Summary
Background
Mpox has spread to many countries around the world. While the existing live attenuated mpox vaccines are effective, advances in 21st century technologies now enable the development of vaccines with more specific antigens, clearer mechanisms, and more controllable side effects.
Methods
We systematically evaluated the immunogenicity and protective efficacy of the A35R, M1R and B6R antigens of the mpox virus (MPXV). With these findings, we designed three single-chain trivalent mRNA vaccines (AMAB-wt, AMAB-C140S and AMB-C140S) by integrating the soluble regions of these antigens into single mRNA-encoded polypeptides based on their protein structures. Then, the immunogenicity and protective efficacy of these single-chain mRNA vaccines were evaluated in mice models against both VACV and MPXV.
Findings
The three single-chain vaccines elicited neutralising antibodies that effectively neutralised both VACV and MPXV. The single-chain vaccines or cocktail vaccine containing mRNAs encoding soluble antigen (sA35R + sM1R + sB6R) exhibited 100% or 80% protection against a lethal dose of VACV challenge, while the cocktail of full-length antigens (A35 + M1 + B6) and the live attenuated vaccine, VACV Tian Tan (VACV-VTT), completely failed to protect mice. Moreover, the single-chain vaccines significantly reduced viral load in the lungs and ovaries of MPXV-challenged mice.
Interpretation
Compared with the cocktail vaccines, our single-chain designs demonstrated similar or superior immunogenicity and protective efficacy. Importantly, the simplicity of the single-chain vaccines enhances both the controllability and accessibility of mpox vaccines. We believe these single-chain vaccines qualify as the next-generation mpox vaccines.
Funding
National Natural Science Foundation of China and Youth Innovation Promotion Association of the CAS.
Keywords: mpox, Trivalent, mRNA vaccine, Single-chain, Chimeric
Research in context.
Evidence before this study
The three currently available mpox vaccines are live attenuated vaccines based on the Vaccinia virus (VACV). Due to the complexity of poxviruses, these vaccines pose potential risks associated with the large number of VACV-encoded immunosuppressive proteins. Among these, the modified vaccinia Ankara (MVA) vaccine, known as JYNNEOS, is considered the safer option because it is replication-deficient. With 21st century technologies, mpox vaccines with more specific antigens and clearer mechanisms can be developed, offering higher effectiveness and more controllable side effects. Since the global mpox outbreak in 2022, a new generation of mpox vaccines has primarily focused on mRNA vaccines which are typically composed of a cocktail of multiple mRNAs that each encodes a single antigen. However, such a cocktail approach is expected to increase the complexity and cost of manufacturing vaccines.
Added value of this study
This study developed single-chain vaccine candidates by integrating the soluble regions of the A35R, M1R and B6R proteins of MPXV into a single chimeric peptide chain based on their protein structures. In mouse models, these single-chain vaccines elicited more balanced immune responses and provided stronger protection against lethal doses of VACV challenge compared to a cocktail of mRNAs encoding full-length single antigens or the live attenuated vaccine, VACV Tian Tan (VACV-VTT). Moreover, these single-chain vaccines significantly reduced viral loads in multiple organs in mice after MPXV challenge.
Implications of all the available evidence
The key features of an ideal vaccine include safety, effectiveness, controllability, and accessibility. Our single-chain vaccine design excels in controllability and accessibility compared to cocktail vaccines. By integrating soluble regions of antigens based on their structures, our vaccines demonstrated similar or superior immunogenicity when compared to cocktail vaccines or the live attenuated VACV-VTT vaccine. Additionally, with only three antigens, our vaccines offer a safety advantage over the last-century attenuated vaccines expressing numerous immune-inhibiting molecules. We believe these single-chain vaccines represent a promising direction for the next-generation mpox vaccines.
Introduction
The causative agent for mpox, mpox virus (MPXV), is a double-stranded DNA virus that belongs to the Poxviridae family of the Orthopoxvirus genus. Since its initial discovery in 1958,1 MPXV has generally spread in West and Central Africa.2, 3, 4 However, a new global mpox outbreak started in the United Kingdom in May 2022, and quickly spread to many countries worldwide.4,5 Mpox was first declared a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO) in July 2022, and this status was lifted in May 2023. However, following a surge in cases in Africa in 2024 and the emergence of a new subtype, Clade Ib, the WHO declared mpox a PHEIC for the second time on August 14, 2024. By August 2024, over 102,000 cases of mpox and 223 deaths had been reported across 121 countries.
Vaccination is the most effective way of controlling transmissible diseases. Currently, only three live attenuated vaccines, JYNNEOS, LC16, and ACAM2000, are available for vaccination against MPXV infection. These vaccines were developed based on live attenuated Vaccinia virus (VACV). All three vaccines were initially smallpox vaccines and were later approved as mpox vaccines in the United States, the European Union, Canada, and Japan. Like their parental pathogens, these attenuated vaccines have genomes as long as 200 kbp that encode up to 190 viral proteins,6 with the biological functions of most viral proteins yet to be elucidated. As a result, these whole-virus vaccines are accompanied by the risk from a large number of VACV-encoded proteins with immunosuppressive functions,7 which is particularly dangerous to certain groups of vaccinees, such as people with immuno-deficiency or other skin diseases.8,9 As a replication-deficient modified vaccinia Ankara (MVA), JYNNEOS is safer than the replication-competent VACV-based vaccines such as ACAM2000 and it has been shown to be safe for immunocompromised patients.10 However, one study found that only 63% of recipients developed MPXV-neutralising antibodies after receiving two doses of JYNNEOS,11 another study showed only 52% of recipients born after 1980 had detectable neutralising antibodies against MPXV.12 The estimated vaccine effectiveness of JYNNEOS was reported to be 35.8% after one dose and 66% after two doses.13 With 21st century technologies such as mRNA-LNP, the safety and effectiveness of mpox vaccines can be substantially enhanced through the development of a new generation of vaccines featuring specific antigens. Given the spread of MPXV, it is prominent to rapidly develop these targeted mpox vaccines.
To design such a vaccine, it is crucial to first pinpoint the proper antigens that elicit potent humoral and cellular immunogenicity. Notably, MPXV has two forms of infectious virus particles, intracellular mature virus (IMV) and extracellular enveloped virus (EEV).14 Previous studies have provided candidates for selecting MPXV antigens, including MPXV M1R (corresponding to VACV L1R, M1R/L1R) and A29L (corresponding to VACV A27L, A29L/A27L) in IMV and A35R (corresponding to VACV A33R, A35R/A33R) and B6R (corresponding to VACV B5R, B6R/B5R) in EEV. These antigens can stimulate humoral immunity and have demonstrated various degrees of protection against poxvirus in mice.15,16 A study of human antibodies cross-neutralising multiple poxviruses also demonstrates that anti-A35R/A33R, anti-M1R/L1R, and anti-B6R/B5R monoclonal antibodies (mAbs) are essential for protecting mice from poxvirus challenge.17 However, the protective efficacy of vaccines composed of a single antigen is often insufficient. Exploration for the optimal combination of poxvirus antigens has been conducted in the past two decades. Compared with single antigens, DNA or protein subunit vaccines composed of multiple antigen mixtures did demonstrate improved protection against poxviruses in mice or monkeys.18, 19, 20, 21 In particular, a cocktail of A35R/A33R, M1R/L1R and B6R/B5R proteins provided complete protection to mice against VACV challenge.20 Recently, several mpox mRNA vaccines have also adopted the multi-antigen strategy by mixing multiple single-gene mRNAs,22, 23, 24, 25, 26, 27 or by connecting multiple antigens in a single mRNA with linkers.28,29 Nevertheless, mRNA cocktails would significantly increase the complexity and cost of manufacturing mRNA vaccines.
In this work, we aimed to develop single-chain vaccine candidates that each encode all antigens in a single polypeptide chain to maintain the simplicity of mRNA vaccines to be more friendly for vaccine quality control during manufacturing and significantly reduce the cost of vaccines. After systematically evaluating the immunogenicity and protective efficacy of MPXV A35R, M1R, and B6R, we designed trivalent mRNA vaccines encoding single-chain chimeric immunogens that integrate these three antigens based on their structures. These vaccines elicited potent neutralising antibodies against VACV and MPXV, achieved complete protection of mice challenged with lethal doses of the VACV Western Reserve strain (VACV-WR), and significantly reduced viral load in mice after MPXV challenge, which exhibited substantial advantage to the cocktail of three full-length (wildtype) antigens or VACV Tian Tan strain (VACV-VTT), a VACV-based live attenuated vaccine. We believe these vaccines represent a new generation of mpox mRNA vaccines and can contribute to the prevention and control of MPXV spread worldwide.
Methods
Ethics
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the Institute of Microbiology, Chinese Academy of Sciences (IMCAS) Ethics Committee. All of the animal experiments were reviewed and approved by the Committee on the Ethics of Animal Experiments of IMCAS (approval number: APIMCAS2022124). Animals at endpoints or lost over 20% weight were euthanised. No data points were excluded because of animals being euthanised.
Plasmids, cells, and viruses
The sequence of mpox A35R, M1R, and B6R genes were optimised, synthesized by GenScript, and inserted into pUC57 plasmids with T7 promoter. The AMAB-wt, AMAB-C140S, and AMB-C140S plasmids encoding single-chain chimeric immunogens were constructed by Golden Gate Assembly. Mouse Igκ signal peptide sequence was used for all soluble antigens and single-chain chimeric immunogens. HEK293T cells (ATCC Cat# CRL-3216, RRID:CVCL_0063) and Vero cells (ATCC Cat# CCL-81, RRID:CVCL_0059) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C under general conditions. Regular tests were conducted on these cells for mycoplasma contamination using the MycoAlert Detection Kit (Lonza Bioscience). VACV Western Reserve strain (VACV-WR) was a kind gift from Prof. Min Fang from the Institute of Microbiology, Chinese Academy of Sciences. VACV Tian Tan strain (VACV-VTT) was kindly provided by Prof. Wenjie Tan (National Institute for Viral Disease Control and Prevention, China CDC). MPXV clade IIb strain (WIBP-MPXV-001) was previously isolated from an mpox patient by Wuhan Institute of Biological Products Co., Ltd.30 Viruses were propagated in Vero cells in DMEM with 2% heat-inactivated fetal bovine serum (FBS), and 100 units/ml penicillin and streptomycin. After 48 h, cells were harvested by centrifugation at 1000 × rpm for 5 min and resuspended in PBS. The cell suspension was frozen and thawed completely three times to release IMV particles.
mRNA in vitro transcription and 5′-capping
Plasmids were linearized by BamHI (NEB) and served as templates for mRNA transcription using T7 High yield RNA transcription Kit (Novoprotein). UTP was replaced by 1-methylpseudourine-5′-triphosphate. 5′-Capping was conducted using a Cap 1 capping system (Novoprotein). mRNA was purified by precipitation with LiCl at −20 °C overnight, centrifugation at 18,800 × g for 20 min at 4 °C, and resuspension with RNase-free water. Purified mRNA was verified by agarose gel and stored at −80 °C until use.
Lipid-nanoparticle encapsulation of mRNA
LNP encapsulation was conducted using the Nanoassemblr Benchtop platform (Precision Nanosystems). Ionizable cationic lipid, phosphatidylcholine, cholesterol, and PEG-lipid were mixed at a ratio of 50:10:38.5:1.5 (mol/mol). LNP encapsulation was conducted by mixing mRNA diluted in 50 mM Sodium acetate (pH = 4.0) with lipids in ethanol in a microfluidic system. The encapsulated mRNA was washed by centrifugation to remove ethanol and the concentration of encapsulated mRNA was measured by Quan-iT Ribogreen RNA reagent (Thermo Fisher).
mRNA transfection and western blot
mRNA was transfected into HEK293T cells with TransIT-mRNA (Mirus Bio). Basically, mRNA (3 μg) was mixed with TransIT-mRNA reagent (6 μl) and booster reagent (6 μl) and then incubated in 100 μl Opti-MEM at room temperature. After 2 min, the mixture was transferred to HEK293T cells cultured in 1% FBS DMEM in 6 well plates. Supernatant and cells were harvested at 48 h post-transfection. The cell pellet was resuspended in cell lysis buffer and stored at −20 °C until use. For Western Blot, the supernatant was added with 5 × SDS loading buffer with dithiothreitol, separated by 10% SDS-phage and transferred to PVDF membrane using a semi-dry apparatus (WIX Technology). The membrane was blocked with 5% non-fat milk diluted in PBS-T buffer, blotted with the serum of AMAB-C140S vaccinated mouse (1:1000) for 1 h and goat anti-mouse IgG-HRP secondary antibody (Easybio) for 1 h. Finally, the membrane was developed using Beyotime BeyoECL Plus (Beyotime Biotech). Western Blot has validated the functionality of these antibodies.
In silico prediction of immunogen structure
The translated structures of AMAB-wt, AMAB-C140S, and AMB-C140S were predicted by AlphaFold Colab (V2.3.2, https://colab.research.google.com/github/deepmind/alphafold/blob/main/notebooks/AlphaFold.ipynb) under default parameters with num_recycles set to 3. The resulting structures were visualised, aligned and recoloured using PyMOL (2.5.4).
Immunisation, poxvirus challenge, and sample collection protocols
All animal experiments were conducted using BALB/c mice (female, 6–8 weeks) purchased from Beijing Vital River Animal Technology Co., Ltd (licensed by Charles River) and housed in an Animal Biosafety Level 2 facility in IMCAS (20 ± 2 °C; 50 ± 10%; light, 7:00–19:00; dark, 19:00–7:00). All vaccines were immunised via i.m. injection in a total volume of 100 μl. For single-gene mRNA vaccines, 2.5 μg was immunised individually or in cocktails (5 μg total for two-antigen and 7.5 μg total for three-antigen cocktails). For single-chain mRNA vaccines, 7.5 μg were immunised. VACV-VTT was immunised once (1 × 107 pfu) through tail scarification on day 0. Mice of all groups were injected with two doses of mRNA vaccine or empty LNP on day 0 and day 14. The VACV-WR challenge was conducted on day 29 (7 LD50, 1.89 × 105 pfu) or day 39 (30 LD50, 8.1 × 105 pfu) via the intranasal route. MPXV challenge was conducted on day 190 (2 × 107 pfu) via both intranasal (20 μl) and intraperitoneal (480 μl) routes. Mice were weighed daily for at least 12 days post–challenge and euthanised after losing over 20% of initial weight. The probability of survival was documented 12 days post–challenge.
For evaluating humoral immunogenicity, blood samples (n = 5) were collected at the indicated time points. Then, sera were separated from blood samples by centrifugation and stored at −80 °C until use. For evaluating cellular immunogenicity, spleens (n = 5) were collected after sacrificing mice on the indicated time points. Then, spleens were homogenised with a tissue grinder and filtered with a 40 μm cell strainer (Corning). Red blood cells were lysed with red blood cell lysis buffer (Solarbio Life Science). Splenocytes were stained with AO/PI and counted using a cell counter (Count star). Live splenocytes were then immediately used for ELISpot assay.
For evaluating viral loads, mice lungs, spleens, or ovaries were collected, weighed, added into 500 μl serum-free RPMI 1640, and ground with tissue homogeniser (NZK) at 4000 rpm for 60s (grind for 10s, pause for 10s, repeat 6 times). Supernatants were isolated by centrifugation and stored at −80 °C until use.
Antibody enzyme-linked immunosorbent assay (ELISA)
Polystyrene 96-well flat bottom plates (Corning) were coated with recombinant antigen proteins (0.2 μg/ml) in 100 μl 0.05 M Sodium Carbonate-Bicarbonate Buffer and incubated at 4 °C overnight. The plates were further incubated at 37 °C for 1 h with 5% non-fat milk. Serum was subjected to a threefold serial dilution starting at 1:200 or 1:1000. Each well was added 100 μl diluted serum and incubated at 37 °C for 1 h. Then, 100 μl of goat Anti-mouse HRP IgG was added as the secondary antibody (1:5000). The plates were developed by 100 μl TMB substrate and incubated for 10 min, and then the reaction was terminated by 100 μl 2 M H2S04. The absorbances at 450 nm and 630 nm were measured using a microplate reader (PerkinElmer, USA). Absorbance values were calculated by subtracting the absorbance at 630 nm from that at 450 nm of the same well. Endpoint titers were defined as the highest reciprocal dilution of serum to yield an absorbance greater than 2.1-fold of the background values. Antibody titer below the limit of detection was determined as one-third of the detection limit.
Plaque assays
(1) For plaque reduction neutralisation test (PRNT) evaluating serum neutralisation, a serum sample from each mouse was subjected to a twofold serial dilution started at 1:20 using DMEM and mixed with an equal volume of DMEM (4% FBS) containing VACV-WR or MPXV. The mixture was transferred into 12-well plates and incubated for 1 h at 37 °C. (2) For viral load titration with plaque assay, supernatants of ground organs (lungs, ovaries, or spleens) were subjected to a tenfold serial dilution starting at 1:10. Then, the serum-VACV mixture or diluted supernatants of ground organs were added on plated Vero cells and incubated for 1 h at 37 °C. After incubation, the inoculum was then removed. The cells were washed once with PBS, added 1 ml DMEM mixed with 1% Carboxymethylcellulose, cultured for 48 h, fixed with 4% Paraformaldehyde (Solarbio Life science) for 2 h, and stained with 0.5% crystal violet overnight. Plaques were captured and calculated by ELISpot reader and BioSpot image analysis software. Viral load (pfu/g) was calculated based on plaque numbers and normalised with organ weight.
Real-time quantitative PCR (qPCR) assay
Viral DNA in the supernatants of ground organs (lungs, ovaries, or spleens) was extracted with QIAamp MinElute Virus Spin Kit (Qiagen) as the template of qPCR. Real-time quantitative PCR assays of VACV-WR were conducted using primers and a probe targeting the E9L gene: forward 5′-CGGCTAAGAGTTGCACATCCA-3’; reverse 5′- CTCTGCTCCATTTAGTACCGATTCT-3’; TaqMan® Minor Groove Binding (MGB) probe: AGGACGTAGAATGATCTTGTA. The qPCR assays of MPXV were conducted using previously established methods targeting the F3L fragment of MPXV.30
Enzyme-linked immunospot (ELISpot) assay
Pre-coated mouse IFN-γ flat-bottom 96 well plates (Dakewe Biotech) were activated by RPMI 1640 for 10 min. Fresh mouse splenocytes (3 × 105) were added to the plates and re-stimulated with each peptide pool (2 μg/ml for each peptide). Phytohemagglutinin (PMA) was added to positive control wells. After 18 h, cells were removed and plates were incubated by biotinylated IFN-γ antibody, streptavidin-HRP conjugate antibody, and substrate. The number of spots was measured by ELISpot reader and ImmunoSpot image analysis software (Immuno Capture 6.5.0).
Statistics
Five mice were included in each group for statistical analysis, as previously established.31 For ELISA and PRNT assays, data were presented as geometric mean ± 95% confidence interval (CI). For ELISpot assay, viral load titration, qPCR, and weight change, data were presented as mean ± standard error of the mean (SEM). Statistical analyses were conducted using the two-way ANOVA with Tukey's multiple comparisons test (ELISA and ELISpot), two-way ANOVA with Dunnett's multiple comparisons test (Weight change), ordinary one-way ANOVA with Dunnett's multiple comparisons test (Viral load titration and qPCR), Kruskal–Wallis test with Dunnett's multiple comparisons test (PRNT). All graphs and statistical analyses were generated with GraphPad Prism version 9.0 software.
Role of funders
The funders had no role in the experiment design, data collection, data analysis, interpretation, manuscript writing or any other aspect of this study.
Results
Systematic evaluation of MPXV antigens with single-gene mRNA vaccines
First, we aimed to determine the essential antigens to be included in an mpox vaccine to ensure optimal immunogenicity and protective efficacy. Thus, single-gene mRNA vaccines were constructed with the A35R, M1R, or B6R gene of the MPXV_USA_2022_MA001 strain (GenBank: ON563414.3) and named A35R, M1R, or B6R. The soluble region of each gene was also constructed and named sA35R, sM1R, and sB6R (Fig. S1). These mRNA vaccines were prepared via a pipeline of in vitro transcription, 5′-capping, and lipid nanoparticle (LNP) encapsulation.32 Modified nucleoside N1-methylpseudouridine was incorporated into the mRNAs during in vitro transcription.
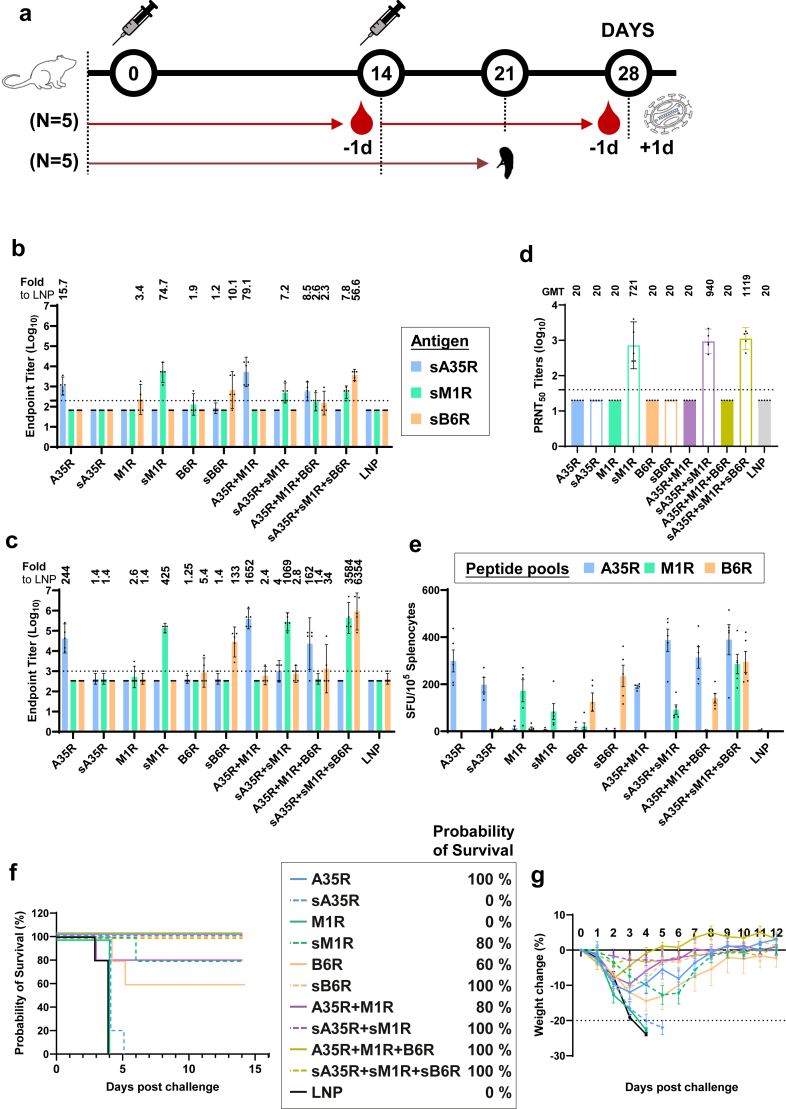
Next, to evaluate the immunogenicity and protective efficacy of the A35R, M1R, and B6R antigens, we vaccinated mice with each of the six single-gene mRNA vaccines, the cocktails of two (A35R + M1R or sA35R + sM1R) or three single-gene mRNA vaccines (full length: A35R + M1R + B6R or soluble region: sA35R + sM1R + sB6R) (Fig. 1a). Using enzyme-linked immunosorbent assay (ELISA), we examined the endpoint titers of IgG in sera from all groups specific to each antigen. We found that by day 27, only the groups vaccinated with A35R, sM1R, or sB6R elicited antibodies specific to the vaccinated antigen(s), but not sA35R, M1R, or B6R (Fig. 1b and c). Consequently, the cocktail of three single-gene mRNA vaccines with soluble regions (sA35R + sM1R + sB6R) elicited the most broad-spectrum antibodies that bind to both sM1R and sB6R. In comparison, the antibodies elicited by the cocktail of two single-gene vaccines could only bind to sA35R or sM1R (Fig. 1b and c).
Fig. 1.
Evaluation of MPXV antigen combinations with single-gene mRNA vaccines. (a) Mice immunisation, challenge and sample collection schedule (n = 5 per group, 110 in total). (b and c) Titers of IgG specific to the indicated MPXV antigens on day 13 (b) and 27 (c). Numbers on top of each graph indicate the fold-increase relative to the cognate antigens in the LNP group. (d) PRNT50 of neutralising antibody against VACV-WR. Numbers on top indicate geometric mean titer (GMT), data are shown as GMT ± 95% confidence interval (CI). (e) ELISpot assay quantifying the IFNγ-secreting splenocytes after re-stimulation by peptide pools of MPXV antigens. Data are shown as means ± SEM (standard error of the mean). (f and g) Probability of survival (f) and percentages of weight change (g) of mice vaccinated with single-gene mRNA vaccine (s) or LNP. Percentages in the legend indicate the probability of survival 12 days post–challenge. Weight change data are shown as means ± SEM. All ELISA and PRNT assays were repeated twice.
Moreover, using the plaque reduction neutralisation test (PRNT), we also examined the neutralising antibodies in each group against IMV of VACV. The 50% plaque reduction neutralisation titer demonstrated that each group vaccinated with sM1R elicited neutralising antibodies against IMV of VACV (Fig. 1d), consistent with the location of M1R in IMV.
In addition to humoral immunogenicity, the cellular immunogenicity of the three antigens was also examined using an enzyme-linked immunospot (ELISpot) assay. Splenocytes from each group were re-stimulated with each of the overlapping peptide pools of the three different proteins (A35R, M1R, and B6R), followed by an examination of the resulting cellular responses. ELISpot assays demonstrated that each single-gene mRNA vaccine and vaccine cocktail elicited robust intracellular IFN-γ after re-stimulation with a peptide pool of the cognate antigen. Notably, although sA35R was unable to elicit an A35R-specific binding antibody, it still elicited a robust cellular response (Fig. 1e).
Finally, the protective efficacy of the single-gene mRNA vaccines and vaccine cocktails were evaluated by the probability of survival and weight change of each group of mice after a lethal dose VACV-WR challenge (7 LD50, 1.89 × 105 pfu). As indicated in Fig. 1, for each antigen in the single-gene mRNA vaccine groups, the ones with stronger antibody-eliciting immunities displayed better protection. Specifically, the A35R group that elicited better antigen-specific antibodies displayed 100% protection with ∼10% weight loss three days post–challenge (Fig. 1f and g). In contrast, the sA35R mRNA that stimulated undetectable antibodies by ELISA provided no protection, and all challenged mice died within five days (Fig. 1f). Similarly, the mRNA vaccine with sM1R, but not M1R, stimulated specific antibodies and provided 80% protection against the lethal challenge (Fig. 1f). Notably, the B6R mRNA elicited no detectable antigen-specific antibody but stimulated a specific T cell response (Fig. 1e). The vaccination of B6R partially protected mice against VACV, with 60% survival, indicating that the protection was likely due to cellular immunity. As a minimal set that covers both IMV and EEV antigens of MPXV, the A35R + M1R cocktail provided 80% protection. When mixed with A35R, the three-antigen cocktail, A35R + M1R + B6R, provided 100% protection. Therefore, we next attempted to design single-chain chimeric immunogens with the three proteins as mpox vaccines to stimulate both humoral and cellular immunities.
Structure-based design and in vitro expression of single-chain MPXV immunogens
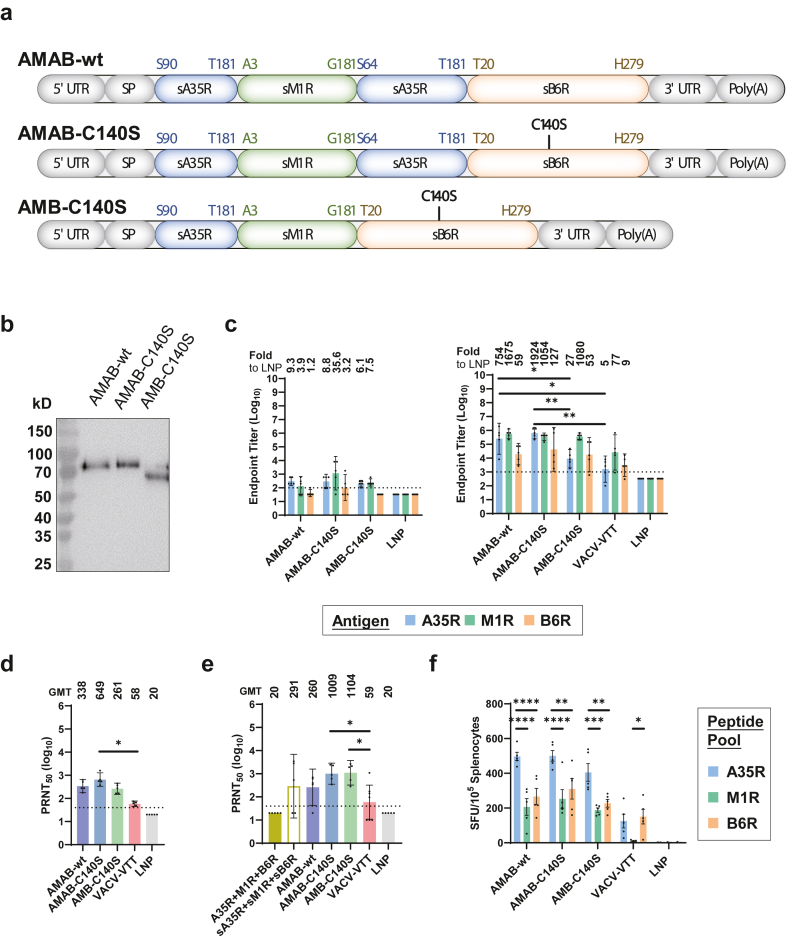
Considering the protective effect of the antigen-specific antibodies, we employed (i) the soluble version of the three antigens (sA35R, sM1R, and sB6R) instead of the full-length versions. (ii) Because sA35R alone was unable to elicit A35R-specific antibodies, we designed two copies of sA35R in the immunogen to boost A35R-specific immunogenicity by mimicking the natural dimeric structure of A35R.33 (iii) Based on AlphaFold2 prediction and our previously developed structure-based vaccine design strategy, we integrated sM1R and sB6R into the sA35R dimer.34 (iv) The soluble B6R contained an odd number of cysteines, which could form interchain disulfide bonds that could lead to large clusters of protein. Thus, we introduced the C140S mutation to remove the free cysteine. With these principles, we designed three mpox mRNA vaccines, AMAB-wt, AMAB-C140S, and AMB-C140S, with single-chain chimeric immunogens containing sA35R, sM1R, and sB6R (Fig. 2a).
Fig. 2.
Design, in vitro expression and immunogenicity evaluation of trivalent mpox mRNA vaccines with single-chain chimeric immunogen. (a) Mpox mRNA vaccines were designed by integrating A35R, M1R, or B6R. Mice were immunised with the same protocol as Fig. 1a, with VACV-VTT as control (n = 5 per group, 100 in total). (b) In vitro expression of the three mRNAs encoding single-chain chimeric immunogens. Each mRNA was transfected into HEK293T cells. Expression of single-chain chimeric immunogens in the supernatant was analysed by western blot. (c) Titers of IgG specific to the indicated MPXV antigens on day 13 (left) and 27 (right). Numbers on top indicate the fold-increase relative to the cognate antigens in the LNP group. Statistical significances were calculated by the two-way ANOVA with Tukey's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01). (d and e) PRNT50 of neutralising antibody against VACV-WR (d) and MPXV (e). Numbers on top indicate geometric mean titer (GMT), data are shown as GMT ± 95% CI. Statistical significances were calculated by the Kruskal–Wallis test with Dunnett's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01). (f) ELISpot assay quantifying the IFNγ-secreting splenocytes after re-stimulation by peptide pools of MPXV antigens. Data are shown as means ± SEM. Statistical significances were calculated by the two-way ANOVA with Tukey's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001). All ELISA and PRNT assays were repeated twice.
Using AlphaFold2, we predicted the translated structures of these three single-chain vaccines. The results revealed that the known binding epitopes for neutralising antibodies in these immunogens were effectively exposed, such as A27D7 in A33R/A35R and 7D11 in L1R/M1R (Fig. S2). Moreover, the two copies of sA35R in AMAB-wt and AMAB-C140S formed a dimeric structure that resembles the corresponding natural structure of the previously reported A33R dimer in VACV,33 which was also consistent with the A35R dimer in the Cryo-EM structure of our recently published mpox protein subunit vaccine that shares the same dimeric A35R design.31 These predictions indicate that our single-chain chimeric immunogens are likely to elicit IgGs specific to the three antigens (sA35R, sM1R, and sB6R).
Then, the 5′-capped mRNAs of these vaccines were transfected into HEK293T cells, and the in vitro expression of single-chain chimeric immunogens was examined by Western Blot. The results showed these immunogens expressed as a single band at their theoretical sizes (Fig. 2b).
Evaluation of the immunogenicity of mpox vaccines with single-chain chimeric immunogens
Next, we evaluated the immunogenicity of the AMAB-wt, AMAB-C140S, and AMB-C140S vaccines, with VACV-VTT as a control for VACV-based live attenuated vaccine. The endpoint titers of IgG antibodies specific to sA35R, sM1R, and sB6R in all vaccinated groups were examined by ELISA. We found that, by day 27, all three single-chain vaccines elicited high levels of IgG specific to sA35R, sM1R, and sB6R (Fig. 2c), which was consistent with the prediction by AlphaFold2. Notably, both AMAB vaccines (AMAB-wt and AMAB-C140S) elicited significantly higher levels of A35R-specific IgG compared to that elicited by AMB-C140S and VACV-VTT. In comparison, the level of A35R-specific IgG elicited by AMB-C140S was not significantly higher than that elicited by VACV-VTT. These data indicated the dimeric sA35R design successfully boosted the immunogenicity of single-chain vaccines (Fig. 2c).
Moreover, using the serum samples collected on day 27, we also examined the neutralising antibodies against VACV-WR as well as MPXV. We observed that potent neutralising antibodies against IMV of VACV-WR and MPXV were elicited by all three single-chain mpox vaccines and the cocktail of sA35R + sM1R + sB6R, but not by the A35R + M1R + B6R cocktail (Fig. 1b, Fig. 2d and e). Notably, compared with AMAB-C140S, VACV-VTT was significantly less effective in eliciting neutralising antibodies against both VACV and MPXV (Fig. 2d and e).
In addition, we also evaluated the cellular immune responses of the mice vaccinated with these vaccines. The ELISpot assay revealed robust cellular immune responses after re-stimulation by the A35R, M1R, or B6R peptide pool in groups vaccinated with the AMAB-wt, AMAB-C140S, and AMB-C140S mRNA vaccines, confirming the cellular immunogenicity of these single-chain vaccines (Fig. 2f). Notably, for all three vaccines, the cellular immune responses were significantly stronger after re-stimulation by the A35R peptide pool compared to the other two peptide pools, suggesting that sA35R may contain potent epitopes for T cell responses worthy of further investigation (Fig. 2f). Interestingly, with VACV-VTT vaccination, similar levels of cellular immune responses were stimulated by the A35R and B6R peptide pool, but the M1R peptide pool failed to stimulate any cellular immune response.
Evaluation of the protective efficacy of mpox vaccines with single-chain chimeric immunogens
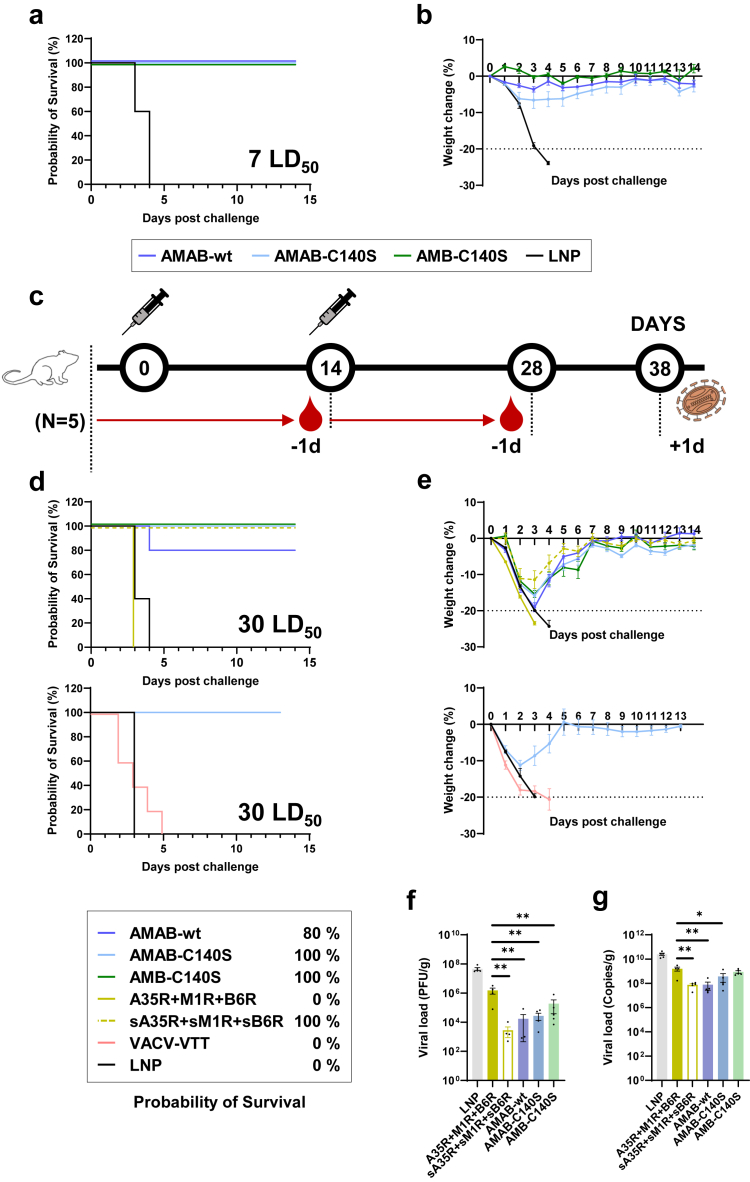
Finally, we evaluated the protective efficacy of the three mpox vaccines by challenging mice with poxviruses including VACV-WR or MPXV. With the groups of mice intranasally challenged with VACV-WR (7 LD50), we found that the group of mice injected with empty LNPs died within four days, while all three single-chain vaccines achieved 100% protection of mice with minimal post–challenge weight loss (Fig. 3a and b), which verified the protective efficacy of the AMAB-wt, AMAB-C140S, and AMB-C140S mpox mRNA vaccines. Moreover, we further examined the protective efficacy of these trivalent mpox vaccines against higher titers of VACV-WR. Compared to previous vaccinations, the dosage of intranasal challenges was increased to 30 LD50 of VACV-WR (∼8.1 × 105 pfu), and additional groups were vaccinated using the A35R + M1R + B6R cocktail, sA35R + sM1R + sB6R cocktail (2.5 μg/mouse for each antigen) or VACV-VTT as controls (Fig. 3c). The humoral immunogenicity elicited by the three trivalent mpox vaccines was also verified by ELISA and VACV neutralisation assays (Fig. S3).
Fig. 3.
Evaluation of protective efficacy of trivalent mpox mRNA vaccines with single-chain chimeric immunogen. (a and b) Probability of survival (a) and percentages of weight change (b) of mice vaccinated with mpox mRNA vaccine challenged with 7 LD50 of VACV-WR. (c) Mice immunisation, challenge, and sample collection schedule (n = 5 per group, 45 in total). (d and e) Probability of survival (d) and percentages of weight change (e) of mice vaccinated with mpox mRNA vaccine or VACV-VTT vaccine challenged with 30 LD50 of VACV-WR. All percentages in the legend indicate the probability of survival 12 days post–challenge. All weight change data are shown as means ± SEM. (f and g) Viral load in the lungs was examined by plaque assay (f) and qPCR assay (g). Data are shown as means ± SEM. Statistical significances were calculated by the ordinary one-way ANOVA with Dunnett's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01). Plaque and qPCR assays were repeated twice.
In contrast to 7 LD50 of VACV-WR, the 30 LD50 challenge led to a >20% weight loss and the consequent euthanisation of all the mice in the A35R + M1R + B6R group within three days, as well as the death of all mice vaccinated with VACV-VTT within four days (Fig. 3d and e). In comparison, the AMAB-C140S and AMB-C140S single-chain vaccines and the sA35R + sM1R + sB6R cocktail provided 100% protection, and mice vaccinated with AMAB-wt were 80% protected (Fig. 3d and e). Using the lungs of these mice, we also examined the viral loads three days post the VACV-WR (30 LD50) challenge (Fig. 3f and g). With both plaque assay and real-time quantitative PCR (qPCR) assays,35 the mice vaccinated with the A35R + M1R + B6R cocktail displayed higher viral loads than those in the other vaccinated groups, which could explain why these mice succumbed to 30 LD50 of VACV-WR (Fig. 3f and g).
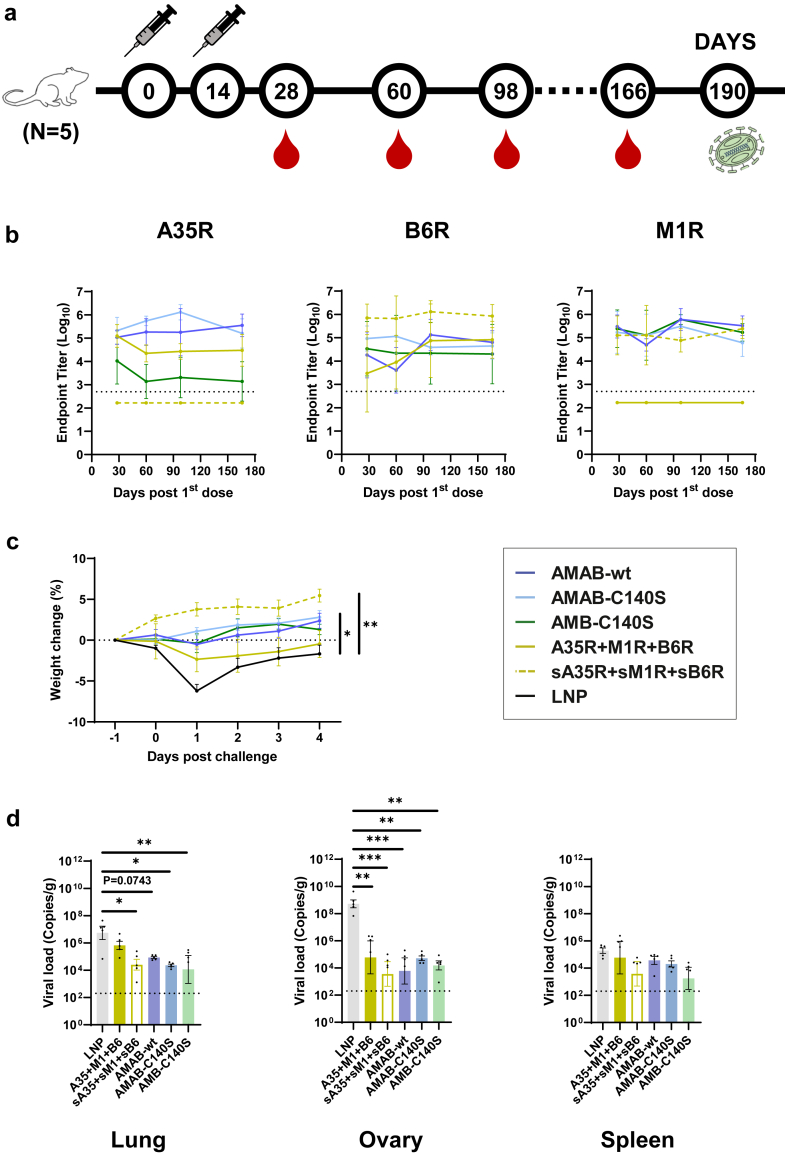
We then evaluated the protective efficacy of the single-chain vaccines against MPXV. Notably, these mice were first monitored for long-term changes in antibody levels by collecting serum samples on days 28, 60, 98 and 166 (Fig. 4a). ELISA results showed that, by day 166, the titers of IgGs specific to the A35R, M1R, or B6R antigens had not significantly decreased in comparison to those on day 28 (Fig. 4b). On day 190, these mice were challenged by MPXV (2 × 107 pfu) via both the intranasal and intraperitoneal routes as previously described (Fig. 4a).30 Then, the mice were weighed daily and euthanised four days after the challenge.
Fig. 4.
Evaluation of the antibody persistency and protective efficacy of trivalent mpox mRNA vaccines against MPXV. (a) Mice immunisation, challenge and sample collection schedule (n = 5 per group, 30 in total). (b) Titers of IgG specific to A35R, B6R and M1R in sera collected 28-, 60-, 98- and 166-days post first dose. Data are shown as GMT ± 95% CI. (c) Percentages of weight change of mice vaccinated with mpox mRNA vaccine challenged with MPXV. All weight change data are shown as means ± SEM. Statistical significances were calculated by the two-way ANOVA with Dunnett's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01). (d) Viral load in lungs, ovaries and spleens were examined by qPCR assay. Data are shown as means ± SEM. Statistical significances were calculated by the ordinary one-way ANOVA with Dunnett's multiple comparisons test (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001).
We found that the LNP group exhibited >5% weight loss after the MPXV challenge, while the groups vaccinated with single-chain vaccines or sA35R + sM1R + sB6R cocktail did not (Fig. 4c). The A35R + M1R + B6R group was the only vaccinated group that showed some weight loss, but not as severe as the LNP group (Fig. 4c). Using qPCR assays, we evaluated the viral loads in the lungs, ovaries, and spleens collected four days post–challenge. We discovered that the MPXV copies in the lungs and ovaries of mice from vaccinated groups decreased dramatically compared with mice of the LNP group. Specifically, the fold-decrease amounted to 2–3 orders of magnitude in the lungs, 4–5 orders of magnitude in the ovaries, and 1–2 orders of magnitude in the spleens (Fig. 4d). Notably, the viral load in the lungs of the A35R + M1R + B6R group was higher than the other vaccinated groups (Fig. 4d), probably due to the advantage of single-chain vaccines over mRNA cocktails of full-length antigens.
Together, these data verified the protective efficacy of single-chain mpox vaccines against the challenge of both VACV-WR and MPXV, and demonstrated the superior protective efficacy of single-chain mRNA vaccine to the cocktail of full-length antigens.
Discussion
The key features of a vaccine include safety, effectiveness, controllability, and accessibility. Hence, an ideal vaccine should possess not only high safety and effectiveness in inducing immune responses but also simplicity in its design and production. This is crucial to enhance the feasibility of quality control, reduce manufacturing costs, and ultimately improve the controllability and accessibility of the vaccine. In recent studies, several mpox mRNA vaccines have been reported, employing antigens from a repertoire of well-defined poxvirus antigens including A35R/A33R, M1R/L1R, B6R/B5R, E8L/D8L, A29L/A27L, or H3L.22, 23, 24, 25, 26,28,36,37 To include multiple antigens in a single vaccine, most of these vaccines took the approach of making a cocktail vaccine comprised of multiple single-gene mRNA vaccines.22, 23, 24, 25, 26,36 Such an approach was straightforward to design, but it would significantly increase the complexity and cost of manufacturing vaccines, which consequently compromises the vaccines’ controllability and accessibility.
Previously, our group developed a structure-based design strategy for vaccines and macromolecular drugs that can effectively integrate multiple antigens as a stable protein. With this strategy, we have developed multiple COVID-19 vaccines, antibodies and peptides, including the widely used ZF2001 vaccine.32,34,38, 39, 40, 41, 42 In this study, we chose the A35R, M1R, and B6R antigens to design single-chain chimeric immunogens for the following reasons: (i) The VACV orthologs of these antigens (A33R, L1R, and B5R) are essential transmembrane proteins on the surface of the IMV or EEV of VACV. Specifically, A35R/A33R and B6R/B5R are EEV-specific glycoproteins that play important roles in the proper formation and cell-to-cell spread of infectious EEV,33,43 while the IMV-specific M1R/L1R is indispensable for the maturation of VACV.44 (ii) A previous study of VACV vaccines demonstrated that the cocktail of soluble A35R/A33R, M1R/L1R, and B6R/B5R proteins was 100% protective to VACV challenge in mice, while the cocktail of soluble A35R/A33R and M1R/L1R was approximately 93% protective.20 (iii) A previous report revealed that 98% of human neutralising mAbs cross-protective against MPXV, VACV, Variola virus (VARV), and Cowpox virus (CPXV) recognise one of the six proteins: A35R/A33R, M1R/L1R, B6R/B5R, E8L/D8L, A29L/A27L or H3L. Removing anti-A35R/A33R, anti-M1R/L1R, or anti-B6R/B5R mAbs hampered the mAb cocktail's protection against poxvirus infection, while mAbs targeting the other three antigens were dispensable in comparison.17
Using the same structure-based vaccine design strategy as our previous COVID-19 vaccines,34 we designed three single-chain mpox vaccines, AMAB-wt, AMAB-C140S, and AMB-C140S, by integrating the M1R and B6R into an A35R dimer. With the structure-based design, we incorporated two sA35R domains in AMAB-wt and AMAB-C140S vaccines to mimic the natural dimeric structure of sA35R to provide the conformational epitopes for antibodies like the A27D7 mAb,33 and further inserted sM1R and sB6R into the sA35R dimer with AlphaFold2 prediction. We also introduced a C140S point mutation to remove the free cystine in sB6R, which could reduce the possibility of forming large clusters by disulfate bonds. Together, as predicted by AlphaFold2, these designs integrated multiple antigens in a single peptide chain while maintaining the natural antibody epitopes, which greatly simplified the manufacturing process and reduced the cost of production. With these single-chain chimeric immunogen designs, our trivalent mpox mRNA vaccines achieved effective neutralisation against both MPXV and VACV-WR. Moreover, these single-chain vaccines completely protected mice against the challenge of a lethal dose of VACV-WR and significantly reduced the viral loads after the challenge of MPXV or VACV-WR.
Compared to the cocktail of multiple full-length antigen single-gene mRNAs or VACV-based live attenuated vaccine, our single-chain vaccines provided more potent and balanced immunogenicity, superior protective efficacy as well as improved controllability. (i) A35R-specific IgGs were elicited by the sA35R integrated in AMAB-wt, AMAB-C140S or AMB-C140S single-chain vaccine, but could not be elicited by the sA35R single-gene mRNA vaccine. Importantly, with a second sA35R incorporated into the single-chain chimeric immunogen, the level of A35R-specific IgG became significantly higher than that elicited by AMB-C140S or VACV-VTT. (ii) M1R-specific and B6R-specific IgGs could only be effectively elicited by sM1R and sB6R instead of the full-length M1R and B6R, respectively. This observation was consistent with a recent report that B6R or M1R antibodies were hardly elicited by an mpox mRNA vaccine that linked the encoding sequence of full-length A35R, M1R, B6R, A29L, and E8 with 2A linkers.28 (iii) The cocktail of three full-length antigens (A35R + M1R + B6R) and VACV-VTT failed to elicit antibodies capable of neutralising the IMV of MPXV or VACV-WR, unlike the single-chain vaccines that exhibited this capability, which may be associated with their weaker protective efficacy against the two poxviruses. (iv) The A35R + M1R + B6R cocktail vaccine and VACV-VTT completely failed to protect mice challenged by a higher dose (30 LD50) of VACV-WR, while the three single-chain mRNA vaccines retained 100% or 80% protection. (v) The viral load in the lungs of the A35R + M1R + B6R vaccinated mice was higher than the other vaccinated groups, which may explain the more severe weight loss in the A35R + M1R + B6R group. These data demonstrated the superior protective efficacy of single-chain chimeric immunogens to the cocktail of full-length antigens and VACV-based live attenuated vaccine. Nevertheless, the B6R-specific IgG levels were not significantly affected among the AMAB-wt, AMAB-C140S, or AMB-C140S groups. More structural studies are needed to reveal the potential mechanism of the C140S mutation.
Interestingly, the sA35R + sM1R + sB6R cocktail vaccine, which coincidentally shares similar amino acid residues as the previously published BNT166c vaccine,26 exhibited slightly higher, albeit not statistically significant, immunogenicity and protective efficacy compared with the single-chain vaccines AMAB-C140S. The A35R + M1R + B6R cocktail was much inferior in comparison. Such a sharp difference in the immunogenicity and protective efficacy between the sA35R + sM1R + sB6R and A35R + M1R + B6R cocktail vaccines underscores the critical role of antigen design, warranting further investigation. Nevertheless, the single-chain vaccines maintain an advantage of maintaining over the sA35R + sM1R + sB6R cocktail vaccine in terms of simplicity. By encoding all three antigens within a single mRNA, the single-chain vaccines facilitate easier quality control during manufacturing and can substantially reduce production costs.
Moreover, our single-chain vaccines exhibited an advantage in antibody persistence when monitoring changes in antibody levels. Both our AMAB-wt and AMAB-C140S vaccines maintained A35R-specific serum antibodies at similar levels from day 28 through day 166. In contrast, a recently reported mpox mRNA vaccine, featuring simpler chimeric immunogens, elicited A35R-specific antibodies in mice that continuously decreased within 5 months after the first dose.37 The persistency of A35R-specific antibodies in the different constructions deserves further study.
Our study has some limitations. Although we have tested the neutralising activities of the single-chain mRNA vaccines-immunised sera against IMV of VACV-WR and MPXV in vitro and evaluated the protective efficacy against the VACV-WR and MPXV in mice, neutralisation test against EEV, which is the other form of infectious poxvirus particles, should be performed in the future study to evaluate the roles that EEV antigens (A35R/A33R and B6R/B5R) played in preventing poxvirus infections. Furthermore, additional research is required to investigate the immunogenic properties of single-chain vaccines and their effectiveness in protecting against MPXV infection in non-human primate models.
In conclusion, our study evaluated the immunogenicity and protective efficacy of the MPXV A35R, M1R, and B6R as single-gene mRNA vaccines and mRNA cocktails. Based on these evaluations, we designed three single-chain mpox vaccines (AMAB-wt, AMAB-C140S, and AMB-C140S) that, with a single polypeptide chain, provide complete protection for mice challenged with a lethal dose of VACV and significantly reduced the viral loads in mice after MPXV challenge. We believe that these single-chain vaccines, with better controllability and accessibility, qualify as potential candidates for future mpox vaccines.
Contributors
Q.W. and G.F.G. initiated and coordinated the project. Q.W., P.D., and H.W. designed the experiments. J.L. conducted mRNA sequence optimisation. T.K. and R.M. prepared mRNA vaccines. R.M. performed Western Blots. T.K., R.M., and X.M. performed animal experiments. T.K., R.M., and R.L. performed ELISA. T.K. and R.M. performed VACV neutralisation assays. H.Z. and F.X. performed MPXV neutralisation assays under the guidance of K.D. and Z.W. Y.L. and R.W. conducted MPXV challenges and viral load titrations. T.K. performed AlphaFold2 prediction and qPCR assay. T.K., R.M., and X.M. performed ELISpot assays. L.Q. prepared recombinant mpox antigen proteins. P.D., T.K., H.W., and Z.G. analysed data. P.D. and T.K. wrote the manuscript. Q.W., H.Q., and G.F.G. revised the manuscript. T.K., P.D., R.M., and H.W. contributed equally to this work. Q.W. and G.F.G. have accessed and verified the underlying data. All authors have read and approved the final version of the manuscript.
Data sharing statement
All relevant data supporting the findings of this study are available within the article or from the corresponding authors upon request.
Declaration of interests
G.F.G., Q.W., P.D., T.K., and R.M. are listed as inventors on patent applications for the three trivalent mpox mRNA vaccines. The other authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 82225021 and 32171428) and the Youth Innovation Promotion Association of the CAS (grant number Y2022037). We thank Dr. Xiao Qu for sharing VACV. We thank Dr. Peng Yin, Ms. Lanju Qin, and Ms. Tingting Zheng for their help with the VACV neutralisation assays, and Ms. Huiting Yang and Ms. Chunli Wu for their help with the ELISpot assay. We thank the Institutional Center for Shared Technology and Facilitates in the Institute of Microbiology, CAS. During the preparation of this work, the authors used AlphaFold2 in order to predict the protein structures of vaccines. The authors have reviewed and confirmed the validity of the text and take full responsibility for the content of the publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105392.
Contributor Information
Qihui Wang, Email: wangqihui@im.ac.cn.
George F. Gao, Email: gaof@im.ac.cn.
Appendix A. Supplementary data
References
- 1.Breman J.G., Steniowski M., Zanotto E., Gromyko A., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165. [PMC free article] [PubMed] [Google Scholar]
- 2.Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalthan E., Tenguere J., Ndjapou S.G., et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Med Mal Infect. 2018;48(4):263–268. doi: 10.1016/j.medmal.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan W., Gao G.F. Neglected zoonotic monkeypox in Africa but now back in the spotlight worldwide. China CDC Wkly. 2022;4(38):847–848. doi: 10.46234/ccdcw2022.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H., Wang W., Zhao L., et al. The first imported case of monkeypox in the mainland of China - chongqing Municipality, China, September 16, 2022. China CDC Wkly. 2022;4(38):853–854. doi: 10.46234/ccdcw2022.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxby D. 1996. Medical microbiology. Galveston (TX) [Google Scholar]
- 7.Smith G.L., Benfield C.T.O., Maluquer de Motes C., et al. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94(Pt 11):2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 8.Interim clinical considerations for use of vaccine for mpox prevention in the United States. 2024. https://www.cdc.gov/poxvirus/mpox/clinicians/vaccines/vaccine-considerations.html
- 9.Rao A.K., Petersen B.W., Whitehill F., et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(22):734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber M.F. Current status of monkeypox vaccines. NPJ Vaccines. 2022;7(1):94. doi: 10.1038/s41541-022-00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaeck L.M., Lamers M.M., Verstrepen B.E., et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023;29(1):270–278. doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubert M., Guivel-Benhassine F., Bruel T., et al. Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals. Cell Host Microbe. 2023;31(6):937–948.e4. doi: 10.1016/j.chom.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deputy N.P., Deckert J., Chard A.N., et al. Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N Engl J Med. 2023;388(26):2434–2443. doi: 10.1056/NEJMoa2215201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts K.L., Smith G.L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16(10):472–479. doi: 10.1016/j.tim.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Pulford D.J., Gates A., Bridge S.H., Robinson J.H., Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22(25-26):3358–3366. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Galmiche M.C., Goenaga J., Wittek R., Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254(1):71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 17.Gilchuk I., Gilchuk P., Sapparapu G., et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167(3):684–694.e9. doi: 10.1016/j.cell.2016.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heraud J.M., Edghill-Smith Y., Ayala V., et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y., Zeng Y., Alexander E., et al. Adsorption of recombinant poxvirus L1-protein to aluminum hydroxide/CpG vaccine adjuvants enhances immune responses and protection of mice from vaccinia virus challenge. Vaccine. 2013;31(2):319–326. doi: 10.1016/j.vaccine.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogg C., Lustig S., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper J.W., Custer D.M., Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng J., Li Y., Jiang L., et al. Mpox multi-antigen mRNA vaccine candidates by a simplified manufacturing strategy afford efficient protection against lethal orthopoxvirus challenge. Emerg Microbes Infect. 2023;12(1) doi: 10.1080/22221751.2023.2204151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R.R., Wang Z.J., Zhu Y.L., et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg Microbes Infect. 2023;12(1) doi: 10.1080/22221751.2023.2192815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang Y., Zhang Z., Liu F., et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct Target Ther. 2023;8(1):172. doi: 10.1038/s41392-023-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia H., He Y.R., Zhan X.Y., Zha G.F. Mpox virus mRNA-lipid nanoparticle vaccine candidates evoke antibody responses and drive protection against the Vaccinia virus challenge in mice. Antiviral Res. 2023;216 doi: 10.1016/j.antiviral.2023.105668. [DOI] [PubMed] [Google Scholar]
- 26.Zuiani A., Dulberger C.L., De Silva N.S., et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell. 2024;187(6):1363–1373.e12. doi: 10.1016/j.cell.2024.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Freyn A.W., Atyeo C., Earl P.L., et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci Transl Med. 2023;15(716):eadg3540. doi: 10.1126/scitranslmed.adg3540. [DOI] [PubMed] [Google Scholar]
- 28.Fang Z., Monteiro V.S., Renauer P.A., et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023;33(5):407–410. doi: 10.1038/s41422-023-00792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su C., Li S., Wen Y., et al. A quadrivalent mRNA immunization elicits potent immune responses against multiple orthopoxviral antigens and neutralization of monkeypox virus in rodent models. Vaccines (Basel) 2024;12(4):385. doi: 10.3390/vaccines12040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang D., Liu X., Lu J., et al. Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from mpox virus challenge. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1203410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Yin P., Zheng T., et al. Rational design of a 'two-in-one' immunogen DAM drives potent immune response against mpox virus. Nat Immunol. 2024;25(2):307–315. doi: 10.1038/s41590-023-01715-7. [DOI] [PubMed] [Google Scholar]
- 32.Han Y., An Y., Chen Q., et al. mRNA vaccines expressing homo-prototype/Omicron and hetero-chimeric RBD-dimers against SARS-CoV-2. Cell Res. 2022;32(11):1022–1025. doi: 10.1038/s41422-022-00720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matho M.H., Schlossman A., Meng X., et al. Structural and functional characterization of anti-A33 antibodies reveal a potent cross-species orthopoxviruses neutralizer. PLoS Pathog. 2015;11(9) doi: 10.1371/journal.ppat.1005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai L., Zheng T., Xu K., et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722–733.e11. doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker J.L., Ward B.M. Development and comparison of a quantitative TaqMan-MGB real-time PCR assay to three other methods of quantifying vaccinia virions. J Virol Methods. 2014;196:126–132. doi: 10.1016/j.jviromet.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N., Cheng X., Zhu Y., et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci China Life Sci. 2023;66:1–13. doi: 10.1007/s11427-023-2378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou F., Zhang Y., Liu X., et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat Commun. 2023;14(1):5925. doi: 10.1038/s41467-023-41628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Y., Li S., Jin X., et al. A tandem-repeat dimeric RBD protein-based covid-19 vaccine zf2001 protects mice and nonhuman primates. Emerg Microbes Infect. 2022;11(1):1058–1071. doi: 10.1080/22221751.2022.2056524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu K., Gao P., Liu S., et al. Protective prototype-beta and delta-omicron chimeric RBD-dimer vaccines against SARS-CoV-2. Cell. 2022;185(13):2265–2278.e14. doi: 10.1016/j.cell.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu S., Wu C., Wu X., et al. Classification of five SARS-CoV-2 serotypes based on RBD antigenicities. Sci Bull (Beijing) 2023;68(23):3003–3012. doi: 10.1016/j.scib.2023.09.048. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Xie Y., Liu H., et al. A broadly neutralizing nanobody targeting the highly conserved S2 subunit of sarbecoviruses. Sci Bull (Beijing) 2023;68(7):684–687. doi: 10.1016/j.scib.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L., Zheng A., Tang Y., et al. Efficient inhibition of SARS-CoV-2 emerging EG.5, EG.5.1 and BA.2.86 variants by fusion inhibitor HY3000 peptide. hLife. 2024;2(1):43–46. [Google Scholar]
- 43.Herrera E., Lorenzo M.M., Blasco R., Isaacs S.N. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72(1):294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su H.P., Garman S.C., Allison T.J., Fogg C., Moss B., Garboczi D.N. The 1.51-Angstrom structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc Natl Acad Sci U S A. 2005;102(12):4240–4245. doi: 10.1073/pnas.0501103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.