Abstract
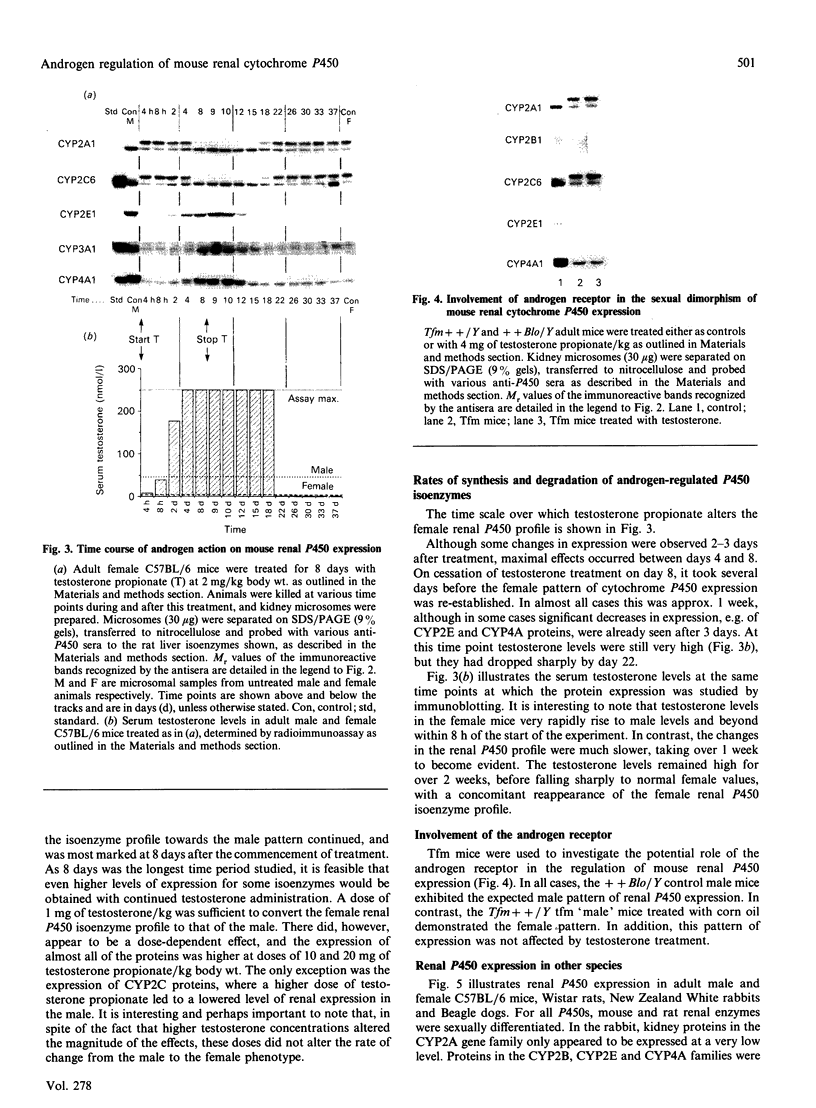
We have previously shown that sexual dimorphism in the expression of mouse renal cytochrome P450s is mediated by androgens, probably at a transcriptional level [Henderson, Scott, Yang & Wolf (1990), Biochem. J. 266, 675-681]. In the present study we show that this effect is already observed for most isoenzymes at only 2-3 weeks of age, as is the ability to induce or suppress expression with exogenous testosterone. The testosterone responsiveness did, however, exhibit age- as well as dose-dependency. Intriguingly, the effects of androgen took up to 8 days to become maximized, and the dose of testosterone needed to convert the female into the male phenotype was much higher than the circulating levels normally found in males. Studies using testicular feminized (Tfm) male mice, which carry an androgen receptor defect, showed them to have the female kidney cytochrome P450 phenotype, and these animals were not responsive to testosterone treatment. These data demonstrate the involvement of the androgen receptor in the regulation process. Taken together, our results indicate that the androgen receptor does not interact directly with the P450 genes, but initiates a cascade of events leading to the changes in cytochrome P450 gene expression. Significant differences were observed in the degree of sexual dimorphism in kidney P450 expression in other mammalian species. The significance of these findings in relation to the observed sexual dimorphism in other species is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T. Sex differences in cytochrome P-450-dependent xenobiotic and steroid metabolism in the mature rainbow trout kidney. J Endocrinol. 1990 Jul;126(1):9–16. doi: 10.1677/joe.0.1260009. [DOI] [PubMed] [Google Scholar]
- Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979 Aug;105(2):555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res. 1988 Jun 1;48(11):2946–2954. [PubMed] [Google Scholar]
- Henderson C. J., Scott A. R., Yang C. S., Wolf C. R. Testosterone-mediated regulation of mouse renal cytochrome P-450 isoenzymes. Biochem J. 1990 Mar 15;266(3):675–681. doi: 10.1042/bj2660675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. N., Clemens T. L., Liu D. K., Vesell E. S., Johnson W. D. Genetic control of chloroform toxicity in mice. Science. 1975 Oct 10;190(4210):159–161. doi: 10.1126/science.1166306. [DOI] [PubMed] [Google Scholar]
- Isomaa V., Pajunen A. E., Bardin C. W., Jänne O. A. Nuclear androgen receptors in the mouse kidney: validation of a new assay. Endocrinology. 1982 Sep;111(3):833–843. doi: 10.1210/endo-111-3-833. [DOI] [PubMed] [Google Scholar]
- Jansson J. O., Ekberg S., Isaksson O., Mode A., Gustafsson J. A. Imprinting of growth hormone secretion, body growth, and hepatic steroid metabolism by neonatal testosterone. Endocrinology. 1985 Nov;117(5):1881–1889. doi: 10.1210/endo-117-5-1881. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer A., Watson C. S., Catterall J. F. Nucleotide sequence of kidney androgen-regulated protein mRNA and its cell-specific expression in Tfm/Y mice. Mol Endocrinol. 1989 Jun;3(6):962–967. doi: 10.1210/mend-3-6-962. [DOI] [PubMed] [Google Scholar]
- Mode A., Wiersma-Larsson E., Gustafsson J. A. Transcriptional and posttranscriptional regulation of sexually differentiated rat liver cytochrome P-450 by growth hormone. Mol Endocrinol. 1989 Jul;3(7):1142–1147. doi: 10.1210/mend-3-7-1142. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Beyer R. Is "1-DNA" derived from nuclear DNA? Nature. 1970 Sep 19;227(5264):1211–1212. doi: 10.1038/2271211a0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Rundlett S. E., Wu X. P., Miesfeld R. L. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol. 1990 May;4(5):708–714. doi: 10.1210/mend-4-5-708. [DOI] [PubMed] [Google Scholar]
- Squires E. J., Negishi M. Reciprocal regulation of sex-dependent expression of testosterone 15 alpha-hydroxylase (P-450(15 alpha)) in liver and kidney of male mice by androgen. Evidence for a single gene. J Biol Chem. 1988 Mar 25;263(9):4166–4171. [PubMed] [Google Scholar]
- Watson G., Paigen K. Progressive induction of mRNA synthesis for androgen-responsive genes in mouse kidney. Mol Cell Endocrinol. 1990 Jan 2;68(1):67–74. doi: 10.1016/0303-7207(90)90171-4. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., LeBlanc G. A., Morrissey J. J., Staunton J., Lapenson D. P. Adult male-specific and neonatally programmed rat hepatic P-450 forms RLM2 and 2a are not dependent on pulsatile plasma growth hormone for expression. J Biol Chem. 1988 Aug 15;263(23):11396–11406. [PubMed] [Google Scholar]
- Yarbrough W. G., Quarmby V. E., Simental J. A., Joseph D. R., Sar M., Lubahn D. B., Olsen K. L., French F. S., Wilson E. M. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990 May 25;265(15):8893–8900. [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Mode A., Norstedt G., Gustafsson J. A. Regulation of sexual differentiation in drug and steroid metabolism. Trends Pharmacol Sci. 1989 Apr;10(4):149–153. doi: 10.1016/0165-6147(89)90167-3. [DOI] [PubMed] [Google Scholar]







