Abstract
Background
Opportunities to minimize inequities in accessing treatments for tricuspid regurgitation disease should be considered.
Objective
The objective of this study was to explore how access to new tricuspid regurgitation technologies change when heart centers are restricted by payer coverage requirements.
Methods
This case series study identified U.S. hospitals with a record of performing transcatheter aortic valve replacement, transcatheter edge-to-edge repair, and tricuspid and mitral valve procedures for the calendar year 2021. Population 65+ years of age and Area Deprivation Index (ADI), were identified by zip code. We created 10 scenarios based on low, medium, and high hospital volumes for combinations of transcatheter aortic valve replacement, transcatheter edge-to-edge repair, tricuspid and mitral valve procedures. Distance from a zip code to scenario eligible hospitals was determined; the closest hospital to a zip code was identified as the distance someone with tricuspid regurgitation would have to travel for care. Each scenario was modeled with the dependent variable as the distance to the nearest scenario eligible hospital by ADI, controlling for population size 65+ years of age.
Results
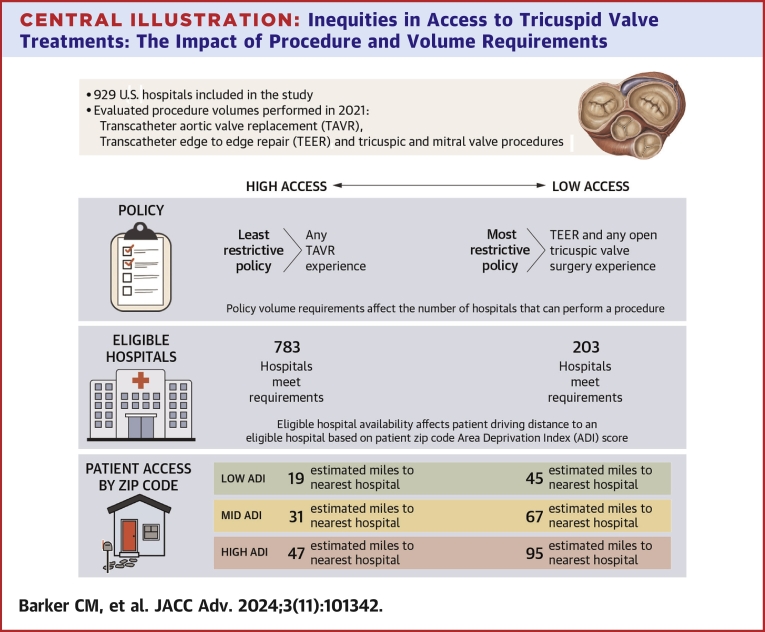
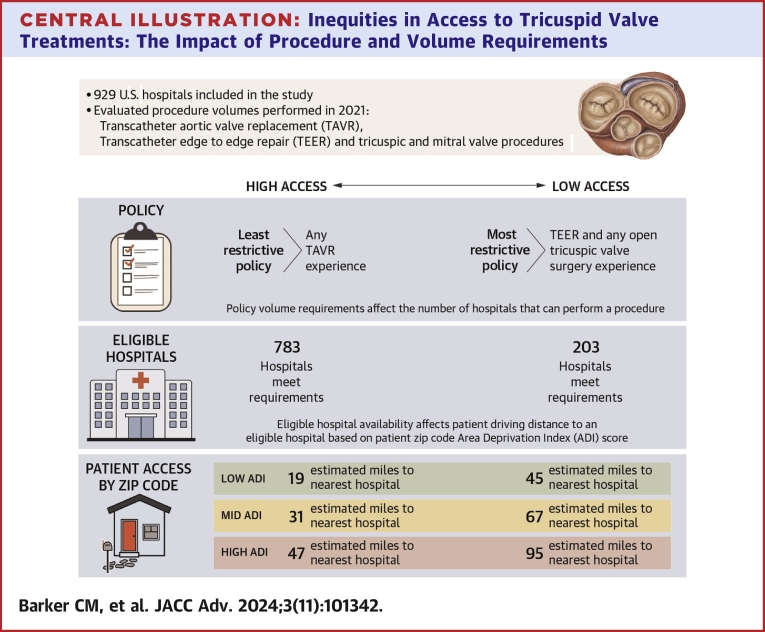
A total of 929 U.S. hospitals met our study inclusion. ADI was statistically significant in every scenario—when ADI goes up (more deprivation), distance to the nearest hospital increases. Patients in zip codes with low ADI travel an average of 15 to 52 miles, medium ADI 31 to 67 miles, and high ADI 47 to 95 miles.
Conclusions
Patients in higher socioeconomic deprivation areas travel longer distances to hospitals meeting procedure volume requirements. Policymakers and patient advocacy groups should consider this to ensure equitable access to potentially life-saving technologies.
Key words: health disparity, health policy, tricuspid regurgitation
Central Illustration
Options for tricuspid valve disease (tricuspid regurgitation [TR])—that is, the “forgotten” valve—have been both limited in number and underutilized in treatment, despite tricuspid valve disease being associated with high morbidity and mortality1, 2, 3 and an increase in health care utilization and expenditures.4,5 Severe TR is a debilitating condition linked to substantial morbidity and poor quality of life. Decreasing TR may reduce symptoms and improve clinical outcomes for patients with this disease.6 Recent advancements in TR treatments, such as transcatheter tricuspid valve replacement (TTVR) and transcatheter edge-to-edge repair (TEER), have shown promising results. Kodali7 (2023) reported that 98.8% of TTVR patients had less than severe TR at 6 months after their intervention compared to 21.6% in medical therapy alone. Sorajja et al6 (2023) demonstrated that TEER significantly reduced the severity of TR, improved patients' quality of life, and was safe compared to medical therapy alone.
The need for effective TR treatments is further emphasized by the findings of Zhan et al8 (2020), who highlighted the prognostic implications of functional TR. Their study revealed that higher TR volumes and fractions, quantified using cardiovascular magnetic resonance, were associated with increased mortality, which underscores the importance of early and accurate assessment and intervention for TR to improve patient outcomes. Similarly, Benfari et al9 (2019) investigated the excess mortality associated with functional tricuspid regurgitation (FTR) complicating heart failure with reduced ejection fraction. Their study found that FTR is common in heart failure patients and is independently associated with increased mortality, pulmonary hypertension, atrial fibrillation, and more severe heart failure presentation. Higher FTR severity was linked to substantially worse long-term survival, emphasizing the critical need for effective intervention strategies for TR.
As new treatments gain approval and come to market, there is concern that restrictive National Coverage Determination (NCD) decisions could potentially limit access to care and disproportionately impact poor and rural communities, similar to what was observed with transcatheter aortic valve replacement (TAVR). When the Centers for Medicare & Medicaid Services made coverage decisions for new transcatheter valve procedures, the conditions placed on hospitals and personnel eliminated many hospitals from coverage. These conditions included requirements for a heart team, appropriate hospital infrastructure, and specific volume requirements. These policy decisions predominantly reflected input from larger centers in major markets and did not account for the interests or concerns of centers or physicians in regions with fewer resources.10, 11, 12
Previous research found that during the initial growth phase of TAVR programs in the United States, hospitals serving wealthier patients were more likely to start programs.13,14 This pattern of growth has led to inequities in the dispersion of TAVR, with lower rates in poorer communities, despite its having been associated with lower mortality rates in patients for whom TAVR would be appropriate, such as those with aortic stenosis.15 This may be part of ongoing geographic and socioeconomic disparities in care.16, 17, 18, 19 Nathan et al attributed the phenomenon to the volume requirements of TAVR’s NCD decisions. The purpose of this research is to explore how access to potentially life-saving new technologies for patients with tricuspid valve disease may change when access to hospitals with heart centers is restricted based on procedure and volume requirements.
Methods
Data sources
The data utilized for this analysis include the following: 1) hospital-level data from the Definitive Healthcare database; 2) census data at the zip code level from the American Community Survey;20 and 3) an index measure of geographical area deprivation.21 The Definitive Healthcare database consists of both Medicare and all-payor hospital billing data aggregated at the hospital level. Annual Medicare data are taken from the Medicare Standard Analytic File and all-payor estimates are generated by Definitive Healthcare from a proprietary algorithm.
Inclusion criteria and variables of interest
Using the Definitive Healthcare database, we identified hospitals across the United States for the calendar year 2021 that met the following criteria: 1) currently have a heart team (have any TAVR volume); and 2) have a record of performing any of the following procedures: TAVR, TEER, as well as tricuspid valve (TV) and mitral valve (MV) (open and transcatheter) procedures. Census data at the zip code level pertinent for this analysis included the number of Medicare eligible (65 years of age or older) people residing in each zip code across the United States. The index measure of geographical area deprivation chosen for this analysis was the Area Deprivation Index (ADI), which describes the relative socioeconomic conditions of neighborhoods at the zip code level based on theoretical domains of income, education, employment, and housing quality. Since the distribution of neighborhood disadvantage varies tremendously across the United States, validated measures of neighborhood disadvantage, are important research tools. We chose ADI because it was available at the zip code level which is the unit of measurement for this analysis and in a recent publication in Health Affairs Forefront (2023) was cited as the most scientifically validated social exposome tool available for policies advancing health equity.22
This is a noninterventional, retrospective, observational study that focused on hospital eligibility, informed consent was not required under an institutional review board exemption status.
A total of 929 hospitals across the United States met these inclusion criteria. For each hospital meeting these criteria, a data set was created with the hospital’s name and location measured by longitude and latitude as well as the number of cases for each procedure of interest performed for the year 2021. For population census data at the zip code level, we accessed the 2019 zip code database23 for the population that is 65 years of age or older. Finally, to define socioeconomic disadvantage at the zip code level, ADI was used to assign a rank to each zip code in the United States, ranging from 0 (least disadvantaged) to 100 (most disadvantaged).
Expert guidance was used to create 10 different scenarios based on low, medium, and high hospital volumes for the following procedural categories: 1) TAVR; 2) TEER; 3) open TV surgery; and 4) any TV or MV procedure. These procedural categories and their volumes were included in 10 scenarios as a proxy for what payers may use to establish procedure and volume requirements for newly approved TTVR procedures. All hospitals meeting the procedure volume requirements for each scenario were flagged and counted, and their location was pinned on a map of the United States. The 10 different scenarios with the number of hospitals meeting the scenario requirements are as follows (note: full scenario descriptions are provided in Table 1): 1) low volume (n = 596), 2) medium volume (n = 537), 3) high volume (n = 323), 4) Any TAVR experience (n = 783), 5) any transcatheter experience (n = 594), 6) TEER and TV/MV procedure experience (n = 396), 7) TAVR and TEER experience (n = 348), 8) TEER and TV/MV experience and open TV surgery experience (n = 295), 9) TAVR and TEER and any open TV surgery experience (n = 287), and 10) TEER and any open TV surgery experience (n = 203). For example, Table 1 shows scenario 1, low volume, in which 596 hospitals across the United States would meet the requirement of having 5 or more TEER procedures and 10 or more of any TV/MV procedures or 10 or more TAVR/TEER procedures and 5 or more open tricuspid valve surgeries.
Table 1.
Ten Scenarios Representing Hospital Experience Based on Procedure Volume for the Year 2021
| Scenarios | Scenario Descriptions | Transcatheter Experience |
Tricuspid or Mitral Valve Experience |
Number of Hospitals | |||
|---|---|---|---|---|---|---|---|
| TAVR (Transcatheter Aortic Valve Replacement) |
TEER (Transcatheter Edge-to-Edge Repair) |
Any Transcatheter Experience TAVR, TEER | Open Tricuspid Valve Surgery | Any Tricuspid or Mitral Valve Procedure (Open and Transcatheter) | |||
| Low volume A or B |
(A) Any tricuspid or mitral valve procedure and transcatheter edge-to-edge repair experience | 0 | ≥5 | 0 | 0 | ≥10 | 596 |
| (B) Open tricuspid valve surgery and any transcatheter experience (TAVR, TEER) | 0 | 0 | ≥10 | ≥5 | 0 | ||
| Medium Volume A or B |
(A) Any Tricuspid or mitral valve procedure and transcatheter edge-to-edge repair experience | 0 | ≥10 | 0 | 0 | ≥20 | 537 |
| (B) Open tricuspid valve surgery and any transcatheter experience (TAVR, TEER) | 0 | 0 | ≥10 | ≥5 | 0 | ||
| High Volume A or B |
(A) Any tricuspid or mitral valve procedure and transcatheter edge-to-edge repair experience | 0 | ≥20 | 0 | 0 | ≥40 | 323 |
| (B) Open tricuspid valve surgery and any transcatheter experience (TAVR, TEER) | 0 | 0 | ≥20 | ≥10 | 0 | ||
| Additional Experience Scenarios of Interest (Sorted by the Number of Hospitals High to Low) | |||||||
|---|---|---|---|---|---|---|---|
| Any TAVR experience | Any TAVR experience | >0 | 0 | 0 | 0 | 0 | 783 |
| Transcatheter experience | High-volume transcatheter experience (TAVR, TEER) | 0 | 0 | ≥40 | 0 | 0 | 594 |
| TEER and tricuspid/mitral valve experience | Medium-volume TEER and any tricuspid or mitral valve procedure experience; however, no open tricuspid valve surgery experience is required | 0 | ≥10 | 0 | 0 | ≥20 | 396 |
| TAVR and TEER experience | Must have both TAVR and medium-volume TEER experience | ≥10 | ≥10 | 0 | 0 | 0 | 348 |
| TEER and tricuspid/mitral valve experience and open tricuspid valve surgery experience | Must have medium volume for TEER, any tricuspid or mitral valve procedure and open tricuspid valve surgery | 0 | ≥10 | 0 | ≥5 | ≥20 | 295 |
| TAVR and TEER and any open tricuspid valve surgery experience | Must have experience in transcatheter aortic valve replacement and medium volume for transcatheter edge-to-edge repair and any open tricuspid valve surgery | ≥10 | ≥10 | 0 | >0 | 0 | 287 |
| TEER and any open tricuspid valve surgery experience | Must have experience in both high-volume TEER and any open tricuspid valve surgery | 0 | ≥20 | 0 | >0 | 0 | 203 |
Using the hospital’s longitude and latitude, the distance between the center of a zip code to the hospital can be calculated. Once the distances from a zip code to hospitals meeting the scenario requirements are determined, the closest hospital to a zip code is identified and included as the distance that a person with TR disease would have to travel to access one of the hospitals meeting the scenario requirements.
Statistical analyses
To explore the hypothesis that restrictive NCD decisions could limit access to TR care and disproportionally impact disadvantaged communities, each of the 10 different scenarios were modeled using ordinary least squares regression with the unit of measurement being the zip code. For each regression model the dependent variable was the distance in miles (from the middle of a zip code) to the nearest hospital meeting the requirements of the scenario, and the 2 independent variables in the regression model were the ADI rank of that zip code and the number of people living in that zip code 65 years of age or older.
Results
Summary statistics
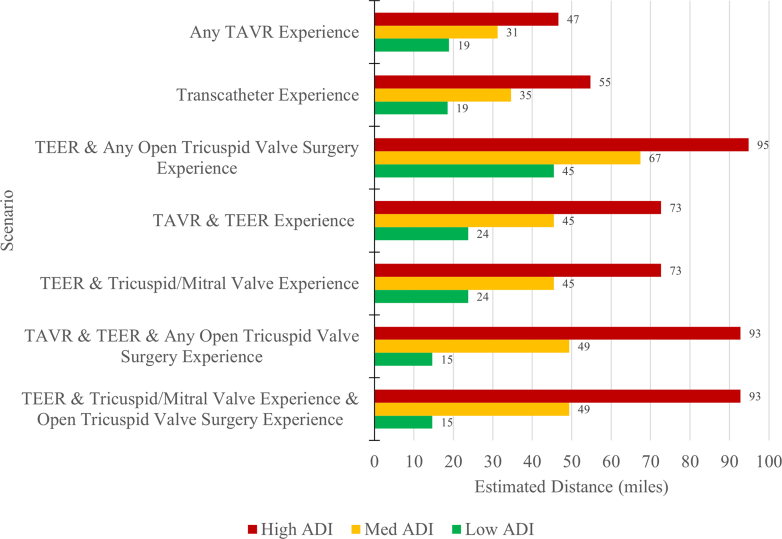
Table 1 provides a description for each of the 10 scenarios along with the number of hospitals that meet the scenario requirement. The first 3 scenarios are based on a combination of low, medium, and high volume requirements (≥5, ≥10, ≥20) for the following procedural categories: 1) TAVR; 2) TEER; 3) open TV surgery; and 4) any TV/MV procedures. The remaining 7 scenarios are listed from the least-restrictive requirements (any TAVR experience n = 783) to the most-restrictive requirements (TEER and any open TV surgery experience n = 203).
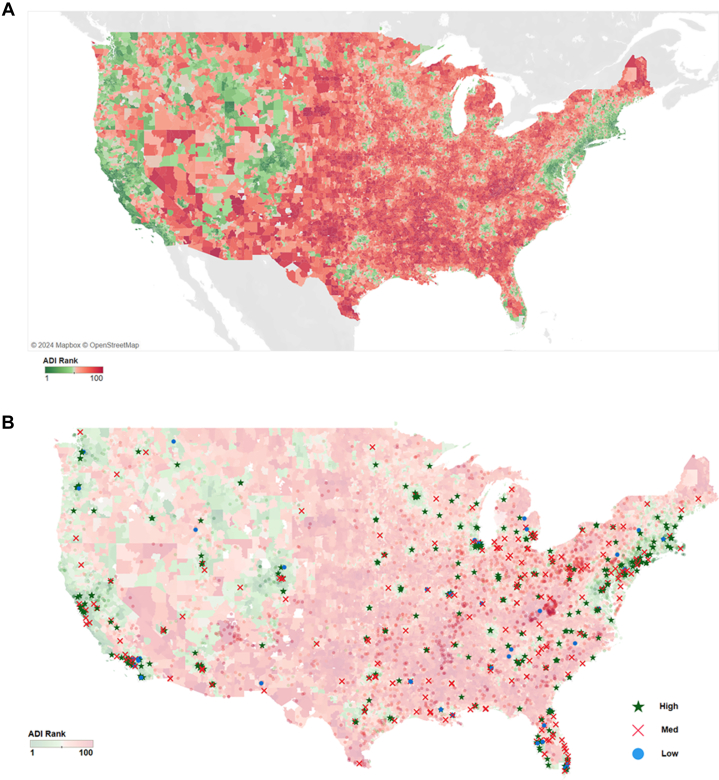
Figure 1A is a heat map of the United States, displaying each zip code’s ADI ranking. Red indicates high ADI and green represents low ADI.
Figure 1.
Geographic Heat Maps of Area Deprivation Index by United States Zip Code
(A) ADI across the USA. Area deprivation index (ADI) was measured for United States zip codes and color coded based on their rank. Green is given for lower ADI and red for higher ADI. (B) Low, medium, and high procedure volume requirements by ADI. U.S. hospitals that met high, medium, and low procedure volume scenario requirements in the 2021 year are mapped over the ADI rank map. Blue dots represent the hospitals ineligible for the medium requirement scenario and red X’s represent the hospitals ineligible for the high volume scenario.
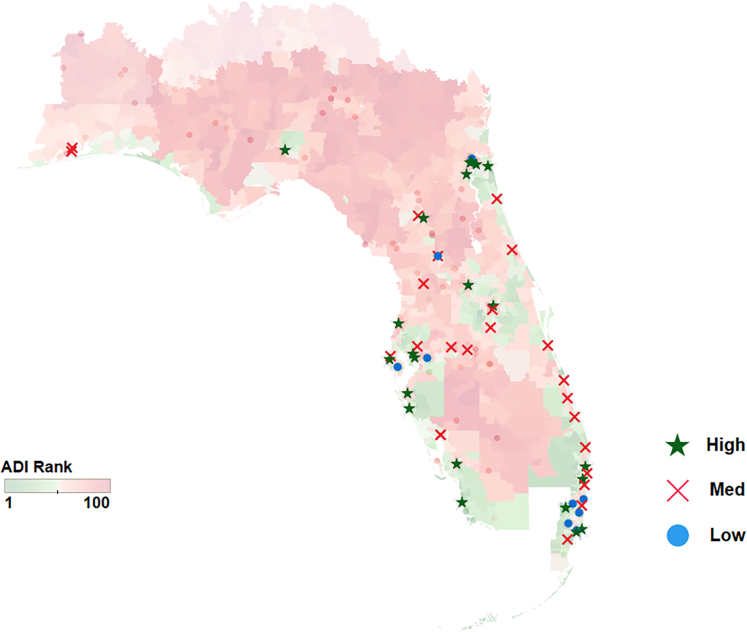
Figure 1B shows the ADI ranking red to green (lighter in transparency) with different symbols representing hospitals meeting the volume scenario requirements for low volume (blue dots), medium volume (red X marks), and high volume or most restrictive are green stars. The red X marks and blue dots are the hospitals that would be ineligible if the most-restrictive scenario was put in place. Figure 2 shows this same information for the state of Florida which has a high Medicare eligible population.
Figure 2.
Procedure Volume Requirement Categories by ADI in the State of Florida
Florida hospitals that met high, medium and low procedure volume scenario requirements in the 2021 year are mapped over the ADI rank map. Blue dots represent the hospitals ineligible for the medium requirement scenario and red X’s represent the hospitals ineligible for the high volume scenario.
Multivariable modeling results
Table 2 reports the multivariable least squares regression results for each of the 10 scenarios. Regressing the distance (miles) from the middle of each zip code to the closest hospital meeting the scenario requirements by ADI and population, the regression results show that ADI is statistically significant with a positive coefficient in every case—that is, when ADI goes up (higher means more deprivation) the distance to the nearest hospital also increases. This is also true when only ADI by itself is in the model (Table 2) and when the model is adjusted for the number of people 65 years and older living in that zip code (Table 3). The results are consistent.
Table 2.
Multiple Least Squares Regression Results for the Relationship Between ADI and Distance Traveled for Each of the 10 Scenarios of Interest
| Scenario | Scenario Description | Number of Hospitals | Estimate | P Value |
|---|---|---|---|---|
| Low volume | TEER ≥5 and any tricuspid or mitral valve procedure (open and transcatheter) ≥10; or any transcatheter experience (TAVR, TEER) ≥10 and open tricuspid valve surgery ≥5 | 596 | 0.5477 | <0.0001 |
| Medium volume | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20; or any transcatheter experience (TAVR, TEER) ≥10 and open tricuspid valve surgery ≥5 | 537 | 0.5615 | <0.0001 |
| High volume | TEER ≥20 and any tricuspid or mitral valve procedure (open and transcatheter) ≥40; or any transcatheter experience (TAVR, TEER) ≥20 and open tricuspid valve surgery ≥10 | 323 | 0.3227 | <0.0001 |
| Any TAVR experience | Transcatheter aortic valve replacement >0 | 783 | 0.4353 | <0.0001 |
| Transcatheter experience | Transcatheter experience (TAVR, TEER) ≥40 | 594 | 0.5355 | <0.0001 |
| TEER and tricuspid/mitral valve experience | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20 | 396 | 0.6956 | <0.0001 |
| TAVR and TEER experience | TAVR ≥10 and TEER ≥10 | 348 | 0.6956 | <0.0001 |
| TEER and tricuspid/mitral valve experience and open tricuspid valve surgery experience | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20 and open tricuspid valve surgery ≥5 | 295 | 0.9099 | <0.0001 |
| TAVR and TEER and any open tricuspid valve surgery experience | TAVR ≥10 and TEER ≥10 and open tricuspid valve surgery >0 | 287 | 0.9099 | <0.0001 |
| TEER and any open tricuspid valve surgery experience | TEER ≥20 and open tricuspid valve surgery >0 | 203 | 0.5856 | <0.0001 |
TAVR = transcatheter aortic valve replacement; TEER = transcatheter edge-to-edge repair.
Table 3.
Multiple Least Squares Regression Results for the Relationship Between ADI and Distance Traveled for Each of the 10 Scenarios of Interest Controlling for Patient Age 65 Years or Older
| Scenario | Scenario Description | Number of Hospitals | Estimate | P Value |
|---|---|---|---|---|
| Low volume | TEER ≥5 and any tricuspid or mitral valve procedure (open and transcatheter) ≥10; or any transcatheter experience (TAVR, TEER) ≥10 and open tricuspid valve surgery ≥5 | 596 | 0.4085 | <0.0001 |
| Medium volume | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20; or any transcatheter experience (TAVR, TEER) ≥10 and open tricuspid valve surgery ≥5 | 537 | 0.4213 | <0.0001 |
| High volume | TEER ≥20 and any tricuspid or mitral valve procedure (open and transcatheter) ≥40; or any transcatheter experience (TAVR, TEER) ≥20 and open tricuspid valve surgery ≥10 | 323 | 0.1777 | <0.0001 |
| Any TAVR experience | Transcatheter aortic valve replacement >0 | 783 | 0.3082 | <0.0001 |
| Transcatheter experience | Transcatheter experience (TAVR, TEER) ≥40 | 594 | 0.4023 | <0.0001 |
| TEER and tricuspid/mitral valve experience | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20 | 396 | 0.5436 | <0.0001 |
| TAVR and TEER experience | TAVR ≥10 and TEER ≥10 | 348 | 0.5436 | <0.0001 |
| TEER and tricuspid/mitral valve experience and open tricuspid valve surgery experience | TEER ≥10 and any tricuspid or mitral valve procedure (open and transcatheter) ≥20 and open tricuspid valve surgery ≥5 | 295 | 0.868 | <0.0001 |
| TAVR and TEER and any open tricuspid valve surgery experience | TAVR ≥10 and TEER ≥10 and open tricuspid valve surgery >0 | 287 | 0.868 | <0.0001 |
| TEER and any open tricuspid valve surgery experience | TEER ≥20 and open tricuspid valve surgery >0 | 203 | 0.5485 | <0.0001 |
ADI = Area Deprivation Index; TAVR = transcatheter aortic valve replacement; TEER = transcatheter edge-to-edge repair.
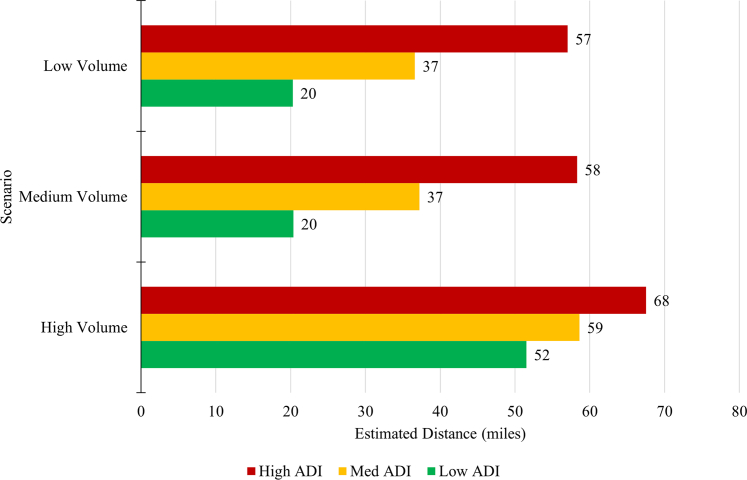
Finally, Figure 3 shows the regression model estimates from the regression equation in miles traveled for the low, medium and high-volume scenarios when ADI is low (10), medium (50), and high (100). For the remaining 7 scenarios, Figure 4 shows these estimates. The distance in high ADI areas is higher than in the medium and low ADI areas. Even in our least-restrictive case of TAVR >0, we see high ADI average distance is estimated at 47 miles, whereas the medium ADI is 31 miles and low ADI is 15 miles. Some of the more-restrictive scenarios yield estimates for high ADI of 95 miles, vs medium ADI at 67 miles and low ADI at 45 miles. The Central Illustration shows in all scenarios, patients in zip codes with low ADI experience estimated travel distances from 15 to 52 miles, while medium ADI areas range from 31 to 67 miles and high ADI areas range from 47 to 95 miles.
Figure 3.
Estimated Distance (Miles) for Procedure Volume Scenarios by ADI
This figure plots the estimated distance traveled (in miles) for low, medium, and high volume scenarios by ADI categories (low, medium, high). Models controlled for medicare eligible population size within each zip code. ADI is statistically significant in each scenario model.
Figure 4.
Estimated Distance (Miles) for Additional Procedure Volume Scenarios by ADI
This figure plots the estimated distance traveled (in miles) for additional procedure volume requirement scenarios by ADI categories (low, medium, high). Models controlled for medicare eligible population size within each zip code. ADI is statistically significant in each scenario model.
Central Illustration.
Inequities in Access to Tricuspid Valve Treatments: The Impact of Procedure and Volume Requirements
Discussion
Results of the study reveal several important findings. First, areas with higher levels of socioeconomic deprivation have longer travel distances to reach hospitals meeting the scenario requirements. This finding suggests that patients residing in economically disadvantaged areas may face greater barriers in accessing specialized care for tricuspid valve disease, a disparity that has been well documented in other medical interventions.24, 25, 26 The positive coefficient of ADI in the regression models indicates a significant association between higher deprivation and increased distance to the nearest hospital, reinforcing the potential disparities in access to care. The research also examines the impact of procedure and volume requirements on access to care. As the criteria in the scenarios become more restrictive, the number of hospitals meeting the requirements decrease, particularly in regions with higher ADI rankings.
These results align with previous studies that have documented similar disparities in access to other cardiovascular interventions. For instance, research on TAVR has shown that restrictive NCD decisions can limit access to care, particularly for patients in socioeconomically disadvantaged areas.10,11 Our study extends these findings to the realm of tricuspid valve disease treatments, highlighting a persistent pattern of healthcare inequality.
The relationship between social determinants of health and access to specialized cardiac care extends beyond mere distance to hospitals. Variations across demographics and urbanicity play crucial roles in shaping healthcare access and outcomes.27, 28, 29 In urban areas, despite potentially shorter distances to hospitals, factors such as transportation infrastructure, traffic congestion, and public transit availability can significantly impact a patient's ability to access care. Conversely, rural patients may face challenges related to limited local healthcare resources and longer travel times, even if straight-line distances appear manageable.30,31
Moreover, social determinants of health can influence procedural volume in complex ways. Hospitals in areas with higher socioeconomic status may attract more patients due to perceived quality of care, better resources, or more comprehensive insurance coverage among the local population. This may create a self-reinforcing cycle where higher-volume centers continue to grow.32,33
Demographic factors such as age, race, and ethnicity can also play a role in access to care and procedural volume. Studies have shown disparities in cardiovascular care across racial and ethnic groups, which may be exacerbated by geographic and socioeconomic factors.34, 35, 36, 37 Older populations, which are more likely to require tricuspid valve procedures, may face additional challenges in traveling for care, further complicating the relationship between hospital proximity and actual access to treatment.38,39
The implications of these findings are significant for policymakers, health care providers, and patient advocacy groups. Efforts should be made to improve the distribution of specialized heart centers and increase access to tricuspid valve disease treatments in underserved areas.
Given the evolving nature of health care policy and hospital capabilities, it is also essential to consider whether social inequalities observed in 1 year are likely to extend into the future. Changes in hospital capabilities or health policies, such as the expansion of insurance coverage, could affect the validity of these findings over time or in different geographic settings. Policymakers need to be proactive in addressing these disparities to ensure equitable access to life-saving technologies for all patients, regardless of socioeconomic status or geographic location.
Study limitations
The limitations of this study should be acknowledged. The analysis relies on data from 2021, and changes in hospital capabilities and geographical distribution may have occurred since then. This study focuses on tricuspid valve disease and does not explore other factors influencing access to care, such as insurance coverage, variation in treatment practices or transportation infrastructure. Our unit of measurement is at the zip code level, and we lack patient-level data for those treated at the hospitals, preventing the analysis of clinical factors such as echocardiograms, hospital staffing levels, patient demographics, comorbidities, insurance types, and direct measures of patient outcomes. Although ADI is a measure of disadvantage it does not account for community/social contextual factors. Furthermore, there are no adjustment for patient level demographics and comorbidities beyond age and the cross-sectional design limits our ability to establish causation using distance to the nearest hospital as a measure of access assumes longer travel correlates with reduced access, which is not always true. Data limitations prevent us from considering transportation availability, patient mobility, or hospital acceptance criteria. We acknowledge that expertise in TAVR or TEER does not automatically translate to expertise in TTVR and volume does not always correlate directly with outcome quality, especially in newer or less-standardized treatments. Additionally, this study does not simulate potential policy changes.
Conclusions
This study highlights the potential impact of restrictive NCDs on access to care for tricuspid valve disease patients, particularly in poor communities. The findings demonstrate that areas with higher levels of socioeconomic deprivation tend to have longer travel distances to hospitals meeting the procedure and volume requirements. Policy makers, health care providers, and patient advocacy groups should consider these findings when ensuring equitable access to potentially life-saving technologies for all patients with tricuspid valve disease. In conclusion, while our findings provide valuable insights, they must be interpreted within the context of our study limitations. Future studies should aim to include more comprehensive variables and patient-level data to better understand the nuances of access to care for tricuspid valve disease.
Perspectives.
COMPETENCY IN SYSTEMS-BASED PRACTICE: Procedure and volume requirements for heart teams are set by health care payers when issuing coverage determinations for new cardiovascular therapies. These requirements can determine availability of access to patients for whom these therapies are intended.
TRANSLATIONAL OUTLOOK: Future studies should explore characteristics of inequities that limit access to emerging TR therapies with the design of developing solution-oriented discussions.
Funding support and author disclosures
This research was sponsored by Edwards Lifesciences. Dr Barker has reported personal fees from Edwards Lifesciences, Medtronic, Boston Scientific and Alleviant. Dr Kemp, Ms. Mancilla, and Ms. Mollenkopf are employees of Edwards Lifesciences. Dr Gunnarsson, Mr. Ryan, and Dr David have reported personal fees from Edwards Lifesciences.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Hahn R.T., Badano L.P., Bartko P.H., et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging. 2022;23(7):913–929. doi: 10.1093/ehjci/jeac009. [DOI] [PubMed] [Google Scholar]
- 2.Barker C.M., Cork D.P., McCullough P.A., et al. Comparison of survival in patients with clinically significant tricuspid regurgitation with and without heart failure (from the optum integrated file) Am J Cardiol. 2021;144:125–130. doi: 10.1016/j.amjcard.2020.12.070. [DOI] [PubMed] [Google Scholar]
- 3.Antunes M.J., Rodríguez-Palomares J., Prendergast B., et al. Management of tricuspid valve regurgitation: position statement of the European society of cardiology working groups of cardiovascular Surgery and valvular heart disease. Eur J Cardio Thorac Surg. 2017;52(6):1022–1030. doi: 10.1093/ejcts/ezx279. [DOI] [PubMed] [Google Scholar]
- 4.Barker C.M., Cork D.P., McCullough P.A., et al. Healthcare utilization in clinically significant tricuspid regurgitation patients with and without heart failure. J Comp Eff Res. 2021;10(1):29–37. doi: 10.2217/cer-2020-0198. [DOI] [PubMed] [Google Scholar]
- 5.Cork D.P., McCullough P.A., Mehta H.S., et al. The economic impact of clinically significant tricuspid regurgitation in a large, administrative claims database. J Med Econ. 2020;23(5):521–528. doi: 10.1080/13696998.2020.1718681. [DOI] [PubMed] [Google Scholar]
- 6.Sorajja P., Whisenant B., Hamid N., et al. TRILUMINATE Pivotal Investigators Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388(20):1833–1842. doi: 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
- 7.Kodali S. TCT 2023 Conference; 2023. TRISCEND II Trial: A Randomized Trial of Transcatheter Tricuspid Valve Replacement in Patients with Severe Tricuspid Regurgitation. San Francisco, CA, United States. [Google Scholar]
- 8.Zhan Y., Debs D., Khan M.A., et al. Natural history of functional tricuspid regurgitation quantified by cardiovascular magnetic resonance. J Am Coll Cardiol. 2020;76(11):1291–1301. doi: 10.1016/j.jacc.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Benfari G., Antoine C., Miller W.L., et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. 2019;140(3):196–206. doi: 10.1161/CIRCULATIONAHA.118.038946. [DOI] [PubMed] [Google Scholar]
- 10.Gandjian M., Verma A., Tran Z., et al. Influence of center surgical aortic valve volume on outcomes of transcatheter aortic valve replacement. JTCVS Open. 2022;11:62–71. doi: 10.1016/j.xjon.2022.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coylewright M., Forrest J.K., McCabe J.M., Nazif T.M. TAVR in low-risk patients: FDA approval, the new NCD, and shared decision-making. J Am Coll Cardiol. 2020;75(10):1208–1211. doi: 10.1016/j.jacc.2019.12.057. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services Transcatheter aortic valve replacement (TAVR), national coverage determination (20.32), CMS Publication No. 100-3. 2019. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=355
- 13.Nathan A.S., Yang L., Yang N., et al. Racial, ethnic, and socioeconomic disparities in access to transcatheter aortic valve replacement within major metropolitan areas. JAMA Cardiol. 2022;7(2):150–157. doi: 10.1001/jamacardio.2021.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy K.P., Groeneveld P.W., Jay G., Fanaroff A.C., Nathan A.S. Economic considerations in access to transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.011489. [DOI] [PubMed] [Google Scholar]
- 15.Bevan G.H., Zidar D.A., Josephson R.A., Al-Kindi S.G. Mortality due to aortic stenosis in the United States, 2008–2017. JAMA. 2019;321(22):2236–2238. doi: 10.1001/jama.2019.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparrow R.T., Sanjoy S.S., Lindman B.R., et al. Racial, ethnic and socioeconomic disparities in patients undergoing transcatheter mitral edge-to-edge repair. Int J Cardiol. 2021;344:73–81. doi: 10.1016/j.ijcard.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Young M.N., Kearing S., Malenka D., Goodney P.P., Skinner J., Iribarne A. Geographic and demographic variability in transcatheter aortic valve replacement dispersion in the United States. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vemulapalli S., Carroll J.D., Mack M.J., et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380(26):2541–2550. doi: 10.1056/NEJMsa1901109. [DOI] [PubMed] [Google Scholar]
- 19.Kundi H., Faridi K.F., Wang Y., et al. Geographic patterns of growth for transcatheter aortic valve replacement in the United States. Circulation. 2019;140(11):969–971. doi: 10.1161/CIRCULATIONAHA.119.040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Census Bureau 2020. http://www.census.gov/2020census/data/ 2020 Census. U.S. Census Bureau.
- 21.Kind A.J.H., Buckingham W. Making neighborhood disadvantage metrics accessible: the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. https://www.neighborhoodatlas.medicine.wisc.edu/9/7/2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Area Deprivation Index Is The Most Scientifically Validated Social Exposome Tool Available For Policies Advancing Health Equity. Health Affairs Health Affairs Forefront; 2023. [Google Scholar]
- 23.Simplemaps US zip codes database. 2023. https://simplemaps.com/data/us-zips
- 24.Hood C.M., Gennuso K.P., Swain G.R., Catlin B.B. County health rankings. Am J Prev Med. 2016;50(2):129–135. doi: 10.1016/j.amepre.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Canedo J.R., Miller S.T., Schlundt D., Fadden M.K., Sanderson M. Racial/ethnic disparities in diabetes quality of care: the role of healthcare access and socioeconomic status. J Racial Ethn Health Disparities. 2018;5(1):7–14. doi: 10.1007/s40615-016-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G.K., Daus G.P., Allender M., et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935–2016. Int J MCH AIDS. 2018;6(2):139–164. doi: 10.21106/ijma.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes Z.H., Hammond M.M., Lewis-Thames M., Sweis R., Shah N.S., Khan S.S. Rural-urban trends for aortic stenosis mortality in the United States, 2008-2019. JACC Adv. 2023;2(8) doi: 10.1016/j.jacadv.2023.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters G.A., Ordoobadi A.J., Panchal A.R., Cash R.E. Differences in out-of-hospital cardiac arrest management and outcomes across urban, suburban, and rural settings. Prehosp Emerg Care. 2023;27(2):162–169. doi: 10.1080/10903127.2021.2018076. [DOI] [PubMed] [Google Scholar]
- 29.Weaver A.M., McGuinn L.A., Neas L., et al. Associations between neighborhood socioeconomic cluster and hypertension, diabetes, myocardial infarction, and coronary artery disease within a cohort of cardiac catheterization patients. Am Heart J. 2022;243:201–209. doi: 10.1016/j.ahj.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe M.K., McDonald N.C., Holmes G.M. Transportation barriers to health care in the United States: findings from the national health interview survey, 1997–2017. Am J Publ Health. 2020;110(6):815–822. doi: 10.2105/AJPH.2020.305579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss D.J., Nelson A., Vargas-Ruiz C.A., et al. Global maps of travel time to healthcare facilities. Nat Med. 2020;26(12):1835–1838. doi: 10.1038/s41591-020-1059-1. [DOI] [PubMed] [Google Scholar]
- 32.Fahrenbach J., Chin M.H., Huang E.S., Springman M.K., Weber S.G., Tung E.L. Neighborhood disadvantage and hospital quality ratings in the Medicare hospital compare program. Med Care. 2020;58(4):376–383. doi: 10.1097/MLR.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuklinski D., Vogel J., Geissler A. The impact of quality on hospital choice. Which information affects patients’ behavior for colorectal resection or knee replacement? Health Care Manag Sci. 2021;24:185–202. doi: 10.1007/s10729-020-09540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolakale-Rufai I.K., Shinnerl A., Knapp S.M., et al. Association between social vulnerability index and admission urgency for transcatheter aortic valve replacement. Am Heart J Plus. 2024;39:100370. doi: 10.1016/j.ahjo.2024.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannu M., Kaltenbach L., Pagidipati N.J., et al. Effects of an intervention to improve evidence-based care for people with diabetes and cardiovascular disease across sex, race, and ethnicity subgroups: insights from the COORDINATE-diabetes trial. Circulation. 2024;150(3):180–189. doi: 10.1161/CIRCULATIONAHA.124.068962. [DOI] [PubMed] [Google Scholar]
- 36.Powell-Wiley T.M., Baumer Y., Baah F.O., et al. Social determinants of cardiovascular disease. Circ Res. 2022;130(5):782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redberg R.F. Gender, race, and cardiac care: why the differences? J Am Coll Cardiol. 2005;46(10):1852–1854. doi: 10.1016/j.jacc.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 38.Du M., Cheng L., Li X., Yang J. Factors affecting the travel mode choice of the urban elderly in healthcare activity: comparison between core area and suburban area. Sustain Cities Soc. 2020;52 [Google Scholar]
- 39.Remillard E.T., Campbell M.L., Koon L.M., Rogers W.A. Transportation challenges for persons aging with mobility disability: qualitative insights and policy implications. Disabil Health J. 2022;15(1) doi: 10.1016/j.dhjo.2021.101209. [DOI] [PubMed] [Google Scholar]