Abstract
Humans become more selective with whom they spend their time, and as a result, the social networks of older humans are smaller than those of younger ones. In non-human animals, processes such as competition and opportunity can result in patterns of declining sociality with age. While there is support for declining sociality with age in mammals, evidence from wild bird populations is lacking. Here, we test whether sociality declines with age in a wild, insular bird population, where we know the exact ages of individuals. Using 6 years of sociality data, we find that as birds aged, their degree and betweenness decreased. The number of same-age birds still alive also decreased with age. Our results suggest that a longitudinal change in sociality with age may be, in part, an emergent effect of natural changes in demography. This highlights the need to investigate the changing costs and benefits of sociality across a lifetime.
This article is part of the discussion meeting issue ‘Understanding age and society using natural populations’.
Keywords: ageing, sociality, house sparrow, social network measures, senescence
1. Introduction
Sociality—the propensity of an individual to form and maintain social associations with others—is an important aspect of animal life history. The last decade has seen an increase [1,2] in studies exploring the causes and consequences, as well as the costs and benefits, of sociality in wild animals, thanks to an increasing availability of sociality data [3]. For instance, animal sociality differs consistently among individuals [4,5] and is correlated with reproductive output [6–8], fitness [6,9,10] and survival [11]. However, we know very little about how patterns of animal social association and social networks change with age and what factors may drive these changes. In humans, with age, people become more selective with whom they spend their time [12]. If social interactions are costly in terms of time and energy, then people may choose the quality of relationships over the quantity of relationships. Indeed, in humans, there is support for social networks expanding to a peak in early adulthood, followed by a decline throughout later life [13,14].
Humans can fill in questionnaires about their emotions, illuminating the psychological, proximate costs and benefits of sociality. The ultimate costs and benefits of social interactions across a lifetime, however, may be more easily studied in other animals [12]. Consequently, the social selectivity hypothesis [15] predicts that ageing animals exhibit decreasing sociality. Several hypotheses have been formulated for why such a decrease might occur [16], suggesting that, and not mutually exclusively, limitations of resources, a change in reproductive value, changes in skill and experience or changes in kinship over time may be responsible. However, an even simpler hypothesis is that social associations are mainly formed early in life in an emergent manner, for instance, through sibships or while sampling for mate choice [16] or by meeting others while seeking and defending a territory [17]. If there is no explicit, proactive process that leads to forming new social associations (akin to actively seeking them out where possible), then a decrease in sociality with age might simply be an emergent process resulting from individuals dying over time. Selection for a behaviour of seeking out new connections later in life (if there are individuals available to choose from) is likely to be weaker in older cohorts, because traits expressed later in life will experience weakened selection compared with traits also expressed in early life [18–20]. So unless high sociality has significant benefits in late life, it is unlikely to evolve; this is defined as the selection shadow [21]. Thus, if the benefits of making new connections are lower than the costs of having smaller social networks, a decrease in sociality with age could be, at least partially, an emergent effect.
However, not much is known about whether, and if so, how costly it is for animals to form new connections at a later age. It has been shown in birds that maintaining social connections is expensive [2]. However, the costs of making new connections may become greater at old age—for example, the higher the risk of aggression and injury from younger, fitter individuals. Indeed, chimpanzee Pan troglodytes males have fewer non-antagonistic relationships in old age and more antagonistic ones when young [15]. The benefits of making new connections could be to have a larger pool from which to choose mates [6,22] or a better knowledge about potential competition [23], but research into this is sparse.
One problem when testing whether sociality changes with age is that one needs to know the precise age and life history of all individuals present at the time sociality is measured. This is relevant to distinguish longitudinal effects [24], where individuals change as they age [25], from more social individuals being prone to higher mortality. Such a distinction is only possible with longitudinal data collected across multiple years with repeated observations on the same individuals. Obtaining longitudinal data is typically difficult in wild populations and particularly difficult in highly mobile organisms like birds. Testing for a longitudinal effect of age requires repeated measures on the same individuals across time, which is not often achieved in open, wild populations. Typically, such studies can only be undertaken in long-term study populations of wild, closed populations to distinguish dispersal from death. Here, we use an island population of songbirds that has been monitored for over two decades to test whether wild birds change sociality with age, distinguish between longitudinal and cross-sectional effects of age on sociality and test for an effect of demography.
2. Methods
(a). Study population
We used a dataset of house sparrows, Passer domesticus, living on Lundy Island in Devon, UK. House sparrows start breeding in their first summer after hatching and can reach ages of up to 13 years (own observations) in the wild. We have records on this population dating back to 1989, and systematic recording began in 2000, since when all birds born on the island have been monitored from egg to death [26]. A bird is considered to have died after observations of it ceased—this assumption is valid owing to house sparrows being poor long-distance flyers and Lundy being 19 km away from the nearest coast [27,28]. We observe individuals, if alive, more than 1.5 times per year with little to no capture bias [29].
When still in the nest, once old enough, all birds receive a numbered metal ring from the British Trust for Ornithology, a unique combination of three colour rings and a subcutaneous passive integrated transponder (PIT) [30]. The latter’s unique code is picked up by several nest box and feeder antennas at the study population site [17,31]. The population is closely monitored during the breeding season each year, from April to the end of August, when each breeding attempt is closely followed and recorded. During each winter, we catch as many birds as possible. The closed nature of the study population, in combination with the multiple ways to record life history of birds on the island, leads to a high annual recording (resighting and recapture) probability and, for a population of highly mobile passerines, unusually precise knowledge of the age of all individuals [25,26,29].
We compiled cohort (year of birth) sizes for each age and year, using the number of birds that either were adult (ages 1+) or had been large enough to receive a metal ring—typically at 14 days of age, which is about a day before they fledge. Age (in calendar years) was calculated as the year of observation minus cohort. We calculated the percentage of the individuals from a given cohort still alive in relation to the total adult population size for each year (from here on: peer age group percentage) to use as a proxy for the number of well known conspecifics, assuming most connections are made early in life. We defined the year of death as the year after the last observation. Sex was determined genetically using blood samples taken from chicks and adults [26]. In any given year, most birds would be between 0 and 3 years old, with the older age classes being much smaller (table 1).
Table 1.
Mean and range of age group sizes in the Lundy house sparrow population between 2000 and 2020.
| age group | mean ± s.e. (%) | range (individuals) |
|---|---|---|
| 0 | 72.49 ± 1.07 | 91−787 |
| 1 | 12.70 ± 0.63 | 13−132 |
| 2 | 6.57 ± 0.55 | 1−80 |
| 3 | 3.78 ± 0.40 | 0−50 |
| 4 | 2.20 ± 0.36 | 0−22 |
| 5 | 1.18 ± 0.20 | 0−11 |
| 6 | 0.54 ± 0.12 | 0−6 |
| 7 | 0.27 ± 0.08 | 0−4 |
| 8 | 0.14 ± 0.06 | 0−3 |
| 9 | 0.07 ± 0.03 | 0−1 |
(b). Social network data
We used sociality data collected between 2013 and 2017. Data were collected using manual annotation of video data between 2013 and 2016, where an edge was defined as two birds identified by their colour ring combination being observed interacting with each other at a feeder [4]. During the years 2015−2017, we recorded birds at a feeder via passive monitoring of PIT tags and defined associations based on arrival time [6,32,33]. This method of using the time difference with which associated individuals arrive together has been found to be the most suitable for a system of gregarious birds like the house sparrows at a feeder [34].
While the method to define association at a feeder differed between videos (actual human-made observations) and automated PIT recordings, we have shown that data from both methods are repeatable and meaningful in this population and others [4]. We computed social network graph traits and variables with package iGraph [35]. We used the variables' degree, betweenness and transitivity to describe house sparrow sociality. Degree describes how many other individuals a focal individual is directly connected with [35]. Betweenness quantifies the number of shortest paths going through a focal node [35]. Betweenness was inversely weighted because iGraph treats high edge weights as a cost [36]. Transitivity describes the probability that adjacent nodes of two individuals are connected [35]—individuals whose associations are also connected with each other have a high transitivity, while individuals with connections that connect out have a low transitivity. Individuals with no connection have no transitivity value defined.
(c). Statistical analysis
All statistical analyses were done in R v. 4.3.1 [37]. We ran three linear mixed models with one of the three scaled sociality traits as response variables. The longitudinal age effect was modelled as the difference of an individual’s age from the average age of the individual in the dataset. We then fitted the average age of the individual to model the cross-sectional age effect. Both were fitted as covariates [24]. We also fitted a squared longitudinal age covariate to account for squared effects of improvements and later declines but removed it from those models where it was not statistically significant. We modelled the peer age group percentage as a covariate to test for a demography effect. We modelled a fixed effect of sex (female as reference group) to account for sex differences [6] and an interaction with age. We removed interactions that were not significant, but not age, average age, method, sex or the peer age group percentage. We fitted the method (videos vs automated data collection) as a fixed factor to account for potential differences occurring from different data collection methods and bird identity (BirdID) as a random effect to account for repeated measures on the same individual.
Fitting models with several closely related covariates can lead to multicollinearity, which can lead to an underestimation of the parameter effect size standard error. Thus, we used the variance inflation factor (VIF) to quantify collinearity between peer age group percentage and age (figure 1b). A VIF of 1 typically indicates no collinearity. A VIF of 5 or higher is considered moderate and may be problematic [38]. Finally, as social network metrics can be skewed, we used package MCMCglmm [39] with a Gaussian link to fit linear mixed models. We report parameter effect sizes, 95% credible intervals (CI) and pMCMC values as an analogue for frequentist p-values, defined as twice the posterior probability that the estimate is directional [40]. We consulted trace plots for the Markov chains for all parameters for unimodal convergence, adjusted chain length and thinning parameters for autocorrelation for each parameter to be below 0.1 and effective sample sizes to be above 1000. All models converged reliably and produced consistent results.
Figure 1.
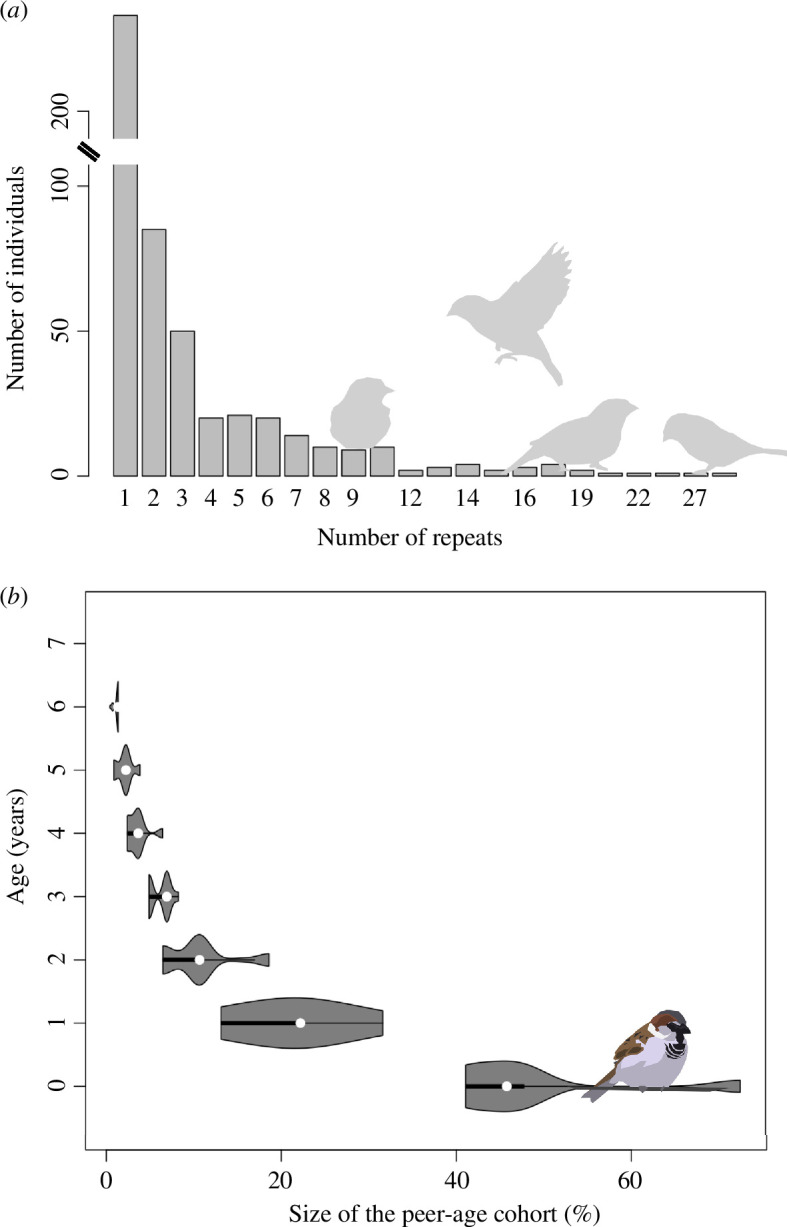
(a) The number of repeated observations of individuals. (b) The ages of Lundy house sparrows (y-axis, in years) in relation to the percentage of fledged individuals of their own cohort that were still alive (x-axis), 2013−2017.
3. Results
We computed 35 social networks (mean 46.97 nodes, s.e. = 7.62). Overall, we recorded 1644 observations of 311 individual females and 304 individual males, with sufficient within-individual repeats (figure 1a). Four hundred and twenty-three individuals were recorded at age 0, 528 at age 1, 332 at age 2, 196 at age 3, 78 at age 4, 53 at age 5, 31 at age 6 and 3 at age 7. No individual older than 7 years was observed in this dataset.
The peer age group percentage relative to the annual population varied from 0.5 to 73% and decreased with age as expected (figure 1b).
Degree, the number of associated individuals, decreased within and across individuals (table 2; figure 2a), and the difference between both slopes was not statistically significant (0.02 (−0.05−0.09 95% CI), p = 0.64). The peer age group percentage was negatively associated with degree—with a smaller peer age group percentage being associated with a lower degree (table 2). There was no sex effect (table 2). There was also a within-individual decrease in betweenness, but not cross-sectionally (table 2; figure 2b). The difference between the slopes of the within-individual and cross-sectional effects was statistically significant (0.07 (0.01−0.15 95% CI), p = 0.03), indicating that an individual’s betweenness decreased with age. There was no effect of the peer age group and no sex effect. We found no age effect with transitivity, but males had lower transitivity (table 2). In all three models, the effect of the method to collect the data was statistically significant, and all VIFs remained lower than 2.4.
Table 2.
Results from three linear mixed models on social network traits of Lundy Island house sparrows. We consider a parameter effect size as statistically significant where the absolute t-value is larger or equal to 2.00 (in bold). VIF, variance inflation factor
| response | degree | betweenness | transitivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| fixed effects | β (95% CI) | p | VIF | β (95% CI) | p | VIF | β (95% CI) | p | VIF |
| intercept | 0.05 (−0.15 to 0.27) | 0.66 | −0.04 (−0.25 to 0.15) | 0.66 | 0.04 (−0.14 to 0.24) | 0.64 | |||
| longitudinal age | −0.13 (−0.24 to −0.03) | 0.01 | 1.27 | −0.10 (−0.19 to 0.00) | 0.04 | 1.26 | −0.02 (−0.13 to 0.07) | 0.63 | 1.26 |
| cross-sectional age | −0.06 (−0.13 to −0.01) | 0.03 | 2.06 | −0.01 (−0.06 to 0.05) | 0.80 | 2.13 | 0.05 (0.00−0.58) | 0.07 | 2.15 |
| peer age group size (%) | −0.49 (−0.92 to −0.05) | 0.03 | 2.26 | −0.17 (−0.58 to 0.22) | 0.42 | 2.35 | 0.18 (−0.21 to −0.02) | 0.42 | 2.38 |
| sex | 0.01 (−0.08 to −0.29) | 0.79 | 1.00 | −0.03 (−0.13 to 0.05) | 0.59 | 1.00 | −0.12 (−0.21 to −0.02) | 0.007 | 1.00 |
| method | 0.19 (0.09−0.29) | <0.001 | 1.00 | 0.16 (0.07−0.25) | 0.01 | 1.00 | −0.16 (−0.26 to −0.05) | 0.002 | 1.00 |
| random effects | variance | variance | variance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| bird identity | 0.08 | 0.01 | 0.01 | ||||||
| residual | 0.91 | 0.97 | 0.98 |
Figure 2.
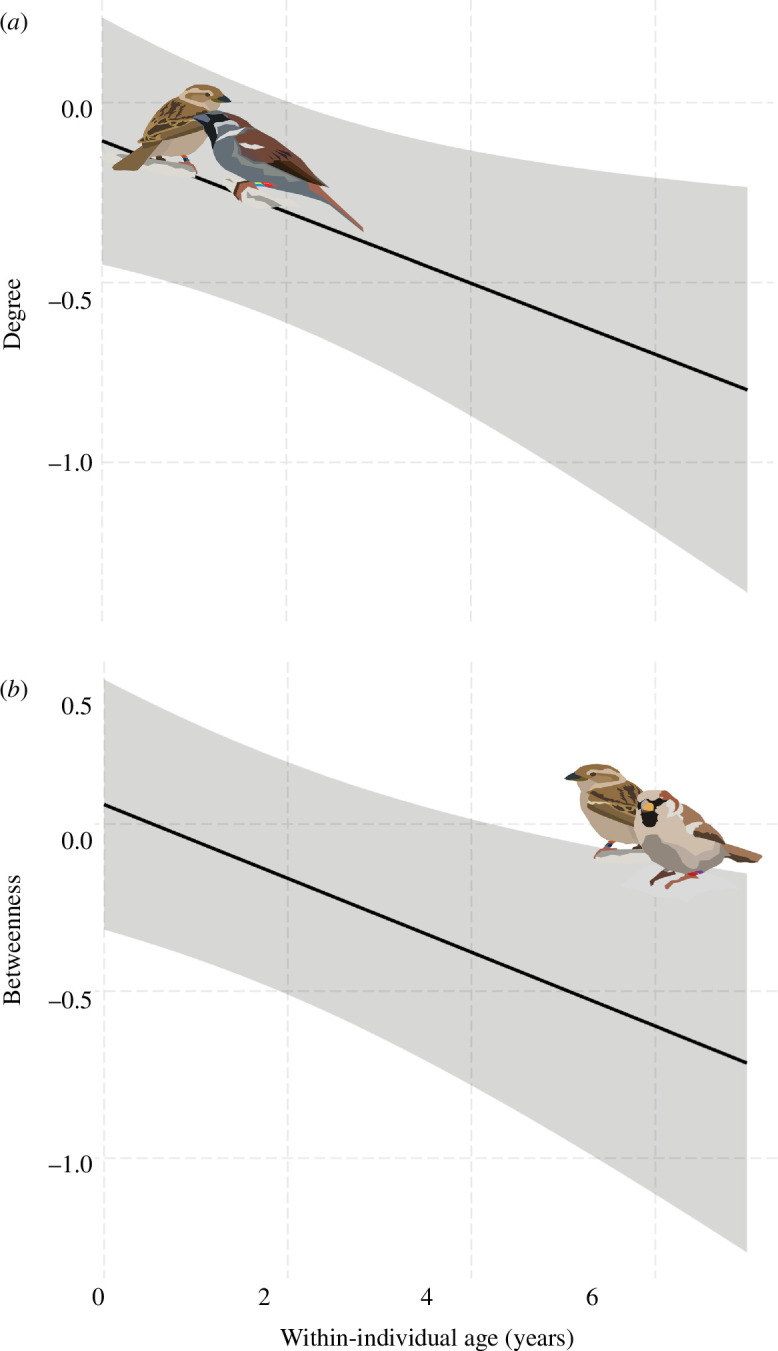
The predicted relationship of degree (a) and betweenness (b) with within-individual age (x-axis) in Lundy Island house sparrows. Predicted regression lines with 95% CI intervals.
4. Discussion
We found that as sparrows got older, their sociality decreased on the longitudinal level. We found supporting evidence suggesting that demography, indeed, may at least partially drive this pattern—the fewer birds in the same peer age group, the lower their sociality was. The decreasing sociality echoes results from studies on primates [15], including humans [13,14], and broadly is a prerequisite for testing hypotheses explaining how and why sociality may decrease also in birds [12,15].
It is intuitive that the social behaviour in birds is also guided by concepts like familiarity with conspecifics. For instance, spatial proximity has been shown to modulate social selectivity [8,23] and to be important for mate choice [41–43]. We have shown in the past that in this sparrow population, the nest boxes visited in the winter are linked to the nest location—and associated mate choice—in the summer, supporting the idea that familiarity with not only location but also individuals is important [17]. In birds, we know that population density can play a role in mate choice [44], with low density possibly limiting the ability to assess several partners. Generally, the idea is that opportunity—how many relevant individuals are available or spatially close—drives these patterns [45]. This suggests that not only spatial distribution but also demography can produce emergent patterns of sociality in addition to other mechanism [16]. In house sparrows, however, it is unlikely that sibships play an important role because in clutches of, on average, four, it is rare for more than one sibling to survive to old age. More research is needed to better understand whether early life interactions can create familiarity and whether and how birds retain individual associations other than mates across a lifetime.
From here on, it is intuitive to consider the influence of demography on social interactions: the older an animal becomes, the more of its initial associates are likely to have died. Thus, the network shrinks unless new connections are made. While finding new mates later in life is a common occurrence, less is known about birds actively seeking out new associates that are not mates. We know that birds can indeed fill in the gaps in the social network left behind by individuals that have died [1] and may be willing to pay costs to maintain familiar social connections [2]. Also, the costs of maintaining and making new connections may increase with age [16], because older individuals may be less able to compete with younger individuals [46]. To fully understand the effect of demography on sociality, we need to better understand the changing costs and benefits across ages of maintaining and making new connections.
We used the number of individuals from the same cohort still alive as a proxy of the group size of associations formed emergently in early life. This is an approximation, and it would be interesting to investigate which early life associates are important and whether older and younger conspecifics might also play a role. We also used two different methods to define edges. Both methods collected data on sparrows visiting a feeder—the first one used manual annotation of videos to confirm interaction between two individuals took place, while the second method considered all individuals that arrived within a certain time span to be interacting [34]. Behavioural data collection is currently undergoing change at a fast pace, from manual ethograms collected in situ, to video recordings annotated manually, to video recording analysed by machine learning algorithms. For research spanning multiple years, changes in technology use may often force researchers to pragmatically bridge between different ways of data collection.
In conclusion, we have found empirical support for decreasing sociality with age in a wild passerine bird, to the best of our knowledge for the first time. Older individuals have smaller social circles and are less well connected, likely owing to the cost of making new connections: an ever-shrinking cohort size as mortality takes its toll. Clearly, demographic processes and sociality are intricately linked [47], and to further our understanding, we must evaluate the costs and benefits of making new connections.
Contributor Information
Julia Schroeder, Email: julia.schroeder@imperial.ac.uk.
Jamie Dunning, Email: jamiedunning8@gmail.com.
Alex Hoi Han Chan, Email: hoi-hang.chan@uni-konstanz.de.
Heung Ying Janet Chik, Email: chikhyjanet@gmail.com.
Terry Burke, Email: t.a.burke@sheffield.ac.uk.
Ethics
Ethical licenses needed for research on wild animals were obtained (Home Office licence numbers PP5873078 and PP7009092).
Data accessibility
Data and code are available at [48].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
J.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, validation, visualization, writing—original draft, writing—review and editing; J.D.: investigation, methodology, writing—review and editing; A.H.H.C.: methodology, writing—review and editing; H.Y.J.C.: data curation, formal analysis, writing—review and editing; T.B.: data curation, project administration, resources, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
No funding has been received for this article.
References
- 1. Firth JA, Voelkl B, Crates RA, Aplin LM, Biro D, Croft DP, Sheldon BC. 2017. Wild birds respond to flockmate loss by increasing their social network associations to others. Proc. R. Soc. B 284, 20170299. ( 10.1098/rspb.2017.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Firth JA, Voelkl B, Farine DR, Sheldon BC. 2015. Experimental evidence that social relationships determine individual foraging behavior. Curr. Biol. 25, 3138–3143. ( 10.1016/j.cub.2015.09.075) [DOI] [PubMed] [Google Scholar]
- 3. Gartland LA, Firth JA, Laskowski KL, Jeanson R, Ioannou CC. 2022. Sociability as a personality trait in animals: methods, causes and consequences. Biol. Rev. Camb. Phil. Soc. 97, 802–816. ( 10.1111/brv.12823) [DOI] [PubMed] [Google Scholar]
- 4. Plaza M, et al. 2020. Repeatable social network node‐based metrics across populations and contexts in a passerine. J. Evol. Biol. 33, 1634–1642. ( 10.1111/jeb.13703) [DOI] [PubMed] [Google Scholar]
- 5. Aplin LM, et al. 2015. Consistent individual differences in the social phenotypes of wild great tits, Parus major. Anim. Behav. 108, 117–127. ( 10.1016/j.anbehav.2015.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunning J, Burke T, Chan AHH, Chik HYJ, Evans T, Schroeder J. 2023. Opposite-sex associations are linked with annual fitness, but sociality is stable over lifetime. Behav. Ecol. 34, 315–324. ( 10.1093/beheco/arac124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cote J, Dreiss A, Clobert J. 2008. Social personality trait and fitness. Proc. R. Soc. B 275, 2851–2858. ( 10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gokcekus S, Firth JA, Regan C, Cole EF, Sheldon BC, Albery GF. 2023. Social familiarity and spatially variable environments independently determine reproductive fitness in a wild bird. Am. Nat. 201, 813–824. ( 10.1086/724382) [DOI] [PubMed] [Google Scholar]
- 9. Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silk JB. 2007. Social components of fitness in primate groups. Science 317, 1347–1351. ( 10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- 11. Lehmann J, Majolo B, McFarland R. 2016. The effects of social network position on the survival of wild barbary macaques, Macaca sylvanus. Behav. Ecol. 27, 20–28. ( 10.1093/beheco/arv169) [DOI] [Google Scholar]
- 12. Carstensen LL. 2006. The influence of a sense of time on human development. Science 312, 1913–1915. ( 10.1126/science.1127488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. English T, Carstensen LL. 2014. Selective narrowing of social networks across adulthood is associated with improved emotional experience in daily life. Int. J. Behav. Dev. 38, 195–202. ( 10.1177/0165025413515404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrzus C, Hänel M, Wagner J, Neyer FJ. 2013. Social network changes and life events across the life span: a meta-analysis. Psychol. Bull. 139, 53–80. ( 10.1037/a0028601) [DOI] [PubMed] [Google Scholar]
- 15. Rosati AG, Hagberg L, Enigk DK, Otali E, Emery Thompson M, Muller MN, Wrangham RW, Machanda ZP. 2020. Social selectivity in aging wild chimpanzees. Science 370, 473–476. ( 10.1126/science.aaz9129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siracusa ER, Higham JP, Snyder-Mackler N, Brent LJN. 2022. Social ageing: exploring the drivers of late-life changes in social behaviour in mammals. Biol. Lett. 18, 20210643. ( 10.1098/rsbl.2021.0643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sánchez‐Tójar A, Winney I, Girndt A, Simons MJP, Nakagawa S, Burke T, Schroeder J. 2017. Winter territory prospecting is associated with life‐history stage but not activity in a passerine. J. Avian Biol. 48, 407–416. ( 10.1111/jav.01055) [DOI] [Google Scholar]
- 18. Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. ( 10.5962/bhl.title.27468) [DOI] [Google Scholar]
- 19. Hamilton WD. 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45. ( 10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 20. Haldane JBS. 1942. New paths in genetics. Nature 149, 317. ( 10.1038/149317a0) [DOI] [Google Scholar]
- 21. Medawar PB. 1957. An unsolved problem in biology. In The uniqueness of the individual, pp. 17–43. London: Methuen & Co. ( 10.4324/9780429299759-2) [DOI] [Google Scholar]
- 22. Oh KP, Badyaev AV. 2010. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 176, E80–E89. ( 10.1086/655216) [DOI] [PubMed] [Google Scholar]
- 23. Albery GF, Clutton-Brock TH, Morris A, Morris S, Pemberton JM, Nussey DH, Firth JA. 2022. Ageing red deer alter their spatial behaviour and become less social. Nat. Ecol. Evol. 6, 1231–1238. ( 10.1038/s41559-022-01817-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van de Pol M, Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 25. Schroeder J, Burke T, Mannarelli ME, Dawson DA, Nakagawa S. 2012. Maternal effects and heritability of annual productivity: maternal effects of annual productivity. J. Evol. Biol. 25, 149–156. ( 10.1111/j.1420-9101.2011.02412.x) [DOI] [PubMed] [Google Scholar]
- 26. Schroeder J, Nakagawa S, Rees M, Mannarelli ME, Burke T. 2015. Reduced fitness in progeny from old parents in a natural population. Proc. Natl Acad. Sci. USA 112, 4021–4025. ( 10.1073/pnas.1422715112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dobson S, Dunning J, Burke T, Chik HYJ, Schroeder J. 2023. Indirect genetic effects increase heritability estimates for male and female extra-pair reproduction. Evolution 77, 1893–1901. ( 10.1093/evolut/qpad100) [DOI] [PubMed] [Google Scholar]
- 28. Alif Ž, Dunning J, Chik HYJ, Burke T, Schroeder J. 2022. What is the best fitness measure in wild populations? A case study on the power of short-term fitness proxies to predict reproductive value. PLoS One 17, e0260905. ( 10.1371/journal.pone.0260905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simons MJP, Winney I, Nakagawa S, Burke T, Schroeder J. 2015. Limited catching bias in a wild population of birds with near-complete census information. Ecol. Evol. 5, 3500–3506. ( 10.1002/ece3.1623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schroeder J, Cleasby IR, Nakagawa S, Ockendon N, Burke T. 2011. No evidence for adverse effects on fitness of fitting passive integrated transponders (PITs) in wild house sparrows Passer domesticus. J. Avian Biol. 42, 271–275. ( 10.1111/j.1600-048X.2010.05271.x) [DOI] [Google Scholar]
- 31. Sánchez-Tójar A, et al. 2018. Meta-analysis challenges a textbook example of status signalling and demonstrates publication bias. eLife 7, e37385. ( 10.7554/eLife.37385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunning J, Burke T, Schroeder J. 2024. Divorce is linked with extra-pair paternity in a monogamous passerine. J. Avian Biol. ( 10.22541/au.168496743.30745415/v1) [DOI]
- 33. Chan AHH, Qi LJ, Burke T, Pearse W, Schroeder J. 2024. Comparison of manual, machine learning, and hybrid methods for video annotation to extract parental care data. J. Avian Biol. ( 10.1111/jav.03167) [DOI]
- 34. Chan AHH, et al. 2024. Animal social networks are robust to changing association definitions. EcoEvoRxiv. ( 10.32942/X2P890) [DOI]
- 35. Csárdi G, Nepusz T. 2006. The igraph software package for complex network research. Complex Syst. 1695, 1–9. [Google Scholar]
- 36. Silk MJ, Croft DP, Delahay RJ, Hodgson DJ, Boots M, Weber N, McDonald RA. 2017. Using social network measures in wildlife disease ecology, epidemiology, and management. Bioscience 67, 245–257. ( 10.1093/biosci/biw175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R Core Team . 2023. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 38. James WD, Hastie T, Tibshirani R. 2013. An introduction to statistical learning: with applications in R. New York, NY: Springer. [Google Scholar]
- 39. Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 40. Hadfield JD, Heap EA, Bayer F, Mittell EA, Crouch NMA. 2013. Intraclutch differences in egg characteristics mitigate the consequences of age-related hierarchies in a wild passerine. Evolution 67, 2688–2700. ( 10.1111/evo.12143) [DOI] [PubMed] [Google Scholar]
- 41. Beck KB, Farine DR, Kempenaers B. 2021. Social network position predicts male mating success in a small passerine. Behav. Ecol. 32, 856–864. ( 10.1093/beheco/arab034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beck KB, Farine DR, Kempenaers B. 2020. Winter associations predict social and extra-pair mating patterns in a wild songbird. Proc. R. Soc. B 287, 20192606. ( 10.1098/rspb.2019.2606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Culina A, Hinde CA, Sheldon BC. 2015. Carry-over effects of the social environment on future divorce probability in a wild bird population. Proc. R. Soc. B 282, 20150920. ( 10.1098/rspb.2015.0920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westneat DF, Sherman PW. 1997. Density and extra-pair fertilizations in birds: a comparative analysis. Behav. Ecol. Sociobiol. 41, 205–215. ( 10.1007/s002650050381) [DOI] [Google Scholar]
- 45. Maldonado-Chaparro AA, Montiglio PO, Forstmeier W, Kempenaers B, Farine DR. 2018. Linking the fine-scale social environment to mating decisions: a future direction for the study of extra-pair paternity. Biol. Rev. 93, 1558–1577. ( 10.1111/brv.12408) [DOI] [PubMed] [Google Scholar]
- 46. McDonald DB. 2007. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA 104, 10910–10914. ( 10.1073/pnas.0701159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shizuka D, Johnson AE. 2020. How demographic processes shape animal social networks. Behav. Ecol. 31, 1–11. ( 10.1093/beheco/arz083) [DOI] [Google Scholar]
- 48. Schroeder J, Dunning J, Burke T. 2024. Data from: Not so social in old age: demography as one driver of decreasing sociality. Figshare. ( 10.6084/m9.figshare.26324557) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code are available at [48].


