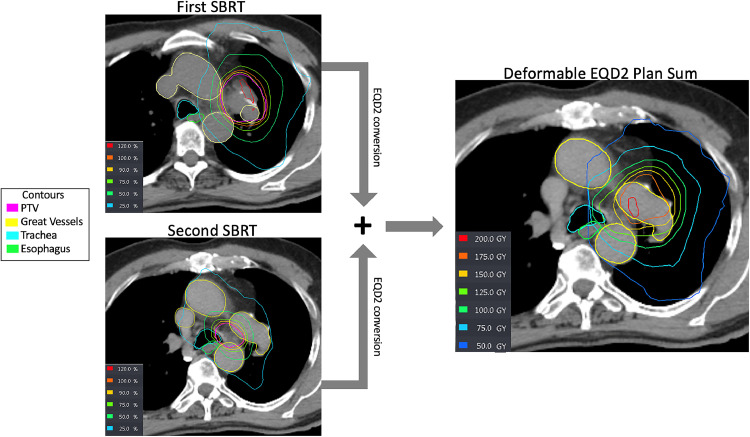
Dosimetric variables were gathered from initial and reirradiation courses. For the construction of the cumulative plan sum, initial and reirradiation treatment plans were imported into the Velocity planning software (Varian). First, plans were converted to equivalent total doses in 2 Gy fractions (EQD2) using the formula d ∗ n ∗ [(d + α/β)], where d represents the dose per fraction in Gy, and n represents the number of fractions. For late treatment-related complications, an α/β value of 3 Gy was used; next, a deformable registration of the initial radiation therapy course to the more recent reirradiation course was performed. The resulting summed plan was manually inspected to confirm the appropriate anatomic alignment of the 2 plans. If significant anatomic variability was noted between the 2 plans, the deformable registration was focused on a more concise region of interest corresponding to the reirradiated tumor volume and adjacent critical normal structures. A representative plan summation can be seen in Fig. 1. Cumulative dosimetric variables for OARs corresponded to delineated structures from the reirradiation course of treatment. These include a total lung percentage of lung receiving a minimum of 5 Gy (V5), 20 Gy (V20), V40, and mean lung dose (EQD2_3). For the esophagus, primary bronchial tree, and great vessels, we report a maximum dose (Dmax) (EQD2_3) and the minimum dose to the most highly irradiated 5 cc (D5cc) and 10 cc (D10cc) of the structure.
Figure 1.
Example of first, second, and composed isodose plans of 1 case. Computed tomography image with superimposed isodose lines and organs at risk of the first treatment stereotactic body radiation therapy (SBRT), second treatment SBRT, and deformable equivalent dose in 2 Gy fractions (EQD2) plan sum.
Abbreviations: PTV = planning target volume.
Statistical analysis
Descriptive statistics was used to summarize cohort data, including patient demographics, tumor characteristics, pretreatment risk factors, and treatment courses, as well as to describe initial, reirradiation, and cumulative treatment dosimetric variables. The Kaplan-Meier method was used to estimate treatment outcomes, including local control (LC), progression-free survival (PFS), and overall survival (OS). Univariate linear regression analysis was performed to evaluate associations between the development of clinically significant pneumonitis and patient, tumor, or dosimetric characteristics. A p value of <.05 was considered significant for all measurements performed. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Patient and tumor characteristics
A total of 15 individuals fulfilled the study inclusion criteria with a median follow-up time of 10 months (range, 2-80 months) following completion of thoracic reirradiation. Of these, 7 (47%) were male and 8 (53%) were female. The median age at the time of reirradiation was 74 years (range, 55-85). The histology of patients consisted of primary lung adenocarcinoma (40%) and squamous cell carcinoma (40%). A minority of patients (13.3%) underwent thoracic reirradiation for recurrent metastatic disease. At the time of initial diagnosis, patients typically presented with locally advanced disease, with 73% presenting with stage III disease. Three patients (20%) were initially diagnosed with early-stage disease, and 1 patient was diagnosed with de novo metastatic disease (Table 1).
Treatment characteristics
For the first radiation therapy course, of the 15 evaluable patients, 11 (73%) had initially undergone a course of conventionally fractionated external beam radiation therapy (EBRT), with the remaining 4 (27%) being treated with an SBRT technique. For those treated with EBRT, the median dose was 61 Gy, corresponding to a BED10 of 72.2 Gy. There was significant variability in the range of initial EBRT dose (45-96 Gy) due to a single patient who underwent 2 conventionally fractionated courses of EBRT (30 Gy in 10 fractions followed by 66 Gy in 33 fractions) prior to a third hfSBRT course. Of the remaining 4 individuals who underwent an initial course of SBRT, 3 were treated with a dose of 50 Gy in 5 fractions corresponding to a BED10 of 100, and a single patient was treated with a 70 Gy in 10 fractions regimen (BED10 = 119 Gy).
For the second hfSBRT course, the median interval between the first course of radiation therapy and the subsequent hfSBRT course was 16 months (range, 4-60 months). All patients were treated using a 10-fraction regimen. Treatment doses ranged from 50 Gy to 70 Gy. The median treatment dose was 63 Gy, corresponding to a BED10 of 103 Gy (range, 75-119 Gy). The reirradiation PTV was 49 cc (range, 12-150 cc) (Table 2).
Dosimetric analysis
Dosimetric data for both initial EBRT and SBRT, as well as hfSBRT, are summarized in Table 3. In addition, a detailed cumulative dosimetric analysis encompassing all radiation therapy courses for critical OARs can be found. All cumulative dosimetric data are represented as an EQD2_3 with an assumed α/β of 3 for normal structures. The median mean lung dose was 24 Gy (EQD2_3) (range, 10-33 Gy). Lung V5, V20, and V40 were 65% (range, 22%-99%), 33% (range, 11%-51%), and 20% (range, 7%-30%), respectively. The median esophageal Dmax was 93 Gy (EQD2_3) (range, 50-148 Gy), while the median D5cc and D10cc were 53 Gy (EQD2_3) (19-112 Gy) and 42 Gy (EQD2_3) (range, 2-89 Gy), respectively. The median primary bronchial tree Dmax was 163 Gy (EQD2_3) (range, 77-204 Gy), with a median D5cc and D10cc of 102 Gy (EQD2_3) (range, 46-167 Gy) and 63 Gy (EQD2_3) (range, 14-130 Gy), respectively. Lastly, the median great vessel Dmax was 191 Gy (EQD2_3) (range, 129-262 Gy). The median great vessel D5cc and D10cc were 139 Gy (EQD2_3) (range, 70-187 Gy) and 113 Gy (EQD2_3) (61-175 Gy), respectively. An overview of individual patient cumulative OAR doses (lung V20, esophageal Dmax, primary bronchial tree Dmax, and great vessel Dmax) can be found in Table 4.
Table 3.
Detailed dosimetric analysis of individual and cumulative radiation courses
| Initial radiation therapy |
Reirradiation/cumulative radiation therapy |
||
|---|---|---|---|
| Organ at risk |
Median value (range) |
Organ at risk |
Median value (range) |
| Conventional fractionation | 10-fraction reirradiation | ||
| Total lung | Total lung | ||
| MLD (Gy) | 18.5 (3.3-22.4) | MLD (Gy) | 17.7 (10.5-21.2) |
| V5 (%) | 57.1 (14.1-87.2) | V5 (%) | 13.8 (10.5-21.8) |
| V20 (%) | 27.3 (4.4-41.7) | V20 (%) | 5.9 (2.2-11.7) |
| V40 (%) | 15.3 (1.2-22.97) | V40 (%) | 2.0 (0.6-4.2) |
| Esophagus | Esophagus | ||
| Dmax (Gy) | 63.8 (33.8-100.1) | Dmax (Gy) | 12.6 (10.3-26.9) |
| D5cc (Gy) | 54.3 (17.1-81.5) | V20 (cc) | 0 (0-1.56) |
| D10cc (Gy) | 32.0 (1.81-75.7) | V40 (cc) | 0 (0-0) |
| Proximal bronchial tree | Proximal bronchial tree | ||
| Dmax (Gy) | 65.2 (24.9-109.7) | Dmax (Gy) | 10.2 (3.18-46.6) |
| D5cc (Gy) | 62.7 (17.7-100.5) | V30 (cc) | 0 (0-0.4) |
| D10cc (Gy) | 61.5 (12.8-97.0) | V50 (cc) | 0 (0-0) |
| Great vessels | Great vessels | ||
| Dmax (Gy) | 66.4 (52.7-109.2) | Dmax (Gy) | 39.9 (9.4-86.2) |
| D5cc (Gy) | 64.9 (51.0-105.0) | V50 (cc) | 7.6 (0-31.8) |
| D10cc (Gy) | 64.7 (50.6-102.6) | ||
| SBRT | Cumulative radiation therapy (EQD2, α/β = 3) | ||
| Total lung | Total lung | ||
| MLD (Gy) | 6.67 (2.4-19.3) | MLD (Gy) | 23.7 (10.1-32.9) |
| V5 (%) | 19.7 (9.0-83.1) | V5 (%) | 64.5 (22.3-99.1) |
| V20 (%) | 7.9 (0.6-20.1) | V20 (%) | 33.1 (11.0-51.2) |
| V40 (%) | 2.3 (0-6.0) | V40 (%) | 19.8 (7.1-29.6) |
| Esophagus | Esophagus | ||
| Dmax (Gy) | 28.2 (7.0-44.8) | Dmax (Gy) | 93.2 (49.7-148.0) |
| V20 (cc) | 1.6 (0-8.6) | D5cc (Gy) | 52.8 (19.2-111.5) |
| V40 (cc) | 0 (0-6.6) | D10cc (Gy) | 41. 7 (1.7-88.5) |
| Proximal bronchial tree | Primary bronchial tree | ||
| Dmax (Gy) | 62.4 (30.0-88.3) | Dmax (Gy) | 163.3 (76.8-203.8) |
| V30 (cc) | 4.4 (0-12.1) | D5cc (Gy) | 102.1 (45.5-167.0) |
| V50 (cc) | 0.8 (0-5.9) | D10cc (Gy) | 63.3 (14.3-130.2) |
| Great vessels | Great vessels | ||
| Dmax (Gy) | 74.9 (54.6-86.5) | Dmax (Gy) | 191. 2 (129.1-262.1) |
| V50 (cc) | 4.2 (0.1-21.3) | D5cc (Gy) | 138.9 (70.0-186.8) |
| D10cc (Gy) | 112.8 (60.9-175.3) | ||
Abbreviations: Dmax = maximum dose; EQD2 = equivalent total doses in 2 Gy fractions; MLD = mean lung dose; SBRT = stereotactic body radiation therapy; V5 = volume receiving 5 Gy; V20 = volume receiving 20 Gy; V40 = volume receiving 40 Gy; D5cc = dose to 5 cc volume; D10cc = dose to 10 cc volume.
Table 4.
Detailed description of individual patient disease status, dosimetry, treatment outcomes, and toxicity
| Patient no. | Disease status prior to re-RT | Recurrent tumor location (PTV size in cc) | Interval from prior RT (mo) | Cumulative dosimetric parameters in Dmax (Gy) or % for V20 (EQD2, α/β = 3) | Late toxicity (grade) (pneumonitis [P], esophagitis [E], airway [A], and hemorrhage [H]) | Patient status (follow-up time, mo) | Local tumor control | Cause of death (treatment relationship) |
|---|---|---|---|---|---|---|---|---|
| 1 | r-LDM | Hilum + mediastinum (43) | 16.2 | L V20:26; E:100; B:155; V:217 | P:0; E:0; A:0; H:0 | Alive (7) | Yes | - |
| 2 | r-NM | Hilum + mediastinum (75) | 23.5 | L V20:33; E:148; B:204; V:193 | P:1; E:0; A:0; H:0 | Dead (2) | Yes | Unrelated |
| 3 | r-NM | Hilum (65) | 60.2 | L V20:24; E:108; B:194; V:220 | P:1; E:0; A:0; H:0 | Dead (47) | No | Unrelated |
| 4 | r-NM | Mediastinum (35) | 3.6 | L V20:11; E:93; B:85; V:262 | P:1; E:0; A:0; H:0 | Dead (33) | No | Unrelated |
| 5 | r-NM | Hilum (149) | 18.3 | L V20:33; E:148; B:197; V:202 | P:2; E:0; A:0; H:0 | Dead (25) | No | Unrelated (POD) |
| 6 | r-NM | Mediastinum (36) | 12.3 | L V20:13; E:93; B:119; V:201 | P:2; E:0; A:0; H:0 | Dead (5) | Yes | Unrelated (COPD) |
| 7 | r-NM | Hilum (17) | 50.1 | L V20:37; E:94; B:114; V:200 | P:1; E:0; A:0; H:0 | Alive (36) | Yes | - |
| 8 | r-NM | Parenchyma (47) | 10.4 | L V20:43; E:74; B:139; V:191 | P:1; E:0; A:0; H:0 | Dead (5) | Yes | Unrelated (Sepsis) |
| 9 | r-NM | Mediastinum (59) | 35.3 | L V20:28; E:80; B:183; V:183 | P:2; E:0; A:0; H:0 | Alive (27) | Yes | - |
| 10 | r-NM | Hilum + mediastinum (85) | 9.6 | L V20:39; E:83; B:170; V:174 | P:0; E:0; A:0; H:0 | Dead (10) | No | Unrelated |
| 11 | r-NM | Parenchyma (85) | 39.2 | L V20:51; E:96; B:77; V:159 | P:0; E:0; A:0; H:0 | Dead (3) | Yes | Unrelated |
| 12 | r-LDM | Hilum (49) | 22.9 | L V20:17; E:56; B:180; V:186 | P:2; E:0; A:0; H:0 | Dead (9) | Yes | Unrelated (POD) |
| 13 | r-NM | Hilum (67) | 4.6 | L V20:45; E:84; B:155; V:150 | P:3; E:0; A:0; H:0 | Dead (3) | No | Unrelated (Cardiac arrest) |
| 14 | r-NM | Mediastinum (25) | 8.2 | L V20:48; E:103; B:187; V:129 | P:0; E:0; A:0; H:0 | Dead (80) | No | Unrelated (POD) |
| 15 | r-NM | Hilum (13) | 13.3 | L V20:38; E:50; B:163; V:168 | P:0; E:0; A:0; H:0 | Dead (14) | Yes | Unrelated |
Abbreviations: Dmax = maximum dose; EQD2 = equivalent total doses in 2 Gy fractions; PTV = planning target volume; RT = radiation therapy; r-LDM = recurrence at local disease and metastasis; r-NM = local recurrence without metastasis; V20 = volume receiving 20 Gy; L = lung; E = esophagus; B = bronchus; V = great vessels.
Treatment outcomes and toxicity
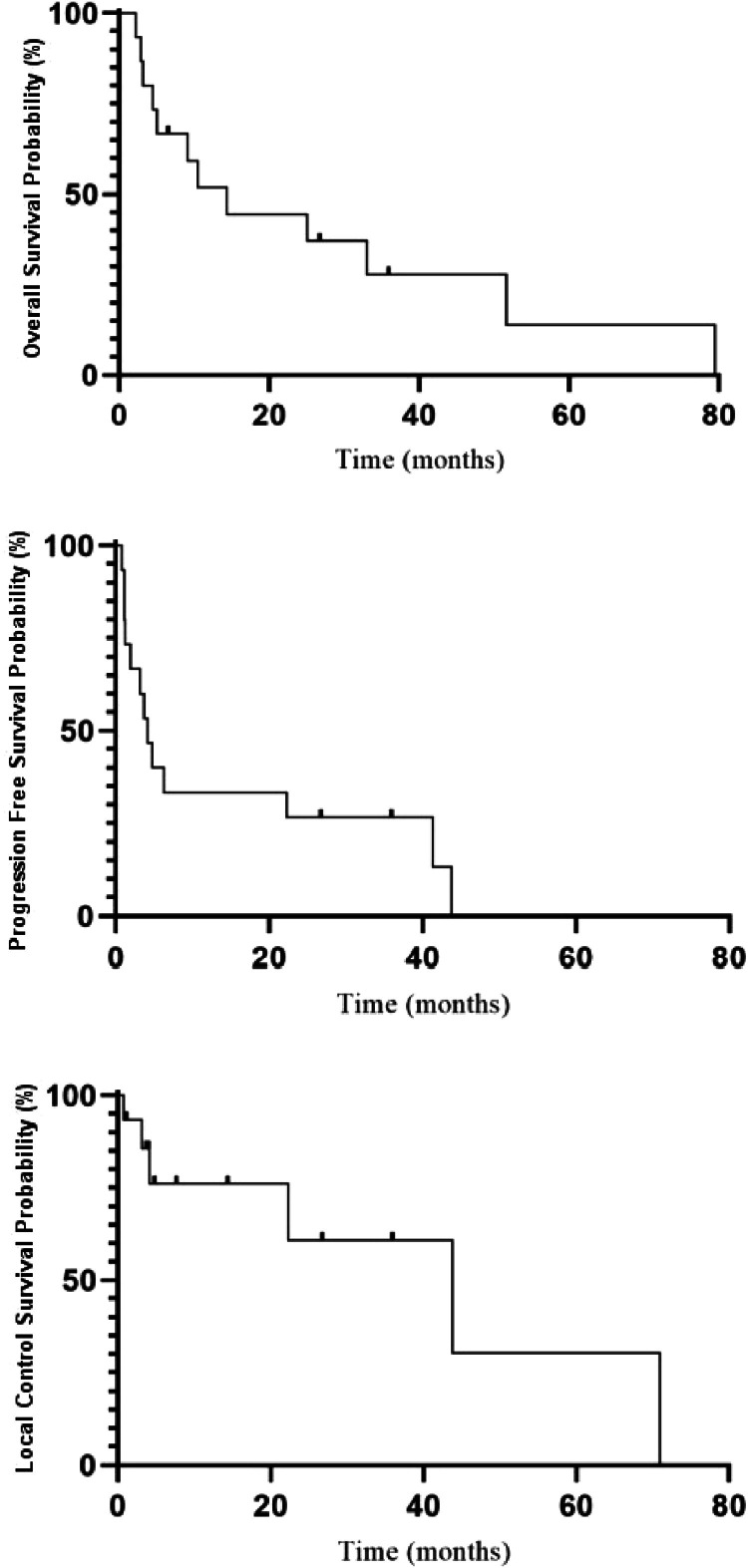
Table 4 provides a detailed overview of outcomes and toxicity profiles for each of the 15 individuals included in this analysis. Cohort OS, PFS, and LC are represented graphically in Fig. 2. The median cohort survival was 14.4 months, with 1- and 3-year survival rates of 51.8% and 27.8%, respectively. The median PFS was 4.1 months, with 1- and 3-year rates of 33.3% and 26.7%, respectively. Lastly, the median tumor control was 43.8 months, with 1- and 3-year LC rates of 76.0% and 60.8%, respectively.
Figure 2.
Kaplan-Meier curves for overall survival, progression-free survival, and local control.
Low rates of clinically significant toxicity were observed. The only observed toxicity was pneumonitis, which was experienced by 5 of 15 individuals (33%). Most of these cases (80%) were grade 2 pneumonitis, with the remaining cases rated as grade 3 (Table 5). The one instance of grade 3 pneumonitis was managed with temporary supplemental oxygen and a course of corticosteroids. The patient promptly recovered and was without additional treatment-related toxicity until the time of death due to an unrelated cause. No cases of esophagitis, airway obstruction, or hemoptysis were observed (Table 5). Univariate analysis did not identify any correlates between clinically significant pneumonitis and patient, tumor, or dosimetric variables (Table 6).
Table 5.
Late adverse events following ultracentral radiation therapy according to the Common Terminology Criteria of Adverse Events v5.0
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Pneumonitis | 4 | 1 | - | - |
| Esophagitis | - | - | - | - |
| Airway toxicity | - | - | - | - |
| Hemoptysis | - | - | - | - |
Table 6.
Univariate logistic regression model for patient and treatment factors associated with the development of clinical pneumonitis
| Characteristic | OR (95% CI) | P value |
|---|---|---|
| Tumor location (reference: lung parenchyma) | ||
| Mediastinum | 5.00 (0.08-334.83) | .45 |
| Hilum | 5.00 (0.09-286.71) | .44 |
| Mediastinum + hilum | 0.71 (0.01-111.87) | .89 |
| COPD | 1.50 (0.17-13.26) | .72 |
| Initial radiation therapy | ||
| Dose (Gy) | 0.97 (0.89-1.08) | .61 |
| BED (Gy) | 0.98 (0.89-1.08) | .61 |
| PTV volume (cc) | 1.01 (0.99-1.01) | .08 |
| Prior RT (reference SBRT) | ||
| EBRT | 1.71 (0.13-22.51) | .68 |
| 10-fraction reirradiation | ||
| Dose (Gy) | 1.02 (0.89-1.17) | .78 |
| BED (Gy) | 1.01 (0.95-1.07) | .81 |
| PTV volume (cc) | 1.02 (0.98-1.05) | .34 |
| Total lung | ||
| MLD (Gy) | 1.09 (0.88-1.35) | .45 |
| V5 (%) | 0.99 (0.93-1.05) | .65 |
| V20 (%) | 0.97 (0.79-1.19) | .78 |
| V40 (%) | 0.97 (0.55-1.70) | .91 |
| Proximal bronchial tree | ||
| Dmax (Gy) | 1.02 (0.96-1.08) | .56 |
| V30 (cc) | 0.96 (0.72-1.32) | .87 |
| V50 (cc) | 1.19 (0.70-2.04) | .52 |
| Cumulative radiation therapy | ||
| Total lung | ||
| MLD (Gy) | 0.90 (0.76-1.07) | .24 |
| V5 (%) | 0.98 (0.94-1.03) | .51 |
| V20 (%) | 0.95 (0.86-1.04) | .25 |
| V40 (%) | 0.83 (0.66-1.03) | .09 |
| Proximal bronchial tree | ||
| Dmax (Gy) | 1.01 (0.98-1.05) | .41 |
| D5cc (Gy) | 1.01 (0.97-1.04) | .71 |
| D10cc (Gy) | 0.99 (0.96-1.04) | .97 |
Abbreviations: BED = biologically effective dose; Dmax = maximum dose; EBRT = external beam radiation therapy; MLD = mean lung dose; OR = odds ratio; PTV = planning target volume; RT = radiation therapy; SBRT = stereotactic body radiation therapy; COPD = chronic obstructive pulmonary disease; V5 = volume receiving 5 Gy; V20 = volume receiving 20 Gy; V30 = volume receiving 30 Gy; V40 = volume receiving 40 Gy; V50 = volume receiving 50 Gy; D5cc = dose to 5 cc volume; D10cc = does to 10 cc volume.
Discussion
Despite significant advances in the management of both early-stage9,10 and locally advanced lung cancer,4, 5, 6 a considerable proportion of those treated will recur. New and innovative techniques are necessary to manage these difficult cases. Due to its highly conformal nature and ability to deliver ablative doses, SBRT is one such technique. This technique, in the recurrent setting, has primarily been investigated in peripheral tumors, away from critical centrally located OARs. Little published data are available for the management of centrally and ultracentrally located tumors where proximity to mediastinal OARs is of great concern. The current investigation provides outcome and toxicity data for a cohort of individuals managed using a 10-fraction hfSBRT technique for recurrent non-small cell lung cancer (NSCLC) or oligometastatic disease. All included patients had dosimetric information available for all radiation therapy courses, allowing a comprehensive dosimetric analysis of individual and cumulative radiation therapy courses, information not currently available in the literature.
Treatment outcomes
This study mainly focused on detailed dosimetry analysis from the patients who had available previous dosimetric records, allowing us to generate composed plans. The cumulative incident analysis from the entire group was published previously.18
In our cohort, the prognosis following thoracic reirradiation was poor despite fair rates of tumor control. At 1 and 3 years, OS rates were 52% and 28%, respectively, a result consistent with the literature. In a population-based setting, Consonni et al8 demonstrated a substantial increase in mortality following NSCLC recurrence. For stage I disease, 1-year mortality dramatically increased from 2.7% to 48.3% (hazard ratio, 34.2). A similar increase was seen with stage III disease, with 1-year mortality increasing from 23.7% to 68.7% (hazard ratio, 4.8). Survival following thoracic SBRT reirradiation follows this trend. Trovo et al,19 who reported outcomes of a cohort of 15 patients with recurrent centrally located tumors, defined using the radiation oncology group (RTOG) 0813 definition,10 treated to a dose of 30 Gy in 5 to 6 fractions, noted a 1- and 2-year survival of 59% and 29%, respectively. Repka et al,20 who is one of the few to report outcomes following SBRT reirradiation for ultracentrally located in-field recurrences (defined as direct recurrent tumor abutment with the trachea, mainstem bronchus, or esophagus), reported a 1-year OS of 45% in a cohort of 20 individuals treated with 5 fractions to a median dose of 35 Gy. They reported significantly improved survival seen in patients treated to a dose ≥ 40 Gy in 5 fractions (1-year OS, 77%).
Despite poor long-term survival, we did demonstrate favorable rates of LC following 10-fraction hfSBRT. In our cohort, the 1- and 3-year LC was 76% and 61%, respectively, with a median LC of 44 months. This is attributable to the high ablative doses we were able to deliver. A BED10 of ≥100 Gy has been firmly established as a benchmark associated with not only improved LC but also superior OS.21 In our cohort, most patients received doses approximating or exceeding this BED10, with 8 of 15 patients (53%) being treated to a BED10 > 100 Gy and 66% to a BED10 of ≥96 Gy. Again, these results are consistent with the literature. Repka et al,20 whose patient cohort most closely approximates our own, confirm the need for ablative dosing to achieve tumor control. At 1 year, an overall LC of only 35% was reported. As with their OS results, a substantial improvement in LC was noted when individuals were stratified to doses above or below 40 Gy in 5 fractions (1-year LC: 66.7% vs 0.0%).
Toxicity
Emerging data are available, suggesting that reirradiation is tolerable and safe in the management of recurrent thoracic tumors. However, the majority of available literature details reirradiation for peripherally located tumors or patient populations with both centrally and peripherally located tumors.22, 23, 24, 25, 26 Data from these single institutional experiences are consistent, with no grade 4 to 5 adverse events. The rate of grade 3 adverse events is also relatively consistent. Three of the 5 cited trials23,24,26 reported 0% grade 3 toxicity, while the remaining 2 trials reported grade 3 toxicity of 18%25 and 33%,22 respectively. In those that reported grade 3 toxicity, treatment-related pneumonitis was most observed. A minority of patients experienced toxicity involving central structures (ie, esophagitis), but these events were rare. Given the abundance of peripherally located tumors in these studies, and therefore, a large spatial distance separating tumor and mediastinum, this lack of central toxicity is expected.
A small minority of the literature reports toxicity for centrally or ultracentrally located tumors following reirradiation. In work by Sumodhee et al,27 toxicity in a cohort of 46 individuals is reported, 52% of which have centrally located tumors. Grade 2 to 5 toxicity was observed in 15%, 2%, 0%, and 4%, respectively. The 2 instances of grade 5 toxicity were a single case of fatal hemoptysis and a case of radiation alveolitis. For univariate and multivariate analysis, centrally located tumors were associated with a higher rate of grade 2 to 5 toxicities, with an incidence of 38% in centrally located tumors and 5% with tumors located in the periphery. The previously described work by Trovo et al19 includes toxicity data for a cohort of patients with exclusively centrally located tumors. Here, 4 patients (23%) experienced grade 3 radiation pneumonitis requiring supplemental oxygen administration. One patient developed grade 5 pneumonitis, and 1 patient developed grade 5 hemoptysis. No other grade 3 or worse toxicity was observed. Lastly, in the exclusively ultracentrally located cohort presented by Repka et al,20 a favorable toxicity profile was observed, with only 2 cases (10%) of grade 2 radiation pneumonitis and 2 cases (grade 2 and grade 3) of recurrent laryngeal nerve paralysis.
The toxicity profile observed in our own cohort of patients was also quite favorable, with only 4 cases (27%) of grade 2 pneumonitis and a single (7%) grade 3 pneumonitis event. We did not experience any adverse events involving central OARs, including the esophagus, primary bronchial tree, or the great vessels. It should be noted, however, that in our previously published work,18 of which a sizable proportion of the current patient cohort was included, there were 2 instances of fatal hemoptysis. Both events were ruled to be “unlikely” related to treatment as both demonstrated radiographic or bronchoscopy evidence of disease progression just prior to the fatal hemoptysis event. In addition, 1 case of grade 3 esophageal stricture, treated with a single esophageal dilation, was seen. These individuals were not included in the current analysis due to a lack of available dosimetric data from their initial radiation courses.
Dosimetry
The current investigation is the first to report dosimetric data in a patient cohort entirely comprised individuals with ultracentrally located tumors. There is, however, available dosimetric data for patients undergoing thoracic reirradiation in general. Liu et al28 analyzed 72 individuals after undergoing thoracic reirradiation following a prior course of conventionally fractionated EBRT. Fourteen patients (21%) developed grade 3 pneumonitis, with a single patient developing grade 5 pneumonitis. In this trial, a total lung V20 > 30% was associated with severe pneumonitis. In our own cohort, the median V20 was 33%, and despite a V20 as high as 51%, only 1 case of grade 3 pneumonitis was observed. In addition, cumulative V20 was not predictive of ≥grade 2 pneumonitis on univariate analysis (p = .25).
We did not see any esophageal adverse events in our patient cohort. Esophageal toxicity in the literature also appears to be rare. Binkley et al29 reported 3 instances of ≥grade 2 esophagitis (grade 2 = 2, grade 3 = 1) in a cohort of 38 individuals undergoing reirradiation. D1cc for those who developed esophagitis ranged from 41 Gy to 100 Gy. A recently published review article detailing reirradiation of recurrent NSCLC from Hunter et al30 suggests an esophageal cumulative Dmax (EQD2_3) < 100 Gy to the esophagus. With a median esophageal Dmax of 93 Gy (EQD2_3), we were able to meet this constraint in most cases and agree that this is a reasonable suggestion. It should be noted, however, that should a recurrent tumor be located directly in the mediastinum where proximity to the esophagus will be much closer, a cumulative Dmax of <100 Gy (EQD2_3) may not be feasible, and efforts to minimize dose and volume to this structure should be taken.
The most feared complication in both the upfront SBRT and reirradiation setting is a fatal hemorrhagic event. This is of particular concern in the management of ultracentrally located tumors, given the intimate association of tumors to either the great vessels or proximal bronchial tree. Evans et al31 reported 2 grade 5 aortic toxicities in a cohort of 35 patients (6%) who were treated with reirradiation. Among patients receiving a cumulative D1cc ≥ 120 Gy (EQD2_3), the rate of grade 5 aortic toxicity was 25%. No patients receiving a cumulative <120 Gy (EQD2_3) experienced any such event, suggesting a D1cc < 120 Gy (EQD2_3) as a safe benchmark. The review from Hunter et al30 concurs with this aortic constraint for reirradiation and recommends a Dmax of <110 Gy (EQD2_3) to the pulmonary artery and a Dmax of <105 Gy (EQD2_3) to the bronchial wall to minimize the risk of severe hemorrhagic events. We were fortunate and did not experience any severe hemorrhagic events in our patient cohort despite significant doses delivered to the great vessels and bronchial tree. The aortic D1cc was not collected in our study, but with a median Dmax of 191 Gy (EQD2_3) and a D5cc of 139 Gy (EQD2_3), this value was exceeded. Likewise, with a median primary bronchial tree Dmax of 163 Gy (EQD2_3), the suggested dose of <105 Gy (EQD2_3) was not met in most cases. This reemphasizes the extreme difficulty imposed by cases of ultracentrally located recurrent tumors and that meeting suggested constraints derived from populations encompassing heterogeneously located tumors may not be feasible. Despite exceeding recommended cumulative dose constraints, ultracentral reirradiation was safely delivered in our patient cohort.
Limitations
There are several limitations to this study apart from its retrospective nature: (1) principle among these is the small patient cohort (n = 15) and the very heterogeneous nature of the patient population, limiting our ability to generalize our dosimetric and toxicity data to all ultracentrally located tumors. The small patient cohort, and therefore, few adverse events, make associations between dosimetric parameters and toxicity difficult to ascertain. (2) The current study is a retrospective study with a short follow-up time following reirradiation, particularly in those who have recently undergone treatment; (3) although the dose of EQD2 was used to convert dose equivalents from different courses of radiation therapy and calculate cumulative dose as suggested by the literature, it is still unclear what is the best way to estimate the true dose conversion from different courses and fractionations of the treatment; (4) in general, the time interval between the courses of treatment could potentially impact the treatment outcome and toxicity, and this small retrospective study is not capable of providing such information; and (5) in 2 cases of patients who died from massive endobronchial bleeding, although bronchoscopy demonstrated endobronchial lesions with bleeding, the other risk factors, such as anticoagulation/antiplatelet/anti-Vascular endothelial growth factor (VEGF) therapy, were not recorded.
Conclusions
Despite these limitations, the current investigation remains the first to provide detailed cumulative dosimetry data from a cohort of patients comprised entirely of ultracentrally located thoracic tumors. Our findings suggest that despite unfavorable anatomic location, given the intimate association to critical OARs, delivering an ablative dose with a 10-fraction hfSBRT course can serve as a feasible option for these extremely challenging cases.
Disclosures
None.
Acknowledgments
Ying Cao performed the statistical analysis.
Footnotes
Sources of support: This work had no specific funding.
Research data are not available at this time. Research data are stored in an institutional repository and will be shared on request to the corresponding author.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 3.Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004-2007. Thorax. 2013;68:551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/nejmoa1709937. [DOI] [PubMed] [Google Scholar]
- 5.Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: A comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. doi: 10.1016/S0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consonni D, Pierobon M, Gail MH, et al. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015;107:djv059. doi: 10.1093/jnci/djv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol. 2019;37:1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol. 2011;6:2036–2043. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89:50–56. doi: 10.1016/j.lungcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11:1081–1089. doi: 10.1016/j.jtho.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Daly M, Novak J, Monjazeb A. Safety of stereotactic body radiotherapy for central, ultracentral, and paramediastinal lung tumors. J Thorac Oncol. 2017;12:S10666. [Google Scholar]
- 15.Li Q, Swanick CW, Allen PK, et al. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: Exploration of clinical indications. Radiother Oncol. 2014;112:256–261. doi: 10.1016/j.radonc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Laba JM, Zayed S, Boldt RG, Palma DA, Louie AV. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: A systematic review. J Thorac Oncol. 2019;14:1332–1342. doi: 10.1016/j.jtho.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Giuliani M, Mathew AS, Bahig H, et al. SUNSET: Stereotactic radiation for ultracentral non–small-cell lung cancer-A safety and efficacy trial. Clin Lung Cancer. 2018;19(4):e529–e532. doi: 10.1016/j.cllc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Sood S, Ganju R, Shen X, ten Napel M, Wang F. Ultra-central thoracic re-irradiation using 10-fraction stereotactic body radiotherapy for recurrent non–small-cell lung cancer tumors: Preliminary toxicity and efficacy outcomes. Clin Lung Cancer. 2021;22:e301–e312. doi: 10.1016/j.cllc.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Trovo M, Minatel E, Durofil E, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:1114–1119. doi: 10.1016/j.ijrobp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Repka MC, Aghdam N, Kataria SK, et al. Five-fraction SBRT for ultra-central NSCLC in-field recurrences following high-dose conventional radiation. Radiat Oncol. 2017;12:162. doi: 10.1186/s13014-017-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 22.Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. 2010;78:1387–1393. doi: 10.1016/j.ijrobp.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijneke TR, Petit SF, Wentzler D, Hoogeman M, Nuyttens JJ. Reirradiation and stereotactic radiotherapy for tumors in the lung: Dose summation and toxicity. Radiother Oncol. 2013;107:423–427. doi: 10.1016/j.radonc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy WR, Gabani P, Nikitas J, Robinson CG, Bradley JD, Roach MC. Repeat stereotactic body radiation therapy (SBRT) for salvage of isolated local recurrence after definitive lung SBRT. Radiother Oncol. 2020;142:230–235. doi: 10.1016/j.radonc.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caivano D, Valeriani M, De Matteis S, et al. Re-irradiation in lung disease by SBRT: A retrospective, single institutional study. Radiat Oncol. 2018;13:87. doi: 10.1186/s13014-018-1041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa Y, Shibamoto Y, Hashizume C, et al. Repeat stereotactic body radiotherapy (SBRT) for local recurrence of non-small cell lung cancer and lung metastasis after first SBRT. Radiat Oncol. 2018;13:136. doi: 10.1186/s13014-018-1080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumodhee S, Bondiau PY, Poudenx M, et al. Long term efficacy and toxicity after stereotactic ablative reirradiation in locally relapsed stage III non-small cell lung cancer. BMC Cancer. 2019;19:305. doi: 10.1186/s12885-019-5542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Zhang X, Vinogradskiy YY, Swisher SG, Komaki R, Chang JY. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:1017–1023. doi: 10.1016/j.ijrobp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binkley MS, Hiniker SM, Chaudhuri A, et al. Dosimetric factors and toxicity in highly conformal thoracic reirradiation. Int J Radiat Oncol Biol Physs. 2016;94:808–815. doi: 10.1016/j.ijrobp.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Hunter B, Crockett C, Faivre-Finn C, Hiley C, Salem A. Re-irradiation of recurrent non-small cell lung cancer. Semin Radiat Oncol. 2021;31:124–132. doi: 10.1016/j.semradonc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Evans JD, Gomez DR, Amini A, et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol. 2013;106:327–332. doi: 10.1016/j.radonc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]