Abstract
Background
Diagnosing drug reaction with eosinophilia and systemic symptoms (DRESS) can be challenging.
Objectives
We sought to identify clinical and laboratory features outside of the Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria that distinguish patients with probable DRESS (RegiSCAR ≥ 4) from those with drug rash and eosinophilia (DRE).
Methods
Using international coding classifications of drug-induced fever, generalized skin eruption due to medications, and eosinophilia, a retrospective audit from 2008 to 2023 of hospitalized patients was performed.
Results
Forty-four cases of DRESS were compared to 80 cases of DRE. In addition to the RegiSCAR distinguishing factors for DRESS were longer drug latency before symptom onset (median 21 vs 5 days, P < .001) and higher alanine transaminase levels (increase by a factor of 2.49 [95% confidence interval, 1.56, 4.00; P = .009]). Follow-up (mean 5.67 years) revealed a low rate of statewide drug alert reporting (29.6%) and drug allergy testing in DRESS (20.5%). Inadvertent reexposure to a culprit or structurally related drug resulted in recurrent DRESS in 3 patients (7.5%), and tolerance of structurally related drugs occurred in 8 patients (17.5%).
Conclusion
In this large study evaluating DRE patients whose disease does not meet the RegiSCAR criteria for DRESS, we found that additional factors outside the RegiSCAR criteria may help clinicians differentiate DRESS, which is critical for optimal and timely patient management. Our study also highlights the need for development of local protocols to ensure appropriate allergy labeling and testing are performed to prevent recurrent DRESS.
Key words: Drug reaction, eosinophilia, systemic symptoms
Adverse cutaneous reactions to drugs are a frequent problem worldwide, affecting up to 2% to 3% of all hospitalized patients.1 Fortunately, most of these reactions are mild and self-limiting; however, a small proportion are severe cutaneous adverse drug reactions (SCARs), one of which is drug reaction with eosinophilia and systemic symptoms (DRESS).1 DRESS is a rare but potentially life-threatening drug hypersensitivity reaction characterized by hematologic abnormalities including eosinophilia and lymphocytosis, skin rash, and internal organ involvement, most commonly hepatitis, typically occurring 1 to 8 weeks after drug exposure.2 DRESS has been reported to have a mortality rate of up to 10% in some cohorts, so early recognition, diagnosis, and withdrawal of offending drugs is critical.3
The clinical presentation is often heterogeneous, with variable courses and severities of differing manifestations.2 Furthermore, many of the cardinal features—fever, rash, and organ involvement—are not specific to this syndrome and may be attributed to a wide range of other causes, such as infections or concomitant or preexisting diseases, often leading to a delay in diagnosis.4
Three diagnostic criteria currently exist for DRESS, which further add to diagnostic complexities;5,6 however, the Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria are the most widely accepted.2 In the original case series of 117 patients, in those with disease that met the criteria for probable or definite DRESS, there was minimal overlap between other SCARs.2
Despite this, in practice, the diagnosis of DRESS is often challenging. In 216 patients hospitalized with a cutaneous adverse drug reaction, 21% of patients were found to have at least 2 possible diagnoses, and the disease of 3 patients was impossible to classify.6 In a study comparing the performance of the 3 different criteria for DRESS, 80% of the patients with disease that met the RegiSCAR probable criteria or more satisfied the Bocquet criteria, but only 27.6% of these patients had disease that met the atypical drug-induced hypersensitivity syndrome criteria.5
Two of the key features of DRESS are rash and eosinophilia; when present, they should prompt clinicians to investigate for DRESS.7,8 However, both of these manifestations are common; cutaneous manifestations are estimated to occur in approximately 45% of all drug reactions,9 and in a prospective evaluation of eosinophilic drug reactions, the incidence was found to be 16.7 per 10,000 hospital admissions.8,10 Furthermore, in a cohort of 824 patients receiving intravenous antibiotic therapy, 25% of all patients developed eosinophilia.8 Therefore, it is not surprising that there is a subset of patients who have a combination of rash and eosinophilia with or without systemic involvement but whose cases do not fulfill the RegiSCAR criteria for probable or definite DRESS.
Methods
A retrospective search of Sir Charles Gairdner Hospital medical record coding classifications from 2008 to 2023 was performed to identify patients with DRESS and drug reaction with eosinophilia not fulfilling DRESS criteria (DRE) using international coding classifications (International Classification of Diseases, Tenth Revision, aka ICD-10): drug-induced fever (R502), generalized skin eruption due to drugs or medicaments (L270), and eosinophilia (D721). Patient details were collected from medical records of inpatient and outpatient visits, and laboratory results were accessed. All DRESS cases were confirmed by RegiSCAR criteria and were included only if they fit probable or definite DRESS classification (RegiSCAR ≥ 4). All other cases were characterized as DRE.
Liver involvement was defined as elevation in alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), or bilirubin >1.5 times the upper limit of normal; renal involvement was defined as a serum creatinine >1.5 times the upper limit of normal. Time of diagnosis was the date of rash development. Baseline laboratory parameters were defined as the lowest result available for ALT, ALP, GGT, bilirubin, creatinine, and eosinophils within 1 year before or after reaction onset; peak parameters were defined as the highest result for ALT, ALP, GGT, bilirubin, creatinine, eosinophils, and C-reactive protein (CRP) that occurred within 1 month before or 3 months after onset of reaction. Clinical characteristics and laboratory parameters of the 2 groups were compared. The study was approved by the Sir Charles Gairdner Hospital Quality and Safety Committee (GEKO 28972).
Data were summarized using means and standard deviations (SDs) for normally distributed variables, medians and interquartile ranges for nonnormally distributed variables, or counts and proportions for categorical variables. Associations between DRESS versus DRE patients and clinical features were assessed by the Fisher exact test where appropriate. For continuous measures, differences between the DRESS and DRE groups were assessed with 2-sample t tests; quantities that exhibited considerable positive skew were log-transformed or symmetric log high-transformed for measures that had zeroes. A large number of hypothesis tests were conducted to compare the DRESS and DRE groups; to mitigate type I error, all reported P values have been adjusted with the Holm procedure.11 All analyses were performed by R v4.3.1 statistical software (R Project; www.r-project.org), and statistical significance was set at P < .05.
Results
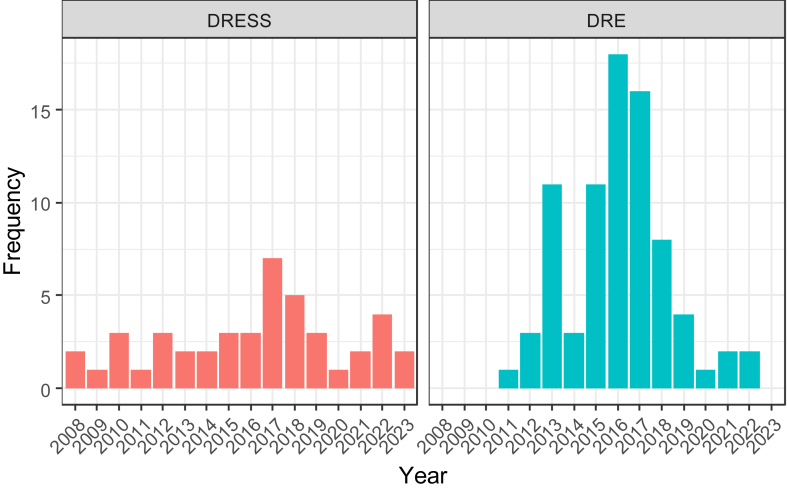
Data of 124 patients were analyzed, 44 (35.5%) of whom had DRESS. The overall mean (SD) age was 61 (19.6) years, with no significant sex predisposition (50.8% male) (Table I). There was no apparent change in the frequency of DRESS diagnosis over the 15-year period studied (Fig 1).
Table I.
Demographic data and outcomes of DRESS versus DRE patients
| Characteristic | DRESS (n = 44) | DRE (n = 80) | P value |
|---|---|---|---|
| Age (years) at diagnosis, mean (SD) | 61.2 (19.8) | 60.5 (19.5) | >.5 |
| Male sex | 66.7% | 33.3% | >.5 |
| Causative drug | >.5 | ||
| Antibiotics | 25 (56.8%) | 55 (68.8%) | |
| Other | 19 (43.2%) | 25 (31.3%) | |
| Underlying diagnosis | >.5 | ||
| Infection | 25 (56.8%) | 55 (68.8%) | |
| Other | 19 (43.2%) | 25 (31.3%) | |
| Unplanned readmission in 6 months | 35 (43.75%) | 26 (59.09) | >.5 |
| Specialist immunology/dermatology review | 37 (84.1%) | 47 (58.8%) | .146 |
Fig 1.
Frequency (cases per year) of DRESS and DRE during study follow-up.
The most common causative drug type was antibiotics (64.5%), which is in keeping with the most common underlying diagnosis of infection (64.5%) (Table I). Causative drug type and underlying diagnoses were similar between the DRESS and the DRE group. Approximately two thirds of patients were reviewed by immunology or dermatology specialist teams during the inpatient stay and specialist review.
Clinical features
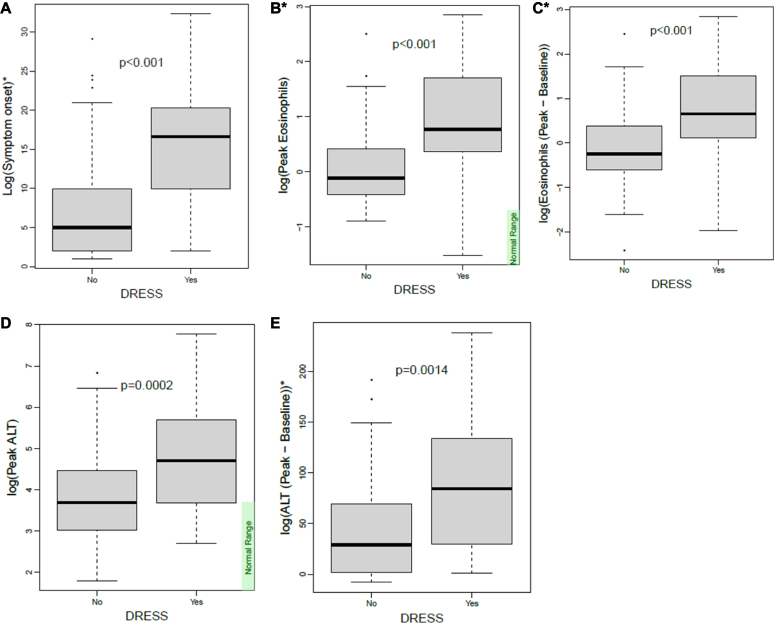
Time to rash onset after causative drug commencement was significantly longer in the DRESS group compared to the DRE group (median 21 vs 5 days, P < .001) (Fig 2). In keeping with the RegiSCAR criteria, patients with DRESS were more likely to have fever, facial swelling, and liver function derangement compared to those with DRE (Table II). Systemic corticosteroid treatment was provided to 65.91% of the DRESS group and 33.06% of the DRE group. Maculopapular rash was the most common rash morphology in both groups (64.9%) but was seen at a higher frequency in the DRESS group compared to the DRE group (odds ratio, 7.29; 95% confidence interval [CI], 2.31, 30.7; P = .005; Table II).
Fig 2.
Clinical and laboratory features in DRESS versus DRE patients. A, Time to symptom onset (days) in DRESS and DRE groups. B, Peak eosinophil count (×109/L) in DRESS and DRE groups. C, Peak minus baseline eosinophil count (×109/L) in DRESS and DRE groups. D, Peak ALT count (U/L) in DRESS and DRE groups. E, Peak minus baseline ALT count (U/L) in DRESS and DRE groups. ∗Variables included in RegiSCAR criteria.
Table II.
Clinical features of DRESS versus DRE
| Characteristic | DRESS (n = 44) | DRE (n = 80) | P value |
|---|---|---|---|
| Symptom onset (median days) | 21 | 5 | <.001 |
| Maculopapular rash | 40 (90.9%) | 46 (57.5%) | .005 |
| Facial swelling (missing data, n = 5) | 18 (40.91%) | 4 (5%) | <.001 |
| Fever∗ | 32 (72.73%) | 24 (30%) | <.001 |
| Liver involvement∗ (missing data, n = 3) | 36 (81.82%) | 73 (58.87%) | .001 |
| Renal involvement∗ | 15 (34.05%) | 30 (24.9%) | >.5 |
Feature included in RegiSCAR criteria.
Laboratory parameters
Patients in the DRESS group had significantly higher ALT levels at peak (increase by a factor of 2.49 [95% CI, 1.56, 4.00; P = .009]). The difference between peak ALT magnitude remained significant when corrected for baseline ALT levels (mean 272 vs 81.1 U/L, P = .0498) (Fig 2). There was no significant difference in GGT, ALP, or bilirubin levels at baseline, diagnosis, and peak between the 2 groups.
In keeping with the RegiSCAR criteria, patients with DRESS had significantly higher peak eosinophil counts (increased by a factor of 2.36 [95% CI, 1.72, 3.23; P < .001]), which remained significant when corrected for the baseline eosinophil level (increase by factor 2.42 [95% CI, 1.66, 3.51; P < .001], Fig 2). Almost all patients had peak eosinophilia, which occurred after the diagnosis was made (87.9%). The median time from diagnosis to peak eosinophilia was 4 days. Atypical lymphocytes were seen in 7 patients (15.9%) in the DRESS group and were not detected in any of the DRE patients (P = .019).
Renal involvement was seen in 11.3% of the DRESS group compared to 8.75% in the DRE group. There was no significant difference in creatinine levels between the 2 groups.
Other laboratory values including lymphocyte, ferritin, CRP, erythrocyte sedimentation rate, sodium, hemoglobin, neutrophil, and platelet levels were not significantly different between the groups (P > .05). There were insufficient numbers to analyze results on virus serology, procalcitonin, or serum IgE levels.
Outcomes
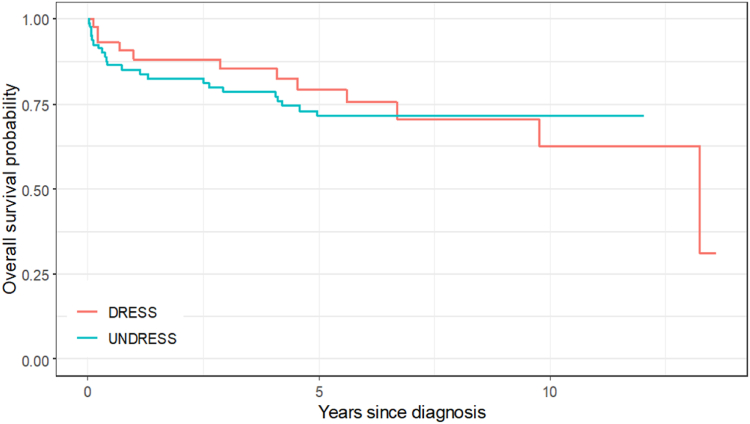
Unplanned readmission rates for all patients 6 months after the diagnosis were high (n = 61, 49.2%), and there were no differences between groups (P = .1823) (Table I). Mortality during admission was higher in the DRESS group, at 3 (7.5%) of 40 versus 1 (1.25%) of 80, but there was no difference in survival throughout the follow-up period (Fig 3). This may have related to the significant comorbidities in the patients in the DRE group. One patient developed chronic relapsing DRESS, and 3 patients experienced recurrent DRESS on inadvertent reexposure to culprit or structurally related drugs.
Fig 3.
Survival over time in DRESS versus DRE groups.
In the DRESS group, 79.6% of patients had their allergy listed on their discharge summary, but only 29.6% patients had a statewide clinical alert completed. Having specialist immunology or dermatology input during the admission did not alter this.
Of the 26 patients with DRESS resulting from antibiotic therapy; only 9 (34.6%) proceeded to have allergy testing performed. Of these 9 tested patients, 7 (77.8%) tested positive to either the culprit or a structurally related drug. In 17.5% of DRESS patients, longitudinal follow-up revealed tolerance of structurally related antibiotics despite testing only being performed in 2 patients (Table III).
Table III.
Structurally related antibiotics tolerated in DRESS
| Patient no. | Culprit | Testing | Tolerated |
|||
|---|---|---|---|---|---|---|
| Penicillin | Cephalosporin | Carbapenem | Glycopeptide | |||
| 1 | Piperacillin/tazobactam | — | Yes (flucloxacillin) | Yes (cephalexin) | Yes (meropenem) | — |
| 2 | Piperacillin/tazobactam, cephazolin, ciprofloxacin | — | — | — | Yes (ertapenem) | — |
| 3 | Ceftriaxone | Patch to ceftriaxone positive | Yes (piperacillin/tazobactam, amoxycillin) | — | — | — |
| 4 | Flucloxacillin, amoxycillin, ciprofloxacin | — | — | — | Yes (meropenem) | — |
| 5 | Meropenem, vancomycin | Patch to meropenem, vancomycin negative; IDT to β-lactams negative | Yes (amoxycillin) | — | — | — |
| 6 | Meropenem, vancomycin | — | — | Yes (ceftriaxone) | — | — |
| 7 | Vancomycin, rifampicin | — | — | — | — | Yes (teicoplanin) |
IDT, Intradermal testing.
Within the DRE group, 33 patients had rash and eosinophilia, but without any organ involvement. Subgroup analysis of these patients compared to the DRESS group did not reveal any additional findings.
Discussion
To our knowledge, this is the largest study examining an intermediate group of drug hypersensitivity reactions characterized by rash and eosinophilia that do not fulfill the criteria for probable or definite DRESS.7,12 Two other studies have previously reported on similar but much smaller cohorts of patients. Pinto Gouveia et al7 reported on 34 patients whose disease appeared to have overlapping features between maculopapular exanthema and DRESS but did not fulfill RegiSCAR criteria for probable DRESS, and Momen et al12 compared 19 patients with RegiSCAR < 3 (minor DRESS) with 26 patients with RegiSCAR > 4 (major DRESS). Both these studies, in keeping with our findings, reported that compared to a cohort of DRESS patients, these patients had a significantly shorter latency period between drug exposure and symptom onset. This is also in keeping with a recent systematic review that evaluated 151 cases of definite DRESS and demonstrated a latency of 24 days.13 In comparison to the other studies in our study, all cases were confirmed by detailed file review.
Consistent with our findings, higher peak eosinophil counts, higher ALT levels, and fever were also found to be significantly different in the major compared to minor DRESS group.12 We found ALT levels, as opposed to ALP or bilirubin levels, to be associated with DRESS, which is in keeping with the literature, which describes a hepatitic pattern of liver derangement to be most common.14 In contrast, our study found lower rates of facial swelling (5.6%) compared to the cohorts of Pinto Gouveia et al7 and Momen et al12 (respectively, 73% and 31.5%).
Numerous studies have reported an association between both the magnitude and presence of eosinophilia with cutaneous drug eruption severity.15 In 206 patients who developed eosinophilia during intravenous antibiotic therapy, patients who went on to develop a drug hypersensitivity reaction had an earlier onset of eosinophilia (11 vs 17 days) and a higher peak eosinophilia (0.857 vs 0.699).8 A number of other studies, both of DRESS and cutaneous adverse drug reactions, have shown a relationship between higher eosinophil counts and greater impairment of liver function, prolonged hospitalization, longer recovery time, and higher cumulative doses of corticosteroids.10,14,16
A new scoring algorithm combining eosinophil count, high-sensitivity CRP, and total body surface area rash involvement, known as the CET score, has recently been evaluated as a diagnostic tool in DRESS syndrome. It has been found to have a positive predictive value of 80.5% for DRESS syndrome compared to maculopapular exanthems. In contrast, we did not find CRP to be significantly different between our cohorts; we were unable to analyze total body surface given the retrospective nature of our study.17
We found that patients with DRESS were also more likely to require systemic corticosteroid therapy compared to those with DRE, but there was no difference in overall survival throughout the follow-up period. This finding is not surprising given the importance of corticosteroid treatment for the management of DRESS syndrome; however, a higher mortality rate may have been anticipated in the DRESS group. During follow-up, 7 patients with DRESS were found to have tolerated structurally related antibiotics. Cross-reactivity patterns in β-lactam DRESS are poorly understood, and as a result, recommendations are to avoid all β-lactam antibiotics until standardized testing in a specialized center is performed.18,19 Similarly, there may be a low but detectable risk of cross-reactivity among the glycopeptide class, but this requires further study.20 We provide some real-world data on future tolerance to structurally related antibiotics in DRESS suggesting that these may be tolerated in some patients, thus highlighting the importance of follow-up drug allergy testing in specialist centers. Of concern, however, is the fact that 3 patients developed recurrent DRESS after inadvertent reexposure to either the culprit drug or a related drug, and although most patients had their adverse drug reaction recorded on their discharge summary, few had a statewide clinical alert for their allergies completed or follow-up allergy testing arranged. In Australia, we have a statewide clinical alert system, which ensures that allergy recording extends beyond individual hospital systems. Development and implementation of standardized management protocols for DRESS may help improve drug allergy labeling, improve specialist testing, and prevent inadvertent reexposure and recurrent DRESS.
Our study has a number of limitations. It is retrospective in nature, so there are missing data on variables, which meant that we were unable to analyze certain parameters, such as the influence of human herpesviruses. The use of ICD-10 codes for retrospective identification of cases may also mean that some cases were missed. The definitions used for liver and renal involvement did not allow consideration of other possible causes, such as toxicity from medications, chronic liver or renal disease, or the underlying infection itself, and therefore are likely to have overestimated the frequency of these organs’ involvement. There were also limited data available on subsequent tolerance of other structurally related antibiotics. Furthermore, there is heterogeneity within the DRE group, which ranges from patients with rash and eosinophilia alone to patients with possible DRESS. Although subgroup analysis of these patients did not identify any additional signal, further studies with larger numbers separating this group out further may be of use.
Our study provides further evidence for a spectrum of eosinophilic drug reactions and suggests that the magnitude of increase in eosinophilia and ALT, as well as the duration of drug therapy before symptom onset, may be useful indexes to aid clinicians in differentiating patients who are more likely to have drug hypersensitivity with eosinophilia alone from DRESS. However, further larger prospective studies are required to further phenotype and define this group, which may allow for the development of useful prognostic and diagnostic biomarkers.
Disclosure statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Wolf R., Orion E., Marcos B., Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–181. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Kardaun S., Sekula P., Valeyrie-Allanore L., Liss Y., Chu C., Creamer D., et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Cabriales S.A., Rodríguez-Bolaños F., Shear N.H. Drug reaction with eosinophilia and systemic symptoms (DReSS): how far have we come? Am J Clin Dermatol. 2019;20:217–236. doi: 10.1007/s40257-018-00416-4. [DOI] [PubMed] [Google Scholar]
- 4.Kardaun S., Sidoroff A., Valeyrie-Allanore L., Halevy S., Davidovici B., Mockenhaupt M., et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.H., Koh Y.I. Comparison of diagnostic criteria and determination of prognostic factors for drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma Immunol Res. 2014;6:216–221. doi: 10.4168/aair.2014.6.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortonne N. Is DRESS syndrome a single entity or within a spectrum of adverse reactions to drug? Br J Dermatol. 2016;175:1142–1144. doi: 10.1111/bjd.14986. [DOI] [PubMed] [Google Scholar]
- 7.Pinto Gouveia M., Gameiro A., Coutinho I., Pereira N., Cardoso J., Goncalo M. Overlap between maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms among cutaneous adverse drug reactions in a dermatology ward. Br J Dermatol. 2016;175:1274–1283. doi: 10.1111/bjd.14704. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal K.G., Youngster I., Rabideau D., Parker R., Manning K., Walensky R., et al. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–1294.e1. doi: 10.1016/j.jaci.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Van D., Hieu C., Craig T. Drug-induced severe cutaneous adverse reactions: determine the cause and prevention. Ann Allergy Asthma Immunol. 2019;123:483–487. doi: 10.1016/j.anai.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez E., Medrano-Casique N., Tong H., Bellon T., Cabanas R., Fiandor A., et al. Eosinophilic drug reactions detected by a prospective pharmacovigilance programme in a tertiary hospital. Br J Clin Pharmacol. 2017;83:400–415. doi: 10.1111/bcp.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 12.Momen S., Diaz-Cano S., Walsh S., Creamer D. Discriminating minor and major forms of drug reaction with eosinophilia and systemic symptoms: facial edema aligns to the severe phenotype. J Am Acad Dermatol. 2021;85:645–652. doi: 10.1016/j.jaad.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Awad A., Goh M.S., Trubiano J.A. Drug reaction with eosinophilia and systemic symptoms: a systematic review. J Allergy Clin Immunol Pract. 2023;11:1856–1868. doi: 10.1016/j.jaip.2023.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Peyriere H., Dereure O., Breton H., Demoly P., Cociglio M., Blayac J.P., et al. Variability in the clinical pattern of cutaneous side effects of drug with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422–428. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Yang X., Li M. Peripheral blood eosinophil counts predict the prognosis of drug eruptions. J Investig Allergol Clin Immunol. 2013;23:248–255. [PubMed] [Google Scholar]
- 16.Drago F., Cogorna L., Agnoletti A., Parodi A. Role of peripheral eosinophilia in adverse cutaneous drug reactions. Eur Rev Med Pharmacol Sci. 2015;19:2008–2009. [PubMed] [Google Scholar]
- 17.Singh S., Vinay K., Bishnoi A., Parsad D., Kumaran M. A prospective observational study validating the CET score as a screening tool in suspected DRESS syndrome. Int Journal Dermatol. 2024;63:1178–1184. doi: 10.1111/ijd.17080. [DOI] [PubMed] [Google Scholar]
- 18.Thompson G., McLean-Tooke A., Lucas M. Cross with caution: antibiotic cross-reactivity and co-reactivity patterns in severe cutanoeus adverse reactions. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.601954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan D., Banerji A., Blumenthal K., Phillips E., Solensky R., White A., et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150:1333–1393. doi: 10.1016/j.jaci.2022.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Nakkam N., Gibson A., Mouhtouris E., Konvinse K.C., Holmes N.E., Chua K.Y., et al. Cross-reactivity between vancomycin, teicoplanin, and telavancin in patients with HLA-A∗32:01–positive vancomycin-induced DRESS sharing an HLA class II haplotype. J Allergy Clin Immunol. 2021;147:403–405. doi: 10.1016/j.jaci.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]