Abstract
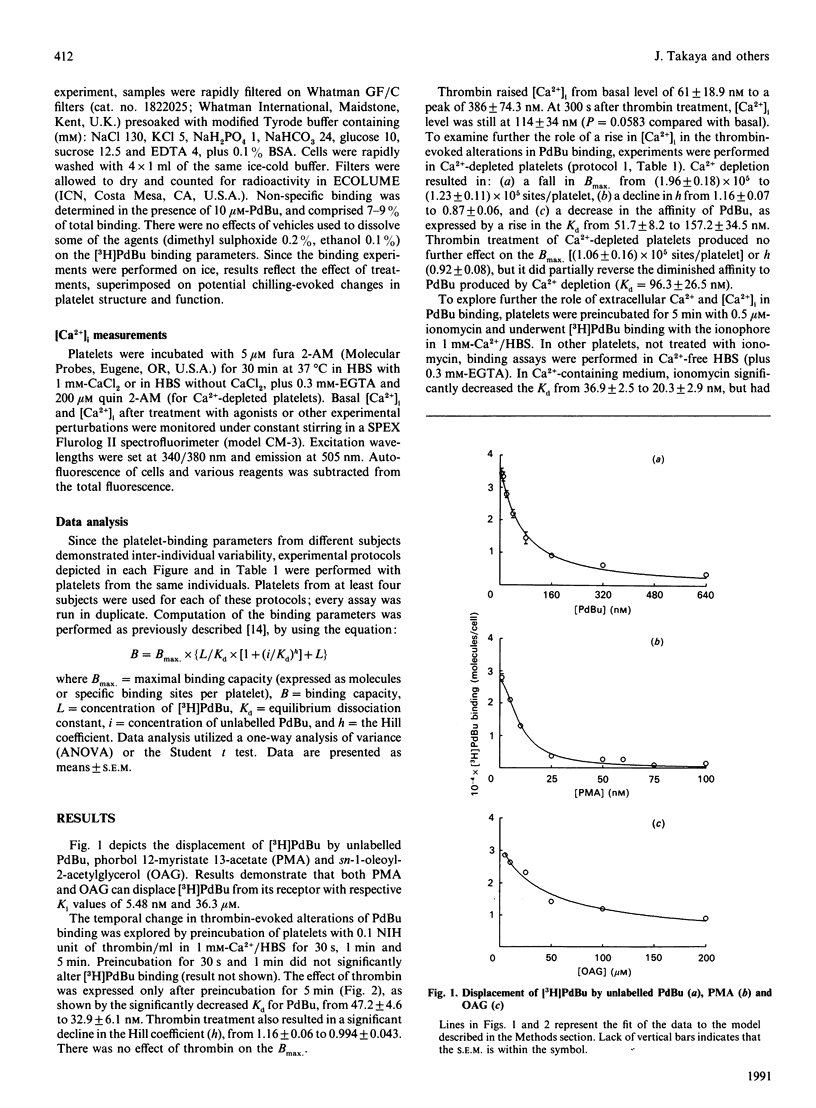
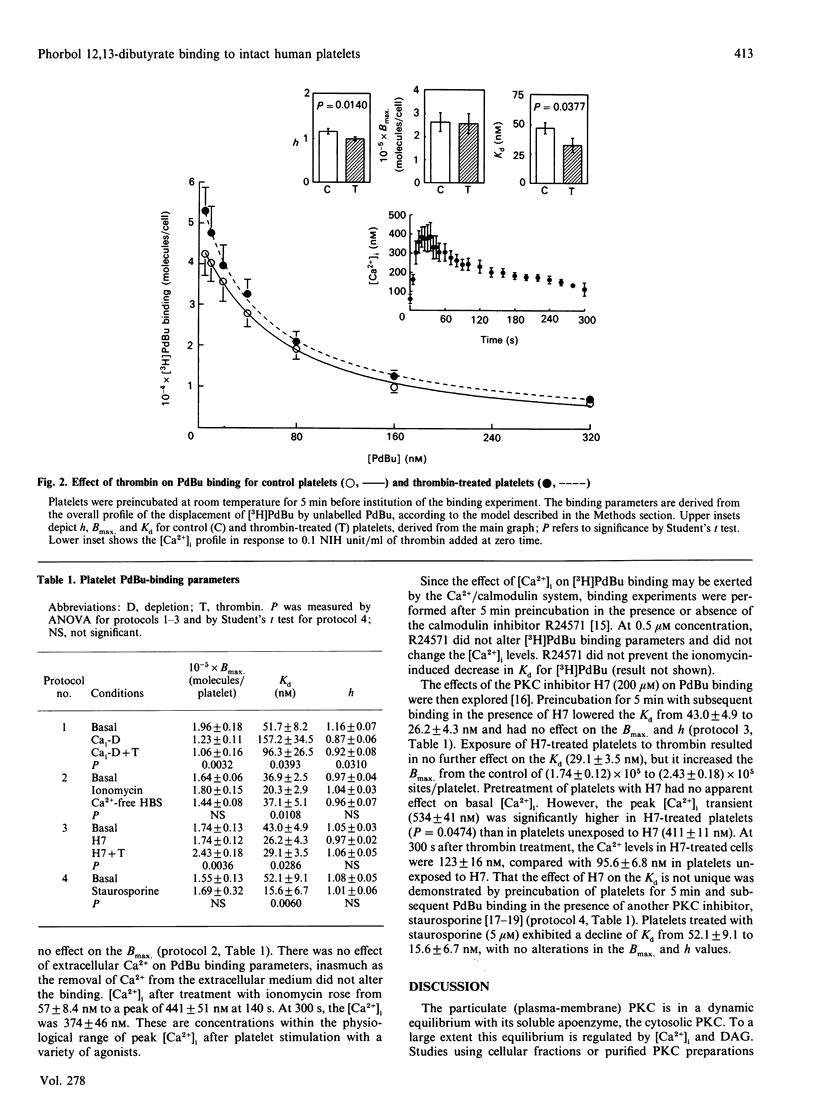
The role of Ca2+ was examined in regulating the binding of phorbol 12,13-dibutyrate (PdBu) to intact human platelets. Alterations in the cytosolic free Ca2+ concn. [( Ca2+]i), but not extracellular Ca2+, substantially influenced the binding parameters of the phorbol ester. Ca(2+)-depleted platelets demonstrated a significant decline in the maximal binding capacity (Bmax), an increase in equilibrium dissociation constant (Kd) and a decrease in the Hill coefficient (h), suggesting the presence of Ca(2+)-sensitive and Ca(2+)-insensitive populations of PdBu-binding sites. In 1 mM-Ca2+ buffer, thrombin (0.1 NIH unit/ml) and ionomycin (0.5 microM) evoked a rise in [Ca2+]i to approx. 300-500 nM, associated with a significant decline in Kd, but without an apparent effect on Bmax. No effect of thrombin was observed on PdBu binding in Ca(2+)-depleted platelets. Inhibition of protein kinase C (PKC) by H7 was associated with a greater thrombin-evoked [Ca2+]i transient and a decline in Kd. Staurosporine also decreased the Kd for PdBu binding. We propose that this effect of the PKC inhibitors on the Kd was also [Ca2+]i-dependent. These observations in intact platelets indicate that the primary role of agonist- or non-agonist-induced rise in [Ca2+]i is to increase the affinity of PKC for PdBu and, presumably, endogenous diacylglycerol. However, in itself a rise in [Ca2+]i does not increase the Bmax, for PdBu binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Dowd J. P., Alila H. W., Hansel W. Phorbol ester receptors in bovine luteal cells: relationship to protein kinase C. Mol Cell Endocrinol. 1990 Mar 5;69(2-3):199–206. doi: 10.1016/0303-7207(90)90013-x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kochenburger R. J., Castagna M., Blumberg P. M. Kinetics and subcellular localization of specific [3H]phorbol 12, 13-dibutyrate binding by mouse brain. Cancer Res. 1981 Jul;41(7):2640–2647. [PubMed] [Google Scholar]
- Gardner J. P., Maher E., Aviv A. Calcium mobilization and Na+/H+ antiport activation by endothelin in human skin fibroblasts. FEBS Lett. 1989 Oct 9;256(1-2):38–42. doi: 10.1016/0014-5793(89)81713-2. [DOI] [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kiley S., Schaap D., Parker P., Hsieh L. L., Jaken S. Protein kinase C heterogeneity in GH4C1 rat pituitary cells. Characterization of a Ca2(+)-independent phorbol ester receptor. J Biol Chem. 1990 Sep 15;265(26):15704–15712. [PubMed] [Google Scholar]
- Kimura M., Gardner J. P., Aviv A. Agonist-evoked alkaline shift in the cytosolic pH set point for activation of Na+/H+ antiport in human platelets. The role of cytosolic Ca2+ and protein kinase C. J Biol Chem. 1990 Dec 5;265(34):21068–21074. [PubMed] [Google Scholar]
- King W. G., Rittenhouse S. E. Inhibition of protein kinase C by staurosporine promotes elevated accumulations of inositol trisphosphates and tetrakisphosphate in human platelets exposed to thrombin. J Biol Chem. 1989 Apr 15;264(11):6070–6074. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lydan M. A., O'Day D. H. Different developmental functions for calmodulin in Dictyostelium: trifluoperazine and R24571 both inhibit cell and pronuclear fusion but enhance gamete formation. Exp Cell Res. 1988 Sep;178(1):51–63. doi: 10.1016/0014-4827(88)90377-1. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Mori T., Takai Y., Minakuchi R., Yu B., Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980 Sep 25;255(18):8378–8380. [PubMed] [Google Scholar]
- Mori T., Takai Y., Yu B., Takahashi J., Nishizuka Y., Fujikura T. Specificity of the fatty acyl moieties of diacylglycerol for the activation of calcium-activated, phospholipid-dependent protein kinase. J Biochem. 1982 Feb;91(2):427–431. doi: 10.1093/oxfordjournals.jbchem.a133714. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Pollock W. K., Sage S. O., Rink T. J. Stimulation of Ca2+ efflux from fura-2-loaded platelets activated by thrombin or phorbol myristate acetate. FEBS Lett. 1987 Jan 5;210(2):132–136. doi: 10.1016/0014-5793(87)81322-4. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Release of superoxide and change in morphology by neutrophils in response to phorbol esters: antagonism by inhibitors of calcium-binding proteins. J Cell Biol. 1985 Sep;101(3):1052–1058. doi: 10.1083/jcb.101.3.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Ca2+ mobilization primes protein kinase C in human platelets. Ca2+ and phorbol esters stimulate platelet aggregation and secretion synergistically through protein kinase C. Biochem J. 1988 Oct 1;255(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Vegesna R. V., Wu H. L., Mong S., Crooke S. T. Staurosporine inhibits protein kinase C and prevents phorbol ester-mediated leukotriene D4 receptor desensitization in RBL-1 cells. Mol Pharmacol. 1988 May;33(5):537–542. [PubMed] [Google Scholar]
- Verme T. B., Velarde R. T., Cunningham R. M., Hootman S. R. Effects of staurosporine on protein kinase C and amylase secretion from pancreatic acini. Am J Physiol. 1989 Oct;257(4 Pt 1):G548–G553. doi: 10.1152/ajpgi.1989.257.4.G548. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Baggiolini M. The protein kinase inhibitor staurosporine, like phorbol esters, induces the association of protein kinase C with membranes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1273–1279. doi: 10.1016/0006-291x(88)90277-x. [DOI] [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]


