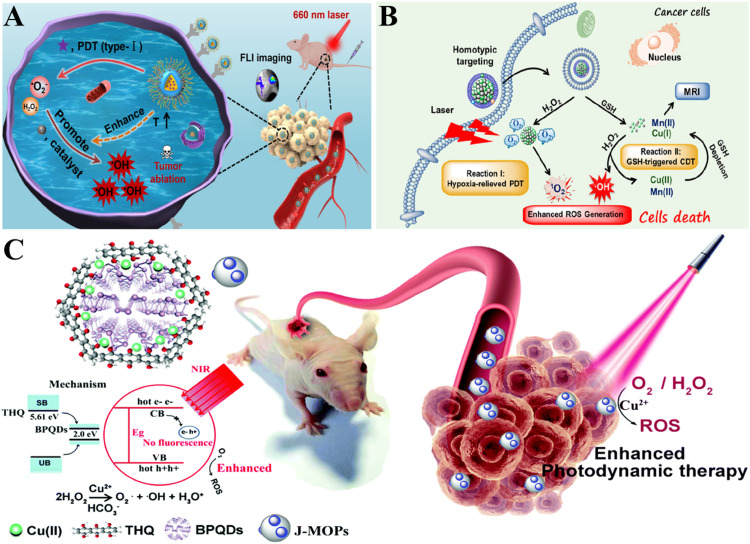
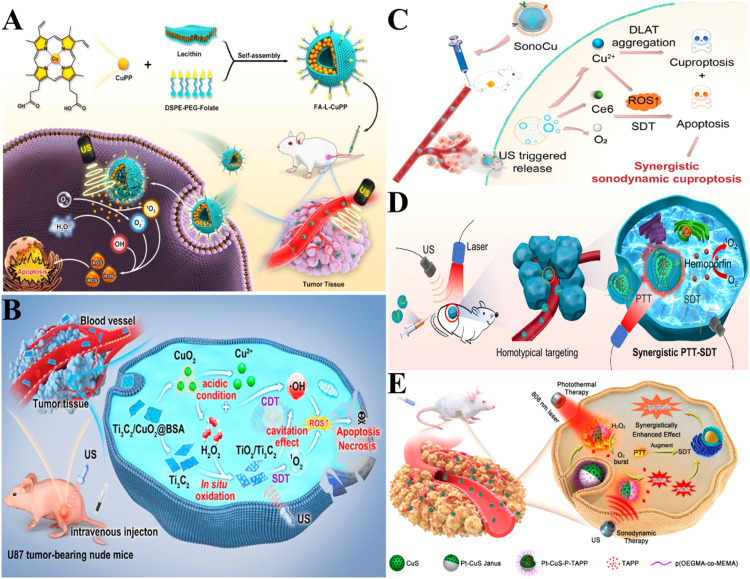
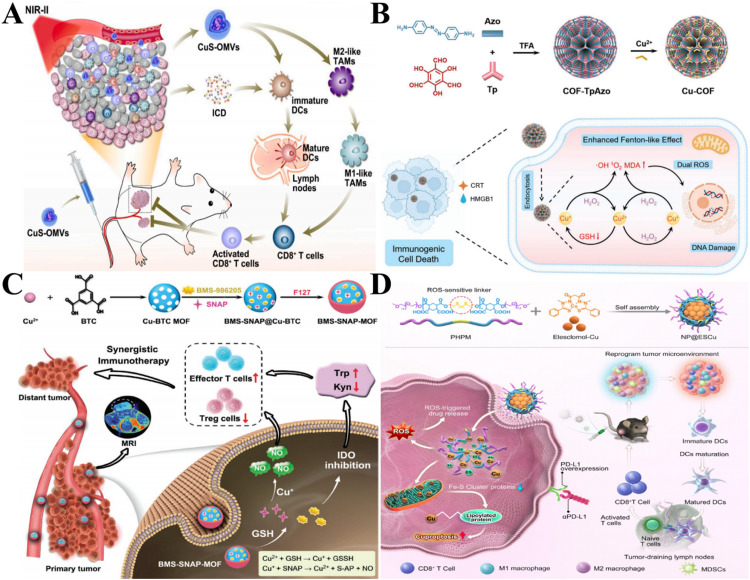
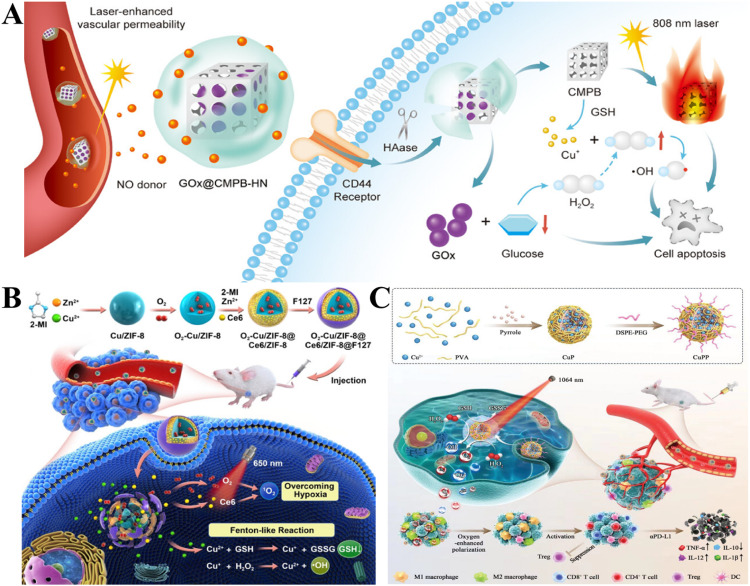
Abstract
As the third essential trace element in the human body, copper plays a crucial role in various physiological processes, which lays the foundation for its broad applications in cancer treatments. The overview of copper, including pharmacokinetics, signaling pathways, and homeostasis dysregulation, is hereby discussed. Additionally, cuproptosis, as a newly proposed cell death mechanism associated with copper accumulation, is analyzed and further developed for efficient cancer treatment. Different forms of Cu-based nanoparticles and their advantages, as well as limiting factors, are introduced. Moreover, the unique characteristics of Cu-based nanoparticles give rise to their applications in various imaging modalities. In addition, Cu-based nanomaterials are featured by their excellent photothermal property and ROS-associated tumor-killing potential, which are widely explored in diverse cancer therapies and combined therapies. Reducing the concentration of Cu2+/Cu+ is another cancer-killing method, and chelators can meet this need. More importantly, challenges and future prospects are identified for further research.
Keywords: Copper homeostasis, Tumor theranostics, Cu-based nanoparticles, Cuproptosis, Chelators
Graphical abstract
1. Introduction
With the development of nanotechnology, the use of metal-based nanoparticles has become increasingly prevalent in industrial, medical and consumer products [1]. In the medical field, tumor theranostics based on metal-based nanoparticles has drawn much attention. Currently, metal nanoparticles such as copper (Cu), iron (Fe), manganese (Mn), gold (Au), silver (Ag), and gadolinium (Gd), are extensively studied for their roles in tumor theranostics. [2]. The advantages and disadvantages of these nanoparticles are listed in Table 1. Among them, copper-based nanoparticles (Cu-based NPs) have drawn extensive attention due to their low cost, easy availability, simple synthesis and wide application.
Table 1.
Comparison between metal-based theranostic agents.
| Metal-based theranostic agents | Advantages | Limitation | Ref. |
|---|---|---|---|
| Cu |
|
|
[3,4] |
| Fe |
|
|
[5,6] |
| Mn |
|
|
[7,8] |
| Ag |
|
|
[9,10] |
| Au |
|
|
[11,12] |
| Gd |
|
|
[13,14] |
Cu has transformed from being a crucial metabolic cofactor to a signaling transducer and metalloallosteric regulator, highlighting its potential applications in tumor theranostics. The complex interplay between Cu and cancer underpins the understanding of Cu's potential therapeutic benefits. As an essential trace element, Cu mediates various critical physiological processes, including angiogenesis, mitochondrial respiration, antioxidant defense, and the biosynthesis of hormones and neurotransmitters [15]. Additionally, Cu is closely linked to cancer through various signaling pathways, such as the PI3K-AKT, MEK-ERK, and RTK pathways. Therefore, Cu dyshomeostasis can lead to oxidative stress, cytotoxicity, and even tumor development. High levels of Cu can inhibit the activity of proteasome and induce apoptosis of human cancer cells. Cu deficiency has been associated with neutropenia, anemia, cardiovascular deficits, acquired perforating dermatosis, osteoporosis, and retinal degeneration, while Cu overload contributes to liver failure and neurodegeneration primarily due to the production of reactive oxygen species (ROS) through the Fenton and Haber–Weiss reactions [16]. Conversely, manipulating Cu contents in tumor cells may provide novel cancer treatment strategies. Cuproptosis, a newly recognized cell death caused by excessive Cu, offers inspiration for cancer treatment. Compared with normal cells, tumor cells require more Cu to maintain the energy requirements of rapid cell division, but it is still insufficient for cuproptosis. Therefore, various methods of supplementing Cu ions to reach the threshold of cuproptosis for treating tumors and the existing challenges are thoroughly discussed. Similarly, studies have demonstrated the angiogenesis-promoting effect of Cu ions. Therefore, reducing Cu concentration will inhibit blood vessel synthesis and starve cancer cells to death. Cu chelators can decrease Cu ion concentrations in cells, and Cu deficiency induced by tetrathiomolybdate can inhibit angiogenesis, thus restraining tumor growth [17].
Inorganic nanomaterials are thriving and equipped with exceptional thermal, optical, catalytic, electrical, tumor-targeting, and magnetic properties. As outstanding photosensitizers and carriers owing to eminent drug-loading capacity and optical characteristics, the inorganic nanomaterials largely fill the gap in biomedicine [18]. Therefore, it is not surprising to witness the thrive of nascent Cu-based NPs, given their earth-abundance, inexpensiveness, photothermal-conversion performance, antimicrobial activity, biocompatibility, and surface activity [19]. Here, commonly used forms of Cu-based NPs, including monoatomic Cu materials, Cu metal derivatives, Cu complexes, and Cu-MOFs, are examined to provide a broad view of the characteristics and potential uses of Cu-based nanoparticles. Moreover, Cu-based NPs are widely applied in tumor imaging, and exhibit the following characteristics: (1) excellent photothermal conversion efficiency and high signal-to-noise ratio for photoacoustic (PA) imaging; (2) existence of unpaired electrons and the proper relaxation efficiency for magnetic resonance (MR) imaging; (3) β-ray emitting ability of 64Cu for positron emission tomography (PET) imaging; (4) high specific activity, high radionuclide purity, and sufficient quantity of 67Cu for single-photon emission computed tomography (SPECT) imaging; (5) prominent X-ray attenuation behavior of Cu for computed tomography (CT) imaging; (6) stable, controllable, and highly luminous Cu-metal nanoclusters (CuNCs) for fluorescence (FL) imaging. Furthermore, Cu-based NPs exhibit potential in various cancer therapies, including photothermal therapy (PTT), photodynamic therapy (PDT), chemodynamic therapy (CDT), sonodynamic therapy (SDT), and immunotherapy. Single therapy is often insufficient for efficient cancer treatment, while combination of these therapies shows more satisfactory anti-tumor results.
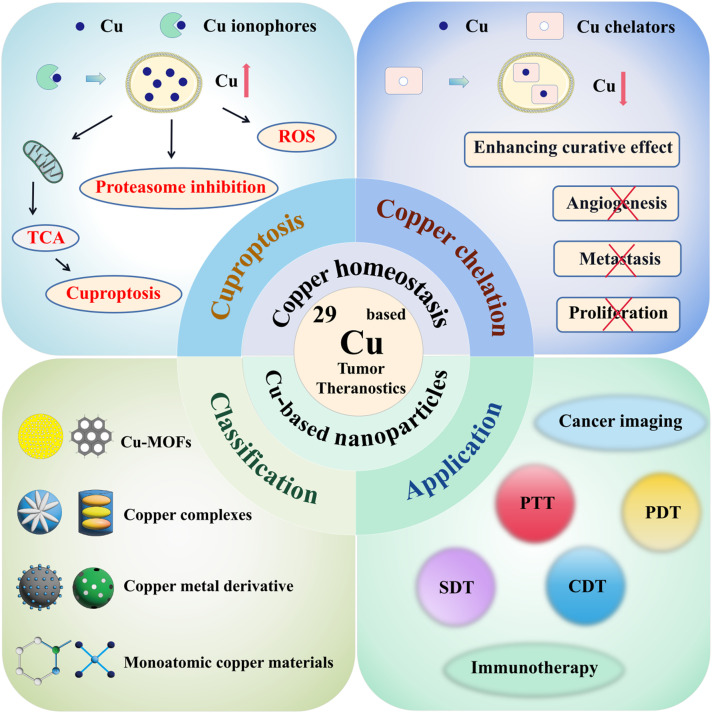
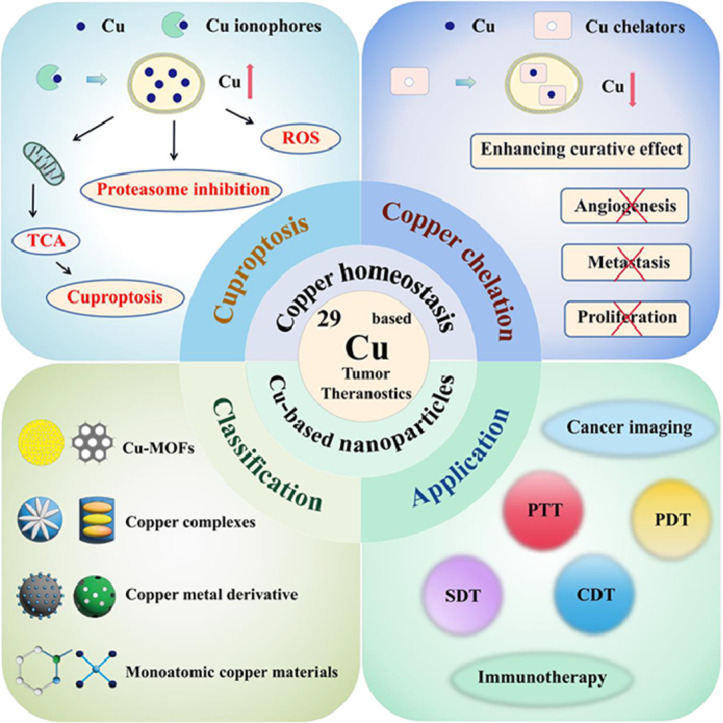
With the increasing diversity and expansion of Cu-based tumor theranostics, a systematic overview of Cu-based tumor theranostics is urgently needed [20]. Here, we review Cu homeostasis and the impact of disrupting Cu homeostasis (cuproptosis and Cu chelation) on cancer, providing a systematic summary of cancer therapies that target Cu homeostasis. We also identify current challenges and propose strategies to improve treatment outcomes. We then summarize Cu-based nanoparticles that may be used for cuproptosis and different cancer treatments, analyzing the advantages and disadvantages of various materials, which can provide guidance for selecting nanomaterials and designing delivery platforms in future research. Finally, we review recent advancements in Cu-based nanoparticles for tumor theranostics and discuss potential key challenges and future opportunities for Cu-based cancer therapies. In conclusion, this review provides a clearer framework for comprehensively studying Cu-based tumor theranostics and proposes new ideas for the clinical translation of Cu-based tumor theranostics. The schematic illustration of Cu-related tumor theranostics is exhibited in Fig. 1.
Fig. 1.
Schematic illustration of regulating Cu homeostasis and Cu-based nanoparticles for tumor theranostics. The mechanisms of cuproptosis and Cu chelation, classification of Cu-based NPs, and application are involved.
2. The relationship between Cu and cancer
Cu is an essential trace element as well as important cofactor for all organisms, occupying an important position in maintaining enzyme activity and transcription factor function. Importantly, the relation between Cu and cancer has long been verified, and many studies have shown that tumors have a higher demand for Cu than normal tissue. Cu promotes tumor angiogenesis, leading to tumorigenesis, growth and metastasis.
2.1. Pharmacokinetics of Cu
The processes of ADME (absorption, distribution, metabolism and excretion) are crucial for maintaining Cu homeostasis in the body:
Absorption: Cu normally enters mammals through the alimentary tract. Dietary Cu is absorbed primarily in the small intestine, with some absorption occurring in the stomach. Cu uptake in the intestine epithelium is mainly facilitated by the high-affinity Cu transporter, CTR1. Following uptake, Cu is transferred through chaperone ATOX1, which delivers the metal ion to Cu-ATPases, ATP7A [21]. In intestinal epithelial cells, ATP7A traffics towards the basolateral membrane, facilitating the absorption of dietary Cu into the bloodstream.
Distribution: Once absorbed, Cu is bound to albumin, transcuprein, and amino acids in the blood, with the majority of the Cu ion being transported by ceruloplasmin. The absorbed Cu is transported to the liver through portal venous circulation and stored, then distributed to various tissues, including the brain, kidneys, and heart, where it is utilized. Within cells, the concentration of Cu is tightly regulated, and its distribution is controlled by metallothioneins, glutathione (GSH), and metallochaperones, which ensure proper delivery to cuproenzymes while avoiding toxic free Cu ions [22].
Metabolism: Cu is incorporated into cuproenzymes, such as cytochrome c oxidase, superoxide dismutase, and ceruloplasmin, during their biosynthesis. This process involves several metallochaperones that transfer Cu to specific proteins, such as COX17 for cytochrome c oxidase and CCS for Cu/Zn-superoxide dismutase. In addition to cytosolic enzymes, Cu is also incorporated into secretory pathway cuproenzymes via ATP7A or ATP7B, which are embedded in the trans-Golgi network [23].
Excretion: Excess Cu is primarily eliminated through the liver via the bile, while only a little fraction is excreted through the kidneys in the urine. The excess Cu in the hepatocytes is transported through endolysosomal vesicles containing ATP7B into lysosomes, which subsequently release Cu across the canalicular (apical) membrane into the bile for excretion [24].
2.2. Cu signal pathway
2.2.1. Cancer-associated signaling pathways
Accumulating studies have proved that Cu is involved in the tumorigenesis of various cancers by modulating multiple signaling pathways. This section will discuss the interplay between Cu and cancer-associated signaling pathways, focusing on the PI3K-AKT and MEK-ERK signaling cascades.
-
(1)
PI3K-AKT signaling pathway: Cu facilitates tumorigenesis through activating the PI3K-AKT oncogenic signaling pathway, thereby facilitating tumorigenesis. Specifically, Cu binds 3-phosphoinositide-dependent protein kinase 1 (PDK1), promoting its interaction with its downstream substrate AKT and subsequently activating AKT [25]. In this way, depleting CTR1 or applying Cu chelators can diminish AKT signaling, therefore reducing tumorigenesis. Abnormally elevated CTR1 levels have been observed in breast cancer, which is subject to negative regulation by the NEDD4-like E3 ubiquitin-protein ligase. Therefore, this protein ligase exhibits tumor-inhibitive ability through inhibiting the CTR1-AKT signaling pathway [26].
-
(2)
MEK-ERK signaling pathway: ERKs are serine/threonine protein kinases that transmit mitogenic signals and serve as downstream targets for various growth factors. Studies have demonstrated that increased Cu ion concentrations lead to elevated expression levels of phosphorylated ERK, indicating that Cu ions can induce ERK phosphorylation within cells, thereby promoting cell proliferation [27]. Cu ions can bind MEK in an antioxidant 1 Cu chaperone (ATOX1)-dependent manner, subsequently activating the MEK-ERK signaling pathway. The absence or mutation of CTR1 in cells can reduce MEK1′s ability to phosphorylate ERK, suggesting that MEK1-ERK interactions require Cu ion involvement [28].
2.2.2. Other signaling pathways
In addition to its involvement in cancer-associated signaling pathways, Cu also plays a role in other cellular signaling processes.
-
(1)
Hypoxia: Adaptation to hypoxia is a driving force for tumor development. Hypoxic stimuli can induce the expression of lysyl oxidase (LOX), which is a secreted Cu-dependent amine oxidase that promotes tumor cell migration and adhesion. ATP7A is required for LOX activity, and the activity is reduced upon cytoplasmic Atox1 silencing. Therefore, Cu ions can influence the ATOX-ATP7A-LOX pathway, thereby promoting cancer cell metastatic expansion [29]. In addition, hypoxia-inducible factor-1 (HIF-1) regulates the expression of the vascular endothelial growth factor (VEGF), a process requiring Cu participation [30].
-
(2)
Autophagy: Cu ions can trigger autophagy, a protective mechanism that allows cells to survive a variety of stress conditions such as nutrient deprivation, hypoxia and DNA damage. Cu can produce ROS through Fenton or Fenton-like reaction, which will trigger autophagy pathway to enhance cellular defense. However, the overactivated autophagy may accelerate cell death. In addition, Cu can induce autophagy through increasing expression of autophagy-related proteins and regulating the AMPK-MTOR pathway. For instance, Cu directly binds to and activates the autophagic kinases ULK1 and ULK2 to induce autophagy [23]. The loss of the Cu exporter ATP7B or Cu imbalance can also lead to autophagy. Moreover, Cu can induce autophagic degradation of glutathione peroxidase 4 to promote ferroptotic cell death [31].
-
(3)
Inflammation: Inflammation is a kind of defensive response to harmful stimuli and is a precursor to several diseases, including cancer. The high expression of CD44 on macrophages is usually a marker of inflammation, while CD44 mediates the endocytosis of Cu bound hyaluronates in cancer cells [32]. The upregulation of CD44 will result in macrophage activation and increase of mitochondrial Cu2+. Chemically reactive Cu2+ largely exists in the mitochondria of inflammatory macrophages, which catalyzes NAD(H) redox cycling by reacting with H2O2. To overcome this dilemma, supformin (LCC-12), a dimer of metformin, was designed to target mitochondrial Cu2+, induce a reduction of the NAD(H) pool, and lead to macrophage deactivation [33].
In conclusion, Cu is crucial in various signaling pathways involved in cancer and other cellular processes. The interplay between Cu and these signaling pathways contributes to the complexity of cellular responses in different contexts.
2.2.3. Toxicological study of Cu
Owing to the regulation mechanism of Cu metabolism, Cu toxicity is relatively low in human body. The concentration of Cu in tissues or body fluids is relatively constant, with a range of less than 50 µg/g. When the intake of Cu in drinking water reaches 6 mg/L [equivalent to 0.14 mg/kg per day (bodyweight)], obvious gastrointestinal reactions can be observed, such as nausea and vomiting [34]. To compare the toxicity of Cu-based NPs with bulk Cu, CuO NPs and CuSO4 were investigated. Results showed that LD50(14) of CuO NPs was 400 mg·b·wt/kg, threefold higher than of CuSO4. Moreover, compared with CuSO4, CuO NPs (≥5 mg·b·wt/kg) induced greater oxidative stress, disrupt blood brain barrier (BBB) and coax toxicity in liver, kidney and spleen [35]. In addition, exposure to CuO NPs at dose concentrations of 200, 133.3 and 100 mg/kg body weights leads to different degrees of developmental deformities in mice [36]. NPs have distinct physicochemical properties, such as high surface‐to‐volume ratio and various surface morphologies, resulting in unique reactivity and toxicological mechanisms owing to abundant reactive sites. Studies have shown that smaller Cu-based NPs had higher surface reactivity and facilitated intracellular transport. The respiratory tract is the most common route for Cu-based NPs exposure, gastrointestinal tract and affected skin are other potential ways [37].
Ionic Cu has been proved to be a carrier of electrons along the mitochondrial electron transport chain. The addition of Cu ions can interfere the electron transmission in the mitochondria to cause mitochondrial damage, and trigger perturbation of cellular cation homeostasis and ROS production, which lead to break of DNA strand and hinder of transcription. Upon Cu-based NPs exposure, MAPK and RTK pathways are activated, resulting in a pro-inflammatory cascade [38]. Moreover, Cu ions can chelate atoms in active regions and deactivate biomolecules, thus impeding physiological processes [39].
Surface modification is a potential method to control toxicity of NPs. For instance, CuO NPs treated with 8-mercaptooctanoic acid exhibited substantial ROS activity [40]. Tween-20-modified Cu2S NPs showed improved biocompatibility and physiological stability, and chemotherapeutic drug Dox can be loaded owing to the modification [41]. In addition, Cu can decrease the toxicity of chemo-drugs. Cu-based preparations can generate oxygen radicals to deactivate cell-free chromatin particles (cfChPs), a substance that can cause chemotherapy toxicity, so as to realize decreased toxicity of docetaxel-based chemotherapy [42].
2.3. Effects of Cu homeostasis on cancer
The intrinsic redox properties of Cu make it a double-edged sword to cells. Cu2+ and Cu+are the two oxidation states of Cu, in which Cu+ is considered to be the main form in the cellular cytosol-reducing environment. Meanwhile, the dysregulation of Cu concentration can lead to oxidative stress and cytotoxicity. Cu is also involved in the different stages of the cancer. Cu at high levels inhibited the activity of proteasome in human cancer cells. It is well known that Cu levels in the human body need to be maintained in a narrow range. From this point, intentionally raising or lowering Cu levels in tumors may be a new strategy for treating tumors.
In past studies, Cu can cause apoptosis, pyroptosis, and even drive ferroptosis [43]. Moreover, the behavior of promoting Cu accumulation in tumors can affect the tumor cells and even induce cell death. However, the underlying mechanism of excess Cu-induced cell death has not yet been revealed, and cuproptosis as an emerging form of cell death offers an acceptable explanation. Studies have shown that cuproptosis relies on mitochondrial respiration. By directly binding to lipoylated proteins in the TCA cycle, Cu ions cause abnormal aggregation of lipoylated proteins and interfere with iron-sulfur cluster proteins in the respiratory chain complex, resulting in a protein-toxic stress response and, ultimately, cell death. FDX1 and six genes involved in protein lipoylation are key genes that promote cuproptosis, which are essential for mitochondrial aerobic metabolism [44]. While excess Cu accumulation can be life-threatening, a more concentrated increase in intracellular Cu can selectively kill cancer cells. At present, Cu ionophores provide the most direct way for the cuproptosis, and the development of Cu-based nanomaterials has also broadened the way for the discovery of new Cu ionophores [45].
Cu is required to meet the energy demands of rapidly dividing cells because mitochondrial cytochrome c oxidase requires Cu as a cofactor. Thus, cancer cells have a higher Cu requirement owing to their strong cell division ability. In addition, the high Cu content is also related to angiogenesis in tumor tissue. Therefore, Cu can promote tumor angiogenesis, leading to tumorigenesis and metastasis [46]. The blood vessels in the tumor are undoubtedly the highways that provide a steady stream of nutrients to support the rapid proliferation of tumor cells. With this as an entry point, reducing Cu in tumors may become a viable strategy to inhibit tumor growth. Cu chelators play a central role in this strategy, which was first used to treat a progressive Cu transport disorder called Wilson's disease. The Cu secretion into bile is reduced, which leads to Cu depletion in the liver. In the treatment of Wilson's disease, Cu chelators bind excess Cu and promote Cu excretion in the urine. The mechanism of Cu chelators in tumor treatment is highly similar to that of Wilson disease. In fact, many studies have reported novel tumor-involved Cu chelators [47].
To achieve a better therapeutic strategy, it is more desirable to see a unilateral killing of tumor cells. Therefore, both Cu ionophore, which triggers various modes of cell death by increasing the Cu content, and Cu chelator, which inhibits tumor growth by decreasing the Cu content, require good selectivity and targeting. Cu ionophores can be designed to release Cu through a TME-responsive mode. Moreover, the concept of pre-chelators is proposed [48], which appear non-toxic in healthy cells, because the chelation is selectively performed in tumor cells. Furthermore, desirable size of Cu ionophores and Cu chelators can realize selective retention in tumors.
3. Cuproptosis—“increasing Cu” to kill cancer
As an essential trace element, Cu is involved in cell proliferation and cell death [23]. In vivo, Cu is mainly transported by binding to ceruloplasmin. Ceruloplasmin has an enzymatic activity and participates in various important biological processes, including antioxidant defense, mitochondrial respiration, and the biosynthesis of pigments, hormones, and neurotransmitters [20]. Cu is involved in almost all stages of cancer development, and undoubtedly Cu homeostasis exists in tumors [45]. When the concentration of Cu2+/Cu+ in cells exceeds the threshold of maintaining the Cu homeostasis mechanism, cytotoxicity appears [49]. Cuproptosis provides evidence that increasing the amount of Cu in tumor tissue kills tumor cells. In addition, cuproptosis has broadened the field of cancer therapy.
3.1. Mechanism of cuproptosis
Cuproptosis is a novel mechanism different from the currently known cell death mechanisms, which is verified through exclusive methods [44]. At present, the discovered mechanism of ferroptosis is through depletion of GSH, the decrease of GSH peroxidase (GPX4) activity and cellular antioxidant capacity, which results in lipid peroxidation, metabolic dysfunction, and the increase of lipid ROS, thereby causing ferroptosis [50]. Unlike ferroptosis, cuproptosis affects cancer cell death more at the mitochondrial respiration level during the TCA cycle.
The mechanism is as follows [44]: First, Cu binds to the lipoylated proteins in the TCA cycle, leading to an aberrant oligomerization of the lipoylated proteins. Protein lipoylation is a highly conserved post-translational modification of a lysine that regulates protein function by attaching lipoic acid groups to the lysine residues of the substrate protein. Therefore, the binding of Cu to lipoylated protein leads to the cell toxicity. A genome-wide CRISPR-Cas 9 loss-of-function screen is performed to identify specific metabolic pathways mediating Cu toxicity. Studies show that the seven related genes, including FDX1, which encodes a known reductase that reduces Cu2+to Cu+, and lipoyl transferase 1, lipoic acid synthase (LIAS), dihydrothiocenamide dehydrogenase, dihydrothiocenamide S-acetyltransferase (DLAT), pyruvate dehydrogenase E1-al subunit and β subunits, are the key genes to promote cuproptosis. Knockdown of the above genes rescues the cells from Cu toxicity. Knockdown of FDX1 represses the expression of the lipoylated proteins in the cells and exhibits a marked TCA cycle inhibition. Such results confirm that FDX1 is an upstream regulator of proteolipid acylation. Thus, FDX1 and proteoliylation are key regulators of cuproptosis.
The above findings are not only verified in the ionophore Elesclomol (ES) application experiment, but also can be proved using CuCl2. Biochemical CuCl2 supplementation also leads to decreased expression of mitochondrial respiratory protein lipodylated protein (DLAT, DLST) and Fe-S cluster protein (FDX1, etc.) in A673 cells. Both FDX1 and LIAS knockdown reversed CuCl2-induced cell death, and depletion of GSH by Cu chelators promoted CuCl2-induced cell death.
Thus, cuproptosis occurs through the direct binding of Cu to the lipoylated components of the TCA cycle, via aggregation of lipoylated proteins and subsequent loss of Fe-S clusters, causing proteotoxic stress and eventual cuproptosis (Fig. 2A) [44]. In addition, cuproptosis kills cancer cells by affecting mitochondrial respiration. However, not all tumor cells perform mitochondrial respiration. Warburg effect shows that the main pathway for most tumor cells is glycolysis. Oxidative phosphorylation, on the other hand, provides a smaller portion of the energy supply. Therefore, it is speculated here that cuproptosis is more effective in tumor cells that use more oxidative phosphorylation capacity.
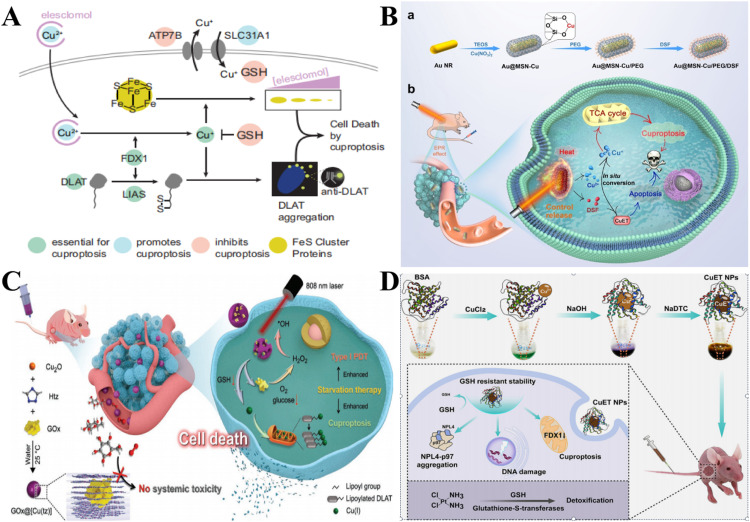
Fig. 2.
Mechanism and application of cuproptosis. (A) Mechanism illustration of cuproptosis [44]. Copyright 2022, Science. (B) The preparation process of Au @ MSN-Cu/polyethylene glycol /DSF and its cuproptosis and other functions in tumor treatment [54]. Copyright 2022, Wiley. (C) The synthesis of nonporous GOx@[Cu(tz)] and starvation enhanced cuproptosis and photodynamic therapy [55]. Copyright 2022, Wiley. (D) The synthetic process and anticancer mechanism of CuET NPs [56]. Copyright 2022, the Royal Society of Chemistry.
Since the concept of cuproptosis was proposed, dozens of genes related to cuproptosis have been discovered so far. Moreover, the study of the role of different genes in different cancers has gradually been enriched. For example, Liu et al. [51] performed genomic analysis on samples from more than 9000 cases of over 30 cancer types. In different types of cancer, the cuproptosis genes that play a role may be different. Among them, brain cancer may be more affected by cuproptosis. Conversely, genes associated with cuproptosis are less expressed in some other types of cancer, such as clear cell carcinoma of the kidney, where cuproptosis's activity is lower.
As the study deepened, more in-depth excavations of cuproptosis genes appeared. More and more studies are conducted to predict cancer prognosis using cuproptosis models. For example, Liu et al. [52] applied a set of bioinformatics tools to probe the expression of cuproptosis genes in lung adenocarcinoma and analyze prognostic results. After analyzing the data of different lung adenocarcinoma patients, 9 genes were found to had prognostic value. Subsequently, the five cuproptosis models constructed are verified and evaluated, which could be well classified. The more important finding was that features associated with cuproptosis might influence tumor prognosis by influencing tumor immunity. The method of predicting small molecule compounds based on differentially expressed genes provides a feasible solution to improve the poor prognosis of high-risk groups. With the deepening of research, the identification of cuproptosis-related genes and the construction of risk models for some cancers with high malignancy and poor prognosis have also emerged. For example, Li et al. [53] tested the cuproptosis gene for ovarian cancer and constructed a risk model based on the cuproptosis genes. Through evaluating the prognostic value and therapeutic sensitivity, specific molecular subtypes were found to have excellent prognostic potential.
The mechanism of cuproptosis is being further studied, and the related genes are still being proposed. For the identification of relevant gene expression, the role of different genes in different cancers is summarized and classified, which is beneficial for personalized, safe and effective tumor theranostics.
3.2. Cu2+/Cu+ ionophores —the method for cuproptosis
Cuproptosis enriches the pathways for inducing tumor cell death, and also encourages research to deeply explore new treatments for cuproptosis to kill tumors. Although tumor cells contain more Cu than normal cells, the Cu content in tumor cells has not yet broken through the threshold of cuproptosis, thus requiring exogenous Cu intake. The upregulation of Cu ions in tumor cells is critical. Cu2+/Cu+ ionophores as anti-cancer agents may have great potential in cuproptosis [57]. They can deliver Cu into cancer cells, so that the concentration of Cu2+/Cu+ in cancer cells exceeds the threshold to initiate and participate in the cuproptosis process.
At present, different kinds of Cu ionophores have already existed as anti-cancer agents such as dithiocarbamates, bis(thiosemicarbazone) ligands, 8-hydroxyquinolines (HQs), flavones, etc. Among them, the most widely studied are pyrrolidine dithiocarbamate and diethyldithiocarbamate (DTC). DTC is the active form of the better-known disulfiram (DSF). Studies have shown that DSF is an excellent anti-cancer drug against many types of cancer cells. The combination of DSF and Cu significantly increased the anticancer effect [58]. Multiple studies have shown that increased Cu concentration results in greater toxicity of DSF. This provides the basis for promoting cuproptosis in cancer cells. Mounting evidence has demonstrated that Cu-DSF has several targets, including ROS levels, the ubiquitin-proteasome system (UPS), NF-κB and NPL4 [59].
Cu2+/Cu+ ionophores break cellular Cu homeostasis and increase Cu levels in tumor cells. This non-selective drawback can cause Cu overload in normal tissues, which in turn leads to the killing of healthy cells. Therefore, improving their selectivity and targeting ability has become a top priority [60]. To solve the above problems, Zhou et al. [54] constructed a mesoporous silica-coated gold nanorod functional carrier doped with Cu ions loaded with DSF, which released Cu2+ and DSF under photothermal trigger for the controlled conversion of non-toxic materials to toxic drugs. The photothermal trigger not only controlled the in-situ release of Cu2+ and DSF in the cell, converted Cu2+ and DSF to generate toxic Cu+ and CuET, but also promoted the cuproptosis and apoptosis of cancer cells. Moreover, by rising the local temperature of the tumor, synergistic treatment was achieved (Fig. 2B).
The application of cuproptosis is still in the less mature stage, and the treating effect of cuproptosis alone has not achieved satisfactory results, while combination with other therapies may be the solution. A glucose oxidase (GOx) engineered non-porous Cu+ 1,2,4-triazolidate ([Cu(tz)]) coordination polymer (GOx@[Cu(tz)]) was developed and used for starvation therapy enhanced cuproptosis and photodynamic synergistic therapy [55]. The GOx was inactive before entering cancer cells and only after being stimulated by GSH in cancer cells would it be “turned on” to efficiently consume glucose, resulting in starvation. The depletion of glucose and GSH sensitized cancer cells to GOx@[Cu(tz)]-mediated cuproptosis, producing aggregation of lipidized mitochondrial proteins, which were targets for Cu-induced toxicity. Intracellular H2O2 levels increased further as glucose was oxidized, activating type I PDT for GOx@[Cu(tz)] (Fig. 2C).
Furthermore, cuproptosis could also be applied to reverse the problem of drug resistance. The coordination compound of Cu and disulfiram, Cu diethyl dithiocarbamate(II) (CuET), had an inert GSH response-ability and its carcinostatic activity was almost unaffected by the high GSH concentration in cancer cells, and could also effectively reverse cisplatin resistance by inducing cuproptosis in cancer cells, providing ideas for apoptotic tolerance of tumor treatment [61]. CuET had a low reduction potential and GSH response inertia, and was not disturbed by high levels of GSH in A549/DDP-resistant cells, thus exhibiting high anticancer activity in cisplatin-sensitive and drug-resistant cancer cells. The toxicity of cisplatin in A549/DDP cells was greatly reduced, and GSH levels in drug-resistant cancer cells were significantly reduced. Lu Yao et al. [56] prepared bovine serum albumin-stable CuET nanoparticles (CuET NPs). In this way, the solubility and bioavailability of CuET were obviously improved. CuET NPs could increase the Cu level in A549/DDP cells and decrease the expression of FDX1. This indicated that CuET NPs can induce cuproptosis in A549/DDP cells and effectively reverse cisplatin resistance (Fig. 2D).
It is urgent to develop safe and more efficient Cu ionophores for cancer treatment. The first strategy is that ionophores can be protected into proionophores. In this strategy, the ionophores exist in the form of proionophores in ordinary cells, thus reducing the killing effect on ordinary cells [60]. GSH highly expressed and H2O2 heavily produced tumor cells can be the trigger condition for proionophores. The redox effect of GSH promotes the release of Cu within cells. Excess GSH in cancer cells can activate proionophores, and the resulting product, ionophores, promote cancer cell death through the accumulation of Cu, GSH depletion and ROS production. In addition, H2O2 can take part in the activation of a proionophore. While activating cuproptosis, Cu2+ released by the transformed Cu ionophores could also react with GSH to obtain Cu+, which triggered a Fenton-like reaction to produce ROS, thus killing tumor cells [57].
In addition to the above method, differences in the types and amounts of enzymes in cancer cells and healthy cells can be utilized to improve the targeting of ionophores. The cell malignant transformation may change some enzymes’ functions and cellular localization [62]. These also provide ideas for improving targeting ability. In addition, the behavioral characteristics of cancer cells provide inspirations. To speed up proliferation, cancer cells take up more glucose than healthy cells and enhance glycolysis, which is one of the hallmarks of cancer called “Warburg effect” [63]. From this, glycol-conjugation also has the potential to improve targeting ability.
The occurrence of cuproptosis depends on Cu accumulation. However, the selectivity of Cu ionophores and the problem of metal overload in the human body still need to be considered [60]. Although high levels of Cu can be observed in tumor cells, the efflux mechanism and high expression of Cu transporters reduce retained Cu in tumor cells. Therefore, simultaneous inhibition of efflux operation and increase of uptake as well as retention of Cu in tumor cells could be a way to achieve safe cuproptosis and effective treatment for cancer.
Cu2+/Cu+ ionophores are Cu-based nanomaterials. With the development of Cu-based nanomaterials, the type, function and size of Cu2+/Cu+ ionophores may be enriched. Current Cu-based nanoparticles are not commonly used in cuproptosis-based therapies. In the future, it is hoped that cuproptosis can be widely applied in tumor treatment.
4. Different forms of Cu-based nanoparticles
Cu-based NPs are promising antitumor agents, which are applied in PTT, PDT, CDT and SDT [64]. Various forms of Cu nanoparticles have been explored in tumor therapy. It is well known that both the single-atomic Cu and the ionic form of Cu can hardly break through the potential energy barrier in the body to reach the target point. Intracellular Cu homeostasis, as well as efflux mechanisms, inhibit exogenous Cu intake, which is precisely the main obstacle of cuproptosis. Nowadays, the continuous progress and development of nanomaterials have gradually overcome these dilemmas. Currently commonly used Cu-based nanoparticles, such as monoatomic Cu materials, Cu metal derivatives, Cu complexes, Cu-MOFs and other Cu-based nanocomposites, are discussed in this section, and their applications are listed in Table 2.
Table 2.
Different kinds of Cu-based nanoparticles and their application.
| Classification | Structures | Composition | Therapeutic profiles | In vivo | Ref. |
|---|---|---|---|---|---|
| Monoatomic Cu materials | Cu-JMCN | N-doped mesoporous carbon nanomotors coordinated with Cu single-atom catalysts | Enhanced uptake and penetration through self-thermophoretic motion triggered by NIR light and catalyzed H2O2 into •OH for CDT with the tumor inhibition rate of 85%, higher than group without NIR light (63%) | MCF-7 | [93] |
| HA-NC_Cu | HA-functionalized single Cu atoms dispersed over Zn (II) boron imidazolate framework-derived nanocubes | Efficient sonothermal energy conversion ability and pH/H2O2/GSH tri-activated •OH generating ability for sonothermal–catalytic synergistic therapy with improved tumor inhibition rate (86.9%) and long-term survival rate (100%) | MDA-MB-231 | [94] | |
| Cu-HNCS | Hollow N-doped carbon sphere doped with a single-atom Cu species | Catalysts with excellent Fenton activity to produce O2•− and •OH at a low metal concentration, achieving relative tumor inhibition rate of 93% of and elevated mice survival rates for over 48 d, better than 34 d of control groups | 4T1 | [95] | |
| Cu metal derivatives/ Cu oxides | LIPSe@CuO2&DHA | Liposomal nanosystem for co-deliver of CuO2 and dihydroartemisinin (DHA) | Catalyzed DHA to generate cytotoxic C-centered radicals, depleted GSH and produced •OH through Fenton-like reaction with the strongest tumor growth suppression rate of 85.9% compared with other groups | 4T1 | [96] |
| DOX@MSN@CuO2 | CuO2-coated and DOX-loaded mesoporous silica nanoparticles | Decomposed into Cu2+ and H2O2 for GSH depletion and ROS generation for combined CDT and chemotherapy | 4T1 | [97] | |
| PEG-CuO@DSF@MTO | DSF and mitoxantrone were embedded into PEGylated mesoporous CuO | In situ complexation and coordination of Cu2+ with DSF and MTO for combined CDT and chemotherapy, achieving the best tumor inhibition rate of 91.27% | H22 | [98] | |
| CBGP NPs | GOx and cationic copolymer PEG2k-PEI1.8k coated CuO-deposited BSA | H2O2 self-supplied and glucose consumption-based tumor-selective CDT | C6 | [99] | |
| Lipo-ART@CPNs | A composite liposomal nanosystem co-loading CuO2 nanodots and artemisinin | ROS generated by breakage of ART endoperoxide bridge and self-supplying H2O2 promoted autophagy-induced ferroptosis with the tumor inhibition rate of 86%, better than group without NIR light (76%) | LLC | [100] | |
| Cu@P-B | Poly amino acids as a platform to bind the DSF prodrug and encapsulate CuO2 NPs | A cascade ROS overproduction and DTC activation | 4T1 | [101] | |
| 64Cu-Cu@CuOx-ECL1i-Gem | Reconstructed 64Cu radiolabeled and gemcitabine loaded Cu@CuOx with ECL1i | Targeted PET imaging, desirable biodistribution, rapid systemic clearance, and substantial tumor inhibition with a statistical difference in tumor size | KPC | [102] | |
| HSCPs | Integrated sulfasalazine into hydroxyethyl starch-doxorubicin conjugates stabilized Cu peroxide nanoparticles | Self-supplied H2O2 for •OH generating, biosynthesis blocking and depletion of GSH, superior stability, longer circulation half-life, and promising tumor inhibition rate of 67.4%, significantly outperforming poly(vinylpyrrolidone) (PVP)-stabilized Cu peroxide (55.5%) | 4T1 | [103] | |
| CuO2/DDP@SiO2 | Silica-coated CuO2 nanoparticles loaded with cisplatin | TME modulation for cuproptosis/chemotherapy/CDT with the tumor volume of 299.1±94.9 mm3, better than the CuO2@SiO2(757.3±154.6 mm3) and DDP@SiO2(629.4±116.0 mm3) | H22 | [104] | |
| CuS-Melanin-FA | Folic acid (FA) modified CuS nanodots using melanin as a template | Extended storage time, enhanced PA imaging performance and PTT efficacy with completely inhibited tumor growth, while tumor recurred and kept growing up to around 20-times of the original one in other groups | 4T1 | [105] | |
| Cu metal derivatives/ Cu sulfide | DOX/PA-Cu/Cu2-xS NPs | In-situ vulcanization of PA-Cu complex followed by loading DOX | Fenton-like activity to generate •OH for down-regulating the expression of HSPs to realize combined mild PTT/CDT/chemotherapy with a tumor inhibition rate of 92.1%, higher than the 15.3% of PA-Cu/Cu2-xS; 52.0% of DOX/PA-Cu/Cu2-xS NPs, and 75.4% of PA-Cu/Cu2-xS+NIR | CT26 | [106] |
| Tf-DSF/CuS | Transferrin (Tf)-modified and DSF-loaded hollow CuS nanoparticles | Chemo-photothermal combined therapy with glioma targeting ability | C6 | [107] | |
| CuS@PDA-TDHP | Polydopamine (PDA)-functionalized CuS nanosheets loaded with three DNA hairpin probes | FL quenching properties and signal amplified capacity for distinguishing cancer cells and realizing enhanced PTT | MCF-7 | [108] | |
| COPIRS&Dox@PDP NPs | Carbonyl manganese modified CuS NPs and Dox were encapsulated in thermal-responsive PDP | Combination of sensitized chemotherapy and penetration-enhanced PTT with severely suppressed tumor volume compared with other groups | 4T1 | [109] | |
| CuS-BSA NPs | BSA as a biological modifier to synthesize sheet-like NPs | Induced necrosis and up-regulated apoptotic proteins with a lower dose of 600 µg/kg at 980 nm NIR | H22 | [110] | |
| CuS/AIPH@BSA | Encapsulate alkyl radical initiator in hollow mesoporous CuS and coated with BSA | Oxygen-independent enhanced free radical treatment with the most prominent tumor cell necrosis manifestation (karyopyknosis, karyorrhexis, and karyolysis) compared with other groups | 4T1 | [111] | |
| Cu complexes | Cuphen | Cu(1,10-phenanthroline)Cl2 | Long-circulating liposomes inhibited AQP3-dependent glycerol permeation and impaired cell migration with the lowest relative tumor growth compared to other groups | CT-26 | [26] |
| [Cu2(HL)2Br2] 3A | Binuclear acylhydrazone Cu (II) complexes | Induced cell cycle arrest in G1 phase and apoptosis with excellent anti-cell migration activity, biocompatibility, and lower systemic toxicity | A549 | [112] | |
| 9-PMAH-Cu | Condensation of 9-anthraldehyde and 2-hydrazinopyrimidine, followed by mixing with CuCl2·2H2O | Arrested the cell cycle at G2/M phase and induced apoptosis, and retained coordination state in human serum albumin with no apparent losses in the body weight and other side effects of the tested mice compared with that of cisplatin | T24 | [28] | |
| CTB | Tri-phenyl-phosphine reacted with Cu-terpyridine complex | Inhibition of aerobic glycolysis and cell acidification, dissipation of mitochondrial membrane potential, activation of mitophagy, and induction of mitochondrial fission. | HCC | [113] | |
| CPT8 | A phenanthroline Cu complex modified with an alkyl chain-linked triphenylphosphonium group | Induced mitophagy and inhibiting angiogenesis and vasculogenic mimicry with a decreased volume of tumor tissue (241.71 mm3), far below that of a saline-treated mouse (428.77 mm3) | MDA-MB-231 | [114] | |
| C4 | Trinuclear Cu thiophene-2-formaldehyde thiosemicarbazone complexes | Induced autophagy and apoptosis of cancer cells and inhibited tumor angiogenesis | T24 | [115] | |
| Cu-MOFs | CuS@Cu-MOF/PEG | In situ vulcanization to grow CuS nanodots on/in the pore structure of Cu-MOF | Fenton-like reaction for CDT, increased NIR photoabsorption for PTT, and PA imaging with desired tumor volumes compared with the group without NIR light | 4T1 | [116] |
| MPDG | Disulfiram prodrug-loaded and GOx conjugated Cu (II)-based MOF | Catalyzed H2O2 generation under high glucose concentration to activate disulfiram prodrug for CDT/chemotherapy with a high tumor inhibition rate of 86.2%, much higher than that of glucose oxidase-loaded (63.7%) and prodrug-loaded (69.8%) groups | 4T1 | [117] | |
| Cu-BTC@DDTC | Nanoscale MOF Cu-BTC loaded with DDTC | Triggered ROS production, increased the lipid peroxidation accumulation and induced ferroptosis in tumor cells | B16F10 | [118] | |
| GOx@Cu MOF | Self-assembly of Cu ion and 4,4′-azobisbenzoic acid in the presence of GOx | GOx induced self-supply of H2O2 and cascade biocatalysis triggered ROS generation for pyroptosis with a tumor suppression down to around 20% of PBS-injected controls | HeLa | [119] | |
| Cu@CPP-800 | Pyrolysis of Cu-BTC at 800°C under an Ar atmosphere | Biocompatible phototherapeutic agent for PTT and photoacoustic bioimaging | Hela | [120] | |
4.1. Monoatomic Cu materials
Monoatomic Cu nanoparticles, characterized by low quantity and high efficiency, are mainly used in biocatalysts in nanocatalytic medicine [65]. They can greatly reduce the use of Cu elements, which guarantees biosafety. Zhu et al. [66] also successfully prepared Cu monoatomic nanoenzyme with peroxidase-like activity by high-temperature carbonization method for self-enhanced nano-catalytic tumor treatment. The PPS was prepared by combining single-atom nanoenzymes with proton pump inhibitors (PPIs) and platelet membrane vesicles. PPIs could regulate the levels of H+, H2O2 and GSH in tumor at the same time.
However, the issues of biocompatibility and safety still need more attention. How to reduce the amount of Cu and achieve a better tumor suppression effect has become the key to the breakthrough. For another example, Lu et al. prepared carbon spheres (Cu-HNCS) doped with monatomic Cu [67]. Cu-HNCS could catalyze the conversion of H2O2 and O2 into ·OH and O2•−without relying on external energy input, and oxidize biomolecules in cells to enhance tumor growth inhibition. Moreover, a low dosage of Cu could achieve excellent antitumor effect, guaranteeing the biological safety.
Despite the progress and prospects, shortcomings still exist. For example, the loading rate and biocompatibility of Cu atoms still need to be considered. In the future, increasing the load rate and biocompatibility and ensuring biological safety may be the key to promoting the wider application of monoatomic Cu nanoparticles [68].
4.2. Cu metal derivatives
In addition to the single atomic form, another widely used type is the Cu metal derivatives. Moreover, the Cu metal derivatives that can be used in tumor theranostics are in various forms, rich in production, cheap and easy to obtain [64]. The main forms of Cu metal derivatives, such as Cu oxides, Cu sulfide, and Cu peroxides, are discussed below.
4.2.1. Cu oxides
As the most common Cu metal derivative, Cu oxide has made dramatic advances in the antitumor field with the support of nanotechnology. For example, Benguigui et al. [69] have demonstrated that CuO NPs can target tumor-initiating cells, generate, accumulate ROS and promote the development of oxidative stress. CuO NPs have been synthesized using Cu acetate and sodium hydroxide at high agitation. PANC1 cells have been implanted subcutaneously into the 7-week-old non-obese diabetic severe combined immunodeficient mice, and treated with intravenous CuO NPs for 7 sequential days. The CuO NPs treatment delayed the tumor growth significantly. Immunofluorescence staining of tumor sections showed that the number of tumor initiation cells decreased significantly. It also suggests that CuO NPs can target tumor-initiating cells to inhibit tumor growth. In addition, CuO NPs synthesized using the extract of Phaseolus vulgaris showed strong cytotoxicity on HeLa cell lines [70]. Cu oxides also play a very important role in PTT and CDT. For example, the modified carbon nanospheres (CuO@CNSs) loaded with biocompatibility and photothermally enhanced Cu oxide have been constructed by Jiang et al. [71]. After CuO@CNSs reached the tumor site, ·OH could be generated by Fenton-like and Haber-Weiss reactions, which induced tumor cell apoptosis. Adsorbing DOX on the surface of this nanosphere could achieve the anticancer effect of pH response release and NIR laser stimulation response. CuO nanoparticles have also been widely studied in the field of pharmacology. Pandurangan's [72] research on anti-human cervical cancer cells also suggested the potential anti-tumor effect of CuO nanorods. After exposing the human cervical cancer cells to CuO, the cell shape changed, and the expression of p53 mRNA, ROS and caspase-3 activities in the cells increased significantly.
4.2.2. Cu sulfide
Cu sulfide nanomaterials have excellent photothermal properties and, therefore, can be applied to PTT [73]. Moreover, the Cu ion valence state transition in Cu sulfide can catalyze the Fenton-like reaction, which also provides the necessary conditions for its participation in CDT. At the same time, Cu sulfide nanomaterials can also be applied to tumor imaging. Cu sulfide nanoparticles have the advantages of low production cost, small size and low cytotoxicity. Most importantly, CuS can generate heat and cause cell damage under NIR irradiation. For example, He et al. [74] proposed a photothermal agent for imaging-guided PTT, namely CuS-deferritin-MBA. It enabled fluorescence imaging and PTT at isolated light wavelengths. Experiments showed that combining CuS-deferriferrin-MBA with 808 nm laser irradiation could ablate tumors. Cu sulfide can also be indirectly involved in PDT as a photothermal agent. Cu sulfide can be used as a heat-triggered switch in the system through strong photothermal effect. Li et al. wrapped drug DOX and photosensitizer Ce6 with PCM and loaded them into hollow mesoporous Cu sulfide nanoparticles [75]. This platform was called the H-CuS@PCM/ DOX/ Ce6 (HPDC) NPs. Under NIR irradiation, Cu sulfide exhibited a strong photothermal effect. After a large amount of heat melts, PCM, Ce6 and DOX were released, thus killing tumors. The limited penetration depth and energy of NIR light require new strategies. Nie et al. [76] developed the CuS-Fe@polymer NPs to enhance the CDT effects by co-delivering Cu sulfide and iron-containing prodrugs. As a catalyst, CuS accelerated the transformation from Fe3+ to Fe2+, so as to continuously provide a higher concentration of Fe2+. This accelerated Fe2+ to catalyze H2O2, thus improving the efficiency of CDT. However, excessive use of Cu may lead to the overload in human body and endanger human health. Therefore, exploring both safe and effective synergistic therapy based on CuS becomes the key to solve the problem. Wang et al. [77] developed a multifunctional nano platform (CuS@COFs-BSA-FA/DOX). The DOX loaded by this platform enhanced the efficiency of CDT in vivo, and the local hyperthermia induced by PTT further improved the CDT efficiency. This enabled the synergy of PTT, chemotherapy, and chemical kinetic therapy.
4.2.3. Cu peroxide
Metal peroxides (CuO2, CaO2, MgO2, ZnO2, etc.) are usually composed of metal ions and peroxide groups, and can be decomposed into O2, H2O2 and metal ions under acidic TME, which have received extensive attention in anti-tumor application. The first preparation case of Fenton-type metal peroxide nanomaterial was the CuO2 nanodot, which exhibited excellent ROS production ability and initiated subsequent flourishing of Cu peroxides [78]. For instance, Lin et al. [79] constructed DMOS@CuO2/ICG-HA (DCI) nanocomplexes, in which the loaded CuO2 could be decomposed at tumor site into O2, H2O2 and Cu2+ in response to acidic TME. Meanwhile, the released Cu2+ acted as a T1-weighted magnetic resonance imaging reagent, achieving MRI-guided anti-tumor effects. Problems exist as the theranostic nanoplatforms often lack specificity. To precisely control the diagnostic signal and therapeutic effect, TMB-CuO2@PLGA@RBCM (TCPR) NPs were fabricated, in which 3,3′,5,5′-tetramethylbenzidine (TMB) owned excellent photothermal efficiency in the NIR-II region after being activated by ·OH. Tactfully, as discussed above, the CuO2 was decomposed into H2O2, and subsequently, Cu+ and H2O2 initiated a Fenton-like reaction to generate ·OH, while the generated ·OH can tactfully oxidize TMB for promoting the application of photothermal effect and NIR-II photoacoustic imaging. CuO2 can provide H2O2 and Cu+for ·OH production, while Cu2+ generated by Fenton-like reaction can deplete GSH, thus avoiding the elimination of ·OH. Foreseeably, the facile integration of CuO2 with other metal peroxides may bring about a better therapeutic effect. Liu et al. [80] modified CaO2 and CuO2 with hyaluronic acid and combined them to prepare CaO2–CuO2@HA NC. Hyaluronic acid specifically bound CD44 protein on tumor cells, thus enhancing permeability and suddenly releasing Ca2+, Cu2+ and H2O2. Undoubtedly, ·OH originated from CuO2 effectively killed tumor cells. In addition, overloaded Ca2+ caused serious damage to mitochondria. The synergy of CuO2 and CaO2 combined both Fenton-like reaction and mitochondrial dysfunction, thus inspiring new ideas for tumor treatment.
4.2.4. Other derivatives
The above metal derivatives of Cu are widely studied and commonly utilized in tumor treatment. Although other copper metal derivatives are less frequently used, they also hold significant potential for cancer therapy. For example, Hu's team [81] built bimetallic nanoparticles based on ruthenium and Cu, which showed catalase and peroxidase actions to produce O2 and ROS. In addition to increasing ROS production in tumor tissue, reducing ROS consumption is another viable strategy for cancer treatment. GSH is one of the most common antioxidants in the antioxidant system of cancer cells, and strategies that deplete GSH can enhance the efficacy of ROS-based therapies by decreasing ROS clearance. Zuo et al. [82] reported an ultra-thin single-point bimetallic (Cu hexacyanocobaltate) nanosheet (CuCo NS). CuCo NSs consumed GSH overexpressed in tumors and performed CDT through Fenton-like reaction. While the discovery of such derivatives has a significant contribution to cancer treatment, their safety remains to be investigated. For example, the impact of different metal intakes on human tissues and organs, as well as their effects on normal physiological functions, needs careful evaluation. Luan et al. [83] developed a pH-responsive reversible self-assembly Cu2-xSe-BSA, which selectively accumulated in the lysosomes of tumor cells, causing lysosomal rupture through swelling and increased lysosomal membrane permeability. Notably, after exerting its effects, aggregated Cu2-xSe-BSA dissociated and subsequently left the acidic tumor microenvironment. In conclusion, diverse metal derivatives of Cu are widely used in cancer therapy, serving as effective PAs for PTT and Fenton reagents for CDT, etc. However, the stability and systemic toxicity of metal derivatives are still worth discussing. The relatively exposed Cu metal derivatives in the blood circulation may lead to ion leakage. To avoid this, researchers often modify or package Cu metal derivatives. Nonetheless, overly tight modification or encapsulation may also pose issues, such as failing to release or activate at the target site, resulting in reduced or lost functionality. Therefore, future research should focus on enhancing the efficacy of new copper metal derivatives while ensuring their safety.
4.3. Cu complexes
Although Cu complexes are currently in the early stages of development, they have great potential for treating cancer. Shen et al. [84] constructed a polymer nanoparticle (GCT NPs) based on phosphorus tree-containing polymer-Cu2+ complex (1G3-Cu) and toyocamycin (Toy) on a surface coated with cancer cell membrane (CM). GCT@CM NPs were stable under physiological conditions, and rapidly dissociated in tumor microenvironment, consuming GSH and releasing drugs, thus inhibiting tumor growth. The feasibility of the idea of converting Cu2+ to Cu+, accelerating the Fenton reaction and generating a large amount of ROS while consuming GSH remains to be tested. Jia et al. [85] designed and synthesized 12 novel complexes, Cu(L1)2 -Cu(L12)2, to improve their chemical therapeutic performance. Cu(L4)2 and Cu(L10)2 showed robust tumor suppression capacity in the T24 xenograft model. Among them, Cu(L4)2 and Cu(L10)2 were reduced by GSH depletion to Cu+, and the generated Cu+ catalyzed excess H2O2 to produce ·OH. Meanwhile, Cu(L4)2 and Cu(L10)2 reduced catalase activity to inhibit the transfer of H2O2 to H2O, thereby enhancing CDT.
In addition, Cu complexes can also function as proteasome inhibitors to treat cancer. Proteasome inhibition can induce cytochrome c into the cytosol, activate the caspase cascade and further induce apoptosis. Interestingly, the rapid proliferation of cancer cells is more dependent on proteasome, which means that they are more sensitive. Studies have confirmed that Cu2+ inhibits the proteasome through direct binding and partial redox action [86]. The ubiquitin-proteasome system (UPS) is a protein catabolic mechanism in the body that maintains the quality and quantity of cellular proteins. Copper complexes, such as CuET and CuHQ, represent a significant class of metal-based anticancer agents. These complexes inhibit the UPS, leading to an imbalance in protein levels and quality within tumor cells, ultimately resulting in the selective killing of cancerous tissue. [87].
However, Cu complexes also have some drawbacks. Enhancing their selectivity will be crucial for advancing this field in the future. Thus, the development of novel Cu complexes that can selectively target cancer cells is highly desirable. It is also important to note that the long-term use of Cu complexes may disrupt the homeostasis of alkali metals, potentially leading to unpredictable adverse effects. Currently, there is a need to improve the selectivity of Cu complexes to achieve more precise targeting effects.
4.4. Cu-MOFs
Metal-organic frameworks (MOFs) are composed of metal ions and organic ligands. Because of the high porosity, regular and adjustable pore size, MOFs possess a large specific surface area while maintaining a low density [88]. Most importantly, the metal ions and ligand structures within MOFs can be modified as needed. Modification of MOFs will result in multifunctional materials. The unique characteristics make MOFs candidates for flexible and efficient drug loading. For instance, in Cheng's work [89], Cu2+ was incorporated into precursor of ZIF-90 to prepare nano-scale Cu/Zn-MOF. Heating the MOF resulted in a Cu+/Cu2+-coexisting hollow porous structure, which was used to load ICG to form a therapeutic diagnostic platform. Owing to the limited anticancer activity of most MOFs (like ZIF-8), it's urgent to develop MOFs with intrinsic anticancer activity. Epigenetic alternation leads to DNA methylation, which contributes to the influence on tumor progression and metastasis. Li et al. [90] designed nanoMOF (Cu-Olsa), in which oxalazin acted as a bioactive ligand for coordination with Cu and DNA hypomethylating agent for epigenetic therapy. Besides, COX-2 expression was down-regulated by Olsa and its organic complex with Cu2+. Cu-Olsa realized efficient production of ·OH and resulted in redox dyshomeostasis. Catechol is a kind of organic ligand that can form a complex with transition metals as well as generate ROS by one- and two-electron oxidation. Interestingly, the coordination of catechol with metal ions results in shielding of ROS production ability. In order to realize TME-responsive ROS generation, Liu et al. synthesized a nano Cu-based MOF (CuHPT) by one-step self-assembly of catechol ligand HPT and Cu2+ [91]. The Cu2+ could be specifically reduced under high GSH concentration at tumors, accompanied by disassemble of CuHPT and release of catechol ligands and Cu+, which triggered and maintained ROS production.
In the process of tumor therapy, the high level of GSH and insufficient H2O2 in tumor cells seriously weaken the efficiency of tumor therapy. To tackle this dilemma, functional core-shell MOFs are widely used as new porous materials. Dong et al. [92] used functional small molecules (aminotriazole, 3-AT) as ligands, and synthesized Cu-MOF by hydrothermal method to prepare the core. Further, a porous silica (bis[3-(triethoxysilyl)propyl]tetrasulfide) layer was grown on the core to obtain Cu-MOF@SMON. After DOX loading, as well as surface modification with hyaluronic acid, a functional core-shell MOF (Cu-MOF@SMON/DOX-HA) was successfully prepared. The MOFs aggregated in cancer cells owing to the specific binding between hyaluronic acid and overexpressed CD44 receptor on cancer cell membranes and then released DOX, Cu2+ and 3-AT in response to GSH and acidic pH. Cu2+ was reduced by GSH to Cu+, which catalyzed the decomposition of overexpressed H2O2 in cancer cells.
Cu-based nanomaterials with rich variety also pave the way for accurate design towards different tumors. However, some Cu-based nanomaterials have poor safety and lack of selectivity, which is the biggest challenge. Combining Cu-based nanomaterials with different therapies may achieve the purpose of safety, high efficiency and low toxicity. This multimodal combination therapy may be the future development of Cu-based nanomaterials.
5. Cu-based nanoparticles for cancer treatment
5.1. Cu-based nanoparticles for PTT
PTT is a crucial method in tumor treatment, and photothermal agents (PTAs) are a crucial component of PTT. On irradiation by an NIR laser, PTAs can be rapidly switched from the ground state to the excited state and then back to the ground state with energy dissipation in the form of heat to raise the local temperature and thus ablate tumor cells [121]. After being injected intravenously into the body, PTAs target and accumulate in the tumor to exert treatment effect.
Metal nanomaterials can act as PTAs through the localized surface plasmon resonance effect, in which Cu-based NPs show great potential owing to their high near-infrared absorbance and good biocompatibility. For instance, Cu-doped CDs synthesized through an eco-friendly, simple, cost-efficient method exhibited excellent PTT effect [122].
Surface modification of Cu-based NPs can achieve many purposes, such as the targeting of Cu-based NPs. CuS nanoparticles are cost-effective agents for PTT, which can strongly absorb NIR light and transform it into heat, thus displaying remarkable photothermal properties. Wu et al. [123] constructed the surface-modified CuS NPs with mitochondrial targeting moiety. In this way, CuS NPs targeted tumor mitochondria and accumulated in tumor mitochondria in large quantities. Modification of the Cu-based NPs could also improve the photothermal efficiency. Wang et al. [124] reported a kind of 2D CuFe2S3 nanosheet obtained by vulcanizing ultra-thin CuFe-LDH NSs. CuFe2S3-PEG modified by PEG showed cracking photothermal conversion efficiency under laser irradiation of 1064 nm. Cu+ and Fe2+ generated by the reaction of CuFe2S3-PEG with GSH triggered Fenton reaction and generated a large number of ROS, thus achieving efficient CDT (Fig. 3A).
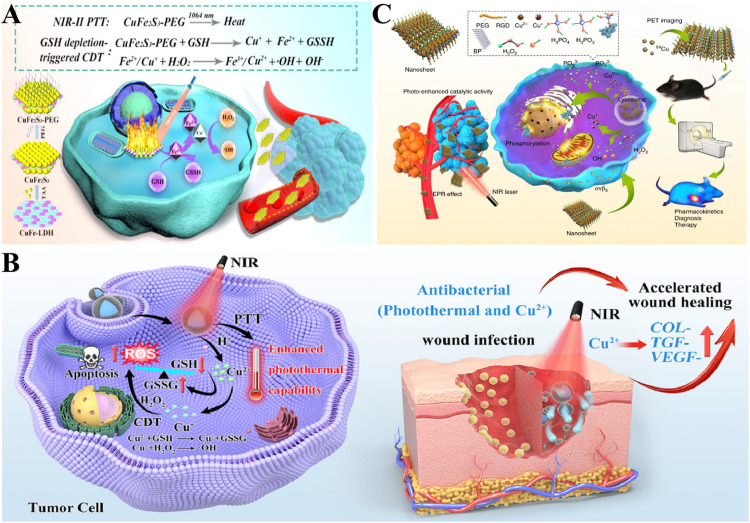
Fig. 3.
Cu-based nanoparticles for PTT. (A) Application of CuFe2S3 nanoplates in synergistic NIR-II PTT and CDT [124]. Copyright 2021 Elsevier. (B) Application of Cu-BTC in the treatment of melanoma by enhancing the PTT and CDT characteristics of PDA [125]. Copyright 2023 Elsevier. (C) Application of BP@Cu in PET-guided PTT cancer treatment [126]. Copyright 2020 Nature Communications.
Incorporation of Cu-based nanomaterials into other PTAs may also enhance the photothermal properties. Liu et al. [125] combined the Cu metal organic framework (Cu-BTC) with polydopamine (PDA) to build the Cu-BTC@PDA nanocomposite material. Density functional theory calculations showed that Cu enhanced the photothermal properties of PDA. Moreover, Cu2+ consumed GSH and the resulting Cu+-initiated Fenton-like reaction promoted the production of large amounts of ROS (Fig. 3B).
Although PTT has shown promising results in cancer treatment, the therapy still has non-negligible drawbacks. For instance, the thermal diffusion caused by high-temperature ablation may seriously damage adjacent normal tissues, and the hyperthermia stimulus would activate the synthesis of heat shock proteins (HSPs), which may cause heat tolerance [127]. Thus, more gentle methods that can relieve the side effects caused by high temperature during PTT are desired. Fortunately, Chang et al. designed Cu SAZs(CuL) containing licofluoxine for mild PTT induced by HSP silencing [128]. Licofluoxine effectively blocked the synthesis of HSPs in cancer cells. In addition, CuL produced a large number of ROS and damaged the existing HSPs in cancer cells. CuL not only inhibited the production of HSPs at the source, but also inhibited the activity of HSPs already existing in cancer cells. The degradation of PTAs has long plagued the wide application of PTT. PTAs with high photothermal stability can be rapidly degraded after use are currently needed. Hu et al. [126] incorporated Cu2+ into black phosphorus (BP) to prepare a BP@Cu nanosystem. The addition of Cu2+ not only accelerated the degradation rate of BP, improved the photothermal stability of BP@Cu system, and introduced CDT, but also realized PET imaging with 64Cu2+(Fig. 3C).
To enhance the efficacy of PTT, combined therapy is necessary, which can provide a broader prospect. Chemotherapy is one of the most effective as well as major means of cancer treatment. However, the growing chemoresistance becomes the main cause of tumor recurrence; therefore, conventional chemotherapy is insufficient for killing resistant cancer cells and may even bolster the unwanted resistance through activating alternative pathways. Fortunately, Liu et al. [91] created a Cu/catechol-based MOF (CuHPT), which exploited high GSH content in drug-resistant cancer cells as a trigger to generate ROS, superior to the general GSH-depleting tactic. Meanwhile, GSH could reduce Cu2+ to Cu+ and Cu+ further catalyzed H2O2 to generate toxic ·OH to kill tumor cells. The selectivity of CuHPT on drug-resistant cells complements chemotherapeutic drugs’ selectivity on drug-sensitive cells, thus completely eliminating tumors.
Further, the limitation of chemotherapy paves the way for chemotherapy combined with PTT. DSF has been proved to have outstanding antitumor activity, which degrades into DDTC and then combines with Cu ions to form Cu(DDTC)2, leading to tumor apoptosis. Tang et al. [129] fabricated CuS–DDTC NDs nanoplatform, which tactfully combined and amplified therapeutic effect of PTT and chemotherapy. The insufficient Cu in human body limited the therapeutic effect of DSF, and the barrier was overcome by introducing CuS NDs as Cu source. The complicated multi-step preparation of nanoplatforms and the low drug loading efficiency are obstacles to PTT. To break through the current predicaments, Pan et al. [130] constructed a facile and tunable nano-system, in which the in-situ synthesis of CuS and Cu(DTC)2 was realized through mixing Cu2+, S2−, DTC solution, followed by loading with a biocompatible nanocarrier BSA. The system realized flexible synergy of PTT and chemotherapy through adjusting ratio of S2−/DTC in case of fixed Cu2+.
In current PTT, the absorption range of many PTAs belongs to the first NIR I region (750–1000 nm). The limited penetration depth regrettably results in insufficient thermal ablation at deep tumors. The laser with deeper tissue penetration in NIR II region (1000–1350 nm) has solved this problem to some extent. Zhu et al. [131] constructed PEG@Cu2-xS@Ce6 nanozymes (PCCNs). Under the irradiation of 1064 nm laser, PCCNs showed good photothermal conversion performance, paving the way for further exploration of NIR II.
5.2. Cu-based nanoparticles for CDT
As a tumor treatment method depending on ROS, CDT introduces transition metals through Fenton or Fenton-like reaction to react with excessive H2O2 produced in TME, and the generated ·OH can effectively kill tumors [68]. The key point of CDT is to find a suitable material, which can effectively convert H2O2 to ROS. Cu+/Cu2+ can exhibit brilliant Fenton-like activity and can be applied in strong acidity, while classical ferric ion shows unsatisfactory catalytic activity under low pH. Basked in the glory of lower redox potential, Cu+/Cu2+ engaged H2O2 catalysis is more efficient than Fe2+/Fe3+ [132]. Pi et al. [133] synthesized natural carrier-free injectable hydrogel consisted of Cu2+-mediated self-assembled glycyrrhizic acid and norcantharidin through an easy, green and economical method, which showed CDT effect and TME regulating ability via apoptosis, cuproptosis and anti-inflammation. However, the high intracellular free Cu will bring serious side effects. To 5 free Cu, Hu et al. [134] chelated GSH and Cu ions to produce Cu-GSSG NPs, which could shield the toxicity of Cu and generate ROS in high-level H2O2 tumor cells.
Moreover, CDT is highly dependent on H2O2 to kill tumors, and the limited H2O2 in the TME seriously limits its therapeutic effect. Meng et al. [135] reported biodegradable Dox@Cu-Met NPs, in which Met reduced O2 consumption, and Dox helped convert the accumulated O2 into O2•− and later into H2O2 by superoxide dismutase (SOD). Then, H2O2 was converted into ·OH by Fenton-like reaction based on Cu+. By exogenous supplementation of H2O2, increasing ·OH production is a good approach. However, most methods of improving the efficiency of CDT depend on the participation of oxygen to some extent, and the hypoxia of tumors hinders the realization of this method. He et al. [136] developed a self-assembled metal-organic coordination nanoparticle Cu-OCNP/Lap. The Cu-OCNP/Lap was prepared by Cu2+ binding ligands 1,4,5,8-tetrahydroxyanthraquinone and banonoanthraquinone dihydrochloride and loading β-rapanone (β-Lap). Under the NIR-II irradiation, Cu-OCNP/Lap generated heat, thus speeding up blood circulation, providing sufficient oxygen for tumors, strengthening the β-Lap circulation reaction and generating a large amount of H2O2. Sufficient H2O2 enhanced the efficiency of CDT and inhibited the growth of tumors (Fig. 4A).
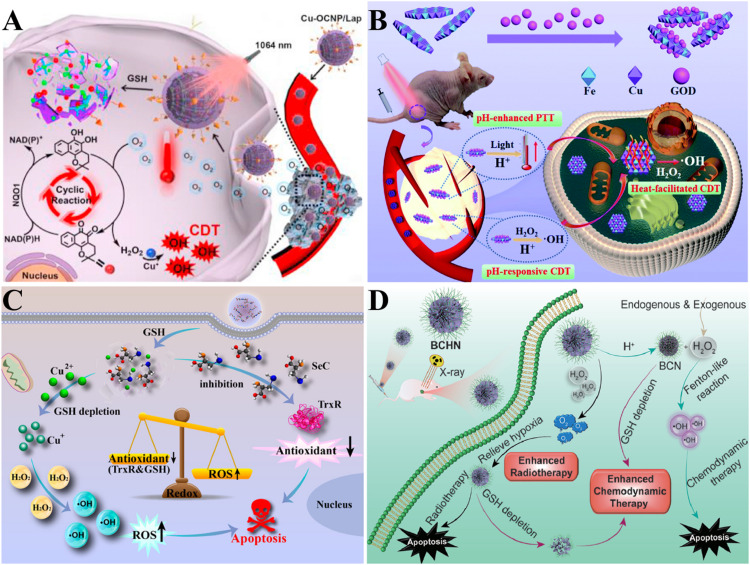
Fig. 4.
Cu-based nanoparticles for CDT. (A) Mechanism of Cu-OCNP/Lap's abundant H2O2 supply enhancing CDT [136]. Copyright 2021, Elsevier. (B) Schematic illustration of tumor-specific therapeutic mechanism of GOD/CuFe-LDHs [137]. Copyright 2021, the Royal Society of Chemistry. (C) Application of Cu-SeC nanoparticles in strengthening CDT [139]. Copyright 2023, American Chemical Society. (D) Schematic illustration of BCHN's synergistic therapy combined with CDT and RT [140]. Copyright 2021, Elsevier.
In addition, the efficiency of CDT was also blocked by the high acidity requirement, which resulted in the insufficient release of catalytic ions. The pH value of TME is higher than the optimum pH value of Fenton reaction. Fortunately, the pH value of lysosomes is suitable for CDT. To maximize the utilization of lysosomes, the escape of nanomaterials from the lysosomes should be inhibited. In order to achieve more accurate and effective treatment, CDT also needs to be sensitive to the low pH value of tumor microenvironment. Hu et al. [137] designed a layered double hydroxide (CuFe-LDH) by immobilizing natural glucose oxidase (GOD) in Cu-based layered double hydroxide. Under acidic tumor conditions, GOD/CuFe-LDH nanosheets converted glucose into ·OH. At the same time, GOD/CuFe-LDH nanosheets also exhibited good photothermal conversion efficiency in acidic environment (Fig. 4B).
The high expression of antioxidant molecules and enzymes in TME will reduce the effect of existing CDT. GSH is the most typical antioxidant in TME, and the excessive expression of GSH greatly limits CDT. Therefore, Wang et al. [138] constructed DOX@Cu2+/ZIF-8@PDA, in which GSH was consumed by reducing Cu2+. The generated Cu+ produced ROS through a Fenton-like reaction. Thioredoxin reductase (TrxR) can also inhibit CDT by ·OH depletion. In order to break the limitations of GSH and TrxR, Chen et al. [139] designed self-assembled Cu-selenocysteine nanoparticles (Cu-SeC NPs). Cu2+ in Cu-SeC NPs consumed GSH, and the generated Cu+ activates Fenton-like reaction, resulting in production of toxic ·OH. At the same time, selenocysteine in Cu-SeC NPs inhibited TrxR activity and reduced its antioxidant effect on ROS (Fig. 4C). Breaking the redox balance of TME and increasing the number of oxidative molecules can increase oxidative stress and correspondingly improve the curative effect of tumor treatment. Liu et al. [140] developed BCHN, a nanoplatform of BiOCl doped with Cu2+ bismuth. BCHN provided H2O2, generated ·OH, realized oxidative stress and enhanced CDT effect. In addition, the TME ray-blocking property of bismuth was crucial for enhancing radiotherapy. BCHN was expected to effectively inhibit the proliferation of tumor cells (Fig. 4D).
Further, the strategy of in situ drug synthesis is beneficial for improving targeting ability and enhancing the therapeutic effect of CDT. The Cu-catalyzed azide–alkyne cycloaddition (CuAAC) reaction shows potential in prodrug activation and in situ drug synthesis. You et al. [141] developed biocompatible Cu-based NPs for CuAAC reaction. The valence state of Cu in Cu-based NPs was adjusted by ROS induced by NIR. However, the limited tissue-penetrating depth restricted the therapeutic effect, therefore, it's urgent to exploit endogenous control of the valence change. In this way, a DNAzyme-augmented catalyst was constructed. Under the condition of high local concentration of H2O2, DNAzyme produced active free radicals and promoted the valence transformation of Cu on Cu-based NPs surface. This greatly enhanced the biological orthogonal catalytic activity and activated the in-situ prodrug.
5.3. Cu-based nanoparticles for PDT
PDT is another emerging cancer treatment method. It uses light with appropriate wavelength to irradiate photosensitizers (PSs) and react with oxygen molecules to produce ROS, which is labeled as the “tumor killer”, thus leading to apoptosis and necrosis of targeted cells. PDT is equipped with less invasion, low systemic toxicity and high selectivity [142,143]. The process is as follows: Firstly, PS molecule absorbs light to switch from the ground state to the first excited singlet state (S1). The PS in S1 state may undergo intersystem crossing to excited triplet state (T1), which has a relatively long lifetime compared to S1, and is necessary for photosensitizer to produce ROS. PSs in T1 undergo two mechanisms: Both the Type I and Type II reactions involve the energy transfer to molecular oxygen in the ground state. The Type I reaction results in the formation of reactive free radicals that cause cellular damage. Type II reaction involves the direct formation of 1O2, which reacts electrophilically with biomolecules such as lipids, proteins, and DNA, leading to oxidative damage and cell destruction. In addition, dead cells caused by photodynamic effects will produce a series of effects in the later stage. In tumor tissue, the irregular distribution of blood vessels makes it difficult to supply oxygen. The high oxygen consumption caused by the rapid proliferation of tumor cells further aggravates the tumor hypoxia. Oxygen is necessary for PDT; thus, hypoxia inhibits its efficacy. To tackle this problem, Cai et al. [144] constructed CuTz-1-O2@F127, which simultaneously relieved hypoxia by acting as photosensitizer to generate O2 as well as adsorbed GSH to promote the efficacy of PDT.
The ROS generated by PDT can also be used as a switch to regulate the efficacy of chemotherapy. Cu(DTC)2 can be used for cancer treatment, but can also cause severe systemic toxicity. To avoid unwanted toxicity, DTC and Cu should be separated during preparation and selectively chelated in tumor cells to produce Cu(DTC)2. Herein, Chen et al. [145] developed a self-amplifying prodrug nano-agent activated by ROS. The ROS-cleavable HA-DQ responsively released DTC in tumor cells of high-level ROS, while remaining no response in normal cells of low-level ROS. Cu(DTC)2 was generated by the released DTC and Cu2+ in MOF. Meanwhile, the original shielding of photosensitizer Zn-TCPP was unfrozen to exert the PDT effect owing to MOF dissociation, which promoted the generation of ROS and further strengthened the ROS-responsive DTC release. In this way, a virtuous circle was formed to achieve the combined therapeutic effect of PDT/chemotherapy.
Another worth noting issue is that ultraviolet, visible light and NIR-induced PDT have the characteristics of low tissue penetration and quick energy attenuation, which penalizes PDT efficacy in treating deep tumors [142]. X-ray owns a strong penetrating ability because of its short wavelength and high energy, paving the way for the thriving of X-ray-induced PDT, which destroys deeply located tumors and reduces damage of radiation on the healthy tissues. Chen's group [146,147] constructed Cu-cysteamine complex (Cu-Cy) NPs, which were novel photosensitizers that could produce ROS not only under ordinary ultraviolet but also under ultrasonic, X-ray, microwave, and cancer-specific pH/elevated H2O2 levels. Moreover, the excellent penetration ability of X-ray indicated that Cu-Cy could be applied in both superficial and deep tumors, ensuring its ability to promote the PDT effect. However, these are insufficient for the clinical translation of X-PDT-based Cu-Cy. In Chen's study, pork layer was used to mimic deep-seated tumor and a clinical linear accelerator represented an X-ray generator. Interestingly, ROS may contribute to tumor cell migration, and Cu-Cy NPs produce abundant ROS that kills tumors [148]. Therefore, extra attention and efforts were paid to clinical investigation. Inversely, no negative effect was observed, and Cu-Cy even showed the ability to inhibit migration and proliferation, demonstrating that ROS produced by X-PDT was conducive to cancer treatment. These results further prove the bright antitumor future of Cu-Cy.
In addition, Cu-Cy-X (X=F, Cl, Br, I) with adjustable luminescence, increased chemical stability and reduced dark toxicity were found, among which Cu-Cy-I showed better performance. This is because the cuprous ion in Cu-Cy belongs to soft acid, while chloride ion belongs to hard base, resulting in weak Cu-Cl covalent bond and reduced stability of Cu-Cy, while soft base I- contributes to higher stability and stronger 1O2 production capacity. These new PSs open up a new way for photodynamic therapy. Recently, the combination of Cu-Cy NPs and KI was proven to enhance the efficacy of PDT, possibly through producing 1O2, I3−, and H2O2, offering a novel Cu-Cy anti-cancer strategy [149].
Cu can also generate ·OH through Fenton reaction to enhance PDT of other PSs. This is the case with Cu-Cy, as mentioned above. Yang et al. [150] designed a nano platform (NSCuCy) assembled by type I photosensitizer NS-STPA and Cu-Cy. This platform generated O2− by irradiation of 660 nm laser and further reacted with Cu+ to generate ·OH (Fig. 5A). It is also a good means to catalyze the decomposition of endogenous H2O2 into ROS in TME. Liu et al. [151] designed mesoporous Cu/manganese silicate nanospheres (mCMSNs) coated with biodegradable cancer cell membranes. Under the irradiation of 635 nm laser, mCMSNs produced 1O2. Cu+ and Mn2+triggered Fenton reaction to catalyze endogenous H2O2 to produce ·OH (Fig. 5B). Adding Cu can improve the stability of PSs and the efficiency of PDT. Zhang et al. [152] designed new Janus nanoparticles (J-MOPs) based on BPQD and tetrahydroxycyanoquinone (THQ)- Cu metal-organic particles (MOPS). BPQD was encapsulated in J-MOP and P-Cu bonds. In this way, BPQD was well separated from air and water, and occupied P solitary pairs, thus enhancing the stability of BPQD. Under the irradiation of 670 nm laser, J-MOPs' strong electron-hole separation and migration ability improved their reactivity to O2, thus improving the generation of 1O2 for cancer. At the same time, Cu2+ produced ·OH from H2O2, which enhanced the anti-tumor effect of J-MOPs (Fig. 5C).
Fig. 5.
Cu-based nanoparticles for PDT. (A) Application of CuCy NPs to enhance PDT of type I photosensitizer NS-STPA with AIE activity [150]. Copyright 2022, American Chemical Society. (B) Application of mCMSNs with GSH depletion and hypoxia relief in the synergetic therapy of PDT and CDT [151]. Copyright 2019, American Chemical Society. (C) Application of J-MOPs in enhancing environmental stability and PDT efficiency [152]. Copyright 2019, the Royal Society of Chemistry.
5.4. Cu-based nanoparticles for SDT
SDT is another non-invasive tumor treatment strategy. While PTT and PDT offer several advantages, their effectiveness is often limited by the relatively poor penetration of NIR light. On the other hand, CDT has the benefit of minimal side effects, but its implementation requires stringent conditions, . The main medium of SDT is ultrasound,which has a strong ability to penetrate biological tissues and is widely used as a guiding tool in various treatment methods. Due to its deep penetration in organizations, SDT can be a promising approach [153]. Sonosensitizers are a key element of efficient SDT. SDT mainly relies on the generation of ROS and cavitation effects caused by ultrasonic irradiation to destroy tumor tissue. Combining the two principles for tumor treatment can achieve better results. Cu-Cy can be activated not only by light, X-ray or microwave, but also by ultrasound as a sonosensitizer of SDT. Wang et al. [154] used ultrasound to activate Cu-Cy and proved that even a small dose of Cu-Cy successfully produced ROS and obvious cavitation. With the progress of materials science, new Cu-based acoustic sensitizers have also been put into the study of SDT. A sea urchin-shaped Cu-based metalloporphyrin liposome nanosystem (FA-L-CuPP) was constructed [155]. The results showed that FA-L-CuPP was an excellent ultrasonic sensitizer, which produced a variety of ROS under the irradiation of ultrasound, such as 1O2 and ·OH. In this process, ROS production and obvious cavitation effect were observed. The above combination killed 4T1 tumor cells and achieved the purpose of inhibiting the growth of tumor tissue (Fig. 6A).
Fig. 6.
Cu-based nanoparticles for SDT. (A) Schematic illustration of anti-tumor SDT mediated by FA–L–CuPP [155]. Copyright 2022, Multidisciplinary Digital Publishing Institute. (B) Schematic illustration of synergistic SDT and CDT of 2D Ti3C2/CuO2@BSA nanosheets under ultrasound [156]. Copyright 2022, American Chemical Society. (C) Schematic illustration of SonoCu's effective combination of SDT and cuproptosis in the treatment of cancer [157]. Copyright 2023, American Chemical Society. (D) Schematic illustration of H-Cu9S8@CCM NPs synergistic PTT-SDT [158]. Copyright 2023, Elsevier. (E) Schematic illustration of antitumor mechanism of PCPT [159]. Copyright 2019, American Chemical Society.
SDT has advantages such as desirable treatment depth and low cost. However, the common problem of SDT at this stage is the insufficient yield of ROS, which seriously affects the treatment effect [153]. The amount of ROS produced in CDT is considerable, and Cu-based materials can catalyze Fenton-like reactions to produce ROS. Therefore, a combination of the above two therapies may achieve better results. The combination of Cu-based Fenton agents and sonosensitizer is delivered to the tumor tissue, and under ultrasound excitation conditions, a large amount of ROS is generated, which induces tumor cell death. Zhang et al. [156] prepared a collective called Ti3C2/CuO2@BSA. Ti3C2 was oxidized to TiO2 by H2O2 in TME. TiO2 was a well-effective inorganic acoustic sensitizer. At the same time as ultrasound irradiation, the Cu-excited Fenton-like response was further enhanced. In this process, a large amount of ROS was generated, achieving the purpose of synergy. It is important to note that, unlike traditional SDT, where the sonosensitizer is delivered directly, the sonosensitizer in this study was generated in situ in TME. This avoided the degradation of the acoustic sensitizer during delivery in traditional SDT. In the nude mouse xenograft U87 tumor model, Ti3C2/CuO2@BSA had good anti-tumor effect and significantly inhibited tumor growth (Fig. 6B).
The inherent permeability of SDT presents significant advantages for the ablation of deep tumors, but the limited production of ROS constrains the overall efficacy of SDT. Improving the hypoxic environment within the tumor is the key to solving the problem. Chen et al. [157] prepared a nanorobot named SonoCu consisting of a carrier-coated Cu doped material, perfluorocarbons, and a sonosensitizer. This system improved tumor cell uptake and enhanced oxygen supply within the tumor. The efficacy of SDT was further amplified by the cuproptosis mechanism, and Cu+ catalyzed the formation of ROS to achieve synergy. Notably, this system had low toxicity to normal cells and exhibited good tumor targeting and selectivity (Fig. 6C).
Additionally, combining SDT with PTT has proven to be a more effective anti-tumor strategy. Zhao et al. [158] developed the hollow nanospheres of haipofen-Cu9S8. Cu9S8 nanospheres were loaded with haipofen sonosensitizers, and then the haipofen-Cu9S8 nanospheres (H-Cu9S8) were disguised by CCM. H-Cu9S8@CCM nanospheres had extensive light absorption in the range of 700–1100 nm. H-Cu9S8@CCM nanospheres showed good photothermal conversion performance under the irradiation of 1064 nm laser. In addition, the loaded propofol generated 1O2 and activated SDT under ultrasonic excitation (Fig. 6D). Without the help of O2, the efficacy of SDT is still unsatisfactory. Liang et al. [159] developed a new Pt-CuS Janus made up of platinum and hollow CuS. Hollow CuS had a large inner cavity and was loaded with sound sensitive molecule TAPP to realize SDT. Pt catalyzed the decomposition of endogenous overexpressed H2O2 to O2. This improved tumor hypoxia and produced ROS, thus effectively making cancer cells apoptosis. Importantly, under the irradiation of 808 nm laser, Pt-CuS Janus generated heat. The catalytic activity of Pt was significantly enhanced, and the O2 production was greatly increased, further promoting SDT (Fig. 6E).
However, the selection of appropriate sonosensitizers remains a critical challenge in SDT. The development of new sonosensitizers requires addressing several key criteria: they should exhibit relatively low toxicity, have prolonged retention time in vivo, possess a relatively simple and well-defined structure, and demonstrate improved targeting capabilities with sufficient ROS production potential.
5.5. Cu-based nanoparticles for immunotherapy
Cancer immunotherapy involves leveraging immune cells for cancer treatment, primarily by bolstering immune cell function or alleviating immune cell inhibition. The integration of Cu materials in immunotherapy was first carried out by labeling immunoconjugates for pharmacokinetic examination. As early as 2002, Novak-Hofer et al. systematically reviewed the application of 67Cu in radioimmunotherapy. This review described the application of 67Cu-labeled antibodies, elucidated quality control methodologies, and discussed relevant clinical data [160]. Compared to traditional radioactive iodine labeling methods, 67Cu-labeled antibodies show higher and longer lasting tumor absorption. However, 67Cu still has some problems with radioactivity. Suzuki et al. [161] proposed to label antibody fragments with 64Cu. Antibody fragments labeled with 64Cu reduced the level of renal radioactivity through urine excretion. This approach solved the problem of high radioactivity level to some extent.
Immunotherapy usually activates the anti-tumor immune response to kill tumors. The process in which tumor cells undergo death by external stimuli and change from non-immunogenic to immunogenic and mediate the body's anti-tumor immune response is called immunogenic cell death (ICD) [162]. Cu sulfide nanoparticles (CuS-OMVs) were developed for synergistic application in systemic photothermal immunotherapy [163]. With advantageous traits such as robust photothermal conversion efficiency and potent tumor-targeting capability, CuS-OMVs demonstrated substantial anti-tumor efficacy under NIR-II light irradiation. Moreover, CuS-OMVs induced robust ICD in tumor cells while concurrently promoted the maturation of DCs and subsequent activation of CD8+ T cells. (Fig. 7A).
Fig. 7.
Cu-based nanoparticles for immunotherapy. (A) Schematic illustration of CuS-OMVs cooperating with PTT and immunotherapy for cancer [163]. Copyright 2022, Elsevier. (B) Schematic illustration of the preparation of Cu-COF and ICD induced by enhanced Fenton-like effect [164]. Copyright 2023, Wiley. (C) Schematic illustration of the preparation of BMS-SNAP-MOF and synergistic enhancement of anti-tumor immunotherapy by IDO inhibition and NO combination therapy [165]. Copyright 2022, Wiley. (D) schematic illustration of the preparation of NP@ESCu and inducing Cu death for enhancing cancer immunotherapy [166]. Copyright 2023, Wiley.
With the rise of CDT, the exploration of Cu+ catalyzing the Fenton reaction tot produce ROS by Cu materials has gained traction increase in the study of immunotherapy. Li et al. [164] developed a covalent organic backbone carrier with Cu+ and Cu2+ at the same time. In this carrier, Cu+ catalyzed H2O2 into ·OH and 1O2. One of the important inducers of ICD was intracellular ROS. This system combined CDT and ICD, which significantly inhibited many types of tumor cells, such as CT26, HeLa and other tumor cells, showing a strong anti-tumor effect (Fig. 7B).
However, the intricate TME remains a formidable obstacle impeding the efficacy of immunotherapy. The development of an immunosuppressive microenvironment is a progressive consequence of sustained tumor antigen stimulation and immune activation. The substances or cells in TME are exhausted, and the related functions cannot be activated, or even the malignant characteristics of tumors cannot be promoted. Du et al. [165] devised BMS-SNAP-MOF, which encapsulated the immunosuppressant enzyme IDO inhibitor BMS-986205 and NO donor. BMS-SNAP-MOF accumulated in tumor tissues through EPR effect. After internalization in tumor cells, enough GSH reacted with MOF, which decomposed and rapidly released BMS-986205 and produced rich NO. Therefore, IDO inhibitor and NO synergistically regulated the microenvironment of immunosuppressed tumor, and produced effective immunotherapy by increasing CD8+ T cells and reducing Treg cells (Fig. 7C).
The discovery of cuproptosis provides another feasible way for Cu materials to participate in immunotherapy. Guo et al. [166] co-embedded selenophenol (ES) and Cu into ROS-sensitive polymer (PHPM), and the prepared nanoparticles were called NP@ESCu. These nanoparticles were triggered by ROS in cancer cells, releasing Cu and ES. NP@ESCu nanoparticles effectively transported Cu and induced cuproptosis. In addition, NP@ESCu nanoparticles were further combined with αPD-L1 for combined immunotherapy (Fig. 7D).
In the latest study, the mechanism of Cu's involvement in immunotherapy has been rationally explored and discussed. Hesemans et al. [167] designed a series of Cu-doped TiO2 systems with different proportions to explore the role of Cu in immunotherapy. Cu-doped TiO2 nanoparticles contributed to promoting the effect of immunotherapy. The 10 % Cu-doped TiO2 system increased tumor-infiltrating lymphocyte levels compared with normal. The 33 % Cu-doped TiO2 system increased the levels of tumor-infiltrating lymphocytes and tumor-associated macrophages. Notably, 33 % Cu-doped TiO2 had a significant activating effect on dendritic cells.
Over years of immunotherapy practice, numerous cases have demonstrated favorable outcomes. These successful cases highlight several advantages, such as a relatively rapid onset of action and a low probability of side effects.Despite these benefits, immunotherapy has not yet become a mainstream cancer treatment. This is primarily because its effectiveness varies greatly among patients, with some experiencing minimal benefits. In addition, the effects of immunotherapy may take longer to manifest compared to more aggressive interventions like chemoradiotherapy. Although immunotherapy drugs can act quickly, the overall treatment duration is often prolonged, which may prevent achieving immediate cancer control.Moreover, effector cell exhaustion is another factor that can impact the effectiveness of immunotherapy. In order to suppress the excess immune response, effector cells may become depleted. Enhancing immunity while simultaneously preventing effector cell exhaustion could be a strategy to improve the efficacy of future immunotherapiesAlthough immunotherapy is not a one-size-fits-all cancer treatment program, its emergence has pointed out a new alternative path for the field of cancer treatment. And immunotherapy can indeed improve the prognosis of some cancers, and some old treatment options cannot achieve the effect, so it is still worth studying and developing.
5.6. Combined cancer therapy
Currently, there are numerous cancer treatment options, with traditional chemotherapy still playing a significant role. However, due to the adverse side effects associated with chemotherapy, there has been a growing interest in new therapies in recent years. Despite this, each therapy has its own limitations and may not be fully effective when used alone. Therefore, combining different therapies could become an effective approach to cancer treatment in the future. This strategy seeks to maximize the strengths and minimize the weaknesses of various treatments, ultimately leading to faster and more effective tumor eradication with fewer side effects.
The combined application of PTT and CDT is common in synergistic therapy. CuAl-LDH@DOX@PDA nanocarrier was constructed, and it showed enhanced absorption at 810 nm, thus having excellent photothermal efficiency [168]. Meanwhile, with the consumption of GSH, Cu2+ was reduced to Cu+, which realized the combination of CDT and PTT. Overcoming endogenous H2O2 deficiency and high GSH concentration in TME is one of the keys to solving the combination therapy. Therefore, Liang et al. [169] designed and constructed a nano-platform consisting of GOx encapsulated by Cu-doped mesoporous Prussian Blue (CMPB) and hyaluronic acid (HA) encapsulated by NO donor (HN). GOx@CMPB-HN nanoparticles realized CDT, PTT and hunger therapy. Cu2+ consumed intracellular GSH in TME. In addition, the by-product Cu+ acted as a substrate to activate the Fenton-like reaction of CDT. In addition, H2O2 enhanced CDT by increasing the intracellular glucose consumption caused by GOx loaded with nanoparticles and further enhancing the acidity of TME. In addition, Cu2+doping greatly improved the photothermal conversion performance of mesoporous Prussian Blue (MPB), and the resulting temperature increase accelerated the CDT catalysis (Fig. 8A).
Fig. 8.
Cu-based nanoparticles for combined cancer therapy. (A) Schematic illustration of GOx@CMPB-HN NPs for in-situ amplification of PTT, CDT and hunger therapy [169]. Copyright 2023, American Chemical Society. (B) Schematic illustration of O2-Cu/ZIF-8 @ Ce6/ZIF-8 @ F127 for synergistic therapy of PDT and CDT [170]. Copyright 2019, American Chemical Society. (C) Schematic illustration of synthesis process of CuPP nanoenzyme and its application in PTT, CDT and immunotherapy [172]. Copyright 2022, Wiley.
In CDT, endogenous H2O2 in tumors is catalyzed to toxic ·OH, which is superior to conventional chemotherapy owing to the site-specific ROS generation. It is possible to obtain better therapeutic effects by using different ways to obtain ROS, such as integrating CDT with PDT or SDT. PDT has been applied in the clinic while SDT is in its infancy. Therefore, the combination of CDT and PDT is worth in-depth study and has promising prospects for clinical application. Cu-based nanoparticles possess GSH oxidase-like and catalase-like activities, which are beneficial to intensifying oxidative stress and relieving hypoxia in cancer cells. However, high Fenton-like reaction activity requires the reduction of Cu2+ to Cu, and the integration of Cu-based Fenton-like agents with photosensitizers inevitably requires a complicated chemical modification process to prepare porous nanocarriers. Hou et al. [132] loaded cisplatin (CDDP) and ICG on the surface of Cu2O nanoparticles modified by hydrazide hyaluronic acid, and obtained a nano-platform named HCCI. HCCI showed a rapid acid reaction dissociation behavior, releasing ICG, Cu and CDDP. ICG transformed O2 into 1O2 under NIR radiation, and CDDP inhibited cell proliferation by cross-linking DNA molecules. Cu consumed GSH, accelerated the production of O2 and ·OH and inhibited ROS clearance. Therefore, HCCI realized efficient PDT, CDT and chemotherapy. Over-expression of GSH and hypoxia in TME seriously hinder CDT. Therefore, Xie et al. [170] developed a therapeutic system named O2-Cu/ZIF-8@Ce6/ZIF-8@F127(OCZCF). OCZCF was biodegradable and realized GSH consumption and O2 intensive combined therapy. Introducing Cu2+ into the system improved O2 reserves of ZIF-8, thus improving the PDT efficiency of Ce6. When GSH was consumed by Cu2+, the reduced Cu+ would trigger an effective Fenton-like reaction to produce ROS, thus realizing CDT (Fig. 8B).
PDT can trigger ROS storms in cancer cells, which further induce an immunogenic cell death cascade to initiate the antitumor immune response. A nano-enzyme-assisted PDT nano-platform HA@Cu(OH)2-ICG(nHACI) was constructed, which could realize efficient ICD by starting ROS storms [171]. Among them, Cu2+ was reduced to Cu+, thus depleting GSH, enhancing the efficacy of PDT and amplifying ICD. ROS contributes to the enhancement of tumor immunogenicity and reverse of immunosuppressive TME [132]. To sum up, combining immunotherapy with ROS-based therapy provides a feasible strategy for cancer treatment. Moreover, this non-invasive cancer treatment has fewer side effects.
The good photothermal properties of Cu-based nanoparticles suggest another approach for synergistic immunotherapy. The combined application of nanocatalytic therapy, immunotherapy and PTT has also been successfully implemented in practice. The novel Cu-doped polypyrrole nanoenzyme (CuP) had three similar enzyme activities of catalase (CAT), GSH peroxidase (GPx) and peroxidase (POD), which can specifically promote the elevation of O2 and •OH in the TME [172]. PEG-modified CuP nanoenzyme (CuPPs) effectively reversed the immunosuppression TME by overcoming tumor hypoxia and polarizing macrophages from the former tumor M2 phenotype to the anti-tumor M1 phenotype. What's more, CuPPs showed high-temperature enhanced enzyme, simulated catalysis and immunomodulatory activity. This led to a strong immune response, and by further combining with αPD-L1, the tumor was almost completely inhibited. CuPPs achieved a high degree of accumulation at the tumor site, and tumor hypoxia levels were significantly alleviated after 1064 nm laser irradiation (Fig. 8C).
6. Cu-based nanoparticles for cancer imaging
Cancer imaging is indispensable in the detection, treatment and prognosis of cancer. Traditional contrast agents (CAs) are less sensitive, accumulate less on the target, and lack specificity for cancer [173]. Therefore, ingenious nanomaterial-based CAs are urgently needed. Cu-based nanoparticles can provide precise anatomical and functional information to determine the stage of cancer. At present, a variety of Cu-based nanoparticles have been applied to cancer imaging. This section mainly introduces the characteristics and applications of Cu-based nanoparticles in photoacoustic (PA) imaging, ultrasound imaging, resonance (MR) imaging, positron emission tomography (PET) imaging, single-photon emission computed tomography (SPECT) imaging, fluorescence (FL) imaging and computed tomography (CT) imaging. Additionally, this section also introduces some Cu-based nanomaterials that can be applied to multimodal combined imaging.
6.1. Cu-based nanoparticles used for cancer unimodal imaging
PA imaging, as a noninvasive modality, is characterized by excellent contrast, spatial resolution, high penetration and sensitivity to tissues. The released heat causes the local temperature to rise, thus resulting in thermal expansion and generating pressure waves, which are the photoacoustic signal. For better PA imaging, the new type of Cu-based nanoparticles can be designed as CAs. There are many types of contrast agents for photoacoustic imaging, including small-molecule organic dyes, organic nanocomposites, carbon nanomaterials, and inorganic nanomaterials. Among them, in addition to gold nanoparticles in inorganic nanomaterials, Cu sulfide has become more and more important in recent years. Photoacoustic imaging is characterized by its ability to provide deep imaging depth and high spatial resolution. The results show that Cu sulfide has high photothermal conversion efficiency and photoacoustic imaging contrast with high signal-to-noise ratio. Therefore, Cu sulfide is increasingly appearing as a contrast agent in photoacoustic imaging. For example, Ouyang et al. [174] wrapped Cu sulfide nanoparticles in functional tree-like molecules (Cu sulfide DENPs) and covalently linked to 1,3- propanesulide and arginine-glycine-aspartic acid (RGD) peptide. Functional RGD-CuS DENPs were applied to PA imaging and inhibited tumor growth and tumor metastasis. In addition, the functional RGD-CuS DENPs had good absorption capacity and good photothermal conversion efficiency.
MRI relies on the principle of nuclear magnetic resonance. MRI is accurate, fast and harmless to human body. Nuclear magnetic resonance contrast agents themselves do not produce resonance signals. These are paramagnetic or superparamagnetic substances, usually containing an ion center containing unpaired electrons, which can interact magnetically with hydrogen nuclei, by changing the relaxation time of hydrogen nuclei in local tissues in vivo and contrasting with surrounding tissues, so as to achieve the purpose of contrast. Common NMR contrast agents include iron oxide, manganese oxide, etc. Relaxation efficiency is one of the key indicators of MRI contrast agents. The unpaired electrons of Cu also meet the requirements for MRI, and the relaxation efficiency is not low. Therefore, research on Cu-based nanomaterials as contrast agents has also been carried out gradually. Li et al. [175] reported ultra-thin Cu tetraketone (4-carboxyphenyl) porphyrin (Cu-TCPP) MOF nanosheets. The D-d band transition and ultra-thin properties of Cu2+ translated into excellent photothermal properties, making it suitable for PTT. The photosensitive properties of TCPP made it suitable for PDT. Cu's unpaired three-dimensional electrons were applied to MRI. Cu-TCPP MOF nanosheets could be applied to the treatment of PTT and PDT, and their MRI could be guided simultaneously.
The basic imaging principle of PET is to label a substance with radionuclides and inject them into the human body to detect its flow and the degree of aggregation in different places, so as to achieve the purpose of imaging. PET is a nuclear imaging technique that can show metabolic processes in the body, providing highly sensitive and quantitative radiotracer readings. PET imaging is an imaging technology that can show the metabolism of biomolecules, receptors and neurotransmitter activities in vivo. It has the advantages of high safety, high sensitivity, high specificity and good whole-body imaging ability. PET imaging is an imaging technique utilizing β+ decay. There are isotopes of β+ decay that can theoretically act as contrast agents. For example, Shah et al. [176] directly added 64Cu into the matrix of Cu sulfide NPs to design radioactive NPs without chelators. Because of the small volume of [64Cu] CuS NPs coated with PEG, they showed high uptake and retention in U87 xenograft tumor cells, and performed well in PET imaging (Fig. 9A).
Fig. 9.
Cu-based nanoparticles involved in cancer imaging. (A) PEG-coated 64CuS NPs for PET imaging of U87 xenograft tumor [176]. Copyright 2016, American Chemical Society. (B) In vivo CT imaging of mice using Cu2-xS:Pt(0.3)/PVP NPs [179]. Copyright 2018, the Royal Society of Chemistry. (C) Application of RGD-CuS-Cy5.5 NPs in fluorescence and CT dual-mode cancer imaging in vivo [183]. Copyright 2018, Elsevier. (D) Application of CuCD NSs in photoacoustic and fluorescence dual-mode cancer imaging [184]. Copyright 2018, American Chemical Society.
Another imaging technique that can be applied to radioactive Cu is SPECT imaging. Unlike PET imaging, SPECT imaging applications utilize γ decay. Its characteristic is that it can reflect information on blood perfusion and material metabolism of tissues and organs. 67Cu has recently made a breakthrough in SPECT imaging, with high specific activity, high radionuclide purity, and sufficient quantity. Hao et al. [177] marked pertuzumab with 67Cu and realized SPECT imaging. All tumors imaged by SPECT were also clearly visualized with the 67Cu-labeled pertuzumab at Day 5 after injection. This also demonstrated the potential of 67Cu radiopharmaceuticals for SPECT imaging. However, its disadvantages are also relatively obvious. For example, the resolution of SPECT imaging is low, and the anatomical structure cannot be clearly displayed, which makes it difficult to locate the lesion. Therefore, SPECT imaging can be combined with other high-resolution, structurally accurate imaging techniques, such as CT imaging.
CT imaging is another anatomical imaging method. It is a sublimation of X-ray technology, and tomography is more three-dimensional. The density resolution of CT display is obviously better than that of X-ray images. However, it is uncertain whether it is harmless to human body. The sensitivity of CT imaging to CAs is low. However, heavy metal elements can solve this problem to some extent because of their X-ray attenuation characteristics. The most prominent feature of CT imaging is the fast scanning time and clear images [178]. The contrast agent for CT imaging must have good X-ray attenuation behavior. A Cu sulfide nanoparticle targeting gastric cancer demonstrated its good CT imaging effect. Dong et al. [179] constructed Cu2−xS:Pt(0.3)/PVP nanoparticles for CT imaging by adding Cu and platinum elements. In tumor cells, Cu2−xS:Pt(0.3)/PVP nanoparticles released Pt4+ by 808 nm laser irradiation. Under such conditions, not only GSH reduced Pt4+ to Pt2+ for chemotherapy, but also due to the addition of Cu, PTT was also achieved. This also realized the synergy of CT imaging, chemotherapy and PTT (Fig. 9B). However, the disadvantages of CT imaging cannot be ignored. For example, during the examination, radiation will be exposed to the body, and when the radiation accumulates to a certain dose, it will cause certain damage to the body [178].
FL imaging is a common functional imaging method. FL imaging has allowed Cu-based nanoparticles to act as CAs. The advantages of fluorescence imaging are high sensitivity, non-invasive, and real-time. Fluorescent metal nanoclusters with relatively suitable sizes are a good choice for fluorescent imaging contrast agents. There are many types of such metal clusters, such as gold, silver and Cu [180]. Among them, Cu is relatively abundant and readily available. The optical properties of Cu nanoclusters are similar to those of gold nanoclusters and silver nanoclusters. The main thing is that Cu is more biocompatible and environmentally friendly than gold and silver. For example, Xia et al. [181] prepared water-soluble and functional Cu nanoclusters (CuNCs). CuNCs had unique photoluminescent properties that could be used for tumor imaging. CuNCs emitted bright red fluorescence in HeLa cells, and with the increase of culture time (1–24 h), it was found that the total fluorescence of cells showed an increasing trend. However, fluorescence imaging also has certain disadvantages, and the significant disadvantage is that the detection depth is relatively low.
Cu-based nanoparticles can also be involved in ultrasound imaging to improve the imaging effect. Ultrasound imaging is a non-invasive, convenient, portable, and cost-effective imaging technique. After receiving and processing the signal reflected by the ultrasonic beam scanning the human body, images of the internal organs can be obtained [182]. A reasonable combination of Cu-containing nanoparticles and ultrasound imaging contrast agents can obtain ultrasound-responsive therapeutic nanoagents that can be used for different applications. CuS nanoparticles were decorated on perfluoropropane aerated microbubbles (MBs) prepared from a micelle solution composed of Span 60 and Tween 80 (CuS-ST68 MBs). After administration in rabbits, CuS-ST68 MBs exhibited good ultrasound imaging functions. Experiments showed that the renal imaging of rabbits was significantly enhanced. This proved that CuS-ST68 MBs effectively enhance the contrast of ultrasound imaging.
6.2. Cu-based nanoparticles used for cancer multimodal imaging
There are many cancer imaging methods in which Cu participates, and each method has advantages and disadvantages in different aspects. Therefore, the advantages of combining and complementing each other may be more reasonable and acceptable. To better improve diagnostic accuracy, multimodal imaging has become a general trend, occupying an increasingly important position and gaining increasing applications in cancer imaging [185]. Multimodal imaging refers to imaging modalities that combine multiple imaging to acquire multiple parameters. Because various imaging modalities vary in principle and reflect information, multimodal imaging is complementary in applications. An increasing number of Cu-based nanoparticles are being developed as CAs.
Combining two or more imaging methods may be the best of both worlds. For example, as a functional imaging method, PET has the advantages of high sensitivity and rapid imaging. However, its imaging spatial resolution is poor, which prevents it from providing a clear anatomical reference frame. The combination of PET and CT imaging can achieve complementary advantages. Comprehensive PET/CT examination has been widely accepted and applied in tumor diagnosis, prognosis, treatment planning and treatment effect evaluation. Based on preclinical results, 64CuCl2 has been used for PET/CT imaging of bladder cancer and prostate cancer. Thus, due to these complementarities, increasing amounts of Cu involved in multimodal imaging have been developed. Shi et al. [183] developed RGD-CuS-Cy5.5 nanoparticles, which made it possible to perform bimodal imaging and PTT on lymph node metastatic gastric cancer. RGD-CuS-Cy5.5 nanoparticles emitted CT and fluorescence signals under NIR irradiation. After injection of RGD-CuS-Cy5.5 nanoparticles, they tended to aggregate in sentinel lymph nodes (SLNs) and entered metastatic gastric MNK45 tumors. Subsequently, the tumor tissue was eliminated by using the photothermal effect produced by 808 nm laser irradiation (Fig. 9C). With the further development of bimodal imaging, the advent of trimodal imaging also became possible. Bao et al. [184] developed Cu/carbon dots cross-linked nanosheets (CuCD NSs). CuCD NSs are small in size and have good photothermal conversion performance and photothermal stability. After laser irradiation at the in-situ tumor site, the tumor accumulation of CuCD NSs was significantly enhanced. When CuCD NSs were coated with fluorescent molecules, multi-mode (PA, PTT and FL) imaging-guided cancer treatment was realized (Fig. 9D).
7. Cu chelators —"reducing Cu" for killing cancer
Chelators are ligands with multiple donor atoms (Lewis bases). Theoretically, metal cations (Lewis acids) can interact with chelating agents to form metal chelates. Under ideal physiological conditions, this metal complex is relatively stable and does not dissociate easily [48]. Cu chelators can reduce Cu levels by chelating Cu ions. Cu ions play an important role in the formation of angiogenesis. Reducing Cu levels in cancer cells can reduce angiogenesis in tumor tissues, block the supply of nutrients to the tumor, and limit tumor growth.
7.1. Application and development of Cu chelators
Cu chelators are mainly used to treat Wilson's disease and other diseases caused by excessive Cu ion concentrations. D-penicillamine is currently the most commonly used Cu chelators. It is the first Cu chelator introduced to the clinic and the longest used Cu chelator. Tristrigraphite and tetrathiomolybdate are anti-cancer drugs developed after the discovery of d-penicillamine. They act as substitutes when patients are manifestly intolerant to d-penicillamine, and both show good anti-cancer ability. Research proved that d-penicillamine, trientine and tetrathiomolybdate all exhibited the ability to inhibit new blood vessel formation (angiogenesis). In a mouse model of BNL hepatocellular carcinoma, d-penicillamine and trientine successfully inhibited tumor growth [48].
The effect of Cu chelators in inhibiting tumor growth and metastasis is studied. Shi et al. [47] synthesized a new thieno [3,2- c] pyridine compound. This compound, called JYFY-001, has an acetamide moiety that can chelate Cu ions. The research showed that the growth of cancer cells was obviously inhibited after JYFY-001 was applied. In another study, a tendency for “starve to death” was observed in tumors. Heuberger et al. [186] used a Cu chelator, PSP-2, to treat H460 human lung cancer cell model transplanted into chick chorioallantoic membrane. With the progress of research, the weight and vascular density of tumor tissue decreased significantly, and the tumor tended to starve (Fig. 10A).
Fig. 10.
Cu chelation for killing cancer. (A) Schematic illustration of PSP-2 chelating Cu ions in tumor, which leads to the obvious decrease of blood vessel density in tumor and tumor [186]. Copyright 2022, Multidisciplinary Digital Publishing Institute. (B) Schematic illustration of PY-TBDP chelates Cu ions and shows high photothermal properties [187]. Copyright 2022, the Royal Society of Chemistry. (C) Schematic illustration of a proposed model of the important role of Nedd4l-CTR 1- Cu -PDK1-AKT signaling pathway in tumorigenesis [188]. Copyright 2021, Wiley. (D) Schematic illustration of the preparation of targeted micelles assembled by CPLP/PLP/ probe X/DOX and tumor therapeutic diagnostic mechanism of targeted drug delivery [189]. Copyright 2022, Elsevier.
PTT is widely applied in the treatment of tumors. Combining PTT with Cu chelators may have a better anti-tumor effect. Therefore, the research on combining Cu chelators with PTT appeared to improve the curative effect of Cu chelators. A dye molecule (PY-TBDP) can quickly chelate Cu ions and has good photothermal conversion performance [187]. The anti-tumor effect was enhanced by combining Cu chelation therapy with PTT (Fig. 10B).
7.2. Cu chelators and signaling pathways
Cu chelators can inhibit tumorigenesis by affecting signaling pathways. Cu chelators have further revealed how they mechanistically affect tumorigenesis by interfering with signaling pathways. For example, the PI3K-AKT signaling pathway has a great influence on regulating many different cellular functions [188]. Cu can activate the PI3K-AKT pathway and send out carcinogenic signals, thus promoting the occurrence of tumors. Reducing AKT signaling by using Cu chelators can reduce tumorigenesis (Fig. 10C).
The TGF-β pathway is another important pathway that affects tumor growth. TGF-β is the key signal factor for inducing epithelial-mesenchymal transition (EMT), which can lead to the growth and metastasis of cancer cells [190]. For example, the chelator tetraethylenepentamine (TEPA) inhibits EMT by reducing Cu. Poursani et al. [191] used three different tumor cell lines as models, including neuroblastoma (NB), diffuse intrinsic pontine glioma (DIPG) and human triple-negative breast cancer (TNBC), to study the role of TEPA. The results showed that TEPA obviously inhibited the growth and metastasis of tumors.
The study of the MEK-ERK pathway is relatively more extensive. In the whole pathway, the upstream BRAFV600E is phosphorylated first. Then MEK1/2 kinases are activated by phosphorylated BRAFV600E and phosphorylated. ERK1/2 kinases are then activated by phosphorylated MEK1/2 kinases [192]. These processes promote the development of cancer. Cu chelators can inhibit signaling by reducing Cu. For example, after the intervention of Cu chelator tetrathiomolybdate, melanoma cell lines using BRAF or MEK1/2 inhibitors showed obvious growth inhibition [193]. It was also found that the anti-tumor activity of BRAF or MEK1/2 inhibitors was improved to some extent after using the Cu chelator tetrathiomolybdate.
Cu can regulate several other pathways, such as receptor tyrosine kinase (RTK)-signaling pathway. DIPG is a cancer that often occurs in children. Many DIPG patients are characterized by altered RTK signaling pathway. Cu is an essential transition metal for cell signaling, and Cu chelators reduce brain Cu levels. Thus, Michniewicz et al. [194] proposed a therapeutic strategy that applies Cu chelators to target Cu in DIPG. The addition of Cu to the culture medium triggered two RTK-mediated downstream signal pathways, and the use of TEPA affected the steady state of Cu and reduced the proliferation of DIPG cells.
7.3. Strategies to increase efficiency and reduce toxicity
Despite Cu chelators’ great potential to be one of the anti-cancer strategies, it is not advisable to apply Cu chelators universally and directly to patients owing to non-negligible side effects. Like the design of selective iron chelators, one strategy is to design selective Cu chelators to achieve the effect of chelating Cu by targeting specific sites in cancer cells [195]. Another strategy is to design pro-chelators based on the characteristics of cancer cells [196]. For example, it is proposed that pro-chelators be designed that can recognize highly active and expressed enzymes in cancer cells. In addition, pro-chelating agents can be designed to take advantage of large amounts of nutrients absorbed by cancer cells. Through the above methods, the side effects of Cu chelators on ordinary cells can be reduced. A poly-l-glutamate penicillamine derivative was discovered as a pro-chelator [197]. It had different degrees of effect on HL-60 leukemia cells and MDA-MB-468 metastatic breast cancer cells. In vivo toxicological studies in P388 leukemia mice carrying CD2F1 showed that a 10 mg kg-1 dose level had low systemic toxicity. This also provided experimental support for the realization of chelators as anti-cancer means.
In addition, Cu chelators are helpful in enhancing the efficacy of other anticancer drugs (e.g., platinum-based drugs) in the combination of Cu chelators and chemotherapy. Platinum drugs are widely used in cancer chemotherapy. However, the impact of resistance to platinum drugs on clinical efficacy is still worthy of attention. Studies show that Cu transport mechanisms are involved in the transport of platinum drugs, such as Cu transporters (hCtr1), Cu chaperone (Atox1), and Cu exporter (ATP7A and ATP7B) [198]. These proteins help cells expel platinum drugs from cells, allowing tumor cells to escape platinum drug-induced cell death. Clinical studies with Cu chelators (tentonic) in carboplatin therapy were performed to reduce cellular Cu bioavailability levels by Cu chelators to overcome Pt resistance in part due to transport defects. Ryumon [199] et al. proved that Cu-chelated ammonium tetramolybdate (TM) could enhance the effect of cisplatin for treating of head and neck squamous cell carcinoma (HNSCC). TM inhibited the expression of ATP7B in HNSCC cells. Cisplatin accumulated more in cancer cells and induced apoptosis. In this way, the amount of Cu chelators is reduced, and the side effects of platinum drugs are fully reduced, thus achieving the purpose of efficiency and attenuation.
In addition, Cu chelators are also used in combination with other chemotherapeutic drugs to have a synergistic therapeutic effect on cancer. A peptide-based micelle targeting tumor neovascularization was developed. The DOX and probe X have an “on-off” program with the micelle-forming system [189]. In vivo Cu+ capture events were reported in real-time by receiving NIR fluorescence and photoacoustic signals. Through anti-angiogenic components and DOX, tumor suppression was achieved. It was worth noting that the toxicity of this system was relatively low and it was a more feasible anti-tumor strategy (Fig. 10D).
7.4. The current limitations and future direction of Cu chelators
Cu chelators have been discovered for decades. From the beginning, they were considered a new breakthrough in the treatment of cancer, but now they are rarely studied owing to the curative effects and side effects. It is assumed that a certain Cu chelator has the ability to lower Cu levels. The first issue to be discussed is the magnitude of Cu reduction. Insufficient Cu-lowering ability will result in no or poor efficacy. Showing strong Cu-lowering ability in tumor tissue and starving tumors to death is the desired outcome. Moreover, examining selectivity and targeting is of great importance [200]. Although selective Cu chelators have been reported, the selectivity and targeting of such chelators are generally unsatisfactory [48]. With poor selectivity and targeting of Cu chelators, the Cu level in the body will be too low, thus leading to a series of problems. Cu deficiency will not only make blood vessels and bones brittle, but also lead to anemia. Cu is also essential for the development of nervous system, intelligence and body. In addition, there may be many problems in the design of Cu chelators. For example, whether Cu chelators will chelate other metal ions, such as zinc ions and iron ions. Another example is whether the chelator or the post-chelated product will conflict with the endogenous substances in the body.
In addition to the above, there are many other factors that can affect the application of Cu chelators. For example, Cu homeostasis will stabilize the Cu level in a certain range, which may regulate the Cu transport mechanism and thus affect the therapeutic effect. From a microscopic point of view, it is also necessary to consider the stability of chelators to transport Cu in tumor tissues in vivo. Although theoretically, the formed Cu chelate is not easily dissociated. However, in the complex tumor microenvironment, the interaction of various conditions can lead to structural dissociation. For example, Cu2+-Cu+ redox conversion may affect the stability of the structure.
In the future, the development of Cu chelators will continue to face significant challenges. On the one hand, ensuring safety is of utmost importance. The toxicity of Cu chelators and the post-chelation products in the body are key concerns. On the other hand, achieving efficacy while ensuring safety is crucial. Designing selective and targeted Cu chelators or pro-chelators is the optimal solution. It may also be a feasible approach to design a system with controlled release of Cu chelators. In short, in the direction of safety and effectiveness, the development of Cu chelators still has a long way to go.
8. Conclusions and outlook
Cu is one of the earliest metals to be utilized by humans, serving as an essential trace element with unique characteristics. It participates in various physiological processes, including angiogenesis, mitochondrial respiration, ROS detoxification, antioxidant defense, and biosynthesis of hormones and neurotransmitters. Maintaining Cu homeostasis is crucial as both excessive and insufficient Cu levels in the body can lead to adverse consequences such as Wilson disease and abnormal cell proliferation. More importantly, Cu's involvement in all stages of cancer highlights its potential application in cancer treatment.
This paper focuses on the relationship between Cu and cancer, the mechanism of cuproptosis and possible application of Cu ionophores in cuproptosis, as well as strategies of disrupting tumor homeostasis through Cu ionophores and Cu chelators for tumor treatment. In addition, this paper provides a comprehensive summary of Cu-based nanoparticles and their tumor theranostics application: (1) multifarious anti-tumor effects: PTT, PDT, CDT, SDT, immunotherapy and combined therapy, (2) imaging function including PAI, PET, SPECT, FL and CT. Besides, the green synthesis approach also provides ideas for improving safety.
With research progressing, Cu-based tumor theranostics has achieved encouraging results. However, there are still challenges and problems to be addressed during clinical translation.
-
(1)
It is crucial and fundamental to investigate the safety of Cu-based nanoparticles. The long-term biocompatibility and biosafety of Cu-based nanoparticles are of paramount concern. Currently, there are no established evaluation principles and standards for the biocompatibility and biological safety of Cu-based nanoparticles, which poses a significant challenge. Additionally, many studies have collected biosafety data over relatively short periods, which fails to fully demonstrate the safety of long-term use of Cu-based nanoparticles. Another safety-related factor is the biodegradation of Cu-based nanoparticles in vivo. Although organic Cu-based nanomaterials are available, most Cu-based nanomaterials still appear in inorganic form. Whether inorganic nanoparticles can be biodegraded is still a topic of interest. Although most inorganic Cu-based nanoparticles have been proved to be excreted and cleared in feces and urine as intact nanoparticles, the low biodegradation rate and prolonged retention time cannot be ignored. One strategy to address this issue is to adjust the size of nanoparticles, enhance their dispersibility, or perform surface modification. Another strategy is to develop organic Cu-based nanoparticles that are more biodegradable than inorganic Cu-based nanoparticles, which may provide a solution to the problem.
-
(2)
Effective and safe synthesis methods of Cu-based NPs are another noteworthy issue. The classic synthesis methods include wet chemical reduction, solvothermal/hydrothermal methods, thermal decomposition methods, and electrochemical approaches. However, toxic raw materials, such as sodium borohydride, are harmful to both experimenters and the environment. Fortunately, green biological synthesis methods exploiting plant extracts and microorganisms have been reported. The phytochemicals in plants can act as reducing, stabilizing, and capping agents to increase the NPs’ stability, without observed significant toxicity, while the microorganisms-based methods can relieve drug-resistance but are restricted by culture contamination and lengthy procedures. Sonochemical synthesis and microwave chemistry are also potential green methods, which can reduce the reaction time by several orders of magnitude. It is expected that a simple, green method can be developed to synthesize stable and industrialization-friendly Cu-based NPs in the future.
-
(3)
Cu ionophores and Cu chelators play key roles in the treatment strategies of “increasing Cu” and “reducing Cu”. While effective agents exist, the lack of selectivity and targeting remains a significant obstacle. As Cu is crucial for the physiological processes of both healthy cells and cancer cells, the targeting of Cu ionophores and chelators has become paramount. It is necessary to design agents that target cancer cells with precision while minimizing effects on normal cells and tissues. Cu ionophores and chelators that function as prodrugs or conditionally gated structures may offer promise in this regard. Additionally, cuproptosis and Cu chelation mechanisms remain understudied, with a better understanding of these processes potentially enabling the design of more effective therapeutic agents, improving their specificity, and reducing adverse effects on healthy tissues. Cu signaling pathway modulation represents a potential new strategy for “increasing Cu” or “reducing Cu” by shielding or strengthening signal transmission, but this approach requires a more advanced drug delivery system to be feasible.
-
(4)
The development of photon nanomedicine has further solidified the status of Cu-based nanoparticles in tumor theranostics. PTT, PDT, and most cancer imaging methods all intersect with the field of photon nanomedicine. Currently, insufficient light penetration is a great challenge that severely impedes theranostics for deep-seated diseases. Careful tuning of nanostructure, composition, and physical and chemical properties can extend the light response wavelength to the NIR II window, thereby effectively enhancing light penetration depth. Additionally, combining photon nanotherapies with other treatment modalities may represent a sound strategy to improve therapeutic outcomes, overcoming the limitations imposed by poor light tissue penetration during phototherapy.
-
(5)
The transformation from laboratory production to mass production represents a significant challenge in the clinical translation process, with numerous aspects to consider in both the design of drug delivery systems and the manufacture of nanoparticles. Diverse functional requirements translate into distinct drug delivery system designs, while nanoparticles share numerous manufacturing considerations. The reproducibility, transparency, and cost of nanoparticle manufacturing have become crucial factors impacting their large-scale production. Nanoparticles are complex composites where component materials, assembly methods, surface properties, ligands, stiffness, and charges each contribute to their overall performance. Successful development of new, effective nanoparticles hinges on optimizing these physical and chemical parameters.
The emergence of Cu-based nanoplatforms has brought increasing hope to cancer treatment, while their development is accompanied by various challenges [201,202]. Current studies have paved the way for more in-depth exploration and clinical translation. It is expected that in vivo effects of Cu-based nanoplatforms, particularly their safety profile should be improved. Classification of Cu-based nanoplatform design and establishment of standards and principles are highly recommended. Different functions require specific designs to be realized. Additionally, laboratory research alone cannot meet the conditions for clinical translation, with many factors requiring control during mass production. The transition from animal to human subjects further increases the difficulty. It is hoped that in the future, these challenges will be fully addressed, enabling Cu-based nanoplatforms to shine in the field of cancer treatment.
CRediT authorship contribution statement
Xinghua Ren: Writing – original draft. Xinyi Luo: Writing – original draft. Fuchang Wang: Resources. Long Wan: Resources. Xiaofan Wang: Software. Jinya Xiong: Resources. Mengwei Ye: Investigation. Shiqiao Rui: Resources. Zhu Liu: Investigation. Siling Wang: Resources, Supervision. Qinfu Zhao: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
Authors declare no conflicts of interest.
Acknowledgements
Grant from Liaoning Provincal National Nature Science Foundation of China (No. 2023-MS-202) is greatly acknowledged.
References
- 1.Makhdoumi P., Karimi H., Khazaei M. Review on metal-based nanoparticles: role of reactive oxygen species in renal toxicity. Chem Res Toxicol. 2020;33(10):2503–2514. doi: 10.1021/acs.chemrestox.9b00438. [DOI] [PubMed] [Google Scholar]
- 2.Roshani M., Rezaian-Isfahni A., Lotfalizadeh M.H., Khassafi N., Abadi M.H.J.N., Nejati M. Metal nanoparticles as a potential technique for the diagnosis and treatment of gastrointestinal cancer: a comprehensive review. Cancer Cell Int. 2023;23(1):280. doi: 10.1186/s12935-023-03115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie W., Guo Z., Zhao L., Wei Y. The copper age in cancer treatment: from copper metabolism to cuproptosis. Prog. Mater Sci. 2023;138 [Google Scholar]
- 4.Wang X., Zhou M., Liu Y., Si Z. Cope with copper: from copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023;561 doi: 10.1016/j.canlet.2023.216157. [DOI] [PubMed] [Google Scholar]
- 5.Luo S., Qin S., Oudeng G., Zhang L. Iron-based hollow nanoplatforms for cancer imaging and theranostics. Nanomaterials. 2022;12(17) doi: 10.3390/nano12173023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K., Qi C., Cai K. Manganese-based tumor immunotherapy. Adv Mater. 2023;35(19) doi: 10.1002/adma.202205409. [DOI] [PubMed] [Google Scholar]
- 8.Hao Y., Zheng C., Wang L., Zhang J., Niu X., Song Q., et al. Tumor acidity-activatable manganese phosphate nanoplatform for amplification of photodynamic cancer therapy and magnetic resonance imaging. Acta Biomater. 2017;62:293–305. doi: 10.1016/j.actbio.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Villalobos Gutiérrez P.T., Muñoz Carrillo J.L., Sandoval Salazar C., Viveros Paredes J.M., Gutiérrez Coronado O. Functionalized metal nanoparticles in cancer therapy. Pharmaceutics. 2023;15(7):1932. doi: 10.3390/pharmaceutics15071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Chang Y., Lian X., Zhou L., Yu Z., Wang H., et al. Silver nanoparticles for enhanced cancer theranostics: in vitro and in vivo perspectives. J Biomed Nanotechnol. 2018;14(9):1515–1542. doi: 10.1166/jbn.2018.2614. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Zou L., Hartono D., Ong C.N., Bay B.H., LY Yung. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv Mater. 2007;20:138–142. [Google Scholar]
- 12.Sakthi Devi R., Girigoswami A., Siddharth M., Girigoswami K. Applications of gold and silver nanoparticles in theranostics. Appl Biochem Biotechnol. 2022;194(9):4187–4219. doi: 10.1007/s12010-022-03963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiraishi K., Kawano K., Maitani Y., Yokoyama M. Polyion complex micelle MRI contrast agents from poly(ethylene glycol)-b-poly(l-lysine) block copolymers having Gd-DOTA; preparations and their control of T(1)-relaxivities and blood circulation characteristics. J Control Release. 2010;148(2):160–167. doi: 10.1016/j.jconrel.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Zeng Y., Zhang H., Gu Z., Gong Q., Luo K. Functional gadolinium-based nanoscale systems for cancer theranostics. J Control Release. 2021;329:482–512. doi: 10.1016/j.jconrel.2020.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Zhao Y., Zhao Y., Zhang Y., Yao X., Hang R. Exosomes secreted by macrophages upon copper ion stimulation can promote angiogenesis. Mater Sci Eng: C. 2021;123 doi: 10.1016/j.msec.2021.111981. [DOI] [PubMed] [Google Scholar]
- 16.Wang M., Zhang W., Ding B., Pa Ma, Lin J. Copper peroxides based multiple tumor microenvironment regulation for enhanced photodynamic/chemodynamic synergistic therapy. Adv Opt Mater. 2023;11(11) [Google Scholar]
- 17.Pan Q., Kleer C.G., Golen K., Irani J., Merajver S.D. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62(17):4854–4859. [PubMed] [Google Scholar]
- 18.Tang L., Zhang A., Zhang Z., Zhao Q., Li J., Mei Y., et al. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Commun. 2022;42(2):141–163. doi: 10.1002/cac2.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naikoo G., Al-Mashali F., Arshad F., Al-Maashani N., Hassan I.U., Al-Baraami Z., et al. An overview of copper nanoparticles: synthesis, characterisation and anticancer activity. Curr Pharm Des. 2021;27(43):4416–4432. doi: 10.2174/1381612827666210804100303. [DOI] [PubMed] [Google Scholar]
- 20.da Silva D.A., De Luca A., Squitti R., Rongioletti M., Rossi L., Machado C.M.L., et al. Copper in tumors and the use of copper-based compounds in cancer treatment. J Inorg Biochem. 2022;226 doi: 10.1016/j.jinorgbio.2021.111634. [DOI] [PubMed] [Google Scholar]
- 21.Lönnerdal B. Intestinal regulation of copper homeostasis: a developmental perspective. Am J Clin Nutr. 2008;88(3) doi: 10.1093/ajcn/88.3.846S. 846s-50s. [DOI] [PubMed] [Google Scholar]
- 22.Linder M.C. Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics. 2016;8(9):887–905. doi: 10.1039/c6mt00103c. [DOI] [PubMed] [Google Scholar]
- 23.Xue Q., Kang R., Klionsky D.J., Tang D., Liu J., Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19(8):2175–2195. doi: 10.1080/15548627.2023.2200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linder M.C. Copper homeostasis in mammals, with emphasis on secretion and excretion. A review. Int J Mol Sci. 2020;21(14):4932. doi: 10.3390/ijms21144932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y., Liu K., Zhang Y., Sun Z., Song B., Wang Y., et al. Hyaluronic acid-modified curcumin-copper complex nano delivery system for rapid healing of bacterial prostatitis. Carbohydr Polym. 2023;310 doi: 10.1016/j.carbpol.2023.120668. [DOI] [PubMed] [Google Scholar]
- 26.Pinho J.O., da Silva I.V., Amaral J.D., Rodrigues C.M.P., Casini A., Soveral G., et al. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int J Pharm. 2021;599 doi: 10.1016/j.ijpharm.2021.120463. [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Deng H., Jian Z., Cui H., Guo H., Fang J., et al. Copper exposure induces hepatic G0/G1 cell-cycle arrest through suppressing the Ras/PI3K/Akt signaling pathway in mice. Ecotoxicol Environ Saf. 2021;222 doi: 10.1016/j.ecoenv.2021.112518. [DOI] [PubMed] [Google Scholar]
- 28.Liu R.X., Wang C.Y., Wu Y.S., Luo R.Y., Jiang X.H., Tang M.T., et al. The copper(II) complexes of new anthrahydrazone ligands: in vitro and in vivo antitumor activity and structure-activity relationship. J Inorg Biochem. 2020;212 doi: 10.1016/j.jinorgbio.2020.111208. [DOI] [PubMed] [Google Scholar]
- 29.Shanbhag V., Jasmer-McDonald K., Zhu S., Martin A.L., Gudekar N., Khan A., et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc Natl Acad Sci. 2019;116(14):6836–6841. doi: 10.1073/pnas.1817473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Z., Ma G., Kong L., Du G. Hypoxia-inducible factor-1: regulatory mechanisms and drug development in stroke. Pharmacol Res. 2021;170 doi: 10.1016/j.phrs.2021.105742. [DOI] [PubMed] [Google Scholar]
- 31.Xue Q., Yan D., Chen X., Li X., Kang R., Klionsky D.J., et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19(7):1982–1996. doi: 10.1080/15548627.2023.2165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller S., Sindikubwabo F., Cañeque T., Lafon A., Versini A., Lombard B., et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12(10):929–938. doi: 10.1038/s41557-020-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solier S., Müller S., Cañeque T., Versini A., Mansart A., Sindikubwabo F., et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023;617(7960):386–394. doi: 10.1038/s41586-023-06017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araya M., McGoldrick M.C., Klevay L.M., Strain J.J., Robson P., Nielsen F., et al. Determination of an acute no-observed-adverse-effect level (NOAEL) for copper in water. Regul Toxicol Pharm. 2001;34(2):137–145. doi: 10.1006/rtph.2001.1492. [DOI] [PubMed] [Google Scholar]
- 35.Kadammattil A.V., Sajankila S.P., Prabhu S., Rao B.N., Rao B.S.S. Systemic toxicity and teratogenicity of copper oxide nanoparticles and copper sulfate. J Nanosci Nanotechnol. 2018;18(4):2394–2404. doi: 10.1166/jnn.2018.14542. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad M., Khan M.K.A., Ahmad N., Parveen M., Shahzad K., Hasan A. Histotoxicity induced by copper oxide nanoparticles (CuO-NPs) on developing mice (Mus musculus) Food Chem Toxicol. 2023;184 doi: 10.1016/j.fct.2023.114369. [DOI] [PubMed] [Google Scholar]
- 37.Javed R., Sajjad A., Naz S., Sajjad H., Ao Q. Significance of capping agents of colloidal nanoparticles from the perspective of drug and gene delivery, bioimaging, and biosensing: an insight. Int J Mol Sci. 2022;23(18):10521. doi: 10.3390/ijms231810521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manke A., Wang L., Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. 2013;2013 doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajjad H., Sajjad A., Haya R.T., Khan M.M., Zia M. Copper oxide nanoparticles: in vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp Biochem Physiol C: Toxicol Pharmacol. 2023;271 doi: 10.1016/j.cbpc.2023.109682. [DOI] [PubMed] [Google Scholar]
- 40.Shi M., Kwon H.S., Peng Z., Elder A., Yang H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano. 2012;6(3):2157–2164. doi: 10.1021/nn300445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S., Wang L., Zhao B., Wang Z., Wang Y., Sun B., et al. Doxorubicin-loaded Cu2S/Tween-20 nanocomposites for light-triggered tumor photothermal therapy and chemotherapy. RSC Adv. 2020;10(44):26059–26066. doi: 10.1039/d0ra03069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostwal V., Ramaswamy A., Bhargava P., Srinivas S., Mandavkar S., Chaugule D., et al. A pro-oxidant combination of resveratrol and copper reduces chemotherapy-related non-haematological toxicities in advanced gastric cancer: results of a prospective open label phase II single-arm study (RESCU III study) Med Oncol. 2022;40(1):17. doi: 10.1007/s12032-022-01862-1. [DOI] [PubMed] [Google Scholar]
- 43.Xue Q., Yan D., Chen X., Li X., Kang R., Klionsky D.J., et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023:1–15. doi: 10.1080/15548627.2023.2165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahlson M.A., Dixon S.J. Copper-induced cell death. Science. 2022;375(6586):1231–1232. doi: 10.1126/science.abo3959. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Chen Y., Zhang J., Yang Y., Fleishman J.S., Wang Y., et al. Cuproptosis: a novel therapeutic target for overcoming cancer drug resistance. Drug Resist Updat. 2024;72 doi: 10.1016/j.drup.2023.101018. [DOI] [PubMed] [Google Scholar]
- 46.Lian L., Li X.L., Xu M.D., Li X.M., Wu M.Y., Zhang Y., et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19(1):183. doi: 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi X., Li Y., Jia M., Zhang Z., Huang L., Zhang M., et al. A novel copper chelator for the suppression of colorectal cancer. Drug Dev Res. 2023;84(2):312–325. doi: 10.1002/ddr.22034. [DOI] [PubMed] [Google Scholar]
- 48.Steinbrueck A., Sedgwick A.C., Brewster J.T., Yan K.C., Shang Y., Knoll D.M., et al. Transition metal chelators, pro-chelators, and ionophores as small molecule cancer chemotherapeutic agents. Chem Soc Rev. 2020;49(12):3726–3747. doi: 10.1039/c9cs00373h. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Li M., Chen G., Liu S., Guo H., Dong X., et al. Dissecting copper biology and cancer treatment: ‘activating cuproptosis or suppressing cuproplasia. Coord Chem Rev. 2023;495 [Google Scholar]
- 50.Kang N., Son S., Min S., Hong H., Kim C., An J., et al. Stimuli-responsive ferroptosis for cancer therapy. Chem Soc Rev. 2023;52(12):3955–3972. doi: 10.1039/d3cs00001j. [DOI] [PubMed] [Google Scholar]
- 51.Liu H., Tang T. Pan-cancer genetic analysis of cuproptosis and copper metabolism-related gene set. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.952290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Lin W., Yang Y., Shao J., Zhao H., Wang G., et al. Role of cuproptosis-related gene in lung adenocarcinoma. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1080985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Fang T., Shan W., Gao Q. Identification of a novel model for predicting the prognosis and immune response based on genes related to cuproptosis and ferroptosis in ovarian cancer. Cancers. 2023;15(3):579. doi: 10.3390/cancers15030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J., Yu Q., Song J., Li S., Li X.L., Kang B., et al. Photothermally triggered copper payload release for cuproptosis-promoted cancer synergistic therapy. Angew Chem Int Ed. 2023;62(12) doi: 10.1002/anie.202213922. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y., Liu S.Y., Zeng L., Ma H., Zhang Y., Yang H., et al. An enzyme-engineered nonporous copper(I) coordination polymer nanoplatform for cuproptosis-based synergistic cancer therapy. Adv Mater. 2022;34(43) doi: 10.1002/adma.202204733. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y., Pan Q., Gao W., Pu Y., He B. Reversal of cisplatin chemotherapy resistance by glutathione-resistant copper-based nanomedicine via cuproptosis. J Mater Chem B. 2022;10(33):6296–6306. doi: 10.1039/d2tb01150f. [DOI] [PubMed] [Google Scholar]
- 57.Oliveri V. Biomedical applications of copper ionophores. Coord Chem Rev. 2020;422 [Google Scholar]
- 58.Kannappan V., Ali M., Small B., Rajendran G., Elzhenni S., Taj H., et al. Recent advances in repurposing disulfiram and disulfiram derivatives as copper-dependent anticancer agents. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.741316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skrott Z., Majera D., Gursky J., Buchtova T., Hajduch M., Mistrik M., et al. Disulfiram's anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene. 2019;38(40):6711–6722. doi: 10.1038/s41388-019-0915-2. [DOI] [PubMed] [Google Scholar]
- 60.Oliveri V. Selective targeting of cancer cells by copper ionophores: an overview. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.841814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Njenga L.W., Mbugua S.N., Odhiambo R.A., Onani M.O. Addressing the gaps in homeostatic mechanisms of copper and copper dithiocarbamate complexes in cancer therapy: a shift from classical platinum-drug mechanisms. Dalton Trans. 2023;52(18):5823–5847. doi: 10.1039/d3dt00366c. [DOI] [PubMed] [Google Scholar]
- 62.Shigeoka M., Koma Y.I., Nishio M., Akashi M., Yokozaki H. Alteration of macrophage infiltrating compartment: a novel view on oral carcinogenesis. Pathobiology. 2021;88(5):327–337. doi: 10.1159/000515922. [DOI] [PubMed] [Google Scholar]
- 63.Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53(6):667–682. doi: 10.1080/10409238.2018.1556578. [DOI] [PubMed] [Google Scholar]
- 64.Zhong X., Dai X., Wang Y., Wang H., Qian H., Wang X. Copper-based nanomaterials for cancer theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(4):e1797. doi: 10.1002/wnan.1797. [DOI] [PubMed] [Google Scholar]
- 65.Lu X., Gao S., Lin H., Shi J. Single-atom catalysts for nanocatalytic tumor therapy. Small. 2021;17(16) doi: 10.1002/smll.202004467. [DOI] [PubMed] [Google Scholar]
- 66.Zhu D., Ling R., Chen H., Lyu M., Qian H., Wu K., et al. Biomimetic copper single-atom nanozyme system for self-enhanced nanocatalytic tumor therapy. Nano Res. 2022;15(8):7320–7328. [Google Scholar]
- 67.Lu X., Gao S., Lin H., Yu L., Han Y., Zhu P., et al. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv Mater. 2020;32(36) doi: 10.1002/adma.202002246. [DOI] [PubMed] [Google Scholar]
- 68.Lu J., Yang Y., Xu Q., Lin Y., Feng S., Mao Y., et al. Recent advances in multi-configurable nanomaterials for improved chemodynamic therapy. Coord Chem Rev. 2023;474 [Google Scholar]
- 69.Benguigui M., Weitz I.S., Timaner M., Kan T., Shechter D., Perlman O., et al. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci Rep. 2019;9(1):12613. doi: 10.1038/s41598-019-48959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagajyothi P.C., Muthuraman P., Sreekanth T.V.M., Kim D.H., Shim J. Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem. 2017;10(2):215–225. [Google Scholar]
- 71.Jiang F., Ding B., Zhao Y., Liang S., Cheng Z., Xing B., et al. Biocompatible CuO-decorated carbon nanoplatforms for multiplexed imaging and enhanced antitumor efficacy via combined photothermal therapy/chemodynamic therapy/chemotherapy. Sci China Mater. 2020;63(9):1818–1830. [Google Scholar]
- 72.Pandurangan M., Nagajyothi P.C., Shim J., Kim D.H. Anti-proliferative effect of copper oxide nanorods against human cervical carcinoma cells. Biol Trace Elem Res. 2016;173(1):62–70. doi: 10.1007/s12011-016-0628-0. [DOI] [PubMed] [Google Scholar]
- 73.Marin R., Skripka A., Besteiro L.V., Benayas A., Wang Z., Govorov A.O., et al. Highly efficient copper sulfide-based near-infrared photothermal agents: exploring the limits of macroscopic heat conversion. Small. 2018;14(49) doi: 10.1002/smll.201803282. [DOI] [PubMed] [Google Scholar]
- 74.He Y., Shen Y., Zhou S., Wu Y., Yuan Z., Wei C., et al. Near infrared dye loaded copper sulfide-apoferritin for tumor imaging and photothermal therapy. RSC Adv. 2018;8(26):14268–14279. doi: 10.1039/c8ra00911b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q., Sun L., Hou M., Chen Q., Yang R., Zhang L., et al. Phase-change material packaged within hollow copper sulfide nanoparticles carrying doxorubicin and chlorin e6 for fluorescence-guided trimodal therapy of cancer. ACS Appl Mater Interfaces. 2019;11(1):417–429. doi: 10.1021/acsami.8b19667. [DOI] [PubMed] [Google Scholar]
- 76.Nie X., Xia L., Wang H.L., Chen G., Wu B., Zeng T.Y., et al. Photothermal therapy nanomaterials boosting transformation of Fe(III) into Fe(II) in tumor cells for highly improving chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11(35):31735–31742. doi: 10.1021/acsami.9b11291. [DOI] [PubMed] [Google Scholar]
- 77.Wang S., Pang Y., Hu S., Lv J., Lin Y., Li M. Copper sulfide engineered covalent organic frameworks for pH-responsive chemo/photothermal/chemodynamic synergistic therapy against cancer. Chem Eng J. 2023;451 [Google Scholar]
- 78.Lin L.S., Huang T., Song J., Ou X.Y., Wang Z., Deng H., et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J Am Chem Soc. 2019;141(25):9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- 79.Wang M., Zhang W., Ding B., Pa Ma, Lin J. Copper peroxides based multiple tumor microenvironment regulation for enhanced photodynamic/chemodynamic synergistic therapy. Adv Opt Mater. 2022;11(11) [Google Scholar]
- 80.Liu B., Bian Y., Liang S., Yuan M., Dong S., He F., et al. One-step integration of tumor microenvironment-responsive calcium and copper peroxides nanocomposite for enhanced chemodynamic/ion-interference therapy. ACS Nano. 2022;16(1):617–630. doi: 10.1021/acsnano.1c07893. [DOI] [PubMed] [Google Scholar]
- 81.Hu B., Xiao X., Chen P., Qian J., Yuan G., Ye Y., et al. Enhancing anti-tumor effect of ultrasensitive bimetallic RuCu nanoparticles as radiosensitizers with dual enzyme-like activities. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121811. [DOI] [PubMed] [Google Scholar]
- 82.Zuo W., Liu N., Chang Z., Liu J., Jin Q., Chen L., et al. Single-site bimetallic nanosheet for imaging guided mutually-reinforced photothermal-chemodynamic therapy. Chem Eng J. 2022;442 [Google Scholar]
- 83.Luan X., Pan Y., Zhou Y., Zhou D., Zhao W., Zeng F., et al. Targeted self-assembly of renal clearable Cu2-xSe to induce lysosome swelling for multimodal imaging guided photothermal/chemodynamic synergistic therapy. Adv Funct Mater. 2022;32(51) [Google Scholar]
- 84.Guo Y., Fan Y., Wang Z., Li G., Zhan M., Gong J., et al. Chemotherapy mediated by biomimetic polymeric nanoparticles potentiates enhanced tumor immunotherapy via amplification of endoplasmic reticulum stress and mitochondrial dysfunction. Adv Mater. 2022;34(47) doi: 10.1002/adma.202206861. [DOI] [PubMed] [Google Scholar]
- 85.Shen W.Y., Jia C.P., Liao L.Y., Chen L.L., Hou C., Liu Y.H., et al. Copper(II) complexes of halogenated quinoline Schiff base derivatives enabled cancer therapy through glutathione-assisted chemodynamic therapy and inhibition of autophagy flux. J Med Chem. 2022;65(6):5134–5148. doi: 10.1021/acs.jmedchem.2c00133. [DOI] [PubMed] [Google Scholar]
- 86.Zhao C., Chen X., Zang D., Lan X., Liao S., Yang C., et al. A novel nickel complex works as a proteasomal deubiquitinase inhibitor for cancer therapy. Oncogene. 2016;35(45):5916–5927. doi: 10.1038/onc.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X., Dou Q.P., Liu J., Tang D. Targeting ubiquitin–proteasome system with copper complexes for cancer therapy. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.649151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z., Yan Z., Di Y., Yang S., Ning Y., Mao Y., et al. Current advances in metal–organic frameworks for cancer nanodynamic therapies. Coord Chem Rev. 2023;497 [Google Scholar]
- 89.Cheng Y., Wen C., Sun Y.Q., Yu H.J., Yin X.B. Mixed-metal MOF-derived hollow porous nanocomposite for trimodality imaging guided reactive oxygen species-augmented synergistic therapy. Adv Funct Mater. 2021;31(37) [Google Scholar]
- 90.Li J., Zhang Z., Li J., Cun J.E., Pan Q., Gao W., et al. Copper-olsalazine metal-organic frameworks as a nanocatalyst and epigenetic modulator for efficient inhibition of colorectal cancer growth and metastasis. Acta Biomater. 2022;152:495–506. doi: 10.1016/j.actbio.2022.08.076. [DOI] [PubMed] [Google Scholar]
- 91.Liu J., Yuan Y., Cheng Y., Fu D., Chen Z., Wang Y., et al. Copper-based metal–organic framework overcomes cancer chemoresistance through systemically disrupting dynamically balanced cellular redox homeostasis. J Am Chem Soc. 2022;144(11):4799–4809. doi: 10.1021/jacs.1c11856. [DOI] [PubMed] [Google Scholar]
- 92.Dong J., Ma K., Pei Y., Pei Z. Core–shell metal–organic frameworks with pH/GSH dual-responsiveness for combined chemo–chemodynamic therapy. Chem Commun. 2022;58(88):12341–12344. doi: 10.1039/d2cc04218e. [DOI] [PubMed] [Google Scholar]
- 93.Xing Y., Xiu J., Zhou M., Xu T., Zhang M., Li H., et al. Copper single-atom jellyfish-like nanomotors for enhanced tumor penetration and nanocatalytic therapy. ACS Nano. 2023;17(7):6789–6799. doi: 10.1021/acsnano.3c00076. [DOI] [PubMed] [Google Scholar]
- 94.Qi Y., Ren S., Ye J., Bi S., Shi L., Fang Y., et al. Copper-single-atom coordinated nanotherapeutics for enhanced sonothermal-parallel catalytic synergistic cancer therapy. Adv Healthc Mater. 2023;12(23) doi: 10.1002/adhm.202300291. [DOI] [PubMed] [Google Scholar]
- 95.Lu X., Gao S., Lin H., Yu L., Han Y., Zhu P., et al. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv Mater. 2020;32(36) doi: 10.1002/adma.202002246. [DOI] [PubMed] [Google Scholar]
- 96.Su G., Xu H., Zhou F., Gong X., Tan S., He Y. Boosting reactive oxygen species generation with a dual-catalytic nanomedicine for enhanced tumor nanocatalytic therapy. ACS Appl Mater Interfaces. 2023;15(51):59175–59188. doi: 10.1021/acsami.3c13882. [DOI] [PubMed] [Google Scholar]
- 97.Tang Z., Jiang S., Tang W., He Q., Wei H., Jin C., et al. H2O2 self-supplying and GSH-depleting nanocatalyst for copper metabolism-based synergistic chemodynamic therapy and chemotherapy. Mol Pharm. 2023;20(3):1717–1728. doi: 10.1021/acs.molpharmaceut.2c00937. [DOI] [PubMed] [Google Scholar]
- 98.Zhou M., Tian B., Bu Y., Wu Z., Yu J., Wang S., et al. Enhanced pH-responsive chemo/chemodynamic synergistic cancer therapy based on in situ Cu2+ di-chelation. ACS Appl Bio Mater. 2023;6(8):3221–3231. doi: 10.1021/acsabm.3c00323. [DOI] [PubMed] [Google Scholar]
- 99.Qiao L., Li X., Xiao Y., Yuan J., Yu D., Zuo M., et al. A component-optimized chemo-dynamic nanoagent for enhanced tumour cell-selective chemo-dynamic therapy with minimal side effects in a glioma mouse model. Biomater Sci. 2022;10(15):4170–4183. doi: 10.1039/d2bm00615d. [DOI] [PubMed] [Google Scholar]
- 100.Li Z., Wang C., Dai C., Hu R., Ding L., Feng W., et al. Engineering dual catalytic nanomedicine for autophagy-augmented and ferroptosis-involved cancer nanotherapy. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121668. [DOI] [PubMed] [Google Scholar]
- 101.Cheng F.T., Geng Y.D., Liu Y.X., Nie X., Zhang X.G., Chen Z.L., et al. Co-delivery of a tumor microenvironment-responsive disulfiram prodrug and CuO2 nanoparticles for efficient cancer treatment. Nanoscale Adv. 2023;5(12):3336–3347. doi: 10.1039/d3na00004d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X., Detering L., Sultan D., Luehmann H., Li L., Heo G.S., et al. CC chemokine receptor 2-targeting copper nanoparticles for positron emission tomography-guided delivery of gemcitabine for pancreatic ductal adenocarcinoma. ACS Nano. 2021;15(1):1186–1198. doi: 10.1021/acsnano.0c08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiong Y., Yong Z., Li S., Wang Q., Chen X., Zhang Z., et al. Self-reliant nanomedicine with long-lasting glutathione depletion ability disrupts adaptive redox homeostasis and suppresses cancer stem cells. Adv Funct mater. 2023;34(8):2310158. [Google Scholar]
- 104.He X., Li M., Fan S., Li Y., Fang L., Xiang G., et al. Copper peroxide and cisplatin co-loaded silica nanoparticles-based trinity strategy for cooperative cuproptosis/chemo/chemodynamic cancer therapy. Chem Eng J. 2024;481 [Google Scholar]
- 105.Qi C., Jiang C., Fu L.H., Sun T., Wang T., Lin J., et al. Melanin-instructed biomimetic synthesis of copper sulfide for cancer phototheranostics. Chem Eng J. 2020;388 [Google Scholar]
- 106.Ren Q., Zhang X., Sheng Y., Yu N., Li M., Chen Z. Phytic acid-Cu2+ framework/Cu2-xS nanocomposites with heat-shock protein down-modulation ability for enhanced multimodal combination therapy. J Colloid Interface Sci. 2023;652:2116–2126. doi: 10.1016/j.jcis.2023.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Lan Q.H., Du C.C., Yu R.J., Zhai J., Shi Y., Kou L., et al. Disulfiram-loaded copper sulfide nanoparticles for potential anti-glioma therapy. Int J Pharm. 2021;607 doi: 10.1016/j.ijpharm.2021.120978. [DOI] [PubMed] [Google Scholar]
- 108.Yang F., Yang Q., Yang L., Li J., Zhang Y., Lu H., et al. Endogenous microRNA accurate diagnostics to guide photothermal therapy. Anal Chem. 2022;94(17):6599–6606. doi: 10.1021/acs.analchem.2c00712. [DOI] [PubMed] [Google Scholar]
- 109.Liu S., Shen C., Jiang D., Qian C., Yang Z., Wang J., et al. Cascade tumor therapy platform for sensitized chemotherapy and penetration enhanced photothermal therapy. Macromol Biosci. 2022;22(3) doi: 10.1002/mabi.202100429. [DOI] [PubMed] [Google Scholar]
- 110.Dun X., Liu S., Ge N., Liu M., Li M., Zhang J., et al. Photothermal effects of CuS-BSA nanoparticles on H22 hepatoma-bearing mice. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1029986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Si P., Yu W., Li C., Chen H., Zhang E., Gu J., et al. Oxygen-independent alkyl radical nanogenerator enhances breast cancer therapy. Nanomed. 2023;48 doi: 10.1016/j.nano.2022.102630. [DOI] [PubMed] [Google Scholar]
- 112.Wu Y., Wu D., Lan J., Li A., Hou L., Xu Y., et al. Assessment of mononuclear/dinuclear copper acylhydrazone complexes for lung cancer treatment. Bioorg Chem. 2024;144 doi: 10.1016/j.bioorg.2024.107122. [DOI] [PubMed] [Google Scholar]
- 113.Li M., Shao J., Guo Z., Jin C., Wang L., Wang F., et al. Novel mitochondrion-targeting copper(II) complex induces HK2 malfunction and inhibits glycolysis via Drp1-mediating mitophagy in HCC. J Cell Mol Med. 2020;24(5):3091–3107. doi: 10.1111/jcmm.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng H., Hu C., Quan Y., Ye X., Shi X., Guo Z., et al. A copper complex that combats triple negative breast cancer by restraining angiogenesis. Dalton Trans. 2023;52(22):7626–7634. doi: 10.1039/d3dt00738c. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., Zhu M., Li S., Xu G., Zhang Z., Yang F. Novel mono-, bi-, tri- and tetra-nuclear copper complexes that inhibit tumor growth through apoptosis and anti-angiogenesis. J Inorg Biochem. 2024;250 doi: 10.1016/j.jinorgbio.2023.112403. [DOI] [PubMed] [Google Scholar]
- 116.Geng P., Yu N., Macharia D.K., Meng R., Qiu P., Tao C., et al. MOF-derived CuS@Cu-MOF nanocomposites for synergistic photothermal-chemodynamic-chemo therapy. Chem Eng J. 2022;441 [Google Scholar]
- 117.Pan Q., Xie L., Liu R., Pu Y., Wu D., Gao W., et al. Two birds with one stone: copper metal-organic framework as a carrier of disulfiram prodrug for cancer therapy. Int J Pharm. 2022;612 doi: 10.1016/j.ijpharm.2021.121351. [DOI] [PubMed] [Google Scholar]
- 118.Li C., Zhou S., Chen C., Zhu L., Li S., Song Z., et al. DDTC-Cu(I) based metal-organic framework (MOF) for targeted melanoma therapy by inducing SLC7A11/GPX4-mediated ferroptosis. Colloids Surf B. 2023;225 doi: 10.1016/j.colsurfb.2023.113253. [DOI] [PubMed] [Google Scholar]
- 119.Shao L., Gao X., Liu J., Zheng Q., Li Y., Yu P., et al. Biodegradable metal-organic-frameworks-mediated protein delivery enables intracellular cascade biocatalysis and pyroptosis in vivo. ACS Appl Mater Interfaces. 2022;14(42):47472–47481. doi: 10.1021/acsami.2c14957. [DOI] [PubMed] [Google Scholar]
- 120.Weng Y., Guan S., Wang L., Lu H., Meng X., Waterhouse G.I.N., et al. Defective porous carbon polyhedra decorated with copper nanoparticles for enhanced NIR-driven photothermal cancer therapy. Small. 2020;16(1) doi: 10.1002/smll.201905184. [DOI] [PubMed] [Google Scholar]
- 121.Xu H., Zhang Y., Zhang H., Zhang Y., Xu Q., Lu J., et al. Smart polydopamine-based nanoplatforms for biomedical applications: state-of-art and further perspectives. Coord Chem Rev. 2023;488 [Google Scholar]
- 122.Yan Z., Zhang H., Chen J., Xu Q., Feng S., Zhao Q., Wang S. Cu(II)-doped mesoporous polydopamine as biodegradable nanoplatforms for photothermal-enhanced multi-mode anti-tumor therapy. Colloid Surf A. 2024;685 [Google Scholar]
- 123.Wu H., Jia P., Zou Y., Jiang J. Cascade targeting tumor mitochondria with CuS nanoparticles for enhanced photothermal therapy in the second near-infrared window. Biomater Sci. 2021;9(15):5209–5217. doi: 10.1039/d1bm00589h. [DOI] [PubMed] [Google Scholar]
- 124.Wang S., Hu T., Wang G., Wang Z., Yan D., Liang R., et al. Ultrathin CuFe2S3 nanosheets derived from CuFe-layered double hydroxide as an efficient nanoagent for synergistic chemodynamic and NIR-II photothermal therapy. Chem Eng J. 2021;419 [Google Scholar]
- 125.Liu L., Zhang H., Peng L., Wang D., Zhang Y., Yan B., et al. A copper-metal organic framework enhances the photothermal and chemodynamic properties of polydopamine for melanoma therapy. Acta Biomater. 2023;158:660–672. doi: 10.1016/j.actbio.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 126.Hu K., Xie L., Zhang Y., Hanyu M., Yang Z., Nagatsu K., et al. Marriage of black phosphorus and Cu2+ as effective photothermal agents for PET-guided combination cancer therapy. Nat Commun. 2020;11(1):2778. doi: 10.1038/s41467-020-16513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu H.J., Wang M., Hu X., Shi S., Xu P. Enhanced photothermal therapy through the in situ activation of a temperature and redox dual-sensitive nanoreservoir of triptolide. Small. 2020;16(38) doi: 10.1002/smll.202003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang M., Hou Z., Wang M., Wen D., Li C., Liu Y., et al. Cu single atom nanozyme based high-efficiency mild photothermal therapy through cellular metabolic regulation. Angew Chem Int Ed Engl. 2022;61(50) doi: 10.1002/anie.202209245. [DOI] [PubMed] [Google Scholar]
- 129.Tang H.X., Liu C.G., Zhang J.T., Zheng X., Yang D.Y., Kankala R.K., et al. Biodegradable quantum composites for synergistic photothermal therapy and copper-enhanced chemotherapy. ACS Appl Mater Interfaces. 2020;12(42):47289–47298. doi: 10.1021/acsami.0c14636. [DOI] [PubMed] [Google Scholar]
- 130.Pan Q., Peng X., Cun J.E., Li J., He B. In-situ drug generation and controllable loading: rational design of copper-based nanosystems for chemo-photothermal cancer therapy. Chem Eng J. 2021;409(9) [Google Scholar]
- 131.Zhu L., Dai Y., Gao L., Zhao Q. Tumor microenvironment-modulated nanozymes for NIR-II-triggered hyperthermia-enhanced photo-nanocatalytic therapy via disrupting ROS homeostasis. Int J Nanomedicine. 2021;6:4559–4577. doi: 10.2147/IJN.S309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hou G., Xu W., Guo M., Wang J., Wang Y., Suo A., et al. Full-active Cu2O/drug core/shell nanoparticles based on “grafting from” drug coordination polymerization combined with PD-1 blockade for efficient cancer therapy. Chem Eng J. 2022;441 [Google Scholar]
- 133.Pi W., Wu L., Lu J., Lin X., Huang X., Wang Z., et al. A metal ions-mediated natural small molecules carrier-free injectable hydrogel achieving laser-mediated photo-Fenton-like anticancer therapy by synergy apoptosis/cuproptosis/anti-inflammation. Bioact Mater. 2023;29:98–115. doi: 10.1016/j.bioactmat.2023.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hu Z.E., Li J., Wu Z.N., Wei Y.J., Yu X.Q. One-pot synthesis-biocompatible copper–tripeptide complex as a nanocatalytic medicine to enhance chemodynamic therapy. ACS Biomater Sci Eng. 2021;7(4):1394–1402. doi: 10.1021/acsbiomaterials.0c01678. [DOI] [PubMed] [Google Scholar]
- 135.Meng X., Zhang X., Lei Y., Cao D., Wang Z. Biodegradable copper-metformin nanoscale coordination polymer for enhanced chemo/chemodynamic synergistic therapy by reducing oxygen consumption to promote H2O2 accumulation. J Mater Chem B. 2021;9(8):1988–2000. doi: 10.1039/d0tb02476g. [DOI] [PubMed] [Google Scholar]
- 136.He Y., Guo S., Zhang Y., Liu Y., Ju H. NIR-II reinforced intracellular cyclic reaction to enhance chemodynamic therapy with abundant H2O2 supply. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120962. [DOI] [PubMed] [Google Scholar]
- 137.Hu T., Yan L., Wang Z., Shen W., Liang R., Yan D., et al. A pH-responsive ultrathin Cu-based nanoplatform for specific photothermal and chemodynamic synergistic therapy. Chem Sci. 2021;12(7):2594–2603. doi: 10.1039/d0sc06742c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang L., Xu Y., Liu C., Si W., Wang W., Zhang Y., et al. Copper-doped MOF-based nanocomposite for GSH depleted chemo/photothermal/chemodynamic combination therapy. Chem Eng J. 2022;438 [Google Scholar]
- 139.Chen Z., Wang X., Ding Y., Xing C., Lu C., Tu X. A self-assembled copper-selenocysteine nanoparticle for enhanced chemodynamic therapy via oxidative stress amplification. ACS Mater Lett. 2023;5(4):1237–1244. [Google Scholar]
- 140.Liu Y., Zhang J., Du J., Song K., Liu J., Wang X., et al. Biodegradable BiOCl platform for oxidative stress injury–enhanced chemodynamic/radiation therapy of hypoxic tumors. Acta Biomater. 2021;129:280–292. doi: 10.1016/j.actbio.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 141.You Y., Liu H., Zhu J., Wang Y., Pu F., Ren J., et al. A DNAzyme-augmented bioorthogonal catalysis system for synergistic cancer therapy. Chem Sci. 2022;13(26) doi: 10.1039/d2sc02050e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wan Y., Fu L.H., Li C., Lin J., Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33(48) doi: 10.1002/adma.202103978. [DOI] [PubMed] [Google Scholar]
- 143.Feng S., Xiao Y., Lu J., Chen Z., Jiang Z., Xu Q., et al. Tumor microenvironment sensitization via dual-catalysis of carbon-based nanoenzyme for enhanced photodynamic therapy. J Colloid Interface Sci. 2024;663:577–590. doi: 10.1016/j.jcis.2024.02.160. [DOI] [PubMed] [Google Scholar]
- 144.Cai X., Xie Z., Ding B., Shao S., Liang S., Pang M., et al. Monodispersed copper(I)-based nano metal-organic framework as a biodegradable drug carrier with enhanced photodynamic therapy efficacy. Adv Sci. 2019;6(15) doi: 10.1002/advs.201900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen J., Tan X., Huang Y., Xu C., Zeng Z., Shan T., et al. Reactive oxygen species-activated self-amplifying prodrug nanoagent for tumor-specific Cu-chelate chemotherapy and cascaded photodynamic therapy. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121513. [DOI] [PubMed] [Google Scholar]
- 146.Chudal L., Pandey N.K., Phan J., Johnson O., Lin L., Yu H., et al. Copper-cysteamine nanoparticles as a heterogeneous Fenton-like catalyst for highly selective cancer treatment. ACS Appl Bio Mater. 2020;3(3):1804–1814. doi: 10.1021/acsabm.0c00098. [DOI] [PubMed] [Google Scholar]
- 147.Sah B., Wu J., Vanasse A., Pandey N.K., Chudal L., Huang Z., et al. Effects of nanoparticle size and radiation energy on copper-cysteamine nanoparticles for X-ray induced photodynamic therapy. Nanomaterials. 2020;10(6):1087. doi: 10.3390/nano10061087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen X., Liu J., Li Y., Pandey N.K., Chen T., Wang L., et al. Study of copper-cysteamine based X-ray induced photodynamic therapy and its effects on cancer cell proliferation and migration in a clinical mimic setting. Bioact Mater. 2022;7:504–514. doi: 10.1016/j.bioactmat.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhen X., Pandey N.K., Amador E.H., Hu W., Liu B., Nong W.X., et al. Potassium iodide enhances the anti-hepatocellular carcinoma effect of copper-cysteamine nanoparticle mediated photodynamic therapy on cancer treatment. Mater Today Phys. 2022;27 [Google Scholar]
- 150.Yang J., Wang Y., Qin G., Tian T., Ran J., Wang H., et al. Photogeneration of hydroxyl radicals based on aggregation-induced emission luminogen-assembled copper cysteamine nanoparticles for photodynamic therapy. ACS Appl Nano Mater. 2023;6(1):533–543. [Google Scholar]
- 151.Liu C., Wang D., Zhang S., Cheng Y., Yang F., Xing Y., et al. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano. 2019;13(4):4267–4277. doi: 10.1021/acsnano.8b09387. [DOI] [PubMed] [Google Scholar]
- 152.Zhang D., Lin Z., Lan S., Sun H., Zeng Y., Liu X. The design of Janus black phosphorus quantum dots@metal–organic nanoparticles for simultaneously enhancing environmental stability and photodynamic therapy efficiency. Mater Chem Front. 2019;3(4):656–663. [Google Scholar]
- 153.Cao X., Li M., Liu Q., Zhao J., Lu X., Wang J. Inorganic sonosensitizers for sonodynamic therapy in cancer treatment. Small. 2023;19(42):2303195. doi: 10.1002/smll.202303195. [DOI] [PubMed] [Google Scholar]
- 154.Wang P., Wang X., Ma L., Sahi S., Li L., Wang X., et al. Nanosonosensitization by using copper–cysteamine nanoparticles augmented sonodynamic cancer treatment. Part Part Syst Charact. 2018;35(4) [Google Scholar]
- 155.Ma A., Ran H., Wang J., Ding R., Lu C., Liu L., et al. An urchin-shaped copper-based metalloporphyrin nanosystem as a sonosensitizer for sonodynamic therapy. Nanomaterials. 2022;12(2):209. doi: 10.3390/nano12020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang M., Yang D., Dong C., Huang H., Feng G., Chen Q., et al. Two-dimensional MXene-originated in situ nanosonosensitizer generation for augmented and synergistic sonodynamic tumor nanotherapy. ACS Nano. 2022;16(6):9938–9952. doi: 10.1021/acsnano.2c04630. [DOI] [PubMed] [Google Scholar]
- 157.Chen K., Zhou A., Zhou X., Liu Y., Xu Y., Ning X. An intelligent cell-derived nanorobot bridges synergistic crosstalk between sonodynamic therapy and cuproptosis to promote cancer treatment. Nano Lett. 2023;23(7):3038–3047. doi: 10.1021/acs.nanolett.3c00434. [DOI] [PubMed] [Google Scholar]
- 158.Zhao Y., Wen M., Yu N., Tao C., Ren Q., Qiu P., et al. Design and synthesis of cancer-cell-membrane-camouflaged hemoporfin-Cu9S8 nanoagents for homotypic tumor-targeted photothermal-sonodynamic therapy. J Colloid Interface Sci. 2023;637:225–236. doi: 10.1016/j.jcis.2023.01.068. [DOI] [PubMed] [Google Scholar]
- 159.Liang S., Deng X., Chang Y., Sun C., Shao S., Xie Z., et al. Intelligent hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19(6):4134–4145. doi: 10.1021/acs.nanolett.9b01595. [DOI] [PubMed] [Google Scholar]
- 160.Novak-Hofer I., Schubiger A.P. Copper-67 as a therapeutic nuclide for radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2002;29(6):821–830. doi: 10.1007/s00259-001-0724-y. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki H., Kise S., Kaizuka Y., Watanabe R., Sugawa T., Furukawa T., et al. Copper-64-labeled antibody fragments for immuno-PET/radioimmunotherapy with low renal radioactivity levels and amplified tumor-kidney ratios. ACS Omega. 2021;6(33):21556–21562. doi: 10.1021/acsomega.1c02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu J., Song L., Feng S., Wang K., Mao Y., Gao Y., et al. Nanozyme-mediated biocatalysis as a mitochondrial oxidative stress amplifier for tumor nanocatalytic immunotherapy. Chem Eng J. 2024;481 [Google Scholar]
- 163.Qin J., Yang T., Li J., Zhan G., Li X., Wei Z., et al. Bacterial outer membrane vesicle-templated biomimetic nanoparticles for synergistic photothermo-immunotherapy. Nano Today. 2022;46 [Google Scholar]
- 164.Li T., Wang D., Meng M., Yang Z., Luo Z., Li Z., et al. Copper-coordinated covalent organic framework produced a robust Fenton-like effect inducing immunogenic cell death of tumors. Macromol Rapid Commun. 2023;44(11) doi: 10.1002/marc.202200929. [DOI] [PubMed] [Google Scholar]
- 165.Du L., He H., Xiao Z., Xiao H., An Y., Zhong H., et al. GSH-responsive metal–organic framework for intratumoral release of NO and IDO inhibitor to enhance antitumor immunotherapy. Small. 2022;18(15) doi: 10.1002/smll.202107732. [DOI] [PubMed] [Google Scholar]
- 166.Guo B., Yang F., Zhang L., Zhao Q., Wang W., Yin L., et al. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater. 2023;35(22):2212267. doi: 10.1002/adma.202212267. [DOI] [PubMed] [Google Scholar]
- 167.Hesemans E., Saffarzadeh N., Maksoudian C., Izci M., Chu T., Rios Luci C., et al. Cu-doped TiO2 nanoparticles improve local antitumor immune activation and optimize dendritic cell vaccine strategies. J Nanobiotechnol. 2023;21(1):87. doi: 10.1186/s12951-023-01844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Busa P., Koutavarapu R., Lee D.Y., Shim J., Kuthati Y. Hierarchical two-dimensional layered double hydroxide coated polydopamine nanocarriers for combined chemodynamic and photothermal tumor therapy. Coatings. 2021;11(8):1008. [Google Scholar]
- 169.Liang J., Sun Y., Wang K., Zhang Y., Guo L., Bao Z., et al. Prussian blue-derived nanoplatform for in situ amplified photothermal/chemodynamic/starvation therapy. ACS Appl Mater Interfaces. 2023;15(14):18191–18204. doi: 10.1021/acsami.2c22448. [DOI] [PubMed] [Google Scholar]
- 170.Xie Z., Liang S., Cai X., Ding B., Huang S., Hou Z., et al. O2-Cu/ZIF-8@Ce6/ZIF-8@F127 composite as a tumor microenvironment-responsive nanoplatform with enhanced photo-/chemodynamic antitumor efficacy. ACS Appl Mater Interfaces. 2019;11(35):31671–31680. doi: 10.1021/acsami.9b10685. [DOI] [PubMed] [Google Scholar]
- 171.Hou G., Qian J., Guo M., Xu W., Wang J., Wang Y., et al. Copper coordinated nanozyme-assisted photodynamic therapy for potentiating PD-1 blockade through amplifying oxidative stress. Chem Eng J. 2022;435 [Google Scholar]
- 172.Zeng W., Yu M., Chen T., Liu Y., Yi Y., Huang C., et al. Polypyrrole nanoenzymes as tumor microenvironment modulators to reprogram macrophage and potentiate immunotherapy. Adv Sci. 2022;9(23) doi: 10.1002/advs.202201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhong X., Dai X., Wang Y., Wang H., Qian H., Wang X. Copper-based nanomaterials for cancer theranostics. WIREs Nanomedicine Nanobiotechnol. 2022;14(4):e1797. doi: 10.1002/wnan.1797. [DOI] [PubMed] [Google Scholar]
- 174.Ouyang Z., Li D., Xiong Z., Song C., Gao Y., Liu R., et al. Antifouling dendrimer-entrapped copper sulfide nanoparticles enable photoacoustic imaging-guided targeted combination therapy of tumors and tumor metastasis. ACS Appl Mater Interfaces. 2021;13(5):6069–6080. doi: 10.1021/acsami.0c21620. [DOI] [PubMed] [Google Scholar]
- 175.Li B., Wang X., Chen L., Zhou Y., Dang W., Chang J., et al. Ultrathin Cu-TCPP MOF nanosheets: a new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics. 2018;8(15):4086–4096. doi: 10.7150/thno.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shah R., Petersburg J., Gangar A.C., Fegan A., Wagner C.R., Kumarapperuma S.C. In vivo evaluation of site-specifically PEGylated chemically self-assembled protein nanostructures. Mol Pharm. 2016;13(7):2193–2203. doi: 10.1021/acs.molpharmaceut.6b00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hao G., Mastren T., Silvers W., Hassan G., Öz O.K., Sun X. Copper-67 radioimmunotheranostics for simultaneous immunotherapy and immuno-SPECT. Sci Rep. 2021;11(1):3622. doi: 10.1038/s41598-021-82812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hopper K.D., Singapuri K., Finkel A. Body CT and oncologic imaging. Radiology. 2000;215(1):27–40. doi: 10.1148/radiology.215.1.r00ap1727. [DOI] [PubMed] [Google Scholar]
- 179.Dong L., Zhang P., Xu X., Lei P., Du K., Zhang M., et al. Simple construction of Cu2−xS:Pt nanoparticles as nanotheranostic agent for imaging-guided chemo-photothermal synergistic therapy of cancer. Nanoscale. 2018;10(23):10945–10951. doi: 10.1039/c8nr02692k. [DOI] [PubMed] [Google Scholar]
- 180.Duan Q.J., Zhao Z.Y., Zhang Y.J., Fu L., Yuan Y.Y., Du J.Z., et al. Activatable fluorescent probes for real-time imaging-guided tumor therapy. Adv Drug Delivery Rev. 2023;196 doi: 10.1016/j.addr.2023.114793. [DOI] [PubMed] [Google Scholar]
- 181.Xia J.M., Wei X., Chen X.W., Shu Y., Wang J.H. Folic acid modified copper nanoclusters for fluorescent imaging of cancer cells with over-expressed folate receptor. Microchim Acta. 2018;185(3):205. doi: 10.1007/s00604-018-2743-4. [DOI] [PubMed] [Google Scholar]
- 182.Freimanis A.K. Ultrasonic imaging of neoplasms. Cancer. 1976;37(S1):496–502. doi: 10.1002/1097-0142(197601)37:1+<496::aid-cncr2820370715>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 183.Shi H., Yan R., Wu L., Sun Y., Liu S., Zhou Z., et al. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomater. 2018;72:256–265. doi: 10.1016/j.actbio.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 184.Bao Y.W., Hua X.W., Li Y.H., Jia H.R., Wu F.G. Hyperthemia-promoted cytosolic and nuclear delivery of copper/carbon quantum dot-crosslinked nanosheets: multimodal imaging-guided photothermal cancer therapy. ACS Appl Mater Interfaces. 2018;10(2):1544–1555. doi: 10.1021/acsami.7b15332. [DOI] [PubMed] [Google Scholar]
- 185.Zhang L., Wang Y., Peng Z., Weng Y., Fang Z., Xiao F., et al. The progress of multimodal imaging combination and subregion based radiomics research of cancers. Int J Biol Sci. 2022;18(8):3458–3469. doi: 10.7150/ijbs.71046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heuberger D.M., Wolint P., Jang J.H., Itani S., Jungraithmayr W., Waschkies C.F., et al. High-affinity Cu(I)-chelator with potential anti-tumorigenic action—a proof-of-principle experimental study of human H460 tumors in the CAM assay. Cancers. 2022;14(20):5122. doi: 10.3390/cancers14205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li C., Hao D., Wang X., Sun T., Xie Z. Copper depletion combined with photothermal therapy suppresses breast cancer. Mater Chem Front. 2022;6(18):2735–2740. [Google Scholar]
- 188.Guo J., Cheng J., Zheng N., Zhang X., Dai X., Zhang L., et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv Sci. 2021;8(18) doi: 10.1002/advs.202004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun T., Zhang G., Guo Z., Chen Q., Zhang Y., Chu Y., et al. Co-delivery of Cu(I) chelator and chemotherapeutics as a new strategy for tumor theranostic. J Control Release. 2020;321:483–496. doi: 10.1016/j.jconrel.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 190.Ungefroren H. TGF-β signaling in cancer: control by negative regulators and crosstalk with proinflammatory and fibrogenic pathways. Cancers. 2019;11(3):384. doi: 10.3390/cancers11030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Poursani E.M., Mercatelli D., Raninga P., Bell J.L., Saletta F., Kohane F.V., et al. Copper chelation inhibits TGF-β pathways and suppresses epithelial-mesenchymal transition in cancer. bioRxiv 2022;2022.10.03.510707.
- 192.Coles L.C., Shaw P.E. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002;21(14):2236–2244. doi: 10.1038/sj.onc.1205302. [DOI] [PubMed] [Google Scholar]
- 193.Brady D.C., Crowe M.S., Greenberg D.N., Counter C.M. Copper chelation inhibits BRAFV600E-driven melanomagenesis and counters resistance to BRAFV600E and MEK1/2 inhibitors. Cancer Res. 2017;77(22):6240–6252. doi: 10.1158/0008-5472.CAN-16-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Michniewicz F., Saletta F., Rouen J., Ziegler D., Vittorio O. DIPG-83. Using copper chelating agents to target receptor tyrosine kinase signalling in diffuse intrinsic pontine glioma (DIPG) Neuro Oncol. 2020;22:iii303. [Google Scholar]
- 195.Rakshit A., Khatua K., Shanbhag V., Comba P., Datta A. Cu2+ selective chelators relieve copper-induced oxidative stress in vivo. Chem Sci. 2018;9(41):7916–7930. doi: 10.1039/c8sc04041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Oliveri V., Vecchio G. Prochelator strategies for site-selective activation of metal chelators. J Inorg Biochem. 2016;162:31–43. doi: 10.1016/j.jinorgbio.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 197.Wadhwa S., Mumper R.J. Intracellular delivery of the reactive oxygen species generating agent d-penicillamine upon conjugation to poly-l-glutamic acid. Mol Pharm. 2010;7(3):854–862. doi: 10.1021/mp1000058. [DOI] [PubMed] [Google Scholar]
- 198.Arnesano F., Nardella M.I., Natile G. Platinum drugs, copper transporters and copper chelators. Coord Chem Rev. 2018;374:254–260. [Google Scholar]
- 199.Ryumon S., Okui T., Kunisada Y., Kishimoto K., Shimo T., Hasegawa K., et al. Ammonium tetrathiomolybdate enhances the antitumor effect of cisplatin via the suppression of ATPase copper transporting beta in head and neck squamous cell carcinoma. Oncol Rep. 2019;42(6):2611–2621. doi: 10.3892/or.2019.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li X., Cui Y., Zhou T., Li J., Lu P., Yuwen L., et al. Nanomedicine targets endogenous copper ions for disease diagnosis and therapy. Chem Eng J. 2023;472 [Google Scholar]