Abstract
Riboflavin or vitamin B2 plays significant roles in metabolic reactions and energy production, establishing it as an important research subject in biology and medicine. While there are numerous riboflavin-related publications in these fields, interrogation of its electronic properties in relation to the physiological function at the cellular level remains obscure due to technological challenges. However, progress in molecular electronics and the discovery of the semiconductor-like behaviour of biomolecules in recent times have initiated growing interest in exploring the electronic properties of these materials for potential bioelectronic device applications. In this work, we demonstrate novel semiconductor-like behaviour in riboflavin within a gold/Riboflavin/gold Schottky junction. We observed the occurrence of two negative differential resistance peaks at low voltages of 1.5 and 2.0 V, probably the first-ever report of this effect in a biomolecule. Interestingly, the proposed mechanism simulates a single Schottky junction behaviour despite the physical existence of two junctions. Solid-state parameters such as turn-on voltage, shunt resistance, and ideality factor were also calculated using Conventional and Cheung and Cheung’s methods. The results were highly characteristic to the riboflavin studied when compared to previous works on biomolecules. This opens up the possibility of developing solid-state sensors for electronically characterising biomolecules like vitamins to help advance our understanding of the electronic properties of these essential nutrients.
Keywords: Riboflavin, Two-terminal PCB, Biosensor, Biomolecule, Electronics
Highlights
-
•
Observations of semiconductive-like behaviours of riboflavin.
-
•
Occurrence of Negative Differential Resistance effects in biomolecule.
-
•
‘Effective single Schottky junction’ behaviour from two-junction structure.
1. Introduction
Riboflavin or vitamin B2 is one of the micronutrients required by the human body to operate optimally. However, it is not naturally synthesized but must be acquired from diet and supplements [1]. Chemically, riboflavin forms an integral part of two major coenzymes, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) [2,3]. These coenzymes assist their respective enzymes in enhancing the metabolism of macronutrient breakdown by increasing the bonded materials’ electron transfer rate [4,5]. Interestingly, riboflavin has also demonstrated antimicrobial properties by inhibiting the growth of various bacteria and fungi, especially when exposed to ultraviolet (UV) radiation [6,7]. These findings underscore the extensive potential of riboflavin in the biomedical and health sectors, making it crucial to understand the fundamental mechanisms that regulate its electron movement.
Numerous researchers have investigated the electron transfer process in riboflavin via standard electrochemical analysis such as cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). A study where square-wave voltammetry was employed on pharmaceutical-grade riboflavin using an unmodified boron-doped diamond electrode depicts that the redox reaction on this material is quasi-reversible at lower pH Britton-Robinson buffer solution (BRBS) [8]. The researchers also reported that the applied analytical procedure could be leveraged for riboflavin determination. Another study suggests that different pH levels of riboflavin in aqueous buffer solution would contribute to different peak currents and diffusion coefficients by using CV, ultraviolet–visible (UV–Vis) spectroscopy, and photoluminescence spectra [9]. The findings showed that both parameters increase with pH levels, indicating that greater diffusion towards the electrode surface occurs at basic pH. While conventional electrochemical studies have provided some insights into the mechanisms of electron transfer, there exist prominent limitations. Specifically, methods such as CV and EIS are time-consuming, require sophisticated equipment and expertise, and do not provide real-time information on the molecular interactions and structural changes of biomolecule during the redox process [10,11].
In one study, Mallik et al., in 1979 discovered that two forms of vitamin A, alcohol and acetate, exhibited semiconductive-like behaviours under certain conditions [12]. An apparent departure from standard electrochemical measurement methods, potential bias was applied to sandwiched vitamin compounds within conducting glass and steel electrodes. The corresponding currents were then measured against exposure to different chemicals such as benzene, toluene and heptane. The result showed that the absorbed vapours influenced the semiconductive current of both forms of vitamin A, suggesting that charge-transfer interactions could affect their semiconductive properties. The same method was employed to investigate the compensational effects of electrical conductivity in different forms of vitamin A (alcohol and acetate), taking into account the molecular geometry [13]. While the semiconductive properties of different initial forms of vitamin A have been established decades ago, there were however to our knowledge no further studies related to semiconductivity or electronic properties of other fundamental vitamins such as riboflavin. A comprehensive review of the literature did not reveal any subsequent studies specifically addressing the semiconductive properties of vitamins in general.
A recent novel approach towards electronically characterizing biological substances has been introduced in recent years using two-metal terminal (2T) printed circuit board (PCB)-based electrode configuration [[14], [15], [16]]. This platform allows for the effective “sandwiching” of biological substances between the metal electrodes upon establishing a potential bias, forming a practical Schottky-like junction. Using this method, current-voltage (I-V) profiles resembling those of a Schottky diode would be obtained if the samples investigated exhibit semiconductive-like behaviours. By utilizing this method, researchers have demonstrated the semiconductor-like properties of several types of proteins, and nucleic acids such as ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) sequences. Following these reports, this Lab-on-PCB electronic characterization method approach was used as a standardized method for biosensing studies to further enable rapid and reproducible results in a cost-effective manner.
In this work, we carried out for the first time, electronic characterization studies on riboflavin using the novel PCB-based 2T electrodes (PCB-2T) platform (metal-riboflavin-metal). Potassium chloride (KCl) buffer solution was used to stabilize riboflavin while potassium hydroxide (KOH) was added to improve the solubility of the vitamin [17]. The proprietary PCB-2T sensor [PI 2017701978; PCT/MY2018/000018; UI2020001868, Japanese Patent Application No. 2019–566680] fabricated for utilization in this work allows for a Lab-on-PCB design approach relevant to current research requirements, while providing standardization for obtaining highly reproducible electronic profiles of the riboflavin.
2. Methods
2.1. Reagents and chemicals
Analytical standard of lyophilized riboflavin (C17H20N4O6) with a molecular weight of 376.36 g/mol was purchased from Sigma-Aldrich (Research and Development (R&D) grade). Blank solutions were prepared by solubilizing 0.372 g of potassium chloride (puriss. p.a., ≥99.5 % (AT)), Sigma-Aldrich) and 1.12 g of potassium hydroxide (pellets for analysis, EMSURE) in 100 mL pure water (Crystal clinic (CL-2910), conductivity < 0.1 μS/cm) each. Preparation of the sample (around 25°C at 55–65 relative humidity (RH)%) was done by solubilizing 10 mg of riboflavin in 1 ml of KOH solution (0.2 M) and topped up with 11.5 mL with pure water. Further, 12.5 mL of KCL solution (0.05 M) was added to form the riboflavin sample solution with an effective concentration of 1.063 mM. The whole process was done under minimal light exposure, and the samples were stored in dark bottles since riboflavin is light-sensitive and used within 30 min of preparation.
2.2. Fabrication of PCB-2T platform
An in-house designed PCB-2T system, as illustrated in Fig. 1, was outsourced to GUH Circuit Industry (Pg) Sdn. Bhd. (Penang, Malaysia) for mass fabrication. To design the PCB, CAD/CAM Software was used to transform circuit film and solder mask layers into Gerber format and generate physical layouts that adhered to the required specifications. This was followed by an imaging process that involved the utilization of a single-sided fiberglass-resin (FR4) laminate panel and a copper layer. Subsequently, the panel was treated with a dry polymer film sensitive to UV light. The circuit film, crafted in accordance with the design, was precisely aligned and exposed to UV light affixing it securely atop the laminated panel. The final step of PCB fabrication involved a chemical photo-developing process to remove unwanted areas of the dry polymer film, leaving the circuit pattern exposed on the panel. The conductivity of the panel was enhanced by electrodepositing a layer of Nickel (Ni) onto the circuit lines and pads. This was followed by electrolytic plating of two Au pads onto the panel. Furthermore, the panel underwent stripping and etching procedures to eliminate the dry polymer and copper sections that were not desired. Finally, an epoxy solder mask ink was used to protect and insulate the circuit lines, while leaving the electrodes and connecting pads exposed.
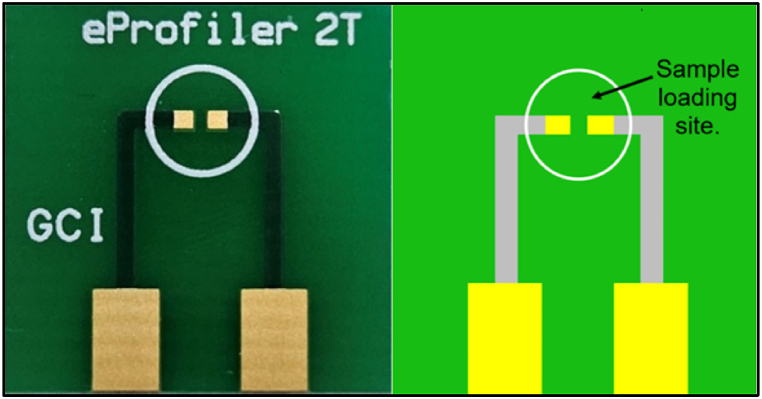
Fig. 1.
The photograph and schematic diagram of the proprietary 2T-PCB platform.
2.3. Cleaning process for PCB-2T platform
The first stage of cleaning the PCB-2T sensors involve fully submerging in acetone at room temperature (around 21°C) for 1 min to remove any organic and dirt contaminants from the surface. The acetone was later discarded, and the sensors were again rinsed with ultrapure water followed by submerging in pure isopropyl alcohol (IPA) for 1 min and subsequently rinsed with ultrapure water one final time. Lastly, the sensors were air-dried at room temperature and sealed in sterile packaging for storage. Each sensor is only removed from the package before loading the riboflavin sample solution. To avoid any risk of contamination, the sample loading area was handled with the utmost precaution and any form of physical contact abstained. The entire process was conducted in a Class 1000 clean room to maintain a minimal dust environment.
2.4. Experimental procedure for PCB-2T setup
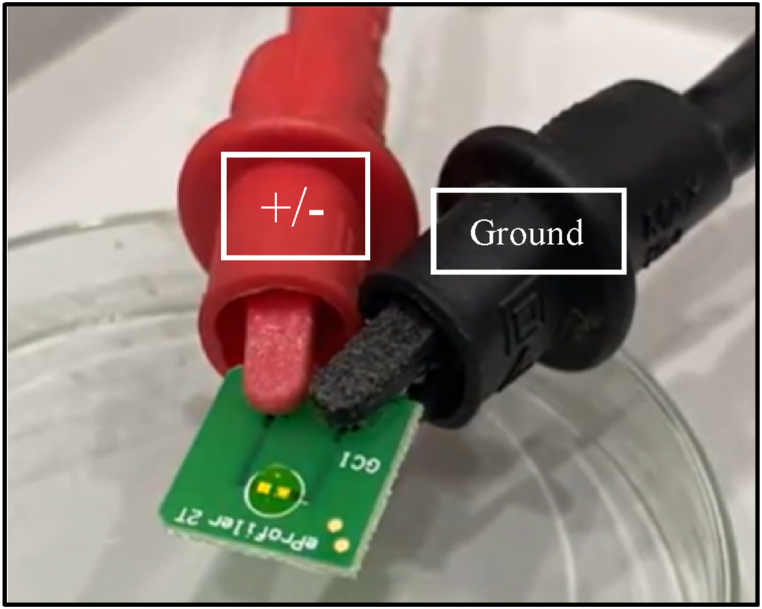
Electronic characterizations were performed by loading 10 μL of freshly prepared riboflavin solution onto the PCB-2T sample loading site. Subsequently, a 5 min incubation period was followed to ensure self-assembling and stabilization of the riboflavin molecules on the gold-electrode surface. To apply voltage and measure current, a B2902A Source Measurement Unit (SMU) model from Keysight was employed. One electrode was connected to ground, while the other was connected to the dual-polarity voltage supply (Fig. 2). The voltage applied ranged from 0 to 3 V in the positive region and from 0 to −3.0 V in the negative region, while its' corresponding current measurements were recorded using fresh PCB-2T sensors each time. This data enabled the generation of I-V characteristics for each riboflavin sample from which specific solid-state parameters were calculated using the Conventional as well as Cheung and Cheung’s methods; both of which consider the thermionic emission theory [18,19].
Fig. 2.
The photograph of the connection between the PCB-2T sensor and the SMU (Keysight, B2902A) via the crocodile clips. Positive and negative sweeps were performed by applying the desired polarity to one electrode, while the other electrode remained connected to ground.
To investigate the effects of negative differential resistance, samples were subjected to two conditions; dark storage in a dark-colored glass bottle and light exposure in a transparent glass bottle under an illumination of 135 lx. Measurements were taken at 24 and 48 hrs for both conditions to assess the effects over time. The entire process was conducted by following stringent aseptic techniques since the samples are of biological nature. Experiments were carried out in at least three replicates to demonstrate measurement reproducibility.
2.5. Field emission surface electron microscopy (FESEM) on the electrodes’ surfaces
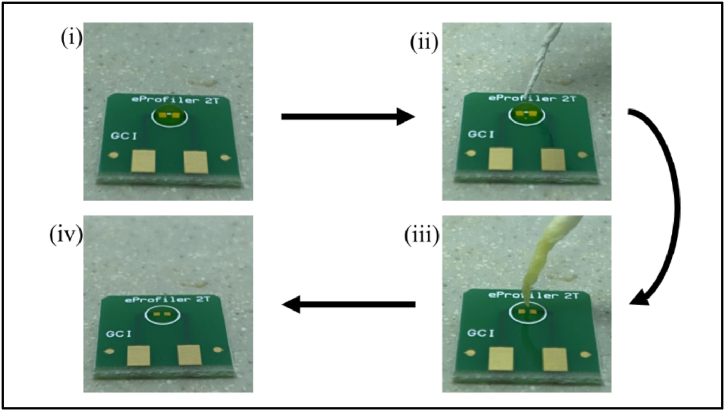
In order to interpret the results obtained from this work, we hypothesized that a self-assembled layer of riboflavin is formed on the surface of the gold electrodes upon 5 min of sample incubation, forming a Schottky-like contact at the Au-riboflavin interface. In order to validate this, the morphology of the deposited riboflavin layer was analysed using FESEM (JEOL, JSM-7600F). Images were recorded at 5 kV under magnifications of 25×, 100X, 500X and 1000X. To prepare the sample for the analysis, instead of air-drying the whole sample, a solution extraction process was performed on the riboflavin sample after a 5-min incubation period. Assuming that riboflavin molecules were deposited to the Au surface, the solution was carefully absorbed as much as possible without touching the riboflavin layer using Kimwipes Delicate Task Wipes tissue (Kimtech Science, USA), and then left to self-dry for half an hour. This process effectively removes most of the solution, potentially leaving only the riboflavin layer on the electrode surfaces. Fig. 3 shows the chronological steps of the solution extraction process.
Fig. 3.
Flow chart of the solution extraction process. Kimwipes Delicate Task Wipes tissue was used to absorb as much of the solution as possible, potentially leaving only the self-assembled riboflavin layer on the sensor. It was carried out with the utmost care to avoid getting too near the electrodes, thereby preventing any damage to the deposited riboflavin layer. From the figure, it can be observed that the solution remains intact in steps (i) and (ii), while most of the solution was already absorbed in step (iii), following step (iv) where it will be air-dried under sterile environment for 30 min.
2.6. Profilometry on the electrodes’ surface
To further demonstrates the presence of the riboflavin layer on the gold electrode surfaces, contact profilometry using a Bruker DektakXT stylus profilometer (Bruker, Germany) was employed to examine the formation of the layer. The profilometer operates with a gold stylus applying a force of 1.5 mg with a resolution of 0.1 μm/s. This procedure was performed on the electrode surface of the PCB-2T sensor under two conditions; (a) bare electrode surface and (b) electrode surface with the riboflavin layer. The sample preparation for condition (b) was similar to the procedures in the FESEM analysis. (Supplementary Fig. S1).
3. Results and discussion
3.1. Riboflavin layer formation on the gold electrode surfaces
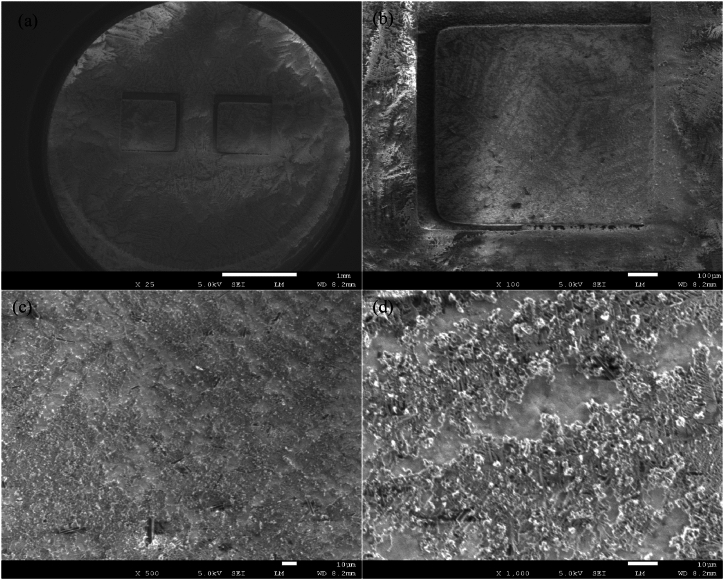
In this study, the primary focus was on the electronic characterization of the naturally self-assembling thin film of riboflavin formed on the surface of the gold electrodes. The FESEM results, as depicted in Fig. 4, confirm the presence of riboflavin deposits on the gold surfaces, supporting the hypothesis of the layer formation. The images at various magnifications (25×, 100X, 500X and 1000X) reveal a layer of riboflavin crystals forming a network over the entire electrode surface. At lower magnifications, the overall distribution of the riboflavin layer is evident, while higher magnifications provide detailed views of its’ crystalline structures. Additionally, the profilometer measurements as shown in Supplementary Fig. S1 confirms the existence of the proposed riboflavin layer.
Fig. 4.
FESEM images of the deposited riboflavin on the gold electrodes at 5 kV under magnification of (a) 25×, (b) 100X, (c) 500X and (d) 1000X. The overall distribution of riboflavin crystals on the electrode surface is visible, showing a layer with crystalline features forming a network over the electrode surface.
This finding aligns with other preceding reports of the self-assembling mechanisms of biomolecules, especially on gold surfaces. This film formation could be attributed to the direct interaction of the biomolecules with the surface [20,21] and gravitational effect of the molecules towards the surface. A morphological study utilizing atomic force microscopy (AFM) also found that biomolecules, such as collagen and hemoglobin naturally self-assembled and formed thin films in the order of nanometer scale when the biomolecules solutions were loaded onto gold surfaces. These biomolecular thin films were observed to be uniformly aligned on the gold electrodes’ surfaces, where its homogeneity also increased significantly when electric field was applied [22].
In this work, the naturally self-assembled riboflavin thin film with potentially significant improved surface uniformity [22] upon the application of a potential bias serves as an effective semiconductor-layer on the gold electrodes, forming Schottky contacts, which resulted in the non-linear Schottky behaviors. Therefore, it can be assumed that the interfacial charge injection process significantly contributes to the charge transfer mechanism occurring at the Schottky contact between the riboflavin layer and the gold electrodes.
3.2. Structure of the Riboflavin/Au junction
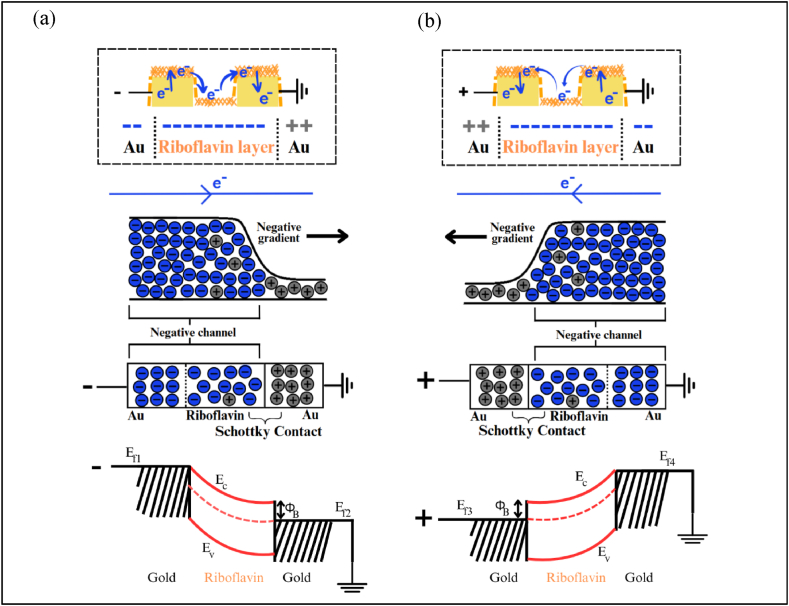
The observation on the formation of the riboflavin layer effectively allows for the visualization of an “interfacial-layer-centric” mechanism, primary to carrier injection process from the proposed Schottky-like junction (Fig. 5) [23]. This particular layer would establish a Schottky contact with the metal, leading to a characteristic semiconductive I-V profile, provided that the layer exhibits semiconductive behaviors. Consequently, the current flowing through the Au/Riboflavin junction is expected to favor unidirectional flow, in conjunction with the principles of a conventional Schottky junction. This behavior is clearly depicted in the Au/Riboflavin interfacial junction shown in Fig. 5.
Fig. 5.
Illustration showing the proposed charge transfer mechanism of the Au/Riboflavin/Au structure depicting the carrier injection process whereby the current direction changes with the polarity. This novel observation highlights a ‘channel-like’ conduction mechanism similar to a transistor whereby (a) demonstrates the flow of electrons from left to right due to the application of negative polarity to the left gold electrode while (b) is the flow of electrons from right to left due to application of negative polarity to the right gold electrode. The proposed band diagram also illustrates the configuration of only one Schottky contact due to the formation of negative channel between the riboflavin and electrodes at the opposite side.
However, the structure of the riboflavin-specific Schottky-like junction in this work differs from conventional designs. In this case, riboflavin establishes a Schottky contact with the electrode that carries opposite polarity. Conversely, the electrode with the same polarity facilitates charge movement within the riboflavin layer, forming a “channel-like structure”. Therefore, applying a different polarity sweep causes the current to flow in the opposite direction while maintaining the same magnitude, resulting in the almost-symmetrical semilog I-V profile observed in this work. Riboflavin tends to act as an electron acceptor in biological systems. This electron-accepting behavior is comparable to the characteristics of n-type materials, where there is an excess of electrons available for conduction [[24], [25], [26]]. While this still preserves the fundamental behaviors of a Schottky junction, where current typically flows in one direction, the direction changes with the applied polarity.
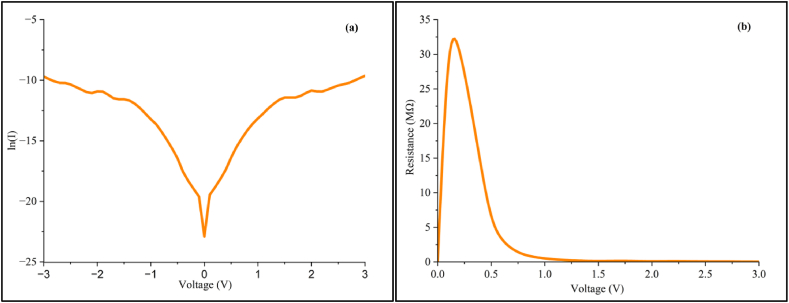
Interestingly, the proposed mechanism resembles a single Schottky junction despite the physical existence of two Au/Riboflavin junctions. In Fig. 5(a), negative polarity was applied, which resulted in the construction of a “negative channel” at the left side. This “negative channel” will form a Schottky contact with the other metal (that have different carrier charges). The concentration gradient of carriers or diffusion drives the charge flow from regions of high to low concentration, resulting in the unidirectional current conduction similar to rectifying effect. While the magnitude of the current remains consistent, the direction changes depending on the applied polarity. The proposed band diagram illustrates the Fermi levels of the two metal electrodes and riboflavin, along with the conduction and valence bands, to further explain the charge transfer mechanisms within our structure. As such, this mechanism effectively explains the almost symmetrical behaviors of the semilog I-V profile (Fig. 7(a)). Although the metal contributes to the formation of the “channel-like” structure with the riboflavin layer, the semiconductive properties arise solely from the riboflavin, as the metal primarily exhibits conductive behaviors.
Fig. 7.
Semi-log I-V characteristic (a) and resistance curve (b) of the riboflavin.
3.3. Electronic characterization of riboflavin
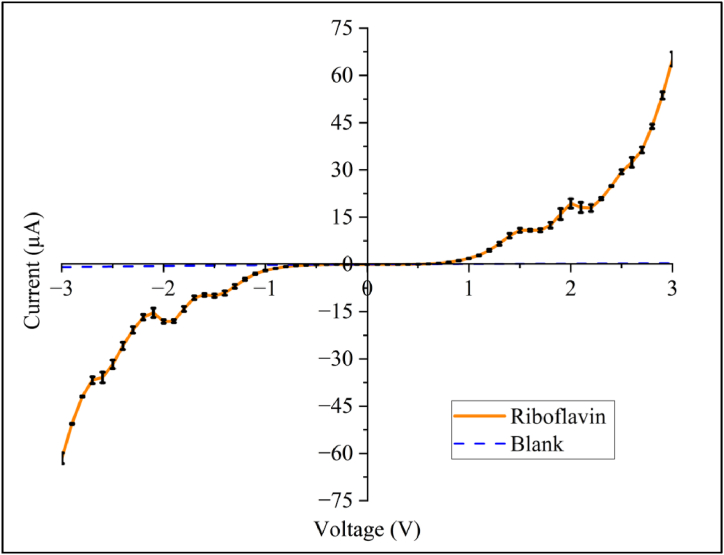
The results of the experimental analysis provide insightful observations regarding the electronic properties of riboflavin. The I-V profile is particularly significant in this investigation, as it enables the derivation of other essential profiles necessary for understanding the material's behaviour and calculating the solid-state parameters. In this work, the I-V profile (Fig. 6) shows a resemblance to conventional I-V characteristic of a typical Schottky diode, suggesting for the first time that riboflavin could exhibit semiconductive-like behaviours when “contacted” within two metal electrodes. The rectifying behaviour is evident, with an insulating region at lower voltages transitioning into a rapid general increase in current once the turn-on voltage is reached. The same characterization studies were also performed on the blank (KCl + KOH solution) used for riboflavin dissolution. As observed from the figure where the blank alone was found to be weakly conducting by nature, the semiconductive-like effect observed could be concluded to be primarily due to the presence of riboflavin. This riboflavin profile aligned with the proposed framework of the Au/Riboflavin/Au structure, whereby interfacial carrier injection would govern the electron transfer mechanisms.
Fig. 6.
I-V characteristics of riboflavin and blank in the positive (0–3 V) and negative (0 to −3 V) biased regions. Measurements were carried out for three technical replicates to validate the reproducibility of the results. Error bars are non-significant in the region of interest (about 0.5–1.8 V),where error bars were calculated using standard deviation method. Error bars seen above 1.8 V were higher and mainly contributed to the perturbation of the molecular structure of the riboflavin molecules due to the high electric field at higher potential bias.
Fig. 7(a) illustrates the ln(I)-V plot of riboflavin-Schottky junction. The nearly symmetrical semilog I-V profile supports the explanation of the Au/Riboflavin junction, whereby the magnitude of the current is expected to be approximately equal for both negative and positive bias. However, it is obvious that non-symmetry does exist primarily due to the minuscule geometrical difference between the two gold electrodes that influences the carrier injection process [27]. At higher voltages, series resistance () effects manifest on the ln(I)-V profile. In organic semiconductors, this parameter would be subjected to its bulk resistance [28]. The homogeneity of the interfacial contact between the riboflavin sample and the metal electrodes of the 2T platform could also affect the values of the organic Schottky-like junctions [29].
Intriguingly, two negative differential resistance (NDR) peaks can be seen occurring at low voltages of 1.5 and 2 V (Fig. 5), demonstrating the phenomenon whereby the current decreases as the voltage increases, indicating negative resistance regions. This phenomenon however has never been reported previously in riboflavin or any other biomolecules. While this effect is not exclusively confined to semiconductive materials, it is commonly associated with semiconductors for the fabrication of low-powered oscillators and amplifiers [30,31]. As probably the case here, the exact potential difference at which NDR occurs vary depending on the specific material and device structure. It is not solely dependent on the applied voltage but also other factors such as the type of semiconductor material, the temperature, and the device configuration [32,33].
The foundation framework of this research leverage thermionic emission theory to analyze and explain the I-V data. The observed semiconductive behaviours resemble that of a metal-semiconductor junction, where charge transfer mechanisms are governed by carrier injection. This can be attributed to the disparity of the work functions between the metal and “semiconductor-like material” at the Schottky contact, which in this report are the Au and the riboflavin layer, respectively. Additionally, the existence of NDR highlights the bulk conduction phenomenon which could also considerably contribute to the non-linear I-V characteristics [34]. Other mechanisms such as electric field-dependent carrier mobility models could also possibly lead to similar non-linear I-V curves [[35], [36], [37]]. However, a significant drawback of such models is that they often fail to accurately represent the complexities of real-world materials and their disordered structures [38]. As it is well known, biomolecules are not static entities but are dynamic in nature. This dynamism, coupled with the inherent complexity of biomolecular structures, poses a significant challenge for electric field-dependent carrier mobility models [39]. These models, which are typically designed for idealized systems, may not fully capture the intricacies of biomolecular behaviour, leading to potential inaccuracies in their predictions.
3.4. Calculation of solid-state parameters
Riboflavin “thin film” acting as the semiconductor-layer on the gold electrodes enables solid-state parameters such as the turn-on voltage () and shunt resistance () to be obtained directly from the I-V and R-V profiles, respectively. The x-intercept of the extrapolated lines, drawn from the non-linear curve segments of the I-V profile (Fig. 6), represents the value which was found to be 0.86 V for riboflavin. This is within previously reported values observed for semiconductive-like biomolecules; about 0.85–1.50 V [[14], [15], [16],29]. The value for shunt resistance or RSH obtained from Fig. 7(b) was determined to be 30.598 MΩ, representing the highest resistance of the Riboflavin/gold Schottky interface junction prior to heavy current conduction typical of semiconductive behaviour. This is significant as the recombination of charge carriers at the Au/Riboflavin junction interface is inversely related to these shunt resistance values [40].
Thermionic emission model was utilized to calculate the other solid-state parameters which were saturation current (), ideality factor based on conventional ( and Cheung and Cheungs’ methods ( and series resistance () [18]. In forward bias (Conventional method), equation expressing the I-V relation in a diode is (1), where I is the forward current, V is the voltage drop across the barrier, q is the charge of electron, k is the Boltzmann constant and T is temperature. Taking natural logarithms on both sides of equation (1), a new expression was obtained; (2) which generates the semi-log profiles in Fig. 7(a). Thus, and can be obtained using the y-intercepts and slopes of the ln(I)-V plots. The calculated saturation current value was 0.016 μA, while ideality factor using Conventional method for riboflavin was 8.23.
However, the Conventional method ignores the effects of series resistance (. Therefore, Cheung and Cheung proposed a new method that considered this effect in the derivation of (3), where expressed the voltage drop due to series resistance [19]. Using equation (3), the and values were calculated taking into consideration the gradient and the y-intercept of the - plot. Based on this, the ideality factor and the series resistance, respectively were calculated to be 17.42 and 1.1773 kΩ. Electron transfer mechanism in inorganic materials can be explained thoroughly as their structures are rigid and predictable. However, the complexity of biomolecules impose a challenge for properly understanding their electrical properties.
Table 1 lists the solid-state parameters calculated for the riboflavin-Schottky junction at their pristine condition and previously reported results for other biomolecules. Undoubtedly, the ideality factor values reported for biomolecules are very high compared to ideal diode. While ideality factor itself is governed by numerous factors such as the interface quality and non-uniformity distribution of the charges, our objective is not to replicate the capabilities of an inorganic diode junction, but rather to utilize the resulting non-ideal diode parameters as means of characterizing the biomolecule being studied. These solid-state parameters define the material in terms of its electronics based on solid-state properties and are currently utilized as a comparison tool to differentiate between genus and even within subspecies [41]. We could hypothesize on its physical meaning, for example relating high conductivity pathway (and therefore lower resistance) within the biomolecules to its adaptability in nature, cell death against live ones and in the case of riboflavin the status of its natural molecular functionalities etc. However, these need further in-depth investigations for conclusions to be made affirmatively, only possible with expansive data collected over long periods of time.
Table 1.
Solid-state parameters of riboflavin compared to previously reported biomolecules studied using PCB-2T platform.
| Biomolecules |
|
|
|
Ref | |||
|---|---|---|---|---|---|---|---|
| Collagen | 1.25 | 0.021010 | 53.944 | 277.500 | 19.80 | 12.76 | [15] |
| Polynucleotide Cytosine | 1.10 | 0.009690 | 61.060 | 360.000 | 17.94 | 13.53 | [14] |
| mRNA | 1.00 | 0.000256 | 106.132 | 917.446 | 20.58 | 3.58 | [16] |
| Riboflavin | 0.86 | 0.016 | 30.598 | 1.1773 | 8.23 | 17.42 | Present findings |
| ±0.02 | ±0.001 | ±4.876 | ±0.6128 | ±0.05 | ±0.74 | ||
| Polynucleotide Guanine | 0.85 | 0.010010 | 52.340 | 25500.000 | 16.34 | 12.34 | [14] |
3.5. Investigation of negative differential resistance against storage and light exposure times
Riboflavin especially in the form of solution is known to be unstable and degrades easily into its’ various photoproducts such as lumichrome (LM) and lumiflavin (LF) when exposed to UV and visible light [42]. This photochemical process is in general reduced when stocked or stored in dark glass bottles. However, chemical decay can still take place during storage, particularly in the presence of alkaline substances in the electrolyte. Such chemical changes have been studied extensively [43,44], however, the electronic properties of the degradation of riboflavin molecules remain inconclusive. Therefore, it would be interesting to explore how these phenomena affect the NDR properties, and in this scenario, involving a vitamin using the current PCB-2T method.
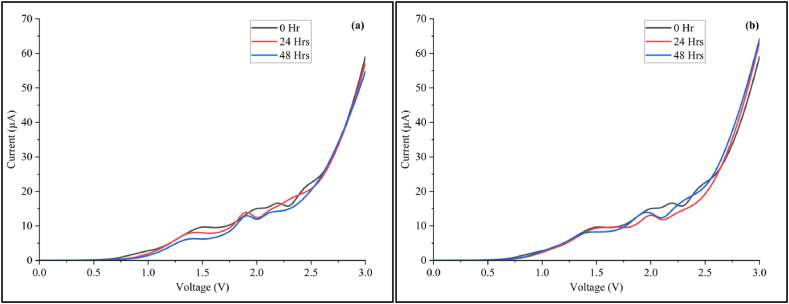
To enable this study, the riboflavin samples were further subjected to time (three days storage) and photodegradation analysis while maintaining aseptic techniques to avoid contamination. The riboflavin solution stored in a clear bottle underwent both photodegradation (due to exposure to visible light) and gradual chemical degradation (in the blank solution due to trace amounts of alkalinity and reactions with oxygen). In contrast, the riboflavin stored in a dark bottle experienced minimal photodegradation but still underwent slow chemical degradation [45]. The results shows that the semiconductive curve is generally maintained for the entire period of storage and exposure to light indicative of the preservation of its intrinsic electronic behaviour. However, attenuation of the NDR peaks or flattening of the curves was observed on the second and third days in both conditions (Fig. 8). A more pronounced shift of peaks is seen for the photodegradation curve suggesting a substantial impact of light-induced time-dependent process on the stability of the riboflavin in agreement with literature [46,47]. It could therefore be concluded that the NDR effect in riboflavin reduces at a faster rate under light environments when compared to dark-storage as anticipated.
Fig. 8.
I-V profiles generated under constant light exposure (a) and against storage time in dark glass bottles (b) over a period of 48 hrs. To increase statistical significance, the experiments were repeated three times for each measurement per day before the average profile was plotted. The experiments were also further repeated the next week to confirm the trend (see Supplementary Figs. S4 and S5 online). Error bars were similar to those obtained in Fig. 2 denoting high repeatability.
The literature extensively documents that NDR could arise as a result of several mechanisms, which include conformational changes of the investigated molecules, trap charges, polarization response, and resonance tunnelling [48,49]. Materials have been shown to demonstrate multiple NDR peaks at different bias voltages due to these mechanisms and are highly influenced by structural changes and therefore its corresponding electronic band structures. Furthermore, a report of NDR on purely polymeric system suggested that this phenomenon occurred due the misalignment and alignment of the energy levels. The alignment provides efficient charge transport leading to strong current conduction with applied potential. A further increase in the applied potential at a particular point then led to misalignment of the energy levels, resulting in a decrease in current flow [50]. This understanding explains the attenuation of the specific peaks observed in extended light and storage conditions in riboflavin. However, it is premature to conclude convincingly the fundamental mechanisms involved prior to a comprehensive energy profile study.
Previous works reported that UV irradiation resulted in higher degradation rates when compared to exposure to visible light [51,52]. Further, it is well-known that the resulting photoproducts are highly pH-dependent [53,54]. The current preliminary studies employed a non-monochromatic light source due to the requirement of this experiment to understand the basic attenuation of the NDR peaks with exposure to light for example. The current findings therefore validate focussed fundamental studies to further enhance our understanding of this exciting discovery of the NDR phenomenon in biomolecules in general.
This electronic approach shows significant potential for quantitatively analysing riboflavin degradation through detailed peak analysis, offering a non-invasive, rapid and real-time solution for quality control in the vitamin industry, particularly via biosensor devices. It could revolutionize vitamin degradation monitoring, enabling on-the-spot detection without extensive lab procedures. By differentiating electronic profiles, especially solid-state parameters, the method may distinguish between various vitamins and assess their stability and potency. Beyond riboflavin, it could offer insights across a wide range of supplements. Additionally, this approach could shed light on the electronic mechanisms behind structural changes in riboflavin, helping to understand deviations from the 'normal' or 'healthy' state, especially during disease progression. Since drug development relies on comprehending abnormalities in biomolecules, this method holds promise for advancing both vitamin stability and disease research.
3.6. Proposed electron transfer mechanisms of the Au/Riboflavin/Au structure
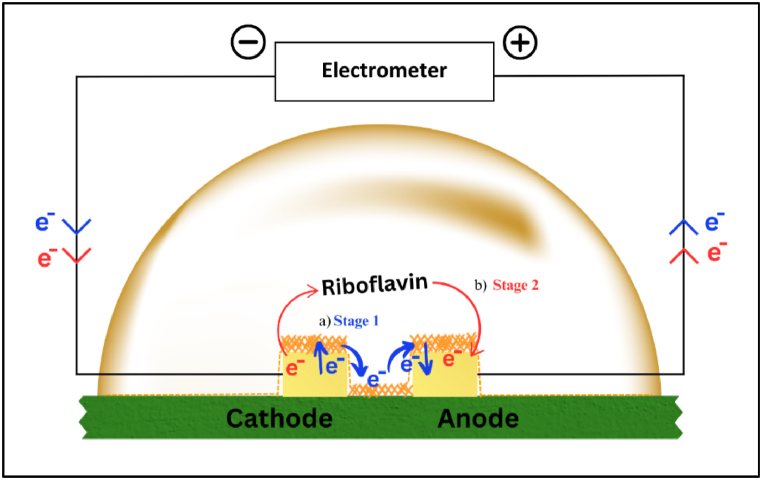
From this work, we therefore postulate that the semiconductive-like properties of riboflavin could emerge from the interfacial carrier injection of the Schottky contact between the riboflavin layers and gold electrodes (in response to the applied external electric field), with expected contribution from bulk electrons conduction within the isoalloxazine ring structure. In correlation with this, we proposed the collective electron mechanism of the Au/Riboflavin/Au structure as representatively shown in Fig. 9. We hypothesize the existence of a 2-stage charge transfer mechanism in conjunction with the solid-state carrier-injection process (Stage 1) as described in Fig. 4. As explained earlier (section 3.2), Stage 1 represents electron movement through (a) interfacial carrier injections of the metal/Riboflavin Schottky contact, driven primarily by the charge gradient.
Fig. 9.
Representative illustrations of electron movements of the Au/Riboflavin/Au structure via (a) carrier injection and (b) ionic conduction.
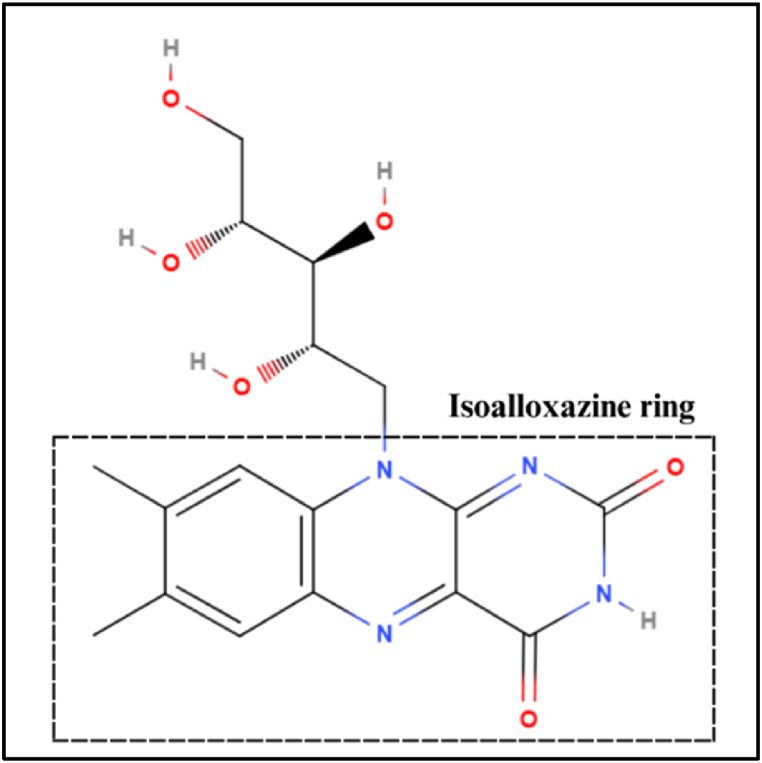
Further, Stage 2 depicts the (b) ionic conduction of the riboflavin molecules, where oxidation and reduction occur at the conjugated-pi system contributing to the bulk properties of the molecules. In general, electron transfer processes of organic molecules can be attributed to mechanisms such as electron hopping, tunnelling, and through-bond interactions [55]. For the case of π-conjugated molecular systems, electron transfer processes could be contributed to the electron “hopping” between the π-electrons [56,57] with significant impact in molecular electronics. Riboflavin’s aromatic ring structures possess 14 conjugated π-electrons. Thus, in our case, intermolecular electron transfer of riboflavin typically occurs at the isoalloxazine ring (Fig. 10) structure that constitutes the vitamin’s molecule and its derivatives, FAD and FMN [58]. Redox reactions take place when the structure receives or donates electron(s), and the stabilization of this process enables the flavins to act as an “electron shuttle” in metabolic processes.
Fig. 10.
Diagram of the molecular structure of Riboflavin. Highlighted is the isoalloxazine ring structure, where redox reactions occur within Riboflavin.
4. Conclusions
The discovery of semiconductive-like behaviour and other interesting electronic properties in biomolecules such as DNA, RNA, and protein molecules in recent times potentially opens up exciting avenues for the development of highly sensitive and selective alternative biosensing methods. Further, it is of great anticipation that other highly characteristic material-dependent electronic properties such as the NDR occurrences could be quantitatively scrutinized to enable further in-depth understandings of fundamental charge mechanisms. While the prospects of electronic characterization and identification of these biomolecules have significant impact on biomedical and molecular biology, especially in the development of various point-of-care (POC) devices, there is a need for miniaturization of sensors like the Lab-on-PCB utilized in this work. This type of sensor effectively achieves the form factor required to allow seamless integration into portable POC devices. The current findings reported were observed to have significantly reduced error bars of the electronic I-V profiles while maintaining the repeatability in regard to previous literatures on the semiconductive-like behaviour of biomolecules. Besides the novel discovery of the semiconductive-like property and the NDR effect in a vitamin, the utilization of the Lab-on-PCB-based platform forms the spotlight of this report.
CRediT authorship contribution statement
Akmal Fathurrahman Zullkifli: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. Mohammad Nofil: Writing – review & editing, Investigation. Chethan C. Thimmarayappa: Writing – review & editing, Investigation. Prince Nishchal Narayanaswamy Elumalai: Writing – review & editing. Sara Talebi: Writing – review & editing, Validation, Investigation. Mitsumasa Iwamoto: Validation, Supervision. Vengadesh Periasamy: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Conceptualization.
Availability of data and materials
The datasets used and/or analysed for the current work are available from the corresponding author on reasonable request.
Funding
The research was supported by the Fundamental Research Grant Scheme (FRGS)[FP065-2023].
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Vengadesh Periasamy has patent licensed to eProfiler Solutions Ltd. (UK). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39411.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Mosegaard S., et al. Riboflavin deficiency-implications for general human health and inborn errors of metabolism. Int. J. Mol. Sci. 2020;21(11) doi: 10.3390/ijms21113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddi A.S., Ho P.K., Camerini-Davalos R.A. The role of riboflavin in carbohydrate metabolism. Adv. Exp. Med. Biol. 1979;119:243–250. doi: 10.1007/978-1-4615-9110-8_35. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Wang Q., Qi Q. Identification of riboflavin: revealing different metabolic characteristics between Escherichia coli BL21(DE3) and MG1655. FEMS Microbiol. Lett. 2015;362(11) doi: 10.1093/femsle/fnv071. [DOI] [PubMed] [Google Scholar]
- 4.Bailey M.R., Schultz Z.D. SERS speciation of the electrochemical oxidation-reduction of riboflavin. Analyst. 2016;141(17):5078–5087. doi: 10.1039/c6an01054g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers H.J., Corfe B.M., Nakano E. Riboflavin in development and cell fate. Subcell. Biochem. 2012;56:229–245. doi: 10.1007/978-94-007-2199-9_12. [DOI] [PubMed] [Google Scholar]
- 6.Ahgilan A., Sabaratnam V., Periasamy V. Antimicrobial properties of vitamin B2. Int. J. Food Prop. 2015;19(5):1173–1181. [Google Scholar]
- 7.Martins S.A., et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest. Ophthalmol. Vis. Sci. 2008;49(8):3402–3408. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 8.Stanković D.M., et al. Sensitive and selective determination of riboflavin (vitamin B2) based on boron-doped diamond electrode. Monatshefte Für Chemie - Chemical Monthly. 2016;147(6):995–1000. [Google Scholar]
- 9.Savan R., et al. Electrochemical and spectroscopic studies of riboflavin. Analytical Chemistry Letters. 2018;8(5):653–664. [Google Scholar]
- 10.Randviir E.P., Banks C.E. A review of electrochemical impedance spectroscopy for bioanalytical sensors. Anal. Methods. 2022;14(45):4602–4624. doi: 10.1039/d2ay00970f. [DOI] [PubMed] [Google Scholar]
- 11.Simoska O.S., K. J Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst (London. 1877. Online)/Analyst. 2019;144(22):6461–6478. doi: 10.1039/c9an01747j. [DOI] [PubMed] [Google Scholar]
- 12.Mallik B., Ghosh A.K., Misra T.N. Pre-exponential factor in semiconducting vitamin A (Alcohol and acetate) Bull. Chem. Soc. Jpn. 1979;52(7):2091–2096. [Google Scholar]
- 13.Mitra B., Misra T.N. Compensation effect in electrical conduction process: effect of substituent group. Bull. Chem. Soc. Jpn. 1978;26(5R):695. [Google Scholar]
- 14.Daraghma S.M.A., Talebi S., Periasamy V. Electronic properties of short polynucleotides studied using Schottky junctions. J. Electron. Mater. 2021;50(3):1267–1274. [Google Scholar]
- 15.Talebi S., et al. Printed-Circuit-Board-Based Two-Electrode system for electronic characterization of proteins. ACS Omega. 2020;5(14):7802–7808. doi: 10.1021/acsomega.9b03831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talebi S., et al. Exploring the electronic properties of ribonucleic acids integrated within a Schottky-Like junction. J. Electron. Mater. 2019;48(11):7114–7122. [Google Scholar]
- 17.Metrohm Determination of riboflavin (vitamin B2) by polarography, in Application Bulletin 219/3 e. https://www.bing.com/ck/a?!&&p=4678c0968be1cda5JmltdHM9MTcyOTAzNjgwMCZpZ3VpZD0yMTc0N2JiZi1mZDM1LTYzMTEtM2UyYy02ZjUwZmM5YzYyOGImaW5zaWQ9NTE5Mw&ptn=3&ver=2&hsh=3&fclid=21747bbf-fd35-6311-3e2c-6f50fc9c628b&psq=bulletin+219%2f3+e+metroohm&u=a1aHR0cHM6Ly93d3cubWV0cm9obS5jb20vY29udGVudC9kYW0vbWV0cm9obS9zaGFyZWQvZG9jdW1lbnRzL2FwcGxpY2F0aW9uLWJ1bGxldGlucy9BQi0yMTlfMy5wZGY&ntb=1
- 18.Rhoderick E.H., Williams R.H. Oxford University Press; USA: 1988. Metal-semiconductor Contacts. [Google Scholar]
- 19.Cheung S.K., Cheung N. Extraction of Schottky diode parameters from forward current-voltage characteristics. Appl. Phys. Lett. 1986;49(2):85–87. [Google Scholar]
- 20.Ditzler L.R., et al. Self-assembled enzymatic monolayer directly bound to a gold surface: activity and molecular recognition force spectroscopy studies. J. Am. Chem. Soc. 2011;133(34):13284–13287. doi: 10.1021/ja205409v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo K.M., et al. DNA–bare gold affinity interactions: mechanism and applications in biosensing. Anal. Methods. 2015;7(17):7042–7054. [Google Scholar]
- 22.Talebi S. Department of Physics. 2020. Electronic Characterization of miRNA, mRNA, hemoglobin and collagen molecules. Universiti Malaya. [Google Scholar]
- 23.Scott J.M., G G. Charge injection and recombination at the metal–organic interface. Chem. Phys. Lett. 1999;299(2) [Google Scholar]
- 24.Richard S.R. Riboflavin metabolism. N. Engl. J. Med. 1970;9:463–472. doi: 10.1056/NEJM197008272830906. [DOI] [PubMed] [Google Scholar]
- 25.Huang L., Tang J., Chen Man, Liu X., Zhou S. Two modes of riboflavin-mediated extracellular electron transfer in geobacter uraniireducens. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giulian D., Chen J., Ingeman J.E., George J.K., Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J. Neurosci. 1989;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Book C., Pham D.V. Improved morphology and charge carrier injection in pentacene field-effect transistors with thiol-treated electrodes. J. Appl. Phys. 2006;100(11) [Google Scholar]
- 28.Güllü Ö., Türüt A. Electrical analysis of organic interlayer based metal/interlayer/semiconductor diode structures. J. Appl. Phys. 2009;106(10) [Google Scholar]
- 29.Azmi S.Z., et al. Electronic profiling of algae-derived DNA using DNA-specific Schottky diode. Appl. Phys. A. 2018;124(8) [Google Scholar]
- 30.Wan H., et al. Switching, dual Spin-Filtering effects, and negative differential resistance in a Carbon-Based molecular device. Switching, dual Spin-Filtering effects, and negative differential resistance in a Carbon-Based molecular device. J. Phys. Chem. C. 2012;116(3):2570–2574. [Google Scholar]
- 31.Chen J., et al. Large On-Off ratios and negative differential resistance in a molecular electronic device. Science. 1999;286(5444):1550–1552. doi: 10.1126/science.286.5444.1550. [DOI] [PubMed] [Google Scholar]
- 32.Gradinaru G., Madangarli V., Sudarshan T.S. On the negative differential resistance effect in high-field semiconductor-dielectric systems. Appl. Phys. Lett. 1992;61(1):55–57. [Google Scholar]
- 33.Sengupta A., Mahapatra S. Negative differential resistance and effect of defects and deformations in MoS2 armchair nanoribbon metal-oxide-semiconductor field effect transistor. J. Appl. Phys. 2013;114(19) [Google Scholar]
- 34.Balkan N., Ridley B., Vickers A. Springer; 2012. Negative Differential Resistance and Instabilities in 2-D Semiconductors. [Google Scholar]
- 35.Ganichev S., et al. Distinction between the Poole-Frenkel and tunneling models of electric-field-stimulated carrier emission from deep levels in semiconductors. Phys. Rev. 2000;61(15):10361–10365. [Google Scholar]
- 36.Li Y., Tjong S.C. Elsevier eBooks; 2010. Nonlinear Current–Voltage Characteristics in Polymer Nanocomposites; pp. 862–890. [Google Scholar]
- 37.Ghosh D.K., Hazra S., Pal P., Misra T.N. Anomalous Poole-Frenkel effect observed in some polyenes in sandwich cell configuration. J. Mater. Sci. 1992;27(15):4184–4188. [Google Scholar]
- 38.Oelerich J.O., et al. Field dependence of hopping mobility: lattice models against spatial disorder. Physical review. B./Physical review. B. 2017;96(19) [Google Scholar]
- 39.Chu W., et al. Physics of biomolecular recognition and conformational dynamics. Rep. Prog. Phys. 2021;84(12) doi: 10.1088/1361-6633/ac3800. [DOI] [PubMed] [Google Scholar]
- 40.Ito M.P., Kumar K., Murugesan A., V. S, et al. Characterization of the organic thin film solar cells with active layers of PTB7/PC71BM prepared by using solvent mixtures with different additives. Int. J. Photoenergy. 2014;2014:1–8. [Google Scholar]
- 41.Atasagun B., et al. Identification and classification of Eremogone species using DNA based Schottky diodes. Inorg. Chem. Commun. 2023;158 [Google Scholar]
- 42.Wong N.G.K., Rhodes C.N., Dessent C.E.H. Photodegradation of riboflavin under alkaline conditions: what can gas-phase photolysis tell us about what happens in solution? Molecules. 2021;26(19):6009. doi: 10.3390/molecules26196009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad I., et al. Photolysis of riboflavin in aqueous solution: a kinetic study. Int. J. Pharm. 2004;280(12):199–208. doi: 10.1016/j.ijpharm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Choe E.K., Huang R., Min D.B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2005;70(1):R28–R36. [Google Scholar]
- 45.Sheraz M.A., et al. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014;10:1999–2012. doi: 10.3762/bjoc.10.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad I., et al. Stability-Indicating photochemical method for the assay of riboflavin: lumichrome method. J. Chem. 2015:1–8. [Google Scholar]
- 47.Janga K.Y., et al. Photostability issues in pharmaceutical dosage forms and photostabilization. AAPS PharmSciTech. 2017;19(1):48–59. doi: 10.1208/s12249-017-0869-z. [DOI] [PubMed] [Google Scholar]
- 48.Galperin M., Ratner M.A., Nitzan A. Hysteresis, switching, and negative differential resistance in molecular junctions: a Polaron model. Nano Lett. 2004;5(1):125–130. doi: 10.1021/nl048216c. [DOI] [PubMed] [Google Scholar]
- 49.Yeganeh S., Galperin M., Ratner M.A. Switching in molecular transport junctions: polarization response. J. Am. Chem. Soc. 2007;129(43):13313–13320. doi: 10.1021/ja0730967. [DOI] [PubMed] [Google Scholar]
- 50.Rathi S., et al. Negative differential resistance in heterojunction polymeric films. Superlattices and Microstructures. 2018;114:15–22. [Google Scholar]
- 51.Ahmad I., Fasihullah Q., Vaid F.H. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. Journal of Photochemistry and Photobiology B-biology. 2004;75(1–2):13–20. doi: 10.1016/j.jphotobiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad I., Fasihullah Q., Vaid F.H. Effect of light intensity and wavelengths on photodegradation reactions of riboflavin in aqueous solution. Journal of Photochemistry and Photobiology B-biology. 2004;82(1):21–27. doi: 10.1016/j.jphotobiol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad I., et al. Effect of pH, buffer, and viscosity on the photolysis of formylmethylflavin: a kinetic study. Aust. J. Chem. 2013;66(5):579. [Google Scholar]
- 54.Treadwell G.E., Cairns W., Metzler D.E. Photochemical degradation of flavins. J. Chromatogr. A. 1968;35:376–388. doi: 10.1016/s0021-9673(01)82399-2. [DOI] [PubMed] [Google Scholar]
- 55.Blumberger J. Recent advances in the theory and molecular simulation of biological electron transfer reactions. Chem. Rev. 2015;115(20) doi: 10.1021/acs.chemrev.5b00298. [DOI] [PubMed] [Google Scholar]
- 56.Luo L., Choi S.H., Frisbie C.D. Probing hopping conduction in conjugated molecular wires connected to metal electrodes. Chem. Mater. 2010;23(3):631–645. [Google Scholar]
- 57.Li L., Meller G., Kosina H. Temperature and field-dependence of hopping conduction in organic semiconductors. Microelectron. J. 2007;38(1):47–51. [Google Scholar]
- 58.Zemplani J., et al. Handbook of Vitamins. CRC Press; 2013. Riboflavin (vitamin B2) pp. 192–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed for the current work are available from the corresponding author on reasonable request.