Abstract
Purpose
This study investigates the rare occurrence of tumor-to-tumor metastasis in Pituitary Neuroendocrine Tumors (PitNETs), also known as pituitary adenomas, aiming to enhance understanding of its diagnostic and therapeutic challenges. We report two cases from our institution of tumor-to-tumor metastasis involving PitNETs, followed by a systematic literature review.
Methods
We conducted a comprehensive literature review using PubMed and Google Scholar databases. This review provides insights into patient demographics, clinical presentations, primary tumor origin, management approaches and outcomes.
Results
We identified 38 documented cases of tumor-to-tumor metastasis involving the pituitary gland in the literature. This revealed a diverse range of primary tumor origins, with lung, breast, and renal carcinomas being the most prevalent. Clinical presentations varied, with visual disturbances emerging as the most frequently reported symptom. Surgical interventions predominantly resulted in subtotal resection. Kaplan–Meier survival analysis demonstrated that endoscopic endonasal approaches (EEA) are associated with longer median survival times compared to other surgical methods.
Conclusion
Tumor-to-tumor metastasis to PitNETs must be considered in differential diagnoses of sellar masses. Prompt and accurate diagnosis, coupled with a multidisciplinary treatment strategy, is essential. Our study contributes to the scarce literature on such metastases, providing a foundation for further understanding of this complex pathological entity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11102-024-01441-9.
Keywords: Tumor-to-tumor metastasis, Pituitary adenomas, Renal cell carcinoma, Prostate adenocarcinoma, PitNET, Collision tumors
Introduction
The occurrence of multiple, histologically distinct tumors at a single anatomical site is an exceptionally rare event in medical practice, presenting unique challenges in diagnosis, treatment, and prognosis. This is particularly uncommon within the sellar region.
Two mechanisms have been described through which distinct tumors might coexist within the same anatomical region. [1] The first, known as a collision tumor, occurs when two neighboring neoplasms grow towards one another until they eventually converge. The sellar region has seen instances of collision tumors, particularly involving Pituitary Neuroendocrine Tumors (PitNETs), also known as pituitary adenomas, coexisting with craniopharyngiomas [2–15]. Likewise, collision tumors linking PitNETs to meningiomas, gliomas, chondromas, and even inverted papillomas have been reported. [16–34]
The second mechanism is tumor-to-tumor metastasis. This process involves the hematogenous spread of malignant cells from its primary site to a distant distinct primary tumor, such as a PitNET. Throughout our review, we found reports on breast adenocarcinoma[35–41], lung carcinoma [42–52], renal cell carcinoma[53–56], colorectal adenocarcinoma[57–60], gastric carcinoma[49, 61], mediastinal cancer [62], melanoma[63, 64], esophageal cancer [65], pancreas and prostate cancer[66] metastasizing to PitNETs. Metastases from an unidentified primary site have also been reported [38, 51, 61, 67, 68].
This paper explores the phenomenon of tumor-to-tumor metastasis within the sellar region, presenting two cases involving PitNETs as the secondary site of metastasis. Additionally, we provide an extensive review of the existing literature on tumor-to-tumor metastasis to PitNETs, seeking to enhance our understanding of its clinical implications, diagnostic challenges, and treatment considerations.
Case reports
Case report #1
A 56 years-old male was referred to our neurosurgery department due to a progressive visual decline over the past two years, which had significantly deteriorated to bilateral blindness. His medical history was notable for renal cell carcinoma, diagnosed 2 years prior, for which he underwent laparoscopic nephrectomy to remove a large right renal mass. His examination revealed pale, dry skin, substantial recent weight loss, and a history of daily smoking.
The patient’s magnetic resonance imaging (MRI) revealed a large, heterogeneous sellar/suprasellar mass, showing diffuse contrast enhancement and signs of intralesional hemorrhages. The lesion was pressing the optic nerves/chiasm and invading the cavernous sinuses and clivus.
Endocrinological evaluation showed hypocortisolism (2.62 μg/dl, NR 5-25 μg/dl), hyponatremia (133 mEq/L, NR 135–145 mEq/L), hypothyroidism (T4F: 0.64 ng/dL, NR 0.9–2.3 ng/dL), hypogonadism (Test < 7 nmol/L, NR 10–35 nmol/L), hyperphosphatemia (4.7 mg/dl, NR 2.5–4.5 mg/dL) and hypomagnesemia (1.5 mg/dL, NR 1.7–2.2 mg/dL). Patient was promptly started on levothyroxine and hydrocortisone.
He subsequently underwent endoscopic endonasal transsphenoidal/transtuberculum approach for tumor resection (Video 1). Intraoperative MRI post-resection confirmed the absence of apoplexy and showed a subtotal resection with a small residual mass in the right cavernous sinus (Fig. 1).
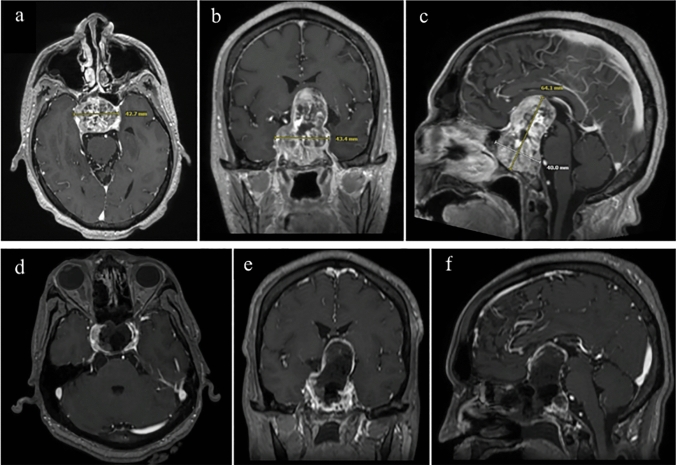
Fig. 1.
The images show radiological studies of the patient from the first case report. a, b & c Axial, Coronal, and Sagittal cuts from brain MRI (T1 C +) showing large sellar/suprasellar heterogenous mass, measuring 43.3 × 64.1×40mm, with diffuse contrast enhancement and signs of intralesional hemorrhages. It causes significant mass effects over the optic nerves/chiasm as well as invasion over the cavernous sinuses and clivus. d, e & f Axial, Coronal, and Sagittal cuts from intraoperative brain MRI (T1 C +) showing subtotal resection of large sellar/suprasellar mass, with little residual tumor on the right cavernous sinus
The postoperative period was uneventful, with no improvement of visual deficit, and the patient was discharged on the third postoperative day. The final histopathological examination confirmed the coexistence of renal cell carcinoma metastasis and gonadotroph PitNET (Fig. 2). Details on the pathology can be found in Supplementary Material 1. On the last follow-up, 6 weeks after surgery, the patient maintained visual deficits, with no additional complaints. He was receiving radiation for the residual tumor and keeping regular follow-ups with Oncology and Endocrinology.
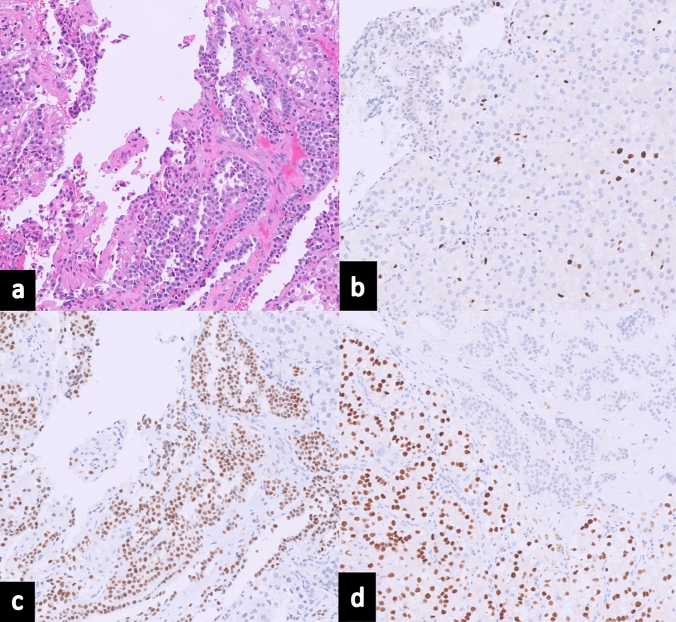
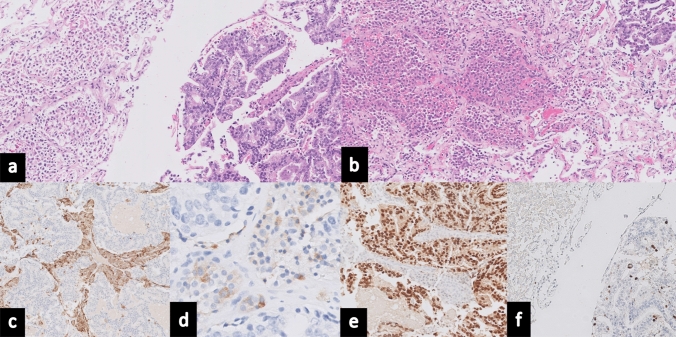
Fig. 2.
Histopathological Analysis from Surgical Resection in Case Report 1: a Hematoxylin and Eosin (H&E) staining demonstrates a dual histological presentation: clear cell renal cell carcinoma is seen in the lower left quadrant with characteristic features such as abundant clear cytoplasm, while the upper right quadrant exhibits a PitNET pattern with epithelioid cells forming expansive lobules. b Ki-67 immunostaining, a marker of proliferation, is shown with a low labelling index, indicating 1% of tumor cells. c Immunohistochemical staining for Steroidogenic Factor 1 (SF-1) reveals positive staining within the PitNET, confirming its nature. d PAX-8 immunostaining, specific for renal lineage, highlights positivity within the metastatic renal cell carcinoma cells
Subsequently, the patient received fractionated stereotactic radiation therapy to the post op bed as well as residual tumor. A dose of 2500 cGy in 5 fractions was prescribed to the planning target volume which consisted of surgical cavity and residual tumor as identified on Post op MRI with a 2 mm margin. Radiation treatment was delivered on a linear accelerator with 4-arc Volumetric Modulated Arc Therapy (VMAT) using 6MV Flatting Filter Free photon beams. Radiation plan is shown in Fig. 3.
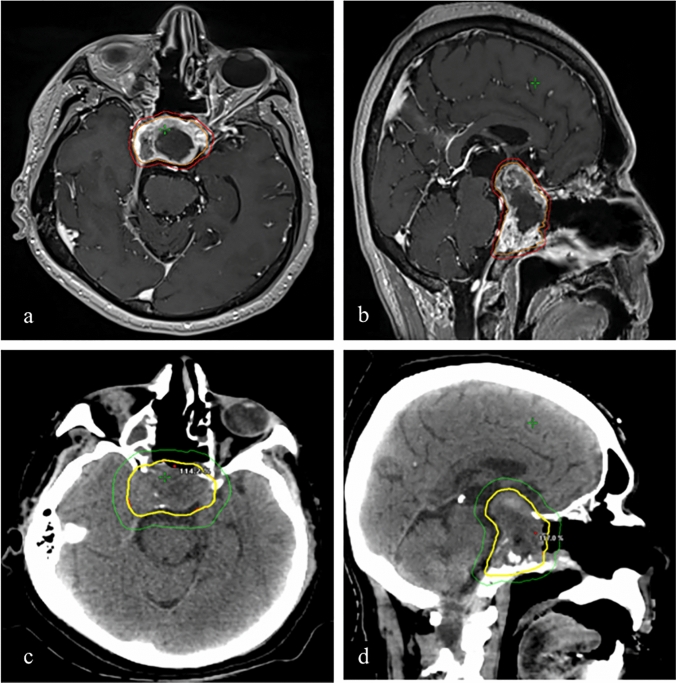
Fig. 3.
a & b Planning Target Volume (PTV) consisted of resection cavity and residual tumor identified on T1 post contrast sequence of post op MRI brain (outlined in orange) plus 2 mm margin (outlined in red). A dose of 2500 cGy in 5 fractions was prescribed to PTV by Stereotactic Radiation Therapy using 4-arc VMAT with 6MV Flatting Filter Free (FFF) photon beams to cover the PTV volume with 100% (2500 cGy) isodose line (in yellow) on planning CT head (c & d)
Case report #2
A 72 years-old male was referred to our clinic after an incidental sellar mass was discovered on imaging studies ordered for the evaluation of memory difficulties. Brain MRI revealed a heterogeneously enhancing sellar and suprasellar mass with asymmetric erosion of the sellar floor, abutting and elevating the optic apparatus. Interestingly though, the patient did not exhibit any cranial nerve deficits and pre-operative hormone levels were within normal range, indicating a non-functional PitNET. The patient underwent endoscopic endonasal transsphenoidal tumor resection without any intra or post-operative complications (Video 2) (Fig. 4).
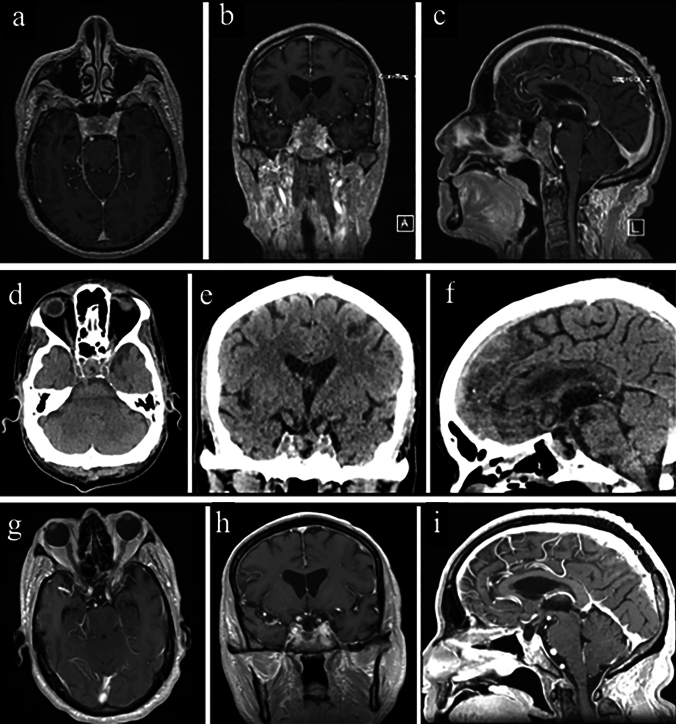
Fig. 4.
The images show radiological studies of the patient from the second case report. a, b & c Axial, Coronal, and Sagittal images from pre-operative brain MRI (T1-weighted post-contrast-enhanced images) revealed a heterogeneously enhancing sellar/suprasellar mass measuring approximately 26 × 16x19 mm. (d, e & f) Axial, Coronal, and Sagittal images from post-operative head CT show no signs of surgical site bleeding, pneumocephalus, or other complications. g, h & i Axial, Coronal, and Sagittal images from postoperative brain MRI (T1-weighted post-contrast-enhanced images) showing a gross total resection of the tumor
During the patient’s hospital course, he reported long-standing difficulty with urination. CT imaging showed a contrast-enhancing prostate mass, whole-body nuclear bone scan revealed diffuse osseous metastases. His prostate-specific antigen (PSA) level was markedly elevated (441 ng/mL, NR < 6.5 ng/mL), consistent with metastatic prostate cancer. The final pathology report on the surgical specimen confirmed concomitant Follicle stimulating hormone (FSH)-secreting PitNET and metastatic prostate adenocarcinoma (Fig. 5). Details on the pathology can be found in Supplementary Material 1. Three months post-surgical brain MRI revealed normal changes with no signs of residual lesions (Fig. 4).
Fig. 5.
Histopathological Analysis from Surgical Resection in Case Report 2: a H&E staining reveals contrasting histology: the left side of the image displays characteristics of prostatic acinar adenocarcinoma, while the right side shows features consistent with PitNET. b Additional H&E staining depicting an intermingled pattern of the two distinct neoplasms, highlighting the juxtaposition of the prostatic acinar adenocarcinoma and PitNET. c Immunohistochemistry demonstrates chromogranin positivity, commonly associated with neuroendocrine differentiation. d Immunohistochemical staining reveals positivity for Follicle-stimulating hormone (FSH), which corroborates the diagnosis of a gonadotroph subtype within the PitNET. e NKX3.1 immunostaining reveals positivity, a marker typically indicative of prostatic tissue. f Proliferation marker Ki-67 staining is shown with a low labelling index, highlighting a proliferation rate of 1% in tumor cells
Five weeks later, the patient underwent an oncological consultation, and was started on chemotherapy and radiotherapy for metastatic prostate carcinoma. However, his clinical course was later complicated by severe weight loss, dysphagia, anemia, acute kidney injury, metabolic acidosis, and liver failure, ultimately leading to his demise less than a year after the surgical intervention.
Methods
Systematic review
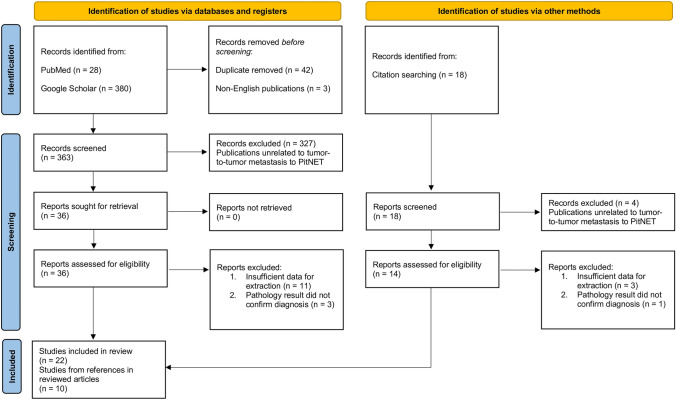
A systemic review was conducted on PubMed and Google Scholar databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [69] (Fig. 6). The search utilized a combination of keywords that included "pituitary adenoma," "metastasis," "tumor-to-tumor metastasis," and "collision tumors". Additionally, referenced papers within identified studies were thoroughly reviewed. The search was restricted to articles published in English, with no time frame limitation. Selected publications were screened by two independent reviewers and a third reviewer adjudicated unresolved discrepancies.
Fig. 6.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review
Inclusion and exclusion criteria
The inclusion criteria for this systematic review were as follows: (1) reported cases of tumor-to-tumor metastasis involving PitNETs, and (2) documentation of clinical, radiological, and histopathological findings. Studies were excluded if they did not meet these criteria or if they were review articles, case series without individual patient data, or non-English publications.
Data extraction
Data was collected and organized into a table to facilitate analysis. Variables encompassed the primary site of the metastasizing tumor, age and gender of the patients, the type of PitNET identified, tumor size measured on the longest axis, and a variety of clinical symptoms presented by the patients. Surgical outcomes and type of surgical approach taken were also documented. In instances where surgical procedures were described in the literature only as transsphenoidal surgery (TSS), they were as such, due to the indistinguishability of whether a microscopic or endoscopic method was employed. Furthermore, gonadotrophin-producing PitNETs have been classified as non-functioning for the purposes of streamlining the presentation.
Risk of bias assessment
We evaluated the risk of bias in the case reports included in this study using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports (https://www.researchgate.net/figure/The-Joanna-BriggsInstitute-JBI-critical-appraisal-checklist-for-studies-reporting_ Fig. 2_322317583). In addition, we assessed the risk of bias in this systematic review using the ROBIS tool (Risk of Bias in Systematic Reviews) (https://abstracts.cochrane.org/introduction-robis-tool-assess-risk-bias-systematicreview).
Statistical analysis
For variables with an approximately normal distribution, means and standard deviations are presented for the sample. Univariate associations between outcomes and the independent variables of interest were evaluated using Fisher's exact test for categorical variables. Non-parametric Kaplan–Meier estimators were calculated to estimate the survival functions for time-to-event data. Survival curves were plotted, and log-rank tests were conducted to assess the difference in survival curves between groups of interest based on the Kaplan–Meier estimates. A significance level of 5% was used to determine statistical significance for all tests. Statistical analyses were performed using JASP version 0.18.3.
Results
Study selection and risk of bias
The initial literature search yielded a total of 408 potentially relevant citations. After removing 42 duplicate records, 366 citations underwent title and abstract screening, resulting in the exclusion of 342 citations. The full texts of the remaining 22 citations were retrieved for further evaluation. Additionally, a manual search of the reference lists of these 22 citations identified 10 more potentially relevant studies. (Fig. 6) The characteristics of each case report, including the two cases presented on this study, are summarized in Supplementary Table 1.
The risk of bias assessment revealed a low risk of bias in 29 studies and a moderate risk in 3 studies (Supplementary Table 2). Given the inclusion of case reports and the small sample sizes, formal evaluations of publication bias and heterogeneity across studies were not conducted due to the inherent limitations of such analyses with this study design. However, the potential for bias within the synthesis and interpretation of findings cannot be entirely ruled out, as mentioned previously.
Patient population characteristics
This systematic review encompassed a total of 38 cases of PitNETs harboring metastasis from various anatomical sites, including the breast (n = 7, 18.4%), colon (n = 4, 10.5%), lung (n = 8, 21.1%), and other sites such as the esophagus, stomach, pancreas, prostate, renal, mediastinum, and melanoma. The mean age of the patients at the time of metastasis diagnosis was 65 ± 11 years, with an equal gender distribution (19 males, 50%). The most common clinical presentations were visual deficits (n = 28, 74%), followed by headache (n = 8, 21%), diplopia (n = 8, 21%), fever (n = 1, 2.6%), and hemiparesis (n = 1, 2.6%). The majority of the cases involved FSH/LH-secreting PitNETs (21%, n = 8), while null cell adenomas and prolactinomas each accounted for 18% (n = 7), growth hormone (GH)-secreting PitNETs for 11% (n = 4), adrenocorticotropic hormone (ACTH)-secreting PitNETs for 8% (n = 3), and FSH-secreting PitNETs for 5% (n = 2) of the cases. In all cases where histology was not provided (18%, n = 7), the tumors were clinically non-functional, as well as in the cases with gonadotroph-secreting PitNETs. The average size of the pituitary tumor was 34.1 ± 16.2 mm in the longest axis. Patients’ demographics and data distribution are summarized in Table 1.
Table 1.
Summary of data extracted from case reports involving malignant tumors metastasis within PitNETs
| Clinical summary | |
|---|---|
| # (%) | |
| Age (mean ± SD) | 65 ± 11 |
| Gender | |
| Male | 19 (50) |
| Female | 19 (50) |
| Types of PitNET | |
| Null cell | 7 (18) |
| Prolactinoma | 7 (18) |
| GH | 4 (11) |
| ACTH | 3 (8) |
| FSH/ LH | 8 (21) |
| FSH | 2 (5) |
| Histology not provided | 7 (18) |
| Clinical presentation | |
| Visual deficit | 28 (74) |
| Headache | 8 (21) |
| Diplopia | 8 (21) |
| Fever | 1 (2.6) |
| Hemiparesis | 1 (2.6) |
| Type of surgery | |
| EEA | 14 (37) |
| TSS | 13 (34) |
| TC | 6 (16) |
| NA | 5 (13) |
| Surgical resection outcomes | |
| Autopsy | 3 (8) |
| Subtotal | 3 (8) |
| GTR | 15 (39) |
| NA | 17 (45) |
| Alive | |
| Yes | 9 (24) |
| No | 24 (63) |
| NA | 5 (13) |
SD Standard Deviation, NA Not Available, GTR Gross Total Resection, EEA Endoscopic Endonasal Approach; TSS Transsphenoidal, TC Transcranial, GH Growth Hormone, ACTH Adrenocorticotrophic Hormone
Treatment and outcomes
Surgical resection was the primary treatment modality employed for the management of metastatic PitNETs. Endonasal endoscopic approaches (EEA) were utilized in 37% of cases (n = 14), transcranial surgeries (TC) in 16% (n = 6), and transsphenoidal surgeries (TSS) in 34% (n = 13). Subtotal resection was achieved in 39% of cases (n = 15), while gross total resection (GTR) was documented in 8% (n = 3). Notably, autopsies were performed in 8% of cases (n = 3), and surgical details were not available for 13% (n = 5) of the cases included in the analysis. Among those studies in which data was available, mean follow-up time was 10.
Overall survival analysis
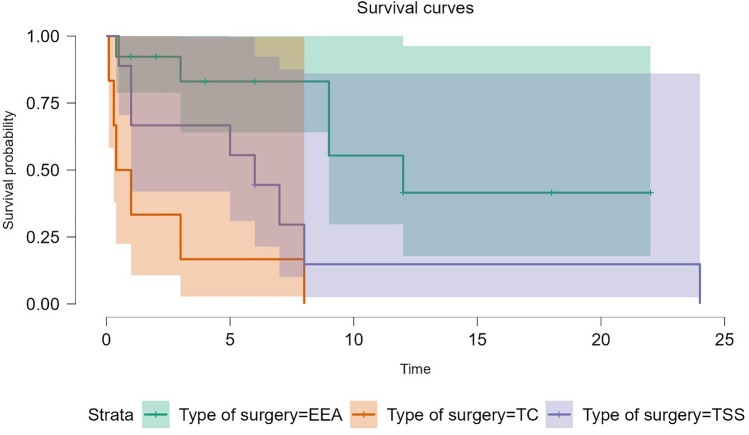
The Kaplan–Meier survival analysis, which included 31 cases with available survival data, revealed a median overall survival of 7 months (95% CI: 3–12 months) in the entire cohort of patients with metastatic PitNETs. Notably, the surgical approach emerged as a significant factor influencing survival outcomes, as demonstrated by the log-rank test (p = 0.009). The choice of EEA as the surgical technique was positively correlated with improved median overall survival. Specifically, patients who underwent EEA had a favorable median survival of 12 months, compared to median survivals of 6 months for those who underwent TSS and 0.7 months for those who underwent TC. (Fig. 7).
Fig. 7.
Kaplan–Meier Survival curves comparing median survival in EEA, TSS and TC approaches. EEA was found to be associated with longer median survival (p = 0.009)
In the overall cohort, the mean restricted survival time was 20.8 months (SE: 10.1 months). No other clinical factors, such as age, gender, tumor functional status, primary metastasis site, tumor size, or clinical presentation, were found to have a significant correlation with survival outcomes in this systematic review.
Discussion
General comments and comparison with related literature
The infrequency of concurrent tumors in the sellar region makes them overlooked in differential diagnoses. Clinically and radiographically, these cases usually do not present with clear signs that indicate the presence of coexisting neoplasms. However, certain clinical indicators such as rapid tumor growth and the swift symptoms progression should raise suspicions of multiple tumor pathology. As exemplified in our first case, diverse enhancement patterns on brain MRI scans may also serve as potential indicator, although such radiologic findings are not pathognomonic for tumor-to-tumor metastasis. This case also presented a substantial surgical challenge due to the lesion's high vascularity, which was likely associated with its malignant component, resulting in significant intraoperative bleeding.
Our systematic review found lung and breast cancer to be the most prevalent primary sources of metastasis to PitNETs, also known as pituitary adenomas, which is consistent with brain metastases overall [70]. The underlying mechanisms leading to the formation of metastases within PitNETs remain unclear. Some hypotheses, however, suggest that abnormalities in the pituitary gland’s vasculature, such as non-portal vessels or neovascularization in the surrounding tissues of the PitNET may provide a route for metastatic cells to invade it [64, 71].
Clinical presentation
Visual impairments were the most frequently encountered symptom among the cases reviewed, with the severity ranging from partial loss, such as typical bitemporal hemianopsia resulting from the pressure on the fibers from nasal retina in the anterior aspect of the optic chiasm, to complete blindness, as observed in the first case we reported. The high incidence of visual symptoms correlates with the predominance of non-functioning tumors, which tend to present clinically only when their growth results in a mass effect on adjacent structures, with the optic chiasm being the most affected. The presence of malignant cells may contribute to more rapid tumor growth, potentially explaining the observed frequency of visual deficits. Furthermore, apoplexy is often observed as a clinical presentation these cases, likely due to this rapid growth, which can overwhelm the pituitary gland's venous drainage capacity. The resultant hemorrhage or infarction within the tumor leads to the sudden onset of symptoms associated with pituitary apoplexy, including severe headache, visual disturbances, and hormonal deficiencies. Headaches are believed to stem from the stretching of the dura mater as the tumor enlarges, a condition that would be anticipated with substantial suprasellar masses that exert pressure on the optic apparatus. However, headaches' more generic nature as a symptom might lead to underreporting in relation to visual disturbances.
Surgical outcomes
The high prevalence of subtotal resection, observed in 39% of cases, reflects the aggressive nature and complexity of those cases. We did not find this high rate to be explained by the used surgical approach or technique. Beside the hypervascularity challenge that was overmentioned, the rapid growth of metastatic cancer disrupts normal anatomical planes. This increases adherence to neurovascular structures, making total resection extremely challenging. On top of that, only 24% of patients remained alive on the latest follow-up, which further emphasizes the severity of this condition.
Strengths and limitations
Our review highlights that the choice of endoscopic approaches over other modalities has been proven beneficial to patients with PitNETs, being associated with less postoperative endocrinological abnormalities, such as diabetes insipidus and hypothyroidism, when compared to the microscopic TSS approach [72]. Mortality rates have also been observed to be higher in cases where a transcranial approach was employed compared to both microscopic TSS and EEA [73]. The finding of a longer median survival in those patients who underwent EEA in our review helps to further solidify the endoscopic approach as the standard of care, although the small number of cases and specific patient subset limit the generalizability of this data for PitNET surgeries overall. Furthermore, as another limitation, the association between the transsphenoidal approach and better outcomes observed in our study may be confounded by a selection bias, where surgeons preferentially use the transsphenoidal approach for less complex cases and reserve the transcranial approach for more challenging cases, potentially skewing the effectiveness comparison.
Conclusion
Tumor-to-tumor metastasis to PitNETs, also known as pituitary adenomas, presents a unique intersection of oncological and endocrinological challenges, characterized by its rarity, diverse origins, and the complexity of surgical management. This study contributes to the growing body of evidence on the subject and the presented cases underscore the importance of multidisciplinary teamwork, including oncological, neurosurgical, and endocrinological, in devising the best plan to optimize provided management quality and outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Guilherme Mansur, Mohammad Bilal Alsavaf, Ludovica Pasquini. Guilherme Mansur, Moataz Abouammo and Chandrima Biswas edited the videos and prepared Figs. 1 and 4. Guilherme Mansur and Mohammad Bilal Alsavaf performed statistical analysis and prepared Figs. 6 and 7. Peter Kobalka participated on Pathological examination of the patients tumors on the case reports and prepared Figs. 2 and 5. Pavnesh Kumar and Raju R. Raval participated on the patient's management in Radiation Oncology (Case Report 1) and prepared Fig. 3. Ricardo Carrau and Daniel Prevedello were the patients' assisting physicians. They performed the surgeries, helped writing the manuscript and provided the final Review. All authors reviewed the manuscript.
Funding
There was no financial or funding support for the project
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
Dr. Daniel Prevedello is a consultant for Stryker Corp., Medtronic Corp., BK Medical and Integra LifeSciences; he has received royalties from Mizuho, KLS-Martin and ACE Medical. Ricardo L. Carrau receives royalties from KLS Corp. All other authors do not have conflict of interest to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Syed S, Karambizi DI, Baker A, Groh DM, Toms SA (2018) A comparative report on intracranial tumor-to-tumor metastasis and collision tumors. World Neurosurg 116:454-463.e2. 10.1016/j.wneu.2018.04.109 [DOI] [PubMed] [Google Scholar]
- 2.Bteich F, El Khoury L, Nohra G, Trak V, Yazbek S, Akiki M (2020) Pituitary adenoma and papillary craniopharyngioma: a rare case of collision tumor and review of the literature. World Neurosurg 139:63–69. 10.1016/j.wneu.2020.03.088 [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Kowari K, Eda H, Kambara M, Maruyama R, Akiyama Y (2019) Ten-year follow-up of collision tumors composed of craniopharyngioma and pituitary adenoma: a case report and literature review. Case Rep Med 2019:8080163. 10.1155/2019/8080163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shareef Z, Kerndt C, Nessel T, Mistry D, Figueroa B (2020) Collision tumor in the pituitary, concurrent pituitary adenoma, and craniopharyngioma. Case Rep Otolaryngol 2020:9584090. 10.1155/2020/9584090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshkin O, Scheithauer BW, Syro LV, Velasquez A, Horvath E, Kovacs K (2009) Collision tumors of the sella: craniopharyngioma and silent pituitary adenoma subtype 3: case report. Endocr Pathol 20(1):50–55. 10.1007/s12022-009-9065-3 [DOI] [PubMed] [Google Scholar]
- 6.Shi X, Jiaqi L, Manxin Z, Tao T, Ye F (2024) Rare collision tumor in the sellar region-pituitary adenoma combined with craniopharyngioma and case review. Case Rep. 10.21203/rs.3.rs-3967491/v1 [Google Scholar]
- 7.Meng X, Xue F, Wang Y, Huang X, Du J, Fu J (2023) Collision tumors of the sella: co-existence of pituitary adenoma with craniopharyngioma, and review of the literature. Res Sq. 10.21203/rs.3.rs-2439253/v138168284 [Google Scholar]
- 8.Jin G, Hao S, Xie J, Mi R, Liu F (2013) Collision tumors of the sella: coexistence of pituitary adenoma and craniopharyngioma in the sellar region. World J Surg Oncol 11:178. 10.1186/1477-7819-11-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gokden M, Mrak RE (2009) Pituitary adenoma with craniopharyngioma component. Hum Pathol 40(8):1189–1193. 10.1016/j.humpath.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Shakally A, Tahara N, Clark B et al (2020) A Rare case of recurrent pituitary collision tumors. J Endocr Soc. 10.1210/jendso/bvaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karavitaki N, Scheithauer BW, Watt J et al (2008) Collision lesions of the sella: co-existence of craniopharyngioma with gonadotroph adenoma and of Rathke’s cleft cyst with corticotroph adenoma. Pituitary 11(3):317–323. 10.1007/s11102-007-0070-6 [DOI] [PubMed] [Google Scholar]
- 12.Kikuta H, Jinguji S, Sato T et al (2023) A Collision tumor of Pit-1/SF-1-positive double pituitary adenoma and a craniopharyngioma coexisting with graves’ disease. NMC Case Rep J 10:169–175. 10.2176/jns-nmc.2022-0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finzi G, Cerati M, Marando A et al (2014) Mixed pituitary adenoma/craniopharyngioma: clinical, morphological, immunohistochemical and ultrastructural study of a case, review of the literature, and pathogenetic and nosological considerations. Pituitary 17(1):53–59. 10.1007/s11102-013-0465-5 [DOI] [PubMed] [Google Scholar]
- 14.Gong L, Chen H, Zhang W et al (2022) Primary collision tumors of the sellar region: Experience from a single center. J Clin Neurosci Off J Neurosurg Soc Australas 100:204–211. 10.1016/j.jocn.2022.04.024 [DOI] [PubMed] [Google Scholar]
- 15.Yoshida A, Sen C, Asa SL, Rosenblum MK (2008) Composite pituitary adenoma and craniopharyngioma?: an unusual sellar neoplasm with divergent differentiation. Am J Surg Pathol 32(11):1736–1741. 10.1097/PAS.0b013e3181753abd [DOI] [PubMed] [Google Scholar]
- 16.Koutourousiou M, Kontogeorgos G, Wesseling P, Grotenhuis AJ, Seretis A (2010) Collision sellar lesions: experience with eight cases and review of the literature. Pituitary 13(1):8–17. 10.1007/s11102-009-0190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aydin MV, Yangi K, Toptas E, Aydin S (2023) Skull base collision tumors: giant non-functioning pituitary adenoma and olfactory groove meningioma. Cureus 15(9):e44710. 10.7759/cureus.44710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhang H, Lian W et al (2017) Collision tumors composed of meningioma and growth hormone-secreting pituitary adenoma in the sellar region: case reports and a literature review. Medicine 96(50):e9139. 10.1097/MD.0000000000009139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tena Suck ML, Balcázar-Padrón JC, Navarro-Garcia Llano JP, Ortíz-Plata A, Gómez-Amador JL (2022) Macro pituitary adenoma and frontal calcified cavernous malformation: a coincidence or a true partnership? Cureus 14(1):e21152. 10.7759/cureus.21152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatain GP, Chee K, Driscoll M, Kleinschmidt-DeMasters BK, Lillehei KO (2024) Pituitary adenoma coexistent with sellar clear cell meningioma unattached to the dura: case report and treatment considerations. J Neurol Surg Rep 85(1):e1–e10. 10.1055/s-0043-1777792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Almeida VA, Lamback EB, Ventura N et al (2020) Collision sellar lesions: coexistence of pituitary adenoma and Rathke cleft cyst-a single-center experience. Endocrine 68(1):174–181. 10.1007/s12020-019-02149-8 [DOI] [PubMed] [Google Scholar]
- 22.Padilla Lichtenberger F, Glerean M, Paissan A, Ajler P (2021) Collision sellar lesions: pituitary adenoma and Rathke cleft cyst. Medicina 81(6):1069–1072 [PubMed] [Google Scholar]
- 23.Jagtap VS, Lila AR, Sarathi V, Bukan AP, Bandgar TR, Shah NS (2018) Coexistent pituitary adenoma with Rathke’s cleft cyst: a case series. J Assoc Physicians India 66(3):42–46 [PubMed] [Google Scholar]
- 24.Gao M, An Y, Huang Z et al (2016) The coexistence of Rathke cleft cyst and pituitary adenoma. J Craniofac Surg 27(2):e128-130. 10.1097/SCS.0000000000002371 [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Qiao L, Zhong C, Yang J, Zhu J, Ma C (2018) The coexistence of growth hormone-producing pituitary adenoma and Rathke cleft cyst: how can we diagnosis preoperation? J Craniofac Surg 29(7):1887–1889. 10.1097/SCS.0000000000004811 [DOI] [PubMed] [Google Scholar]
- 26.Tajika Y, Kubo O, Takeshita M, Tajika T, Shimizu T, Kitamura K (1989) An intracranial collision tumor composed of intrasellar gangliocytoma and pituitary adenoma. No Shinkei Geka 17(12):1181–1186 [PubMed] [Google Scholar]
- 27.Jukes A, Allan R, Rawson R, Buckland ME (2016) Growth hormone secreting pituitary adenoma with admixed gangliocytoma and ganglioglioma. J Clin Neurosci Off J Neurosurg Soc Australas 31:202–204. 10.1016/j.jocn.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 28.Tanriover N, Aydin O, Kucukyuruk B et al (2014) Endoscopic approach to a collision tumor of growth hormone-secreting adenoma and gangliocytoma in the pituitary gland. J Craniofac Surg 25(4):1277–1279. 10.1097/SCS.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 29.Heng LJ, Jia D, Gong L, Zhang W, Ma J, Qu Y (2017) Endoscopic endonasal resection of a mixed lesion of gangliocytoma and nonfunctioning pituitary adenoma. World Neurosurg 106:1050.e1-1050.e6. 10.1016/j.wneu.2017.05.064 [DOI] [PubMed] [Google Scholar]
- 30.Levitus CF, Charitou MM (2019) AN incidental collision tumor Of the sella turcica. AACE Clin Case Rep 5(4):e247–e249. 10.4158/ACCR-2019-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matyja E, Maksymowicz M, Grajkowska W et al (2015) Ganglion cell tumours in the sella turcica in close morphological connection with pituitary adenomas. Folia Neuropathol 53(3):203–218. 10.5114/fn.2015.54421 [DOI] [PubMed] [Google Scholar]
- 32.Naik H, Vernon V, Gade P, Bhople L, Guha A (2018) Anaplastic astrocytoma and pituitary macroadenoma within the same patient: a rare case of intracranial collision tumor. Neurol India 66(3):857–860. 10.4103/0028-3886.232341 [DOI] [PubMed] [Google Scholar]
- 33.Menéndez RH, Thompson P, Barea H et al (2020) Simultaneous resection of pituitary macroadenoma and sphenoid sinus inverted papilloma: the challenge of operating sinonasal and skull base pathologies through a single-stage endoscopic endonasal approach. World Neurosurg 133:260–265. 10.1016/j.wneu.2019.09.159 [DOI] [PubMed] [Google Scholar]
- 34.Rivera J, Alves S, Bianchi CC, Al-Mutawa N, Guiot MC, Zeitouni A (2010) An unusual collision tumor comprising a prolactinoma and a plasmocytoma originating from the sellar region. Pituitary 13(2):189–193. 10.1007/s11102-008-0145-z [DOI] [PubMed] [Google Scholar]
- 35.Andreev DN, Kim DS, Shishkina LV et al (2020) Breast cancer metastasis into a giant hormone-inactive pituitary adenoma adenoma. Zh Vopr Neirokhir Im N N Burdenko. 84(1):55–61. 10.17116/neiro20208401155 [DOI] [PubMed] [Google Scholar]
- 36.Castle-Kirszbaum M, Beng Phung T, Luen SJ, Rimmer J, Chandra RV, Goldschlager T (2020) A pituitary metastasis, an adenoma and potential hypophysitis: a case report of tumour to tumour metastasis in the pituitary. J Clin Neurosci Off J Neurosurg Soc Australas 81:161–166. 10.1016/j.jocn.2020.09.033 [DOI] [PubMed] [Google Scholar]
- 37.Bret P, Jouvet A, Madarassy G, Guyotat J, Trouillas J (2001) Visceral cancer metastasis to pituitary adenoma: report of two cases. Surg Neurol 55(5):284–290. 10.1016/s0090-3019(01)00447-5 [DOI] [PubMed] [Google Scholar]
- 38.Richardson JF, Katayama I (1971) Neoplasm to neoplasm metastasis An acidophil adenoma harbouring metastatic carcinoma. Arch Pathol 91(2):135–139 [PubMed] [Google Scholar]
- 39.van der Zwan A, Luyendijk W, Bots GT (1971) Metastasis of mammary carcinoma in a chromophobe adenoma of the hypophysis. Psychiatr Neurol Neurochir 74(5):369–377 [PubMed] [Google Scholar]
- 40.Mills MT, Wharton SB, Connolly DJ, Mirza S, Sinha S (2022) Pituitary apoplexy secondary to metastatic breast carcinoma into a gonadotroph cell adenoma of the pituitary. Br J Neurosurg 36(5):643–646. 10.1080/02688697.2018.1540766 [DOI] [PubMed] [Google Scholar]
- 41.Zager EL, Hedley-Whyte ET (1987) Metastasis within a pituitary adenoma presenting with bilateral abducens palsies: case report and review of the literature. Neurosurgery 21(3):383–386. 10.1227/00006123-198709000-00018 [DOI] [PubMed] [Google Scholar]
- 42.Sogani J, Yang W, Lavi E, Zimmerman RD, Gupta A (2014) Sellar collision tumor involving metastatic lung cancer and pituitary adenoma: radiologic-pathologic correlation and review of the literature. Clin Imaging 38(3):318–321. 10.1016/j.clinimag.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 43.Heritage S, O’Donovan D, Das T, Mannion R, Bulusu VR (2021) BRAF V600E mutant lung adenocarcinoma presenting with a skull base metastasis and pituitary adenoma collision tumour. Cureus 13(9):e18180. 10.7759/cureus.18180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki K, Tahara S, Hattori Y et al (2024) Lung adenocarcinoma metastasis within a pituitary neuroendocrine tumor: a case report with review of literature. Endocr J 71(3):295–303. 10.1507/endocrj.EJ23-0372 [DOI] [PubMed] [Google Scholar]
- 45.Borhan MK, Tan FHS, Basry NSA (2022) Collision of two tumors: a case report of a lung adenocarcinoma with metastasis to a pituitary adenoma. J ASEAN Fed Endocr Soc 37(2):89–94. 10.15605/jafes.037.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotondo F, Kovacs K, Macdonald RL, Prud’Homme GJ, Latta E, Munoz D (2013) Non-small cell bronchial carcinoma metastasizing into a prolactin-producing pituitary adenoma. Int J Surg Pathol 21(1):68–71. 10.1177/1066896912449478 [DOI] [PubMed] [Google Scholar]
- 47.Hoellig A, Niehusmann P, Flacke S, Kristof RA (2009) Metastasis to pituitary adenoma: case report and review of the literature. Cent Eur Neurosurg 70(3):149–153. 10.1055/s-0028-1082063 [DOI] [PubMed] [Google Scholar]
- 48.Hanna FW, Williams OM, Davies JS, Dawson T, Neal J, Scanlon MF (1999) Pituitary apoplexy following metastasis of bronchogenic adenocarcinoma to a prolactinoma. Clin Endocrinol (Oxf) 51(3):377–381. 10.1046/j.1365-2265.1999.00717.x [DOI] [PubMed] [Google Scholar]
- 49.Molinatti PA, Scheithauer BW, Randall RV, Laws ER (1985) Metastasis to pituitary adenoma. Arch Pathol Lab Med 109(3):287–289 [PubMed] [Google Scholar]
- 50.Post KD, McCormick PC, Hays AP, Kandji AG (1988) Metastatic carcinoma to pituitary adenoma Report of two cases. Surg Neurol 30(4):286–292. 10.1016/0090-3019(88)90301-1 [DOI] [PubMed] [Google Scholar]
- 51.Fujimori T, Okauchi M, Shindo A et al (2014) Intrapituitary adenoma metastasis from lung cancer with progressive cranial nerve palsies: a case report and literature review. No Shinkei Geka 42(10):943–949. 10.11477/mf.1436200010 [DOI] [PubMed] [Google Scholar]
- 52.Nasr C, Mason A, Mayberg M, Staugaitis SM, Asa SL (2006) Acromegaly and somatotroph hyperplasia with adenomatous transformation due to pituitary metastasis of a growth hormone-releasing hormone-secreting pulmonary endocrine carcinoma. J Clin Endocrinol Metab 91(12):4776–4780. 10.1210/jc.2006-0610 [DOI] [PubMed] [Google Scholar]
- 53.James RL, Arsenis G, Stoler M, Nelson C, Baran D (1984) Hypophyseal metastatic renal cell carcinoma and pituitary adenoma case report and review of the literature. Am J Med 76(2):337–340. 10.1016/0002-9343(84)90798-8 [DOI] [PubMed] [Google Scholar]
- 54.Weber J, Gassel AM, Hoch A, Spring A (2003) Concomitant renal cell carcinoma with pituitary adenoma. Acta Neurochir (Wien) 145(3):227–231. 10.1007/s00701-002-1060-0 [DOI] [PubMed] [Google Scholar]
- 55.Burns WA, Kadar AT (1973) Unusual metastases from a transitional-cell carcinoma of the renal pelvis and ureter. Med Ann Dist Columbia 42(2):65–66 [PubMed] [Google Scholar]
- 56.Magnoli F, Finzi G, Riva C, Capella C (2014) Renal cell carcinoma metastatic to a pituitary FSH/LH adenoma: case report and review of the literature. Ultrastruct Pathol 38(6):430–437. 10.3109/01913123.2014.937843 [DOI] [PubMed] [Google Scholar]
- 57.Thewjitcharoen Y, Shuangshoti S, Lerdlum S, Siwanuwatn R, Sunthornyothin S (2014) Colorectal cancer manifesting with metastasis to prolactinoma: report of a case involving symptoms mimicking pituitary apoplexy. Intern Med Tokyo Jpn 53(17):1965–1969. 10.2169/internalmedicine.53.2353 [DOI] [PubMed] [Google Scholar]
- 58.Donofrio CA, Pizzimenti C, Djoukhadar I, Kearney T, Gnanalingham K, Roncaroli F (2023) Colorectal carcinoma to pituitary tumour: tumour to tumour metastasis. Br J Neurosurg 37(5):1367–1370. 10.1080/02688697.2020.1823937 [DOI] [PubMed] [Google Scholar]
- 59.Noga C, Prayson RA, Kowalski R, Sweeney PJ, Mayberg M (2001) Metastatic adenocarcinoma to a pituitary adenoma. Ann Diagn Pathol 5(6):354–360. 10.1053/adpa.2001.29344 [DOI] [PubMed] [Google Scholar]
- 60.Skulsampaopol J, Klaisuban W, Hansasuta A (2017) Colon metastasis to residual pituitary macroadenoma causing accelerated growth: Case report and review of the literature. Interdiscip Neurosurg 8:26–32. 10.1016/j.inat.2017.01.004 [Google Scholar]
- 61.van Seters AP, Bots GT, van Dulken H, Luyendijk W, Vielvoye GJ (1985) Metastasis of an occult gastric carcinoma suggesting growth of a prolactinoma during bromocriptine therapy: a case report with a review of the literature. Neurosurgery 16(6):813–817. 10.1227/00006123-198506000-00014 [DOI] [PubMed] [Google Scholar]
- 62.Abe T, Matsumoto K, Iida M, Hayashi M, Sanno N, Osamura RY (1997) Malignant carcinoid tumor of the anterior mediastinum metastasis to a prolactin-secreting pituitary adenoma: a case report. Surg Neurol 48(4):389–394. 10.1016/s0090-3019(97)00002-5 [DOI] [PubMed] [Google Scholar]
- 63.Yang C, Liu L, Lan X, Zhang S, Li X, Zhang B (2017) Progressive visual disturbance and enlarging prolactinoma caused by melanoma metastasis: A case report and literature review. Medicine 96(14):e6483. 10.1097/MD.0000000000006483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung SM, Hsu YY, Chuang CC, Chang CN, Hsueh C, Kuo T, tong. (2007) A man in his mid-70s with a sellar mass. Brain Pathol Zurich Switz. 10.1111/j.1750-3639.2007.00044_1.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gariépy C, Champagne PO (2023) Metastatic seeding from a gastrointestinal neoplasia in a pituitary adenoma: a case report and literature review. Cureus 15(2):e34676. 10.7759/cureus.34676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsay J, Kovacs K, Scheithauer B, Ezrin C, Weiss M (1988) Metastatic carcinoma to pituitary adenomas: a report of two cases. Exp Clin Endocrinol Diabetes 92(04):69–76 [DOI] [PubMed] [Google Scholar]
- 67.Hurley TR, D’Angelo CM, Clasen RA, DiGianfilippo A, Ryan WG (1992) Adenocarcinoma metastatic to a growth-hormone-secreting pituitary adenoma: case report. Surg Neurol 37(5):361–365. 10.1016/0090-3019(92)90004-7 [DOI] [PubMed] [Google Scholar]
- 68.Nassiri F, Cusimano M, Rotondo F, Horvath E, Kovacs K (2012) Neuroendocrine tumor of unknown origin metastasizing to a growth hormone-secreting pituitary adenoma. World Neurosurg 77(1):201.e9-201.e12. 10.1016/j.wneu.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 69.Page MJ, McKenzie JE, Bossuyt PM et al (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112. 10.1016/j.jclinepi.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 70.Sacks P, Rahman M (2020) Epidemiology of Brain Metastases. Neurosurg Clin N Am 31(4):481–488. 10.1016/j.nec.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 71.Jin LONG, & Lloyd RV (1993) Metastatic neoplasms to the pituitary gland. Surgical Pathology of the Pituitary Gland. Philadelphia: WB Saunders, p 137–140. [Google Scholar]
- 72.Chen J, Liu H, Man S et al (2021) Endoscopic vs microscopic transsphenoidal surgery for the treatment of pituitary adenoma a meta-analysis. Front Surg. 10.3389/fsurg.2021.806855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komotar RJ, Starke RM, Raper DMS, Anand VK, Schwartz TH (2012) Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary 15(2):150–159. 10.1007/s11102-011-0359-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.