Abstract
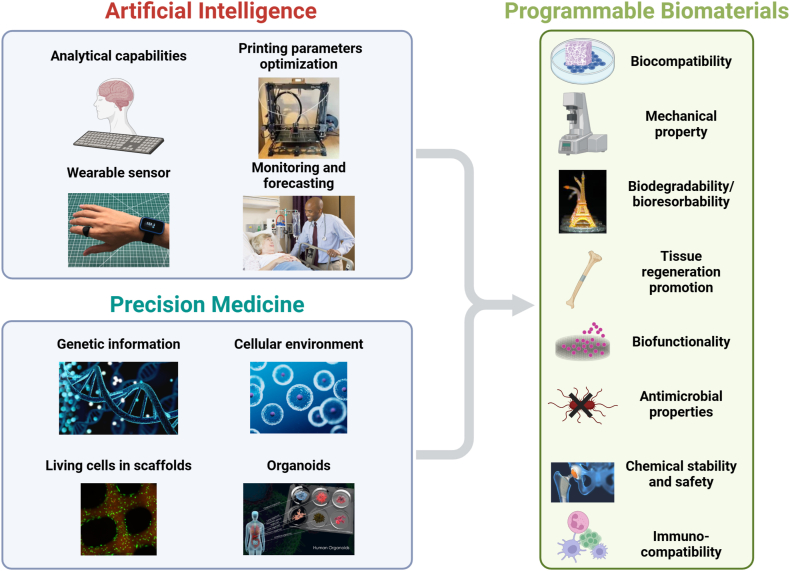
Programmable biomaterials are distinguished by their ability to adjust properties and functions on demand, in a periodic, reversible, or sequential manner. This contrasts with traditional biomaterials, which undergo irreversible, uncontrolled changes. This review synthesizes key advances in programmable biomaterials, examining their design principles, functionalities and applications in bone regeneration. It charts the transition from traditional to programmable biomaterials, emphasizing their enhanced precision, safety and control, which are critical from clinical and biosafety standpoints. We then classify programmable biomaterials into six types: dynamic nucleic acid-based biomaterials, electrically responsive biomaterials, bioactive scaffolds with programmable properties, nanomaterials for targeted bone regeneration, surface-engineered implants for sequential regeneration and stimuli-responsive release materials. Each category is analyzed for its structural properties and its impact on bone tissue engineering. Finally, the review further concludes by highlighting the challenges faced by programmable biomaterials and suggests integrating artificial intelligence and precision medicine to enhance their application in bone regeneration and other biomedical fields.
Keywords: Programmable biomaterials, Bone regeneration, Development of biomaterials, Bone tissue engineering, Artificial intelligence
Graphical abstract
1. Introduction
Bone and cartilage diseases, induced by factors such as aging, trauma and poor lifestyle choices, including osteoarthritis, bone defects and cartilage degeneration, significantly compromise human health. The most common treatment for bone defects is bone grafting, which involves using bone from the patient (autografts), from donors (allografts), from other species (xenografts), or synthetic materials. Autografts are preferred for their compatibility and bone-growth properties. However, they are costly, require additional surgery, and have limited availability, along with risks like immune rejection and disease transmission [[1], [2], [3]]. The development of biomaterials has significantly improved this clinical scenario, offering a broader range of functionalities to support bone tissue mechanical stability and cellular repair processes. However, most biomaterials offer limited functionality, focusing either on mechanical support or targeting specific cellular functions in bone repair. Bone tissue repair is a complex, dynamic and long-term biological process that encompasses inflammation, repair and remodeling phases. Each stage is important for successful bone tissue healing [[4], [5], [6]]. Consequently, biomaterials capable of dynamically responding to and regulating these biological processes, tailored to specific needs, hold the greatest promise for advancement and clinical application potential in the field of regenerative medicine [7].
Programmable materials can change their morphology, physical properties, or chemical functions in a predetermined sequence in response to external stimuli or environmental changes. This programmability enables time-dependent control methods, thereby offering extensive application potential in various fields such as drug delivery, tissue engineering, regenerative medicine, smart medical devices, and biosensors [8]. In bone regeneration, it means that implant materials can dynamically respond and regulate on demand based on the natural bone repair process or microenvironmental characteristics and finally achieve the ideal bone repair [9,10]. Resveratrol (Res) is a polyphenol with antioxidant, anti-inflammatory, and cardiovascular protective effects. It also boosts the osteogenic potential of bone marrow mesenchymal stem cells (BMSC), showing potential for treating osteoporosis and bone repair. Due to its poor water solubility and rapid decomposition upon exposure to oxygen, liposomes are frequently employed to enhance Res's stability and bioavailability. Moreover, bone norphogenetic protein-2 (BMP-2) can stimulate deoxyribonucleic acid (DNA) synthesis and cell replication, thereby promoting the directional differentiation of mesenchymal cells into osteoblasts. It is an important growth factor for bone repair and regeneration. Although BMP-2 is crucial for osteogenesis, its clinical use is limited by its short half-life, high cost, and potential side effects. Cai et al. develops novel delivery systems using film dispersion and static loading to prepare chitosan-coated resveratrol liposomes (CS-Res@Lipo) and HAMA@HepMA hydrogel microspheres (MS) via a chemical grafting condensation reaction. These systems utilize non-covalent interactions at MS binding sites to efficiently anchor BMP-2, forming a programmed release system. This strategy uses Res to control the immune response, while BMP-2 is released slowly to aid bone healing. This dual-release system not only targets inflammation management but also enhances the osteogenic process, leveraging the coordinated release profiles to maximize therapeutic efficacy and optimize bone regeneration outcomes [11]. In addition, a silk fibroin (SF)-based scaffold mimicking cartilage can programmatically regulate the timed release of bioactive factors to enhance in-situ cartilage regeneration. Initially, transforming growth factor-β1 (TGF-β1) is incorporated into the SF cryogel scaffolds through physical adsorption, subsequently followed by the encapsulation of E7 within a rapidly degrading SilMA/HAMA coating. This setup allows for rapid release of E7 in the initial days and a slow, sustained release of TGF-β1 over several weeks, synergistically promoting BMSC recruitment and their chondrogenic differentiation in vitro. These SF scaffolds maintain outstanding structural integrity and mechanical properties similar to cartilage, offering an optimal 3D microenvironment for cartilage reconstruction [12]. Programmable biomaterials are engineered to respond dynamically to the physiological environment of the injury site, allowing for tailored therapeutic actions based on the specific needs of the tissue repair process. These materials hold substantial clinical advantages in the field of bone repair, presenting a promising approach to regenerative medicine.
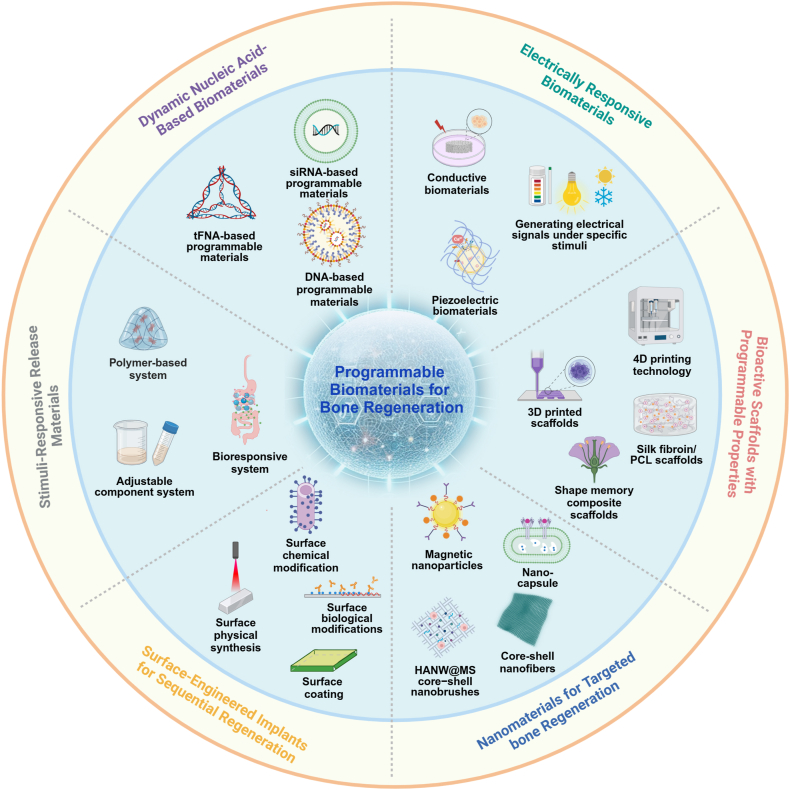
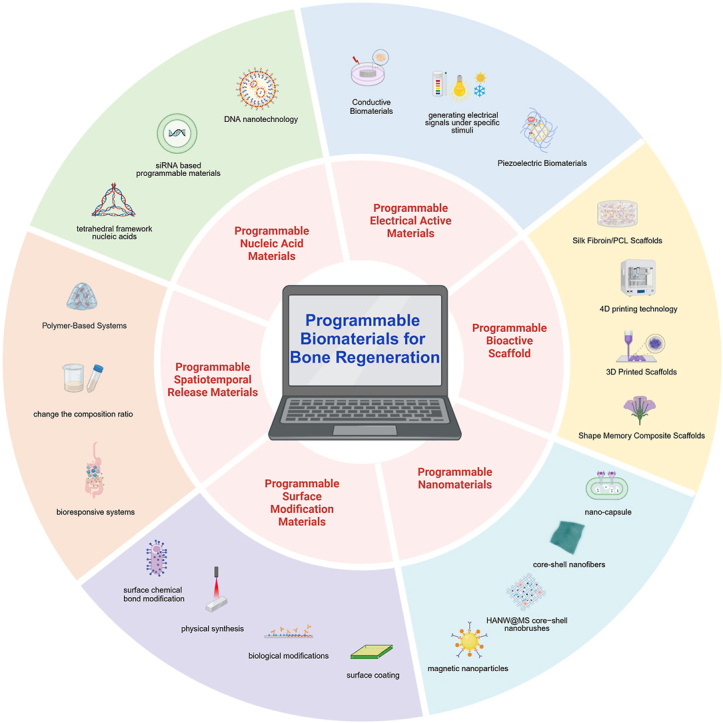
Research on programmable biomaterials for bone repair is flourishing and shows considerable clinical potential. However, there is currently a lack of comprehensive overviews on the use of these materials for bone healing. This review begins by briefly outlining the develop history of biomaterials, then contrasts the notable advantages of programmable biomaterials over traditional ones from clinical application and safety perspectives. It categorizes programmable materials based on their structural properties into six types: dynamic nucleic acid-based biomaterials, electrically responsive biomaterials, bioactive scaffolds with programmable properties, nanomaterials for targeted bone regeneration, surface-engineered implants for sequential regeneration and stimuli-responsive release materials (Fig. 1), systematically summarizing the cutting-edge research in bone repair. Furthermore, we address its existing challenges and forecast future trends by integrating artificial intelligence and precision medicine to enhance their application in bone regeneration and other biomedical fields. Overall, the review lays a theoretical foundation for the development of programmable biomaterials and underscores their impact on bone tissue engineering, focusing on the capacity of programmable biomaterials to fulfill the complex requirements of bone regeneration.
Fig. 1.
Classification of programmable biomaterials.
2. The development of biomaterials and the emergence of programmable biomaterials
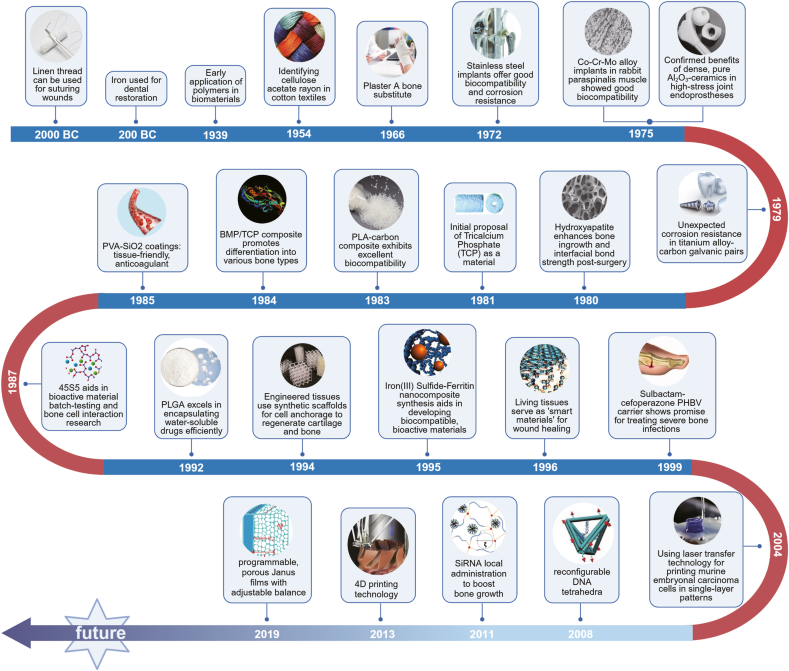
The development of biomaterials has been a journey of continuous innovation, evolving through three distinct generations, each marked by significant scientific advancements and shifting paradigms in materials science (Fig. 2) [[13], [14], [15]]. The first generation of biomaterials, primarily used from the 1950s to the 1980s, focused on materials that were biologically inert. These included gypsum, various metals, rubber and cotton. The primary objective during this era was to create materials that would not react adversely with biological tissues. However, the inert nature of these materials often led to host reactions and long-term compatibility issues, prompting the need for more sophisticated solutions. Despite their limitations, these materials laid the groundwork for future developments by establishing the fundamental criteria for biocompatibility [16,17]. The second generation, spanning from the 1980s to the 1990s, saw a shift towards bioactive materials. This era was characterized by an interdisciplinary approach, combining insights from medicine, materials science, biochemistry and physics. The development of technologies like advanced polymer materials science and enhanced physical testing methods allowed for the creation of materials that interacted beneficially with biological tissues. Key materials from this period include hydroxyapatite, tricalcium phosphate, polyhydroxy acids, hydroxyethyl methacrylate polymers, collagen and fibrin. These materials were designed not just to be compatible with the body but also to actively participate in biological processes, such as tissue regeneration and healing. The focus on bioactivity opened up new avenues for medical applications, including more effective implants and scaffolding for tissue engineering [[18], [19], [20], [21]]. The advent of the third generation of biomaterials, beginning in the 1990s and continuing to the present day, marked a revolutionary shift towards materials that could actively interact with and influence biological systems. This generation focuses on cell, protein and gene-activated materials, which are essentially biomedical composites designed to enhance the body's inherent healing and regenerative capabilities. These materials are a blend of active components that promote physiological responses and inactive components for control and stability. They are designed to achieve an optimal balance between material properties and biological function [[22], [23], [24], [25], [26]]. Third-generation biomaterials are characterized by their ability to adapt to physiological conditions, respond to cellular environments and facilitate the natural regenerative processes of the body. Representative materials include BMP and other biologically active compounds, which have found extensive applications in regenerative medicine and tissue engineering.
Fig. 2.
The evolution of biomaterials: from simple replacements to advanced regeneration.
Programmable biomaterials mark a pivotal advancement, merging disciplines such as materials science, biology and computer science. These biomaterials are engineered to integrate computational principles, such as coding and data processing, into their structure and functionality. This innovative approach enables the meticulous modulation of material characteristics via targeted molecular interactions, tailored chemical alterations and sensitivity to external stimuli. Programmable biomaterials are adept at altering their attributes or behavior in reaction to environmental fluctuations, including changes in temperature, pH levels, or mechanical forces, rendering them exceedingly versatile and responsive. The application of programmable attributes within biomaterials has catalyzed the creation of groundbreaking medical technologies. These include intelligent drug delivery mechanisms capable of precisely timing and locating the release of therapeutic agents, as well as sophisticated tissue engineering scaffolds that can adjust and progress in harmony with recuperating tissues. Such materials are engineered to be dynamic, in terms of both structure and functionality, fostering a new era of research in materials science. Programmable materials can respond to specific stimuli in several ways. In some materials, a stimulus may cause changes in non-covalent interactions within the material, leading to a reversible physical change [27]. Another type of programmable material is shape memory materials. Shape memory is a property that both organic materials and alloys can exhibit, and it may occur in one direction, two directions or multiple directions [28]. Programmable biomaterials epitomize the fusion of materials science, biology and computer science, yielding materials that are not only biocompatible but also equipped for dynamic interactions with their surroundings. This synergy between material characteristics and biological systems is key to devising more efficacious and customized medical interventions. It holds immense promise for advancing regenerative medicine, targeted drug delivery systems and other medical fields, heralding a new chapter in healthcare innovation [8,29,30].
As the research and development of programmable biomaterials continue to advance, we can expect these materials to play an increasingly vital role in addressing complex medical challenges. The future of biomaterials is likely to see even more sophisticated integration of biological and synthetic components, leading to innovative solutions that can adapt and respond to the body's needs in real-time. The evolution from inert to bioactive and programmable materials shows our growing understanding of interactions between materials and biological systems. This evolution heralds the onset of a new era of bio-inspired and bio-integrated materials science. As our understanding of these complex interactions continues to deepen, we can design more intelligent biomaterials that can not only interact precisely with biological systems, but also adapt self-regulation and repair in specific biological environments, greatly promoting the development of regenerative medicine and tissue engineering.
3. Programmable biomaterials versus traditional biomaterials
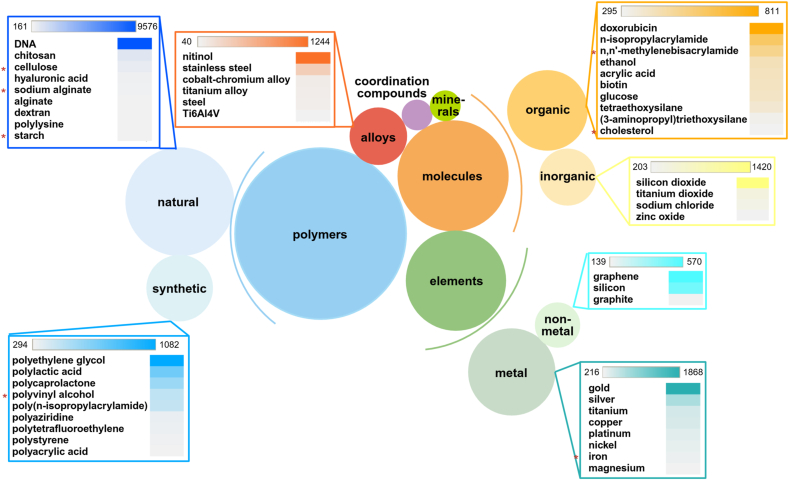
There is a significant difference between programmable materials and traditional material preparation and synthesis processes. Traditional large-scale material manufacturing often struggles to precisely create required components, leading to defects, deformations, or functional deviations [[31], [32], [33], [34]]. The programmability of programmable biomaterials comes from their ability to respond to small changes in the environment, such as pH, temperature, light, electric or magnetic fields, or specific chemical or biological signals. DNA-based materials represent a special class of programmable biomaterials. Their particularity lies in the fact that DNA can achieve precise structural adjustability through Watson-Crick base pairing [35,36]. The directed self-assembly of single-stranded DNA can produce different two-dimensional and three-dimensional structures, whose formation and dynamics can be controlled at the molecular level [37]. In addition to the ability to generate specific structures, DNA can also be modified to respond to specific chemical environments and further modified by using CRISPR technology. Currently, commonly used programmable biomaterials mainly include polymers (natural and synthetic), inorganic and organic small molecules, metal and non-metal elements, minerals, coordination compounds and alloys [[38], [39], [40]] (Fig. 3).
Fig. 3.
Distribution of publications (journals and patents) on substances used in programmable biomaterials from 2003 to 2023. Larger circles represent more publications, and materials with relatively faster growth rates are marked with asterisks.
Programmable biomaterials have increasingly become a part of our daily lives. Take, for instance, Nitinol, an alloy composed of nickel and titanium, known for its shape-memory properties. This alloy can be molded into a specific shape and then alter its form when exposed to heat. This characteristic has led to its designation as a shape-memory alloy. In the realm of orthodontics, the archwires in braces, made from Nitinol, contract upon exposure to the warmth of the human body, exerting the necessary force to correct the alignment of teeth [41,42]. Additionally, Nitinol finds extensive applications in medical devices such as stents used in heart surgeries, temperature controllers and mechanisms controlling the stable configuration of space systems [43]. Since its discovery in 1959, new applications of Nitinol have been identified almost every year, demonstrating its versatility and utility across various fields [44].
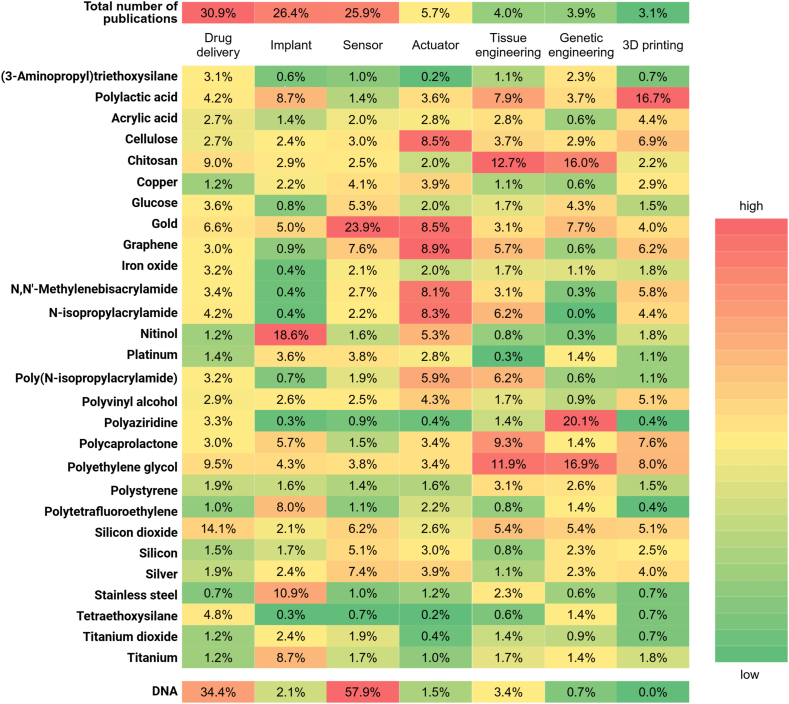
Another representative example of programmable materials is Jahn-Teller metals, which exhibit varying electrical properties depending on environmental changes. Named after the Jahn-Teller effect, which describes the distortion of molecules and ions with geometrically arranged electrons under low pressure environments, these metals enable scientists to transform insulators into conductors simply through the application of pressure. The Jahn-Teller effect allows for the manipulation of electronic states, opening up possibilities for innovative applications in various technological domains. This adaptability to external conditions underscores the significant potential of Jahn-Teller metals in advancing material science and engineering [[45], [46], [47]]. As shown in Fig. 4, the heatmap illustrates the primary applications of programmable biomaterials in biomedicine and the relationships between these applications and the substances used. Because DNA is not a single chemical substance, it is treated separately in this figure, representing DNA-based materials. The color gradient from green to red reflects the relative frequency of each substance mentioned in each application.
Fig. 4.
Heatmap showing the relationship between commonly used substances in programmable biomaterials (left column) and common applications (second row). The percentages indicate the frequency of each substance used in the respective applications.
The burgeoning research in programmable materials has brought a paradigm shift in materials science and engineering, encompassing synthetic biology, chemistry and computational design. These materials, with their unique adaptability for various applications, have opened new opportunities in processing technology, particularly in terms of uniformity and scalability. Recent advancements in programmable materials and associated manufacturing technologies hold great promise, potentially impacting both research and industrial applications significantly [48].
4. Different types of programmable biomaterials and their functions
4.1. Dynamic nucleic acid-based biomaterials
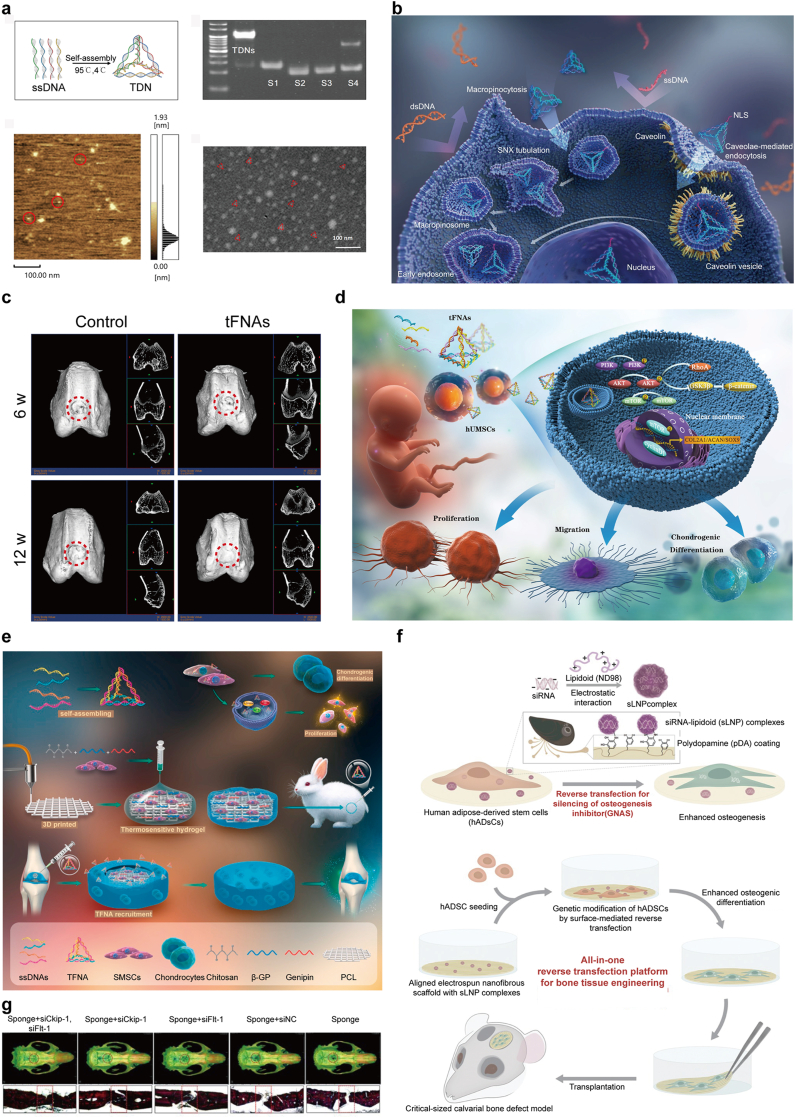
Since the advent of DNA nanotechnology in the 1980s, self-assembled DNA nanostructures have gained global interest for their inherent biocompatibility, remarkable programmability and diverse functionalities. These dynamic DNA nanostructures, which respond to alterations in temperature, pH, metal ion concentration, enzymes and specific oligonucleotides, can be meticulously designed for self-assembly into well-ordered, precisely defined systems [25,[49], [50], [51], [52]]. This is achieved through the reversible nature of hydrogen bonds, following the Watson-Crick base pairing principle. DNA's unique properties facilitate the design and creation of intricate, dynamic and functional nanostructures. Being composed of DNA, these nanostructures exhibit low cytotoxicity, are biocompatible and biodegradable and minimally trigger immune responses. This compatibility enables their use both in vivo and in vitro. Leveraging the reversible hydrogen bonding principle, researchers can engineer various DNA nanostructures with customizable structures and potent functionalities. These structures can adapt their conformation in response to different external stimuli [53] (see Table 1). A particularly noteworthy development in this field is tetrahedral framework nucleic acids (tFNAs). tFNAs, known for their excellent biocompatibility, have been shown to positively influence cellular behaviors such as proliferation, migration, differentiation and preservation of cell phenotype. Due to these properties, tFNAs are extensively utilized in the biomedical domain as three-dimensional DNA nanomaterials, marking significant advances in the application of DNA-based structures in medicine and biotechnology (Fig. 5a and b).
Table 1.
Dynamic nucleic acid-based biomaterials for bone repair.
| Materials | Signaling pathway | Relevant gene/protein expression | Cell type | Function | Ref. |
|---|---|---|---|---|---|
| tFNAs | Wnt/β-catenin | ALP↑, Runx2↑, OPN↑, β-catenin↑, Lef-1↑, cyclin-D↑ | Adipose-derived stem cells | Increased osteogenic potential and proliferation | [70] |
| tFNAs | notch | Runx2↑, OPN↑, NOTCH 1↑, HES 1↑, HEY 1↑ | Dental pulp stem cells | Enhanced proliferation and osteogenic differentiation | [71] |
| tFNAs | Wnt/β-catenin | ALP↑, Runx2↑, OPN↑, β-catenin↑, Lef-1↑, GSK-3β↓ | Periodontal ligament stem cells | Enhanced proliferation and osteogenic differentiation | [72] |
| tFNAs | Wnt/β-catenin | C-Fos↑, NFATc1↑, p-AKT↑, GSK-3↓ | Osteoclasts | Protective effect on the viability | [73] |
| tFNAs | Notch, Wnt/β-catenin | β-catenin↑, Lef-1↑, cyclin-D↑, collagen II↑ | Chondrocytes | Enhanced chondrocyte phenotype and proliferation | [74] |
| tFNAs | Wnt/β-catenin | β-catenin↑, Lef-1↑, cyclin-D↑, Col 2↑, Acan↑, Sox 9↑ | Synovial mesenchymal stem cells | Promoted the proliferation and chondrogenic differentiation | [75] |
| tFNAs | / | RhoA↑, Rock2↑, vinculin↑ | Chondrocytes | Promote chondrocyte motility, migration and chondrogenic differentiation | [76] |
| tFNAs | Tiam1/Rac1, Rhoa/Rock2 | Tiam1↑, Rac1↑, Rhoa↑, Rock2↑, Vcl↑ | Adipose-derived stem cell | Promote cell migration | [77] |
| tFNAs | PI3K/Akt | β-catenin↑, cyclin D1↑, GSK3β↓, RhoA↑, Sox9↑, ACAN↑, COL2↑ | Human umbilical cord mesenchymal stem cells | Enhance the proliferation, migration and chondrogenic differentiation | [62] |
| tFNAs | Wnt/β-catenin, TGF | β-catenin↑, Lef-1↑, cyclin-D↑, CD73↑, CD105↑, collagen II↑, SOX9↑, Smad2/3↑ | Synovium-derived MSCs | Enhanced proliferation, migration and regeneration | [61] |
| tFNAs | MAPK/ERK | ALP↑, RUNX 2↑, OPN↑, TNF-α↓,IL-6↓,IL-1β↓, ERK↓,JNK↓,P38↓ | Periodontal ligament stem cells | Decreased the release of pro-inflammatory cytokines, promoted osteogenic differentiation | [59] |
| tFNAs, miR-2861 | / | HDAC5↓, Runx2↑, ALP↑ | Bone marrow mesenchymal stem cells | Promote osteogenic differentiation | [58] |
| tFNAs-miR(miR-2861) | / | HDAC5↓, ALP↑, Runx2↑ | Bone marrow mesenchymal stem cells | Promoting osteogenic differentiation | [58] |
| tFNAs- clindamycin (CLI) | / | ALP↑, OCN↑, OPN↑, Runx2↑ | Bone mesenchymal stem cells | Outstanding osteogenic and antimicrobial Activity |
[78] |
| tFNAs, Curcumin | MAPK | ALP↑, Runx2↑, Osx↑, p-JNK↓, Bax↓, Caspase 3↓, Bcl 2↑, cytochrome c↓ |
Bone marrow mesenchymal stem cells | Antioxidant, anti-Apoptotic, promote bone regeneration | [57] |
| hydrogel-blended scaffold, CRISPR/Cas9 | / | CD 31↑, vWF↑, OCN↑ | Ob-like cells (mg-63), HUVECs | Promote osteogenesis and angiogenesis | [63] |
| polydopamine-siRNA(lipidoid nanoparticle)-poly(lactic-co-glycolic acid) | / | GNAS↓, OPN↑, COL↑ | Human adipose-derived Stem cells |
Enhanced Osteogenesis and mineralization |
[66] |
| PLLA scaffold, siRNA- semaphorin4d | / | / | Femur osteoporotic defect model | Did not affect osteoclasts, increased osteoblasts, improved new bone formation | [79] |
| chitosan sponge, siCkip-1, siFlt-1 | / | VEGF↑, ALP↑, OCN↑, vWF↑ | Primary rat bone marrow-derived MSCs | Promote osteogenesis and angiogenesis | [67] |
| MiR@TDNs/Li-hep-gel | Wnt/β-catenin | VEGF↑, β-catenin↑, DKK1↓, ALP↑, OCN↑ | Bone mesenchymal Stem cells |
Promote osteogenesis and angiogenesis | [56] |
| hybrid nanoparticle (NP), PEG)-based hydrogel, siRNA(WW domain-containing E3 ubiquitin protein ligase 1) | / | Wwp1↓, Runx2↑, OC↑, ALP↑, type 1 collagen↑, | Mesenchymal stem cells | Promote bone regeneration | [80] |
| poly-D,L-lactic acid-p-dioxanone-polyethylene glycol block co-polymer (PLA-DX-PEG), siRNA(Noggin) | / | Noggin↓ | Mouse dosal muscle pouches | Promote bone regeneration | [81] |
| stearylamine/cholesterol sterosome, siRNA(Noggin) | / | Noggin↓, ALP↑, Runx2↑, OCN↑ | Mesenchymal stem cells | Promoted differentiation | [82] |
Fig. 5.
a) Characterization of TDNs. Reproduced with permission [72]. Copyright 2019, Wiley-VCH Verlag. b) tFNAs can enter cells, unlike naked DNA which cannot cross cell membranes. tFNAs with nuclear localization sequences (NLSs) also reach the nucleus, showcasing their targeted delivery potential. Reproduced with permission [53]. Copyright 2022, Springer Nature. c) tFNAs enhance transformation of SMSC into cartilage cells and boost joint cartilage repair in living organisms. Reproduced with permission [61]. Copyright 2021, KeAi Communications Co. d) Bone regeneration in the rat skull defect model. Reproduced with permission [67]. Copyright 2014, Dove Medical Press Ltd. e) TFNA boosts SMSC growth and aids their transformation into cartilage cells. Combined with a CS hydrogel and 3D-printed polycaprolactone (PCL) scaffold, it effectively repairs rabbit cartilage defects. Reproduced with permission [75]. Copyright 2021, Elsevier BV. f) pDA helps attach sLNP complexes to PLGA scaffolds for efficient siRNA delivery, boosting bone formation from ADSCs. This method offers a unified solution for stem cell engineering, differentiation and implantation, effectively repairing large bone defects in mice. Reproduced with permission [66]. Copyright 2016, Wiley-VCH Verlag.
tFNAs are highly effective biological carriers. They have a very stable tetrahedral structure that can maintain its shape under various physiological conditions, which is crucial for carrying drugs or genetic material in vivo. Additionally, the surface of tFNAs can be functionalized through chemical or biological methods, allowing them to attach various drugs, gene fragments, proteins, or other bioactive molecules, thereby achieving multifunctionality [54]. tFNAs can also efficiently enter cells and release their payloads, making them highly promising for biomedical applications [55]. Wang et al. developed tFNAs to act as delivery vehicles for MiR335-5p and synthesized a heparin lithium hydrogel (Li-hep-gel) as a dual delivery agent for lithium and MiR@tFNAs. Following the insertion of MiR@tFNAs/Li-hep-gel into a steroid-associated osteonecrosis (SAON) model, considerable bone regeneration was noted within the osteonecrotic defect through modulation of the Wnt signaling pathway [56]. Bai et al. developed tFNAs/Cur complexes, an innovative nucleic acid drug system designed to enhance bone regeneration. This system delivers Curcumin (Cur) into BMSC, providing antioxidant and anti-apoptotic benefits. The tFNAs/Cur complexes effectively neutralize the osteogenic suppression induced by TNFα, markedly enhancing the expression of ALP, Runx2 and Osx proteins. Additionally, these complexes reduce the levels of proapoptotic proteins such as Bax and caspase3, while increasing the levels of the anti-apoptotic protein Bcl2 [57]. Li et al. used an RNase H-responsive sequence to link a sticky-end tetrahedral framework nucleic acid (stFNA), a special type of tFNAs characterized by sticky ends designed at the termini of its DNA single strands, with miR-2861, a targeted microRNA designed to regulate histone deacetylase 5 (HDAC5) expression in bone marrow mesenchymal stem cells (B-MSCs). This novel approach culminated in the development of a bioswitchable nanocomposite (stFNA–miR), which effectively unloads and deploys miR-2861 upon intracellular delivery. This targeted mechanism leads to the suppression of HDAC5 expression, thereby enhancing osteogenic differentiation [58].
tFNAs have been shown to suppress the release of pro-inflammatory cellular factors (including IL-6, TNF-α and IL-1β) and in decreasing the production of cellular reactive oxygen species (ROS). This method promotes the migration of periodontal ligament stem cells (PDLSCs) in vitro. It also facilitates their osteogenic differentiation. Additionally, in rat models suffering from periodontitis, tFNAs substantially decreased the infiltration of inflammatory cells and significantly lowered the levels of IL-6 and IL-1β. This led to the inhibition of osteoclastogenesis [59,60]. In rabbit models with articular cartilage defects, injecting tFNAs into the joint cavity enhanced the therapeutic outcomes for cartilage repair compared to control treatments that did not use tFNAs (Fig. 5c) [61]. Human umbilical cord mesenchymal stem cells (hUMSCs) are increasingly recognized as a valuable choice for regenerating cartilage. However, the ongoing challenge remains in developing suitable biomaterials that can reliably guide their self-renewal and differentiation. Fu et al. have explored the use of tFNAs as an innovative method in vitro to influence hUMSC behavior. Their research reveals that tFNAs alter the transcriptome and several signaling pathways in hUMSCs, particularly activating the PI3K/Akt pathway. Moreover, tFNAs modulate the expression of various proteins, including glycogen synthase kinase-3β (GSK3β), RhoA and (mechanistic target of rapamycin) mTOR, along the PI3K-Akt axis. This leads to improved cell proliferation, migration and chondrogenic differentiation of hUMSCs, offering fresh insights on augmenting the chondrogenic potential using tFNAs [62].
CRISPR technology is a revolutionary RNA-guided genome editing method utilizing a nuclease, such as Cas9, in conjunction with a single guide RNA (sgRNA). The sgRNA is structured with a scaffold domain and a spacer region that identifies and attaches to the protospacer adjacent motif (PAM) on the target DNA. This interaction allows the Cas9/sgRNA complex to bind to genomic DNA, causing a double-strand break that facilitates gene editing. In the realm of regenerative medicine, CRISPR has emerged as a pivotal tool for regulating gene expression in pluripotent stem cells, enabling precise modifications in cellular function and characteristics, which is crucial for advancing therapies and understanding cellular mechanisms. Shahabipour and colleagues engineered a hydrogel-based scaffold by blending gelatin methacryloyl (GelMA) with alginate and enhancing it with hydroxyapatite nanoparticles (HAP) to construct an in vitro prevascularized bone model. They utilized CRISPR/Cas9 technology to integrate GFP into the human-like ROSA locus within the genome of human umbilical cord vascular endothelial cells (HUVECs). GFP-labeled HUVECs were then co-cultured with osteoblast-like cells (MG-63) within this 3D hydrogel scaffold to study the interactions between osteoblasts and endothelial cells in a three-dimensional environment. In contrast to mono-cultures, these cells arranged themselves into vessel-like structures and the cells in the co-culture model exhibited actin extensions and spike-like filopodia. Moreover, genes associated with angiogenesis and osteogenesis, such as CD31 and osteocalcin (OCN), showed higher expression in the co-culture compared to the mono-culture [63].
Small interfering RNAs (siRNAs) are double-stranded non-coding molecules that regulate gene expression with high specificity, capable of inactivating a single gene. siRNA therapy targets and suppresses genes hindering osteogenesis, thus promoting bone repair and growth. It modulates osteoblast activity, enhancing differentiation and mineralization and is particularly effective when used with scaffolds in bone tissue engineering. Critical inhibitors of bone regeneration, such as Noggin, WW domain-containing E3 ubiquitin-protein ligase 1, Semaphorin4d (Sema4d) and casein kinase-2 interacting protein-1 (Ckip-1), represent promising molecular targets for siRNA therapies aimed at enhancing bone growth [64,65]. Traditional siRNA delivery methods into stem cells, which depend on solution-based transfection, encounter obstacles such as low transfection efficiency and minimal interaction time between cells and siRNA during prolonged culture periods. To address these limitations, a new method inspired by biology has been developed using polymer-based reverse transfection. This approach utilizes implantable poly(lactic-co-glycolic acid) (PLGA) scaffolds that are modified with siRNA-lipidoid nanoparticle (sLNP) complexes through a polydopamine (pDA) coating. This novel pDA-sLNP-PLGA system is non-toxic and effectively silences genes inhibiting osteogenesis in ADSCs, thereby substantially promoting their osteogenic differentiation [66]. Jia et al. engineered a chitosan sponge scaffold that incorporates two targeted siRNAs: siCkip-1, which targets casein kinase 2 interaction protein 1 and siFlt-1, aimed at the soluble Vascular Endothelial Growth Factor Receptor 1 (VEGFR1). Both siRNAs are recognized for their efficacy in enhancing osteogenesis and angiogenesis. This scaffold is designed to steadily release siRNAs in a neutral phosphate buffer solution (PBS) for over two weeks. Additionally, in the presence of lysozyme, the scaffold demonstrates enhanced degradation capabilities. This characteristic ensures the scaffold's optimal biodegradability within a simulated in vivo environment, facilitating both effective drug delivery over the intended period and subsequent biological clearance once drug release concludes, thus enhancing the implant's biocompatibility and functionality. The application of this scaffold significantly suppressed the expression of targeted genes while elevating levels of osteocalcin, alkaline phosphatase and vascular endothelial growth factor (VEGF)-key factors in the mineralization of the extracellular matrix. Immunofluorescence analysis further verified that the siRNA-modified scaffold amplified the expression of bone and vascular health markers, specifically osteocalcin and von Willebrand factor. In vivo experiments employing a critical-size skull defect model in rats demonstrated substantial bone regeneration following the administration of siCkip-1 and siFlt-1, confirming the scaffold's potential for clinical applications in bone repair and regeneration (Fig. 5d) [67].
The ability of dynamic nucleic acid-based biomaterials to target specific genes and provide a sustained, tailored release of bioactive molecules makes them highly effective in promoting bone regeneration while minimizing side effects. In addition, engineered cells for bone regeneration represent a cutting-edge approach in regenerative medicine, utilizing genetic modification and cellular engineering to enhance the body's natural healing processes. These cells, often derived from mesenchymal stem cells (MSCs), are modified to overexpress osteogenic factors such as BMP-2 or VEGF, which promote bone formation and vascularization [68,69]. By integrating with bioactive scaffolds or delivering targeted gene therapies, engineered cells significantly improve the efficiency of bone repair, offering a promising strategy for addressing complex bone injuries and diseases.
4.2. Electrically responsive biomaterials
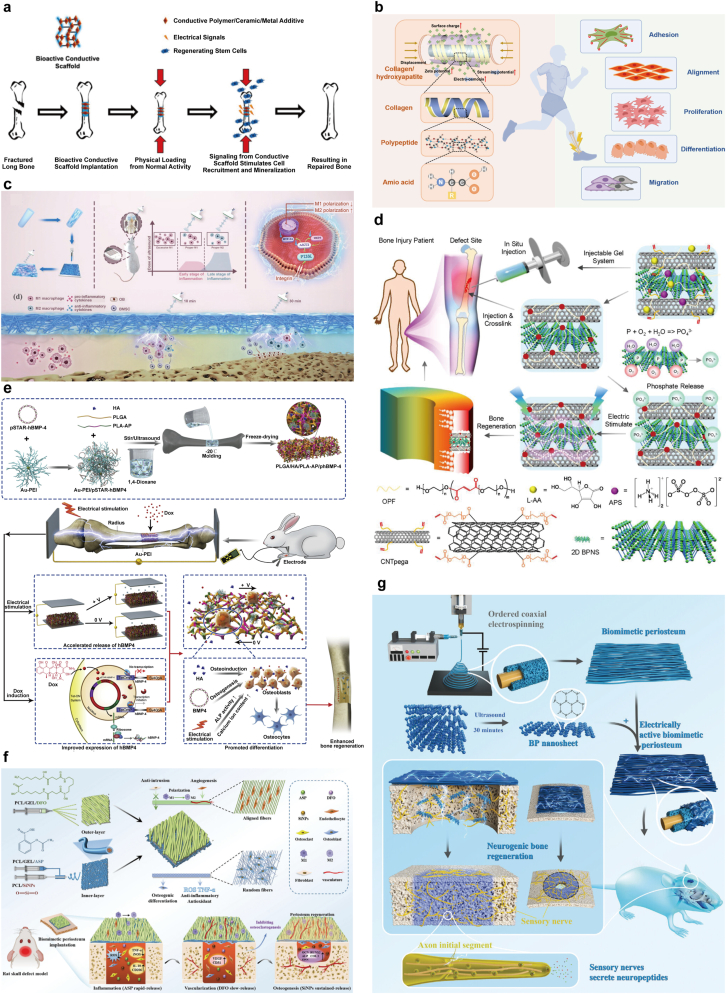
The discovery that endogenous electric fields (EnEFs) are important biophysical cues for maintaining bone homeostasis and promoting regeneration has led to the emergence of electrical stimulation as an external intervention to accelerate bone defect healing. EnEFs are critical in various biological processes such as tissue remodeling and cellular stability. As a biophysical indicator within the extracellular matrix (ECM), EnEFs are recognized for their ability to facilitate the regeneration of multiple tissues and organs, including nerves, bones, skin, muscles and the heart. While bone tissue itself does not conduct electricity, EnEFs are crucial for its growth, homeostasis, remodeling and metabolic processes. The electric fields within bones primarily arise from the piezoelectric effects within the collagen matrix, which makes up 85–90 % of the ECM and about 22 % of the bone structure. They have a non-centrosymmetric polar hexagonal crystal structure and a nanoscale helical morphology that can convert physiological pressure into electrical potential. Based on this, electroactive biomaterials (EABMs) that can change their physical properties in response to electrical stimulation have been developed and these changes can be precisely programmed and controlled by electrical signals. This programmability allows scientists to dynamically adjust the behavior and function of materials to suit different biomedical applications. EABMs consist of various types, including conductive biomaterials like carbon-based materials, conductive polymers, metal nanomaterials and MXenes. They also comprise piezoelectric biomaterials like ceramics and polymers that respond to mechanical stress, as well as other intelligent biomaterials capable of producing electrical signals in response to specific environmental triggers like changes in pH, light, or temperature. The development of these materials not only promotes innovation in biomedical engineering but also provides new strategies and tools for future regenerative medicine and tissue repair (Fig. 6a, b and 6e) [83].
Fig. 6.
a) Under compressive stress, the bone scaffold creates electrical dipoles, attracting osteoblasts to the negative side. There, osteoblasts produce new ECM and minerals, forming healthy bone tissue. Reproduced with permission [93]. Copyright 2022, Multidisciplinary Digital Publishing Institute (MDPI). b) Piezoelectric properties of bone and cellular responses to electrical signals. Reproduced with permission [83]. Copyright 2023, Elsevier. c) Schematic of a smart electroactive tissue engineering scaffold enabling controlled release and expression via electrical stimulation. Reproduced with permission [94]. Copyright 2023, American Chemical Society. d) Schematic of the innovative injectable hydrogel (BP-CNTpega) showcasing its superior mechanical and electrochemical traits. It continuously releases phosphate ions and responds electrically, enhancing cell osteogenesis and bone healing. Reproduced with permission [97]. Copyright 2020, American Chemical Society. e) Schematic of a Smart Electroactive Tissue Engineering Scaffold Enabling Controlled Release and Expression via Electrical Stimulation. Reproduced with permission [83]. Copyright 2023, Elsevier. f) Schematic diagram of the preparation and mechanism of a biomimetic periosteum to programmatically promote bone regeneration. Reproduced with permission. Copyright 2024, John Wiley and Sons Ltd [98]. g) Schematic of PD@BP Enhancing Neurogenic Bone Repair. The electrically active periosteum fosters nerve-stimulated bone healing, offering a promising approach for bone regeneration in clinical settings. Reproduced with permission [99]. Copyright 2023, John Wiley and Sons Ltd.
In vitro studies have shown that electrical stimulation applied via direct, capacitive and inductive coupling can activate key cellular signaling pathways that promote osteogenesis, particularly through calcium/calmodulin-dependent pathways [84]. Direct coupling and capacitive coupling mainly affect the cell membrane, thereby increasing intracellular Ca2+ concentration and promoting the synthesis of prostaglandin E2. This process occurs through voltage-gated calcium channels that activate calcium transport across the membrane. In contrast, inductive coupling stimulation acts on cells through electromagnetic fields, mainly targeting the cytoplasm, triggering the release of intracellular calcium ions in calcium storage areas inside the cell, such as the endoplasmic reticulum. These different electrical stimulation methods further affect cell physiology and metabolic processes by regulating the dynamic balance of intracellular calcium ions and have an important regulatory effect on cell growth and differentiation processes, especially in the osteogenic activity of bone cells and their biological effects. These external stimulations collectively result in cellular activities that elevate calcium levels, in turn, enhancing calmodulin activation. This process is crucial for the proliferation of osteoblasts. Furthermore, these stimulations boost the synthesis of crucial proteins, including VEGF and transforming growth factor-beta1 (TGF-β1), which are vital for bone formation [85,86]. Additionally, membrane proteins, which function as signal integrators, respond to variations in extracellular signals and shifts in transmembrane potential. The mitogen-activated protein kinase (MAPK) signaling pathway is central to cellular signal transduction, mediating critical cellular responses to a variety of external stimuli. This pathway influences key processes such as cell growth, differentiation and apoptosis, thereby playing an indispensable role in cellular function and communication [87]. ATPases on the membrane utilize energy from electrical stimulation at specific frequencies and amplitudes to control the activity of membrane proteins [88,89]. Moreover, the production of ROS may also represent an alternative mechanism through which cells to react to electrical signals [90,91].
Cui and colleagues developed a composite scaffold composed of PLGA/HA/PLA-AP/pSTAR-hBMP-4 (phBMP-4), specifically designed for the controlled and programmable release and expression of growth factors. In this study, the plasmid vector (pSTAR) was utilized to control the expression of human bone morphogenetic protein 4 (hBMP-4) with doxycycline present. This vector is embedded into a triblock copolymer (poly(L-lactic acid)-block-aniline pentamer-block-poly(L-lactic acid), PLA-AP) combined with poly(lactic-co-glycolic acid) and hydroxyapatite to form a composite scaffold (PLGA/HA). In vitro experiments showed that hBMP-4 gene release can be regulated under electrical stimulation, thereby promoting cell proliferation and osteogenic differentiation. In vivo experiments were conducted using a rabbit model with a radial bone defect, in which the scaffold promoted effective bone healing. Research results indicate that the scaffold not only promotes the controllable expression and release of genes, but also holds considerable promise in boosting bone regeneration through the combined influence of biochemical and electrical stimulation. This provides a promising approach for combining gene therapy and electroactive materials in tissue engineering to treat large-scale bone defects [92]. Electroactive scaffolds with electrical conductivity are known to enhance intercellular communication. This capability promotes osteogenesis, especially in the presence of an electric field effect (EnEF) [93]. Sun et al. designed an electroactive membrane combining PCL with potassium-sodium niobate (KNN) to form a biodegradable 3D scaffold featuring efficient nanogenerators. This advanced membrane enables the creation of programmable electrical signals by adjusting the timing and duration of ultrasound stimulation (US) treatment. The customized electric output is intended to precisely control macrophage polarization. Their research introduces a temporal immunomodulation strategy in vivo, aimed at promoting stem cell recruitment by initially activating M1 macrophages and subsequently enhancing osteogenic differentiation through a proliferation of M2 macrophages, driven by reduced AKT2 expression and phosphorylation (Fig. 6c) [94]. Castro et al. developed a bioreactor that electromechanically stimulates piezoelectric scaffolds. This system, through a biomimetic approach, effectively mimics the microenvironment essential for the development and differentiation of bone cells. A distinctive aspect of the bioreactor is its ability to magnetically stimulate magnetoelectric scaffolds, providing mechanical and electrical stimuli to cells via magnetomechanical or magnetoelectrical actions, which rely on the scaffold's piezoelectric characteristics. The proposed magnetic bioreactors enable remote stimulation without direct contact with the material. This experiment validates the effectiveness of these magneto-responsive scaffolds in fostering the adhesion and growth of pre-osteoblasts [95]. Electro-active scaffolds are key in tissue engineering for areas like bone and cartilage repair, where Their ability to provide targeted electrical stimulation enhances the efficacy of these regenerative processes. Lei and colleagues developed a chitosan/hydroxyapatite (HAp) composite Janus film, which functions effectively as a scaffold for guided bone regeneration. By varying the salt concentrations in the electrolyte under an electric field, the internal pore structure of the polysaccharide film can be precisely adjusted. Additionally, the use of bioactive, partially soluble calcium phosphate (CaP) salts helps to create a porous structure within the Janus film, making it well-suited for bone regeneration applications [26]. Panda et al. developed a composite platform using poly(vinylidene difluoride) (PVDF) and barium titanate (BaTiO3, BT) to satisfy stem cell differentiation. The research indicated that direct current (DC) stimulation promoted early osteogenesis in human mesenchymal stem cells (hMSCs), accompanied by an elevated level of intracellular ROS. Conversely, square wave stimulation was observed to guide late osteogenesis, characterized by reduced ROS regeneration [96]. Liu et al. adeptly combined carbon nanotube (CNT)-poly(ethylene glycol)-acrylate (CNTpega) with black phosphorus (BP) in osteoinductive peptide-functionalized (OPF) hydrogel, creating an injectable BP-CNTpega hydrogel. This hydrogel exhibited exceptional mechanical strength and electrical conductivity, making it suitable for bone tissue engineering. The introduction of cross-linkable CNTpega in the hydrogel provided both mechanical support and electrical conductivity. When subjected to electrical stimulation, the hydrogel significantly enhanced the osteogenesis of preosteoblast cells, evident by the upregulated expression of critical genes involved in osteogenic pathways. In vivo applications have shown that the BP-CNTpega hydrogel is capable of efficient in situ gelation and cross-linking, as confirmed by X-ray imaging (Fig. 6d) [97]. Inspired by the natural structure and functionality of the periosteum, Zhao et al. created a biomimetic periosteum designed for the controlled release of multiple agents, aimed at enhancing bone regeneration (Fig. 6f) [98]. Su et al. developed a biomimetic periosteum using coaxial electrospinning, consisting of a PCL core and a DNM shell (PD), enhanced with 2D black phosphorus (BP) for electrical activity (PD@BP). This periosteum promotes nerve regeneration, aided by 2D BP and endogenous electric fields, with DNM providing the necessary extracellular matrix. Its primary function is to stimulate axon growth and neurotransmitter secretion, fostering osteogenesis. Demonstrated through both in vivo and in vitro studies, the PD@BP biomimetic periosteum effectively induces neurogenic osteogenesis, primarily via the Fanconi anemia pathway, offering a novel strategy for bone regeneration with significant clinical application potential (Fig. 6g) [99].
Electrically responsive biomaterials offer significant advantages in bone regeneration due to their ability to respond dynamically to electrical stimuli, promoting cellular activities crucial for osteogenesis. These materials, such as conductive polymers, piezoelectric biomaterials, and metal nanomaterials, enhance intercellular communication and stimulate osteoblast proliferation through calcium/calmodulin pathways and other signaling mechanisms. Electrically responsive biomaterials enable the controlled and sustained release of bioactive molecules and growth factors, thereby improving the precision of tissue engineering approaches. By integrating electrical and biochemical cues, electrically responsive biomaterials facilitate the regeneration of bone tissue, providing an adaptable and programmable platform that enhances bone healing and promotes cellular differentiation.
4.3. Bioactive scaffolds with programmable properties
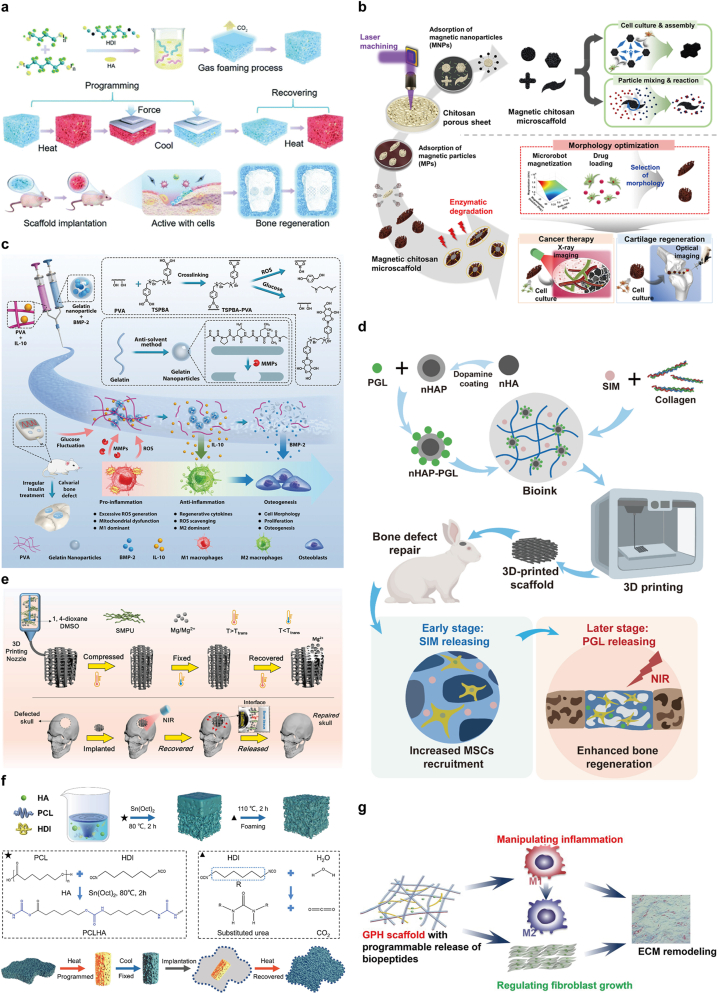
Bioactive scaffolds with programmable properties combine structural support with dynamic, tailored biological functionality. This programmability allows the scaffold to respond to different biological phases of healing, ensuring that signals are delivered at the right time to enhance cellular activity and promote efficient bone repair (see Table 2). Wang et al. designed and synthesized a series of biomimetic hydroxyapatite/shape memory composite scaffolds. These scaffolds feature programmable pore structures, exhibiting diverse parameter and high connectivity, along with adjustable mechanical properties and excellent shape memory capabilities. By altering the amount of hydroxyapatite (HA), the microstructure and pore configuration of these composite scaffolds can be precisely regulated, which further enhances the creation of perforated pores. In addition, changing the HA content can also improve the hydrophilicity, expansion rate, melting point and mechanical properties of the scaffold, making it more suitable for biomedical applications (Fig. 7a) [100]. A magnetic chitosan microscaffold (Mag-C) was designed for adaptability in shape and movement for various biomedical applications, demonstrating its versatility. Mag-C comprises a chitosan microscaffold (CMS) and surface-attached magnetic particles (MPs). The CMS is rapidly and precisely shaped using laser micromachining on a porous chitosan sheet, capitalizing on chitosan's biocompatibility and biodegradability. Adsorption of MPs onto the CMS surface imparts magnetic responsiveness. This surface modification maintains chitosan's inherent properties while enhancing Mag-C's magnetic actuation and cell adhesion capabilities. Mag-C is capable of performing specific roles based on its shape, enabling particle manipulation and assembly by loading various cells and magnetic fields, thus making it suitable for in vitro biomedical applications. The effectiveness of the developed microscaffold was demonstrated in both in vitro and in vivo, especially in the regeneration of knee cartilage. The optimal design and fabrication of this microscaffold are expected to significantly enhance the development of biopolymer-based microscaffolds and micro/nanorobots (Fig. 7b) [101].
Table 2.
Bioactive scaffolds with programmable properties for bone repair.
| Materials | Key Features | Applications | Ref. |
|---|---|---|---|
| Biomimetic Hydroxyapatite/Shape Memory Composite Scaffold | Programmable pore structure, adjustable mechanical properties, shape memory, hydrophilicity | Bone regeneration, adaptable scaffolds | [100] |
| Magnetic Chitosan Microscaffold | Magnetic responsiveness, biocompatibility, biodegradability, enhanced cell adhesion | Knee cartilage regeneration, micro/nanorobots | [101] |
| Injectable Hydrogel Systems | Supports cell viability, differentiation, angiogenesis, bioactive molecule release, native ECM mimicry | Bone tissue engineering, osteoblast promotion | [102,103] |
| Dual-Network Composite Hydrogel | Viscoelastic properties, stress relaxation, biodegradable, ROS and glucose-responsive | Diabetic bone regeneration, tissue repair | [104,105] |
| 3D-Printed Dual-Drug Biomimetic Scaffold | NIR light-responsive, on-demand drug release (SIM, PGL), promotes osteogenesis and stem cell migration | Bone regeneration, rabbit skull defect model | [106] |
| Shape Memory Polyurethane Scaffold | NIR light response, shape recovery, Mg ion release for osteogenesis, strong mechanical properties | Bone repair, minimally invasive applications | [107] |
| Composite SMP Scaffold with Hydroxyapatite | Shape memory, enhanced mechanical properties, promotes bone-like mineralization and tissue repair | Minimally invasive bone repair | [108] |
| Silk Fibroin (SF) Cartilage Repair Scaffold | Sequential release of TGF-β1 and E7, BMSC recruitment | Cartilage regeneration | [12] |
| PCL-Based Electrospun Nanofibrous Scaffold | Programmable release of aprotinin and Tβ4, reduces inflammation, promotes ECM reconstruction | ECM remodeling, tissue engineering | [109] |
| Alginate/Calcium Phosphate Scaffold | Sequential release of PDGF and BMP-2, promotes osteoblast differentiation, cellular infiltration | Bone regeneration, stem cell differentiation | [110] |
| Biodegradable Polyurethane Scaffold with HA | Shape memory, tunable porosity, HA integration for osteoconductivity, in vivo biocompatibility | Bone defect implantation, bone repair | [114] |
| NIR-Responsive PLMC Scaffold with PDA | Shape recovery under NIR, self-fitting for irregular defects, enhanced osteogenesis, minimally invasive | Bone regeneration, cranial bone defects | [115] |
| ZnSr.TCP-SF Scaffold | Bone-mimicking architecture, tunable pore size, enhanced scaffold integrity and bone regeneration potential | Osteoregeneration, in vitro bone modeling | [116] |
| Functionally Graded TPMS Scaffold | Programmable pore size, tailored mechanical properties, smooth interconnectivity, bone-mimicking structure | Bone scaffolds, tissue engineering | [117] |
Fig. 7.
a) A range of biomimetic hydroxyapatite/shape-memory composite scaffolds with programmable pore designs were developed, utilizing poly(ε-caprolactone), polytetrahydrofuran (PTMG) and osteoconductive hydroxyapatite (HA). These programmable porous scaffolds show promising potential for bone regeneration applications. Reproduced with permission [100]. Copyright 2021, Royal Society of Chemistry. b) Schematic of a multifunctional, biodegradable magnetic chitosan microscaffold (Mag-C) with customizable shape for medical uses. These microscaffolds have tunable pores and sizes for specific needs, offering multiple functions. Their versatility was shown in lab tests and real-world treatments for liver cancer and knee cartilage repair. Reproduced with permission [101]. Copyright 2021, American Chemical Society. c) Diagnostic Strategy for Sensing Pathological Signals (like glucose changes, ROS, MMPs) in Diabetes, Guiding Timed Drug Release for Enhanced Tissue Repair. Reproduced with permission [105]. Copyright 2022, Wiley-Blackwell. d) Schematic of a 3D-printed scaffold responsive to NIR light, enabling controlled drug release and improved bone healing [106]. e) Infrared-responsive scaffold, made with low-temperature rapid prototyping (LT-RP) 3D printing, supports bone growth in both in vitro and in vivo studies. Reproduced with permission [107]. Copyright 2022, KeAi Communications Co. f) Developed a porous, biocompatible bone scaffold from shape memory polymers (SMP), using poly(ε-caprolactone) diol, hexamethylene diisocyanate (HDI)and hydroxyapatite (HA). These scaffolds can be programmed to a temporary shape and then return to their original form to fit bone defects. Reproduced with permission [108]. Copyright 2022, Elsevier Ltd. g) Bioactive PCL scaffolds designed for controlled release of aprotinin and thymosin β4 in a programmable manner. Reproduced with permission [109]. Copyright 2023, Oxford University Press.
Injectable hydrogel systems have advanced significantly in biomedical applications, notably in tissue engineering. These hydrogels play a key role in supporting the cell survival environment, not only improving cell viability and adhesion, but also promoting cell differentiation and effective integration with host tissues. They replicate the native ECM, offering a biocompatible and regenerative environment, particularly for bone tissue engineering. Hydrogels have shown unique potential in promoting bone formation and repair, primarily by promoting osteoblast differentiation, angiogenesis and the controlled release of bioactive molecules. Their porous, dilute crosslinked structure and high water content, akin to the natural ECM, enable them to effectively tailor cell activities through architectural, chemical and drug delivery features [102,103]. In recent decades, hydrogels have received increasing attention for their application potential in tissue engineering, especially when combined with advanced technologies such as microfluidic platforms and 3D bioprinting. These high-tech methods allow researchers to precisely control polymer precursors within microscale channels, thereby greatly improving the ability to build complex structures and broadening design possibilities. Scaffold biomaterials are designed to mechanically mimic the viscoelastic properties of the native ECM to accommodate complex local defects that may include irregular shapes, shear, tension and the structural integrity of surrounding tissues. Recent studies have shown that stem cells are extremely sensitive to the mechanical environment and that the viscoelastic properties of the hydrogel matrix, such as stress relaxation and creep behavior, can significantly promote bone regeneration. Based on these findings, a dual-network composite hydrogel was developed in this study, whose design not only reproduces the mechanical properties of natural ECM but also provides more physiologically relevant viscoelastic cues to optimize the cell regeneration and tissue repair environment. A dual-logic hydrogel, both diagnostic and therapeutic, was created for diabetic bone regeneration. It comprises a double network of phenylboronic-acid-crosslinked poly(vinyl alcohol) (PVA) and gelatin colloids. The PVA network, responsive in the diagnostic logic, can reversibly degrade upon exposure to ROS or high glucose levels. The gelatin colloidal network offers bioactive patterns that enhance cell affinity and allow for matrix metalloproteinase (MMP)-induced degradation, enabling cargo delivery initiation in response to the dynamic diabetic microenvironment. Therapeutically, the hydrogel, loaded with interleukin 10 (IL-10) and bone morphogenetic protein-2 (BMP-2), releases IL-10 initially for immune regulation, followed by BMP-2 delivery later, aligning with osteoblast activation during the later stages of tissue regeneration (Fig. 7c) [104,105].
A 3D-printed dual-drug-loaded biomimetic scaffold utilizing near-infrared (NIR) light-responsive properties was developed to optimize the bone regeneration process. The scaffold effectively delivers the bone formation-promoting drug pargyline (PGL) via polydopamine-coated hydroxyapatite nanoparticles. In addition, to enhance its drug release capability and therapeutic effect, the small molecule chemoattractant drug simvastatin (SIM) is integrated into the scaffold and is added directly to the hydroxyapatite/collagen bioink used for 3D printing. This scaffold was designed for an on-demand, sequential drug release where SIM is rapidly released in the initial stages, followed by PGL released in a NIR light-responsive manner. The SIM-loaded scaffold effectively accelerated stem cell migration. Furthermore, the results of the rabbit skull defect model showed that the on-demand sequential release mechanism of the biomaterial effectively enhanced the activity of alkaline phosphatase (ALP), significantly increased the gene expression of markers related to osteogenesis and promoted new bone formation (Fig. 7d) [106]. Taking advantage of the thermal response properties of shape memory polyurethane (SMPU) and the photothermal effect and bioactive properties of magnesium (Mg), a scaffold with near-infrared light response function was developed. This scaffold, produced using low-temperature rapid prototyping (LT-RP) 3D printing technology, recovers its shape under NIR light post-implantation, ensuring tight contact with surrounding tissues. Gradual release of Mg ions, due to the degradation of Mg particles, aids in promoting osteogenesis, essential for bone repair. Significantly, this compressed composite scaffold exhibited the ability to lift a weight of 100 g under NIR light, an amount over 1700 times its own weight (Fig. 7e) [107]. A porous, composite, biocompatible bone scaffold utilizing shape memory polymer (SMP) materials was developed, incorporating poly(ε-caprolactone) diol, hexamethylene diisocyanate (HDI) and hydroxyapatite (HA). Programmed to adopt a temporary shape, these scaffolds are capable of reverting to their original form, effectively occupying the site of a bone defect. The incorporation of HA not only enhances the mechanical properties of the scaffolds but also fosters cell adhesion and modulates the speed of shape recovery. During in vitro mineralization studies, HA has been shown to facilitate the formation and deposition of bone-like hydroxyapatite, thereby accelerating the repair of damaged tissues. Importantly, no inflammatory issues were observed following in vivo implantations. This innovative approach to programmable scaffolds shows great promise for minimally invasive bone repair applications (Fig. 7f) [108].
Mao et al. developed a cell-free cartilage repair scaffold based on silk fibroin (SF) that can programmatically release bioactive molecules specifically targeting cartilage regeneration. The scaffold is designed to sequentially release two bioactive factors: transforming growth factor-β1 (TGF-β1) and a BMSC-specific affinity peptide (E7). TGF-β1 was initially loaded onto the SF scaffold via physical absorption and E7 was subsequently incorporated through a gradient degradation coating of silk fibroin methacryloyl (SilMA) and hyaluronic acid methacryloyl (HAMA). This biomimetic scaffold was shown to maintain excellent structural and cartilage-like mechanical properties, creating a favorable 3D microenvironment for cartilage reconstruction. In vitro studies demonstrated that the scaffold effectively induced BMSC recruitment and chondrogenic differentiation due to the initial rapid release of E7 followed by the slow and sustained release of TGF-β1. Further in vivo experiments using a rabbit cartilage defect model showed that the scaffold significantly enhanced in situ cartilage regeneration. The synergistic release of E7 and TGF-β1, combined with the intrinsic properties of the SF scaffold, provides a promising approach to improving cartilage tissue engineering [12].
Xiang et al. developed a PCL-based electrospun nanofibrous scaffold with a core-shell structure designed for the programmable release of aprotinin and thymosin β4 (Tβ4). The core of the scaffold contains hyaluronic acid (HA) and Tβ4, while its shell is composed of PCL and the outer layer is coated with a heparin/gelatin/aprotinin layer. This design allows aprotinin to be gradually released from the shell after implantation, effectively reducing the inflammatory response caused by excessive recruitment of inflammatory cells on the scaffold surface. Within the first three days after implantation, Tβ4 is released, a process that not only hinders the fusion of macrophages to form multinucleated foreign giant cells but also promotes the transformation of macrophages from M1 to M2 types, thereby contributing to the ECM remodeling and tissue repair. At the same time, the gelatin in the scaffold is conducive to cell proliferation and migration due to its gradual degradation, while the heparin coating effectively prevents fibrosis of the ECM around the implant and maintains the health of the implanted area. This PCL-based scaffold not only effectively controls local inflammatory responses through its strategic, programmable biopeptide release mechanism but also promotes normal ECM reconstruction of damaged tissues, showing great potential in the fields of regenerative medicine and tissue engineering due to its application potential (Fig. 7g) [109].
A novel biomaterial scaffold, integrating alginate matrices with calcium phosphate scaffolding, was designed to enable a programmed release for growth factors. This scaffold consists of a strategic blend of alginate microspheres, alginate hydrogels and a novel resorbable calcium phosphate-based cement (ReCaPP). Within this structure, platelet-derived growth factor (PDGF) and BMP-2 were sequentially released, achieving a desired three-day overlap in the delivery of PDGF followed by BMP-2. Investigations using a three-dimensional coculture model revealed that this specific sequence of PDGF and BMP-2 release significantly influenced cellular infiltration into the scaffold and the expression of ALP. These findings suggest that the strategically timed presentation of PDGF followed by BMP-2 effectively promotes the differentiation of human mesenchymal stem cells (hMSCs) toward an osteoblast phenotype while concurrently enhancing cellular infiltration within the scaffold [110]. Microcapsules containing various bioactive molecules were immobilized on scaffold surfaces, allowing for multimodal activation through physical (ultrasound, laser radiation) and biological (enzymatic treatment) stimuli. This arrangement facilitates controlled release of the encapsulated substances from the scaffolds [111].
While 3D printing offers exciting possibilities in biofabrication, it faces challenges in creating complex, non-linear shapes and in varying the properties of multi-material structures over time. Compared to 3D bioprinting, 4D bioprinting can construct dynamic active structures that accurately mimic the intrinsic dynamics and conformational changes of natural tissues, thus meeting higher application demands in biomedical engineering. Since the advent of 4D printing technology in 2013, the field has garnered extensive attention. Two key factors for achieving ideal 4D printing outcomes are smart materials and intelligent design. Smart materials refer to materials that can change their shape or properties under external stimuli, while intelligent design aims to achieve programmable transformations by fully considering the time-dependent characteristics of the printed objects [112,113]. In the future, 4D printing technology is expected to further develop to handle biocompatible smart materials, biochemical substances, and living cells, thereby generating dynamic 3D living structures. Programmable active scaffold materials are designed to provide not just physical support for tissue regeneration but also to actively participate in the healing process. Their "programmability" lies in their ability to respond to biological signals and environmental changes, making them dynamic participants in the regeneration of tissues.
Bioactive scaffolds with programmable properties offer significant advantages in bone regeneration by providing not only mechanical support but also actively participating in the healing process. Their programmability allows them to release bioactive molecules in a controlled and sequential manner, tailored to the different phases of bone healing. This ensures that growth factors such as BMP-2, IL-10, and PDGF are delivered at the optimal time to promote osteoblast differentiation, angiogenesis, and immune regulation. Furthermore, these scaffolds can be designed with customizable pore structures and materials that mimic the natural extracellular matrix, enhancing cellular infiltration, proliferation, and differentiation. The dynamic and responsive nature of these scaffolds makes them highly effective in promoting the regeneration of complex bone structures while minimizing inflammation and ensuring tissue integration.
4.4. Nanomaterials for targeted bone regeneration
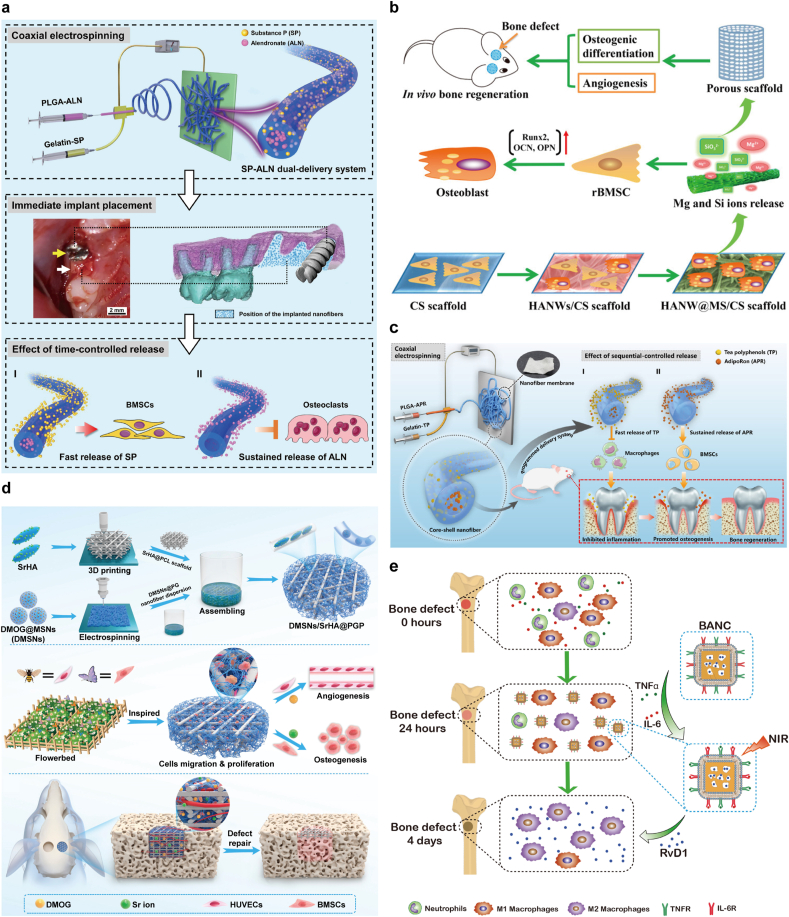
Nanomaterials for targeted bone regeneration for bone repair represent a cutting-edge convergence of nanotechnology and bone tissue engineering. These materials are engineered at the nanoscale to interact specifically with bone tissues, offering innovative approaches to promote bone repair and regeneration. A dual delivery system was developed using coaxial electrospinning to create a core-shell structure with alendronate (ALN) in the core and Substance P (SP) in the shell, ensuring a programmed release consistent with treatment needs. SP aimed to promote bone regeneration while ALN was intended to inhibit bone resorption, thereby enhancing implant osseointegration. The dual-delivery system successfully controlled the release rates of SP and ALN, thereby enhancing mesenchymal stem cell recruitment and osteogenesis while reducing osteoclast activity (Fig. 8a) [118]. Developing novel strategies that utilize magnetic nanoparticles (MNPs), magnetic field technology and stem cells is enhancing development of bone tissue engineering. These innovative strategies significantly enhance osteogenic differentiation, angiogenesis and bone regeneration by combining magnetic nanoparticles (MNPs) and scaffolds with magnetic fields and stem cells. This approach has been shown to increase the effects of bone tissue engineering by 2–3 times compared to the control group. This approach not only enhances the interaction between cells and scaffolds, but also regulates the local microenvironment through magnetic fields, promoting the directional differentiation of stem cells and new bone formation, thereby accelerating the bone repair process. The potential clinical applications of these strategies significantly improve bone repair and regeneration outcomes [119]. Sun et al. synthesized hydroxyapatite nanowire@magnesium silicate nanosheet core-shell structured hierarchical nanocomposites, referred to as nanobrushes. They were integrated into a chitosan matrix to form a scaffold. Nanobrushes exhibit large specific surface areas and pore volumes, which benefit high-performance drug loading and sustained release. The biological performance of the scaffolds was evaluated both in vitro and in vivo. In vitro, the scaffold demonstrated enhanced attachment and proliferation of rat bone marrow mesenchymal stem cells (rBMSCs) and promoted the expression of osteogenic differentiation-related genes and VEGF. In vivo studies using a rat bone defect model demonstrated that the scaffold significantly stimulated bone regeneration and angiogenesis. This ability is attributed to the scaffold's provision of a favorable environment for cell attachment, proliferation and differentiation, facilitated by the sustained release of bioactive ions necessary for bone tissue regeneration (Fig. 8b) [120]. He et al. developed core-shell nanofibers designed for programmed, sequential release of tea polyphenols (TP) and AdipoRon (APR), targeting inflammation control and bone regeneration enhancement. These nanofibers, synthesized through electrospinning, feature controlled sequential release functionality. The release profiles revealed an initial rapid release of TP, followed by a sustained release of APR. This design effectively diminished proinflammatory cytokine levels and augmented osteogenic differentiation in an inflammatory microenvironment (Fig. 8c) [121].Controlling pro-inflammation triggered by cytokines and promoting the anti-inflammatory response of M2 macrophages are crucial for osteogenesis during bone tissue repair. Zhou et al. used 3D printing and electrospinning technology to develop a biomimetic scaffold that mimics the extracellular matrix for bone regeneration. The scaffold adopts a core-shell structure incorporating dimethyloxalylglycine (DMOG)-loaded mesoporous silica nanoparticles and a 3D-printed framework containing strontium-enriched hydroxyapatite and PCL. This design promotes the sequential release of DMOG and strontium ions, enhancing angiogenesis and osteogenesis. In vitro tests showed that the scaffold enhanced cell attachment, proliferation and differentiation. It significantly upregulated genes related to osteogenic differentiation and promoted the expression of VEGF, which is critical for blood vessel formation. In vivo experiments using a rat bone defect model demonstrated that the scaffold effectively supported new bone formation and vascularization, performing significantly better than controls and showing promising applications in bone defect repair (Fig. 8d) [122]. Yin et al. synthesized and characterized biomimetic anti-inflammatory nanocapsules (BANCs) designed to reduce inflammation and promote M2 macrophage polarization for bone tissue repair. These nanocapsules were coated with lipopolysaccharide-treated macrophage membranes containing cytokine receptors and were encapsulated in gold nanocages containing the resolving agent Resolvin D1 (RvD1). This structure allows the controlled release of RvD1 by near-infrared laser irradiation, consistent with the healing phase of bone tissue repair. Experimental results showed that in vitro, BANCs effectively block pro-inflammatory cytokines and promote M2 polarization of macrophages, which is critical for bone tissue regeneration and repair. In vivo testing was performed using a rat model with a femoral defect. These studies demonstrated that BANCs significantly enhanced bone regeneration and angiogenesis compared to controls. This is attributed to the ability of the nanocapsules to modulate the inflammatory environment and promote beneficial cellular activity at the defect site. Using biomimetic nanomaterials with anti-inflammatory properties, this approach offers a potential treatment for bone defects caused by trauma or associated with inflammation (Fig. 8e) [123]. Programmable nanoparticle materials offer a promising and innovative approach for enhancing bone repair. Their ability to deliver targeted therapies, support bone regeneration and provide diagnostic capabilities positions them at the forefront of advancements in bone tissue engineering. As research in this field progresses, these nanoparticles are expected to play an increasingly significant role in the treatment of bone-related injuries and diseases.
Fig. 8.
a) The SP-ALN dual-delivery system created by coaxial electrospinning and its application in an implant model with dual-delivery fibers in the tooth socket. Reproduced with permission [118]. Copyright 2021, Elsevier BV. b) Synthesis of hydroxyapatite nanowire@magnesium silicate sheet (HANW@MS) core-shell nanocomposites is detailed. The HANW@MS/CS scaffold significantly promotes bone healing by enhancing rBMSC osteogenesis and angiogenesis. Reproduced with permission [120]. Copyright 2017, American Chemical Society. c) Core-shell nanofibers designed for sequential release of tea polyphenols (TP) and AdipoRon (APR) to manage inflammation and enhance bone healing in periodontitis-related alveolar bone defects. Reproduced with permission [121]. Copyright 2021, American Chemical Society. d) Schematic of DMSNs/SrHA@PGP Scaffold Creation for Dual Delivery of Angiogenic Drugs and Osteogenic Ions, Using 3D Printing and Electrospinning. Reproduced with permission [122]. Copyright 2023, American Chemical Society. e) Biomimetic anti-inflammatory nano-capsules (BANC) with surface cytokine receptors neutralize pro-inflammatory cytokines, easing inflammation. BANC encourages M2 macrophage polarization and inhibits M1 with Resolvin D1 (RvD1) released under NIR light, enhancing femoral bone repair. Reproduced with permission [123]. Copyright 2020, Elsevier BV.
Nanomaterials for targeted bone regeneration provide precise and controlled delivery of therapeutic agents, enabling enhanced bone regeneration by targeting specific cellular processes. Their nanoscale design allows for effective interaction with bone tissues, promoting osteogenesis, angiogenesis, and inflammation control. By utilizing systems such as core-shell nanofibers, magnetic nanoparticles, and biomimetic nanocapsules, these materials can sequentially release bioactive molecules, regulate the local microenvironment, and support mesenchymal stem cell differentiation. This advanced level of programmability, combined with their ability to modulate immune responses and promote cell attachment and proliferation, positions nanomaterials for targeted bone regeneration as a tool in bone tissue engineering and regenerative medicine.
4.5. Surface-engineered implants for sequential regeneration
Surface-engineered implants for sequential regeneration represent a cutting-edge class of materials in the field of materials science and engineering. These materials are uniquely designed to alter their surface characteristics in response to specific stimuli, thereby providing tailored functionalities for various applications. The programmable sequential bone repair function is achieved by modifying the surfaces of implants or biomaterials. Poly(aryl-ether-ether-ketone) (PEEK), known for its excellent mechanical properties, intrinsic radiolucency and biostability, is a promising material in orthopedics. This widely utilized thermoplastic is favored in engineering applications for its high temperature resilience and advantageous mechanical characteristics. PEEK is a semi-crystalline polymer whose molecular structure consists of an aromatic backbone where ketone and ether groups are linked to each other. PEEK, a high-performance polymer, demonstrates superior chemical stability, a high melting point of 340 °C and robust resistance to radiation and sterilization processes. Its elastic modulus ranges from 3.7 to 4.0 GPa, with a tensile strength of 103 MPa. In addition, the radiolucency of PEEK allows implants to be effectively evaluated by X-ray imaging. Due to its unique physical and chemical properties, PEEK is extensively applied in the field of biomedicine, including spinal fusion, cranial reconstruction and dental implants [124,125]. A programmed surface on PEEK is designed and fabricated to sequentially guide osteoimmunomodulation and bone regeneration. Xie et al. modified PEEK implants using a combined strategy of humanized interleukin-10 (IL-10) and dexamethasone (DEX), a widely used glucocorticoid. This modification takes advantage of IL-10-activated immunomodulatory pathways and a small amount of DEX released early after implantation to initiate an immune response. The PEEK surface is engineered to swiftly release IL-10 during the initial week and to methodically release DEX across the subsequent four weeks. The synergistic effect of IL-10 and DEX triggered a controllable, mild inflammatory response in the early stages. After this stage, significant polarization of macrophages toward the M2 type was observed, accompanied by an upregulation of autophagy-related factors. This precisely regulated immune response not only promotes the initial osteogenesis process but also further enhances subsequent bone tissue regeneration through the sustained release of DEX (Fig. 9a) [126]. Zheng et al. developed a degradable hybrid coating for PEEK implants, which consists of poly(lactide-co-glycolide) and nanohydroxyapatite (nHA) loaded with sodium ALN. IL-4 was effectively attached to the hybrid coating's surface through the use of nitrogen plasma-enhanced ion implantation followed by soaking in an IL-4 solution. Within the first few days after implantation, the significant release of IL-4, ALN and Ca2+ synergistically alleviated the early acute inflammatory response, thereby creating an osteoimmunomodulatory microenvironment conducive to bone regeneration. Subsequently, the slow and sustained release of ALN and Ca2+ continued for several weeks, which not only enhanced osteogenic activity but also inhibited osteoclast formation. This dual-action strategy significantly improved the integration of bone implants into the surrounding bone tissue and effectively enhanced bone integration even under osteoporotic conditions (Fig. 9b) [127]. Wei et al. enhanced bone integration for PEEK materials through the use of a porous structure and surface activation methods. They crafted porous PEEK scaffolds using fused deposition modeling, then applied a magnesium-enriched polydopamine (PDA) coating. This surface treatment improved the osseointegration at the interface of the scaffolds and as the PDA coating degraded, the magnesium released promoted early bone formation by stimulating vascularization (Fig. 9c) [128].
Fig. 9.
a) Engineered surfaces are designed for immune-driven bone growth, with a PTMC coating that degrades to programmatically release IL-10 and DEX. Reproduced with permission [126]. Copyright 2021, Elsevier. b) Engineered surface of PEEK, designed to improve bone-implant integration in osteoporotic conditions. It promotes a beneficial osteoimmune environment initially, followed by enhanced bone healing. Reproduced with permission [127]. Copyright 2022, KeAi Communications Co. c) Magnesium-enhanced 3D-printed PEEK scaffolds support blood vessel and bone growth. These porous structures are engineered for repairing bone loss due to periodontitis. Reproduced with permission [128]. Copyright 2023, KeAi Communications Co.
Surface-engineered implants for sequential regeneration offer significant advantages in bone regeneration by allowing for the precise release of bioactive substances that influence cellular behavior and immune responses. This targeted approach helps create a favorable environment for healing, promoting the repair and integration of bone tissue with implants. By strategically controlling the release of these substances, these materials can enhance the regenerative process, especially in complex situations like osteoporosis, ultimately improving the success rates of orthopedic implants.
4.6. Stimuli-responsive release materials
Stimuli-responsive release materials represent a major advancement in material science, particularly for controlled and precise substance release. These materials are engineered to release therapeutic agents at specific times or in response to environmental stimuli, such as changes in pH, temperature, or enzymes, enhancing treatment efficacy, reducing side effects, and improving patient compliance.
BMP-2 plays a key role in promoting osteogenesis. However, traditional delivery methods face challenges due to safety concerns with viral vectors [129]. To address this, Ding et al. designed a biomimetic scaffold combining silk fibroin (SF) and hydroxyapatite (HA) nanoparticles, creating a nanocomposite that precisely controls BMP-2 release. By modifying the BMP-2 ratio, the scaffold maintains biological activity and regulates release kinetics, optimizing bone regeneration (Fig. 10a) [130]. Additionally, BMP-2 loaded onto biphasic calcium phosphate (BCP) with a multilayer coating allows sustained BMP-2 release, reducing the initial burst phase and enhancing osteogenesis. This strategy improves bone healing by controlling BMP-2 release over 3–7 days while maintaining BCP's porous structure, essential for bone repair (Fig. 10b) [131]. To facilitate the delivery of multiple bioactive factors with programmed release kinetics, diverse populations of microspheres carrying different factors are utilized [132]. Ding et al. developed new SF microspheres measuring approximately 1.5 ± 0.3 μm in diameter, containing BMP-2 and VEGF. They utilized capillary technology to ensure controlled production, enabling programmable and sequential release of bioactive factors. The rapid release of VEGF enhances angiogenesis early in the bone healing process by mimicking natural vascularization. Simultaneously, the sustained release of BMP-2 aids in supporting bone cell differentiation and enhancing bone tissue formation. After 12 weeks of in vivo trials on rat skull defects, the microsphere system proved to significantly enhance bone regeneration (Fig. 10c) [133,134]. In order to precisely control the release of recombinant human bone morphogenetic protein 2 (rhBMP-2) during bone regeneration, rhBMP-2 was attached to mesoporous bioglass nanoparticles (MBGN) through amide bonds. These particles were then mixed with gelatin methacrylate (GelMA) and photo-cross-linked to form a GelMA/MBGNs-rhBMP-2 hydrogel membrane. The hydrogel membrane is designed to manage the release of rhBMP-2 during the initial stages of bone regeneration and as treatment progresses, calcium and silicon ions are slowly released to enhance the osteogenesis process. This design not only fosters the maturation and growth of bone tissue, but its unique ion release mechanism accelerates cell differentiation into osteoblasts and mineralization of bone tissue, boosting the efficiency of bone regeneration (Fig. 10d) [135]. Other innovative approaches include chitosan liposomes combined with microfluidic-technology-prepared hydrogel microspheres for resveratrol and BMP-2 release, promoting immune regulation and osteogenesis. Similarly, a dual-drug release system using electrospun fiber mats (EFM) provided rapid deferoxamine (DFO) release for angiogenesis and sustained dexamethasone (DEX) release for osteogenesis, facilitating vascular and skeletal regeneration (Fig. 10e) [136]. Interleukin 4 (IL4) was embedded into nanofibrous, heparin-enhanced gelatin microspheres (NHG-MS). Heparin within these microspheres created specific sites for IL4 attachment, preserving its activity and allowing controlled release. The delivery of IL4 through NHG-MS successfully shifted pro-inflammatory M1 macrophages towards a healing M2 type, effectively managing inflammation and promoting osteoblast differentiation. This approach markedly enhanced bone repair, demonstrating the NHG-MS's potential in tissue regeneration [137].
Fig. 10.
a) A programmed biomimetic design boosts bone growth in HA and SF scaffolds, enabling precise BMP-2 release for enhanced osteogenesis. Reproduced with permission [130]. Copyright 2016, American Chemical Society. b) A multi-layered biphasic calcium phosphate (BCP) scaffold is designed for programmed release of BMP-2. This design aims to prevent the initial burst release and instead promote a controlled release during the osteogenic cells' differentiation phase. Reproduced with permission [131]. Copyright 2021, Elsevier BV. c) A cell-free bone tissue engineering system using a SF/nanohydroxyapatite (nHAp) scaffold was created. This system embeds low doses of BMP-2 and VEGF, which are released in a controlled way to support bone formation and vascularization. Reproduced with permission [133]. Copyright 2017, Royal Society of Chemistry. d) rhBMP-2 bonded to mesoporous bioglass nanoparticles (MBGNs) forms a GelMA/MBGNs-rhBMP-2 hydrogel for staged release: initial rhBMP-2 release for early bone healing, followed by calcium and silicon ion release for sustained osteogenesis. Reproduced with permission [135]. Copyright 2020, American Chemical Society. e) DFO and DEX synergistically promote osteogenic differentiation through the Wnt/β-catenin pathway. Their combined release from EFM leads to enhanced vascularized bone regeneration in rat skull defects. Reproduced with permission [136]. Copyright 2022, John Wiley and Sons Ltd. f) A smart hydrogel programmed for on-demand, pulsatile and sustained release of PTH, enhanced with magnetic actuation to improve osteogenesis in critical-sized defects. Reproduced with permission [138]. Copyright 2023, American Chemical Society.
A new magnetized hydrogel was created to promote bone development by controlled release of parathyroid hormone (PTH) and magnetic activation. The hydrogel consists of a GelMA-PVA (GP) biphasic system, infused with magnetic nanoparticles (GPM) and PTH (GPMP). Early testing showed that PTH is released in a pulsating manner, initially triggered by magnetization during the first four days, followed by steady release for a month managed by the GP structure. Biocompatibility was confirmed by in vitro tests for all versions. Notably, the presence of PTH considerably boosted the growth of MC3T3-E1 pre-osteoblasts. In vivo experiments showed significant bone regeneration improvements, with notable increases in bone volume and density in both GPM and GPMP groups (120 % and 251 % increases, respectively, compared to untreated controls). These results highlight the strong osteogenic capacity of these hydrogels and their effectiveness in enhancing bone repair (Fig. 10f) [138].
Osteochondral scaffolds offer mechanical support and an appropriate cellular microenvironment. This environment facilitates the growth and differentiation of BMSC, thus enhancing osteochondral regeneration. BE-PSA's composite scaffold is prepared using 3D printing technology and multiple material modifications and it features a gradient structure and programmed release of molecules. The scaffold design contains different micron-sized pores above and below to accommodate various conditions for bone and cartilage regeneration. To sequentially regulate BMSC activity, a fast-degrading sodium alginate (SA) hydrogel initially releases the E7 peptide, enhancing BMSC migration within the first 72 h. At the same time, a more slowly degrading SFporous matrix enables a prolonged release of the B2A peptide, supporting the differentiation of BMSCs into both bone and cartilage lineages for over 300 h, due to the varying degradation rates of the SAhydrogel and SFmatrix [139,140].
Stimuli-responsive release materials by tailoring release profiles, these materials can optimize conditions for osteogenesis and angiogenesis, mitigating initial inflammatory responses while sustaining the necessary signals for cell differentiation and tissue integration. Such innovative systems not only improve the efficacy of treatments but also enhance the biocompatibility and functional integration of implants within the host environment, ultimately leading to more successful regeneration of bone tissue.
5. Conclusion and future perspectives
The application of programmable biomaterials in bone regeneration addresses the complex, multi-phase process of bone healing, which includes inflammation, repair, and remodeling. Each type of programmable material plays a specific role in supporting these stages. Dynamic nucleic acid-based biomaterials, for example, are designed to precisely control the expression of osteogenic genes, such as BMP-2, by delivering nucleic acids in a spatiotemporal manner. This targeted gene regulation enhances osteoblast differentiation and bone formation. Bioactive scaffolds with programmable properties provide both mechanical support and biological signals to the injury site, promoting cell adhesion, proliferation, and osteogenesis. These scaffolds can be engineered to release growth factors like VEGF and TGF-β1, which are essential for angiogenesis and bone repair. Stimuli-responsive release materials offer controlled and sustained delivery of bioactive molecules, ensuring that osteoinductive factors are available throughout the healing process. This controlled release minimizes the initial burst effect and aligns the delivery of growth factors with the body's natural healing phases, optimizing bone tissue regeneration. Together, these programmable materials meet the specific demands of bone regeneration by dynamically responding to the microenvironment and supporting cellular activities essential for effective bone healing.
The exploration of programmable biomaterials in bone tissue engineering presents intriguing challenges that necessitate an interdisciplinary approach, encompassing biology, chemistry, physics and engineering. Despite their vast potential in numerous fields, the development and application of programmable biomaterials are still in the stages of continuous growth and optimization. Particularly in bone repair, precise control over the dosage and release kinetics of growth factors or other bioactive molecules is crucial. Factors such as the rate of material degradation, varying environmental conditions and the specific implantation site can significantly affect this process, often leading to unforeseeable outcomes [141]. Moreover, the application of materials like shape-memory polymers (SMP), which require precise positioning and fitting to the surgical site following temperature regulation, poses higher demands on surgical techniques and medical conditions [142]. The long-term stability of biomaterials within the body is crucial. Ideally, these materials should gradually degrade after completing bone repair, with degradation products that are harmless to the body [143,144]. Materials used in bone repair must have sufficient mechanical strength to support skeletal reconstruction and withstand the pressures generated by body weight and movement. However, to enhance bioactivity, some programmable biomaterials may compromise on strength and stiffness. This means they might not meet the necessary mechanical standards under prolonged stress or heavy loads [145,146]. Additionally, the production and acquisition of advanced programmable biomaterials are expensive, which restricts their widespread use in clinical settings [147,148]. It is challenging to integrate multiple independent functions within a single material, such as responding to a single stimulus simultaneously or sequentially, or responding differently to different stimuli, while reducing interference between the functions [149,150]. Therefore, while programmable biomaterials present promising solutions for bone repair and regeneration, their development and application are subject to the need for technological advancements (Fig. 11).
Fig. 11.
Artificial intelligence and precision medicine will drive the development of programmable biomaterials.
Integrating artificial intelligence (AI) into the field of programmable biomaterials for bone tissue engineering promises a prospective development. Predictive capabilities of AI can significantly enhance the design of programmable biomaterials, tailoring their properties for specific applications like bone repair [151]. From design to clinical applications, AI's involvement can lead to more precise and effective treatments for bone defects [[152], [153], [154]]. AI's analytical capabilities enable the prediction of material behaviors under various physiological conditions, which is essential in customizing biomaterials for specific bone repair needs. This includes identifying the optimal mix of mechanical and biochemical properties, such as porosity, biodegradation rate and controlled release of bioactive molecules, to enhance bone regeneration [155]. AI also plays a vital role in the manufacturing process, especially in techniques like 3D printing [156]. It can optimize printing parameters to fabricate scaffolds with intricate structures that closely resemble natural bone architecture, crucial for providing mechanical support and facilitating cell growth and nutrient diffusion [[157], [158], [159]]. In clinical, AI aids in personalizing treatment plans by analyzing patient-specific data to recommend suitable biomaterials and scaffold designs, thus enhancing the likelihood of successful integration and regeneration. Post-implantation, AI's monitoring and adaptive capabilities are invaluable. Although clinical outcomes remain somewhat unpredictable for clinicians, AI can effectively predict these outcomes [160,161]. AI systems equipped with wearable sensor can track bone healing and biomaterial performance in real-time. This data enables the system to adjust biomaterial properties, like altering bioactive molecule release or mechanical properties, in response to the evolving needs of the healing bone [[162], [163], [164], [165]]. Furthermore, the synergy between AI and programmable biomaterials extends to predictive maintenance and failure prevention. AI systems can analyze implant data to foresee potential issues, allowing for preemptive measures to ensure implant longevity and effectiveness. In the process of transitioning from cell culture-based experiments to clinical applications, animal experiments are often unavoidable. However, predicting the results of in vivo experiments based on in vitro data can be challenging. Therefore, AI as an emerging tool, can effectively predict experimental results while reducing the number of animal experiments. This marks a major step towards more efficient and humane research methods in biomedical research [155].
In addition, the emerging precision medicine model not only improves treatment outcomes by utilizing detailed information from individual patients to customize clinical treatment plans, but also plays a key role in advancing the development of bone tissue engineering. Integrating precision medicine with programmable biomaterials offers a synergistic approach to bone repair and regeneration [166]. This integration leverages the unique genetic and biological information of each patient to customize biomaterials that are specifically designed for their individual healing and regenerative needs [167]. For example, biomaterials can be programmed to release growth factors or drugs in response to the specific cellular environment of a patient's injury site. Such customization not only maximizes the efficacy of the treatment but also minimizes potential side effects or complications [163,168]. And bioprinting technology furthers this advancement by enabling the direct printing of living cells within scaffolds, thus fabricating tissue constructs that more closely mimic natural organoids. Integrating bioprinting technology with programmable biomaterials allows for the precise placement of stem cells within these structures. This precise control is essential for creating complex, functional organoids that closely replicate the intricate architecture and cellular diversity of native tissues. By combining stem cells with programmable biomaterials in bioprinting, it's possible to tailor the microenvironment, guiding stem cell differentiation and maturation into specific cell types. This approach not only enhances the functionality of the engineered organoids but also ensures that they accurately represent the physiological conditions of the tissues they are designed to emulate [[169], [170], [171], [172], [173]].
Interestingly, the combination of precision medicine and AI applied to bone tissue engineering can greatly promote the development of personalized treatment and regenerative medicine. Precision medicine can customize biomaterials that are suitable for a patient's specific needs based on their genetic information, cell characteristics, and pathological environment. By analyzing the gene expression data and pathological characteristics of each patient, it can be determined which materials, drugs, or growth factors are most suitable for their bone repair process. Combined with AI technology, the design and combination of materials can be optimized through big data modeling and machine learning algorithms to ensure that the mechanical properties, degradation rate, and drug release of the materials meet the individual's treatment requirements. At the same time, during the bone repair process, AI can be used to monitor the patient's healing progress in real time to optimize treatment outcomes [[174], [175], [176]]. In addition, AI can predict the effects of different treatment options and evaluate their potential risks by learning from a large amount of clinical data on bone repair and biomaterial use. Combined with information from precision medicine, AI can predict the patient's response before treatment begins, help doctors choose the most appropriate treatment strategy, and reduce the risk of complications and treatment failure [155].
The future of programmable biomaterials in bone tissue engineering is promising, potentially revolutionizing medical treatments and regenerative medicine systems. Ongoing research is expected to lead to innovative applications, significantly impacting healthcare and improving patient outcomes. As the field progresses, programmable biomaterials will undoubtedly navigate and overcome various challenges, moving steadily towards realizing their vast potential in bone tissue engineering. This evolution from traditional to programmable biomaterials marks a shift from static to dynamic solutions, opening new possibilities in bone repair and regeneration.
CRediT authorship contribution statement
Peiran Song: Writing – original draft. Dongyang Zhou: Writing – original draft. Fuxiao Wang: Writing – original draft. Guangfeng Li: Writing – review & editing. Long Bai: Writing – review & editing, Supervision. Jiacan Su: Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This work was financially supported by National Natural Science Foundation of China (32471396, 82230071, 82172098), Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303), Shanghai Committee of Science and Technology (23141900600, Laboratory Animal Research Project), Shanghai Clinical Research Plan (SHDC2023CRT01), Shanghai Municipal Demonstration Project for Innovative Medical Device Applications (23SHS05700), Young Elite Scientist Sponsorship Program by China Association for Science and Technology (YESS20230049).
Contributor Information
Guangfeng Li, Email: dlgf84@163.com.
Long Bai, Email: bailong@shu.edu.cn.
Jiacan Su, Email: drsujiacan@163.com.
Data availability
Data will be made available on request.
References
- 1.Tang D., Tare R.S., Yang L.Y., Williams D.F., Ou K.L., Oreffo R.O. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Stahl A., Yang Y.P. Regenerative approaches for the treatment of large bone defects. Tissue Eng Part B Rev. 2021;27:539–547. doi: 10.1089/ten.TEB.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201903279. [DOI] [Google Scholar]
- 4.Delaviz Y., Finer Y., Santerre J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand A., Buchalla W., Attin T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007;23:343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 8.Chen X., Gianneschi N., Ginger D., Nam J.M., Zhang H. Programmable materials. Adv Mater. 2021;33 doi: 10.1002/adma.202107344. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro D.M., Mandava G., Yalcin S.E., Arranz-Gibert P., Dahl P.J., Shipps C., Gu Y., Srikanth V., Salazar-Morales A.I., O'Brien J.P., Vanderschuren K., Vu D., Batista V.S., Malvankar N.S., Isaacs F.J. Protein nanowires with tunable functionality and programmable self-assembly using sequence-controlled synthesis. Nat. Commun. 2022;13:829. doi: 10.1038/s41467-022-28206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu W., Li X., Li Y., Luo R., Ou C., Huang D., Liang X., You Y., Wu Q., Gong C. A programmable releasing versatile hydrogel platform boosts systemic immune responses via sculpting tumor immunogenicity and reversing tolerogenic dendritic cells. Biomaterials. 2024;305 doi: 10.1016/j.biomaterials.2023.122444. [DOI] [PubMed] [Google Scholar]
- 11.Cai W., Xu X., Jiang Y., Cheng K., Liu F., Song C., Guo D., Hu Z., Liu Z., Liu Z. Programmed release of hydrogel microspheres via regulating the immune microenvironment to promotes bone repair. Mater Today Adv. 2023;18 doi: 10.1016/j.mtadv.2023.100381. [DOI] [Google Scholar]
- 12.Mao Z., Bi X., Wu C., Zheng Y., Shu X., Wu S., Guan J., Ritchie R.O. A cell-free silk fibroin biomaterial strategy promotes in situ cartilage regeneration via programmed releases of bioactive molecules. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202201588. [DOI] [PubMed] [Google Scholar]
- 13.Prasad A. Bioabsorbable polymeric materials for biofilms and other biomedical applications: recent and future trends. Mater. Today. 2021;44:2447–2453. doi: 10.1016/j.matpr.2020.12.489. [DOI] [Google Scholar]
- 14.Allo B.A., Costa D.O., Dixon S.J., Mequanint K., Rizkalla A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012;3:432–463. doi: 10.3390/jfb3020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todros S., Todesco M., Bagno A. Biomaterials and their biomedical applications: from replacement to regeneration. Processes. 2021;9 doi: 10.3390/pr9111949. [DOI] [Google Scholar]
- 16.Griss P., Heimke G., von Andrian-Werburg H.F. The alumina oxide ceramic-metal composite endoprosthesis. A new hip endoprosthesis for partially cement free implantation. Arch. Orthop. Unfall-Chir. 1975;81:259–266. doi: 10.1007/BF00416952. [DOI] [PubMed] [Google Scholar]
- 17.McFadden J.T. Tissue reactions to standard neurosurgical metallic implants. J. Neurosurg. 1972;36:598–603. doi: 10.3171/jns.1972.36.5.0598. [DOI] [PubMed] [Google Scholar]
- 18.Ducheyne P., Hench L.L., Kagan A., 2nd, Martens M., Bursens A., Mulier J.C. Effect of hydroxyapatite impregnation on skeletal bonding of porous coated implants. J. Biomed. Mater. Res. 1980;14:225–237. doi: 10.1002/jbm.820140305. [DOI] [PubMed] [Google Scholar]
- 19.Iwata M., McGinity J.W. Preparation of multi-phase microspheres of poly(D,L-lactic acid) and poly(D,L-lactic-co-glycolic acid) containing a W/O emulsion by a multiple emulsion solvent evaporation technique. J. Microencapsul. 1992;9:201–214. doi: 10.3109/02652049109021237. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K., Mizuno H., Okada K., Katoh H., Hitomi S., Teramatsu T., Shimizu Y., Hino T. Experimental application of polyvinyl alcohol-silica for small artificial vessels. Biomater. Med. Devices Artif. Organs. 1985;13:133–152. doi: 10.3109/10731198509118847. [DOI] [PubMed] [Google Scholar]
- 21.Douglas T., Dickson D.P., Betteridge S., Charnock J., Garner C.D., Mann S. Synthesis and structure of an iron(III) sulfide-ferritin bioinorganic nanocomposite. Science. 1995;269:54–57. doi: 10.1126/science.269.5220.54. [DOI] [PubMed] [Google Scholar]
- 22.Yagmurlu M.F., Korkusuz F., Gursel I., Korkusuz P., Ors U., Hasirci V. Sulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: in vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitis. J. Biomed. Mater. Res. 1999;46:494–503. doi: 10.1002/(sici)1097-4636(19990915)46:4<494::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Sabolinski M.L., Alvarez O., Auletta M., Mulder G., Parenteau N.L. Cultured skin as a 'smart material' for healing wounds: experience in venous ulcers. Biomaterials. 1996;17:311–320. doi: 10.1016/0142-9612(96)85569-4. [DOI] [PubMed] [Google Scholar]
- 24.Ringeisen B.R., Kim H., Barron J.A., Krizman D.B., Chrisey D.B., Jackman S., Auyeung R.Y., Spargo B.J. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004;10:483–491. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 25.Goodman R.P., Heilemann M., Doose S., Erben C.M., Kapanidis A.N., Turberfield A.J. Reconfigurable, braced, three-dimensional DNA nanostructures. Nat. Nanotechnol. 2008;3:93–96. doi: 10.1038/nnano.2008.3. [DOI] [PubMed] [Google Scholar]
- 26.Lei M., Qu X., Liu H., Liu Y., Wang S., Wu S., Bentley W.E., Payne G.F., Liu C. Programmable electrofabrication of porous Janus films with tunable Janus balance for anisotropic cell guidance and tissue regeneration. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201900065. [DOI] [Google Scholar]
- 27.Meng F.H., Zhong Z.Y., Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2009;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 28.Wang K., Si H., Wan Q., Wang Z., Qin A., Tang B.Z. Luminescent two-way reversible shape memory polymers prepared by hydroxyl–yne click polymerization. J. Mater. Chem. C. 2020;8:16121–16128. doi: 10.1039/d0tc03888a. [DOI] [Google Scholar]
- 29.Wang Y. Programmable hydrogels. Biomaterials. 2018;178:663–680. doi: 10.1016/j.biomaterials.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu R.Y., Wu L.W., He S., Liu S., Cui T.J. Programmable metamaterials. Programmable Materials. 2023;1:e4. doi: 10.1017/pma.2023.1. [DOI] [Google Scholar]
- 31.Montoya C., Du Y., Gianforcaro A.L., Orrego S., Yang M., Lelkes P.I. On the road to smart biomaterials for bone research: definitions, concepts, advances, and outlook. Bone Res. 2021;9:12. doi: 10.1038/s41413-020-00131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez M.M., Liu J.C., Trujillo-de Santiago G., Cha B.H., Vishwakarma A., Ghaemmaghami A.M., Khademhosseini A. Delivery strategies to control inflammatory response: modulating M1-M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–363. doi: 10.1016/j.jconrel.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Pharr M., Salvatore G.A. Lab-on-Skin: a review of flexible and stretchable electronics for wearable health monitoring. ACS Nano. 2017;11:9614–9635. doi: 10.1021/acsnano.7b04898. [DOI] [PubMed] [Google Scholar]
- 34.Skardal A., Murphy S.V., Devarasetty M., Mead I., Kang H.W., Seol Y.J., Shrike Zhang Y., Shin S.R., Zhao L., Aleman J., Hall A.R., Shupe T.D., Kleensang A., Dokmeci M.R., Jin Lee S., Jackson J.D., Yoo J.J., Hartung T., Khademhosseini A., Soker S., Bishop C.E., Atala A. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017;7:8837. doi: 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan S., Wang D., Kenaan A., Cheng J., Cui D., Song J. Create nanoscale patterns with DNA origami. Small. 2019;15 doi: 10.1002/smll.201805554. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Tu J., Wang D., Zhu H., Maity S.K., Qu X., Bogaert B., Pei H., Zhang H. Programmable and multifunctional DNA-based materials for biomedical applications. Adv Mater. 2018;30 doi: 10.1002/adma.201703658. [DOI] [PubMed] [Google Scholar]
- 37.Speed S.K., Gupta K., Peng Y.H., Hsiao S.K., Krieg E. Programmable polymer materials empowered by DNA nanotechnology. J. Polym. Sci. 2023;61:1713–1729. doi: 10.1002/pol.20230160. [DOI] [Google Scholar]
- 38.Guo B., Chen T., Hu X., Yang C., Shi Z., Wang Z., Wu X., Shen S., Ding W., Huang F., Zhu Z., Xu R.X. Programmable photoswitchable microcapsules enable precise and tailored drug delivery from microfluidics. ACS Appl. Mater. Interfaces. 2024;16:6447–6461. doi: 10.1021/acsami.3c17621. [DOI] [PubMed] [Google Scholar]
- 39.English M.A., Soenksen L.R., Gayet R.V., de Puig H., Angenent-Mari N.M., Mao A.S., Nguyen P.Q., Collins J.J. Programmable CRISPR-responsive smart materials. Science. 2019;365:780–785. doi: 10.1126/science.aaw5122. [DOI] [PubMed] [Google Scholar]
- 40.Keller A., Linko V. Challenges and perspectives of DNA nanostructures in biomedicine. Angew Chem Int Ed Engl. 2020;59:15818–15833. doi: 10.1002/anie.201916390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen O.T., Jansen C.E., Seo Y., Yellich G. Guided nitinol-retained (smileloc) single-tooth dental restorations. Oral Maxillofac Surg Clin North Am. 2019;31:437–446. doi: 10.1016/j.coms.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y., Mao H., Li H., Lei L. Uprighting horizontally impacted third molars by super-elastic nickel-titanium wire in patients with an extracted first molar. J. Clin. Pediatr. Dent. 2024;48:41–51. doi: 10.22514/jocpd.2024.006. [DOI] [PubMed] [Google Scholar]
- 43.Au P.K. Use of the nitinol .014 coronary guidewire during directional coronary atherectomy. Cathet. Cardiovasc. Diagn. 1993;30:182–183. doi: 10.1002/ccd.1810300224. [DOI] [PubMed] [Google Scholar]
- 44.Laubach M., Kobbe P., Hutmacher D.W. Biodegradable interbody cages for lumbar spine fusion: current concepts and future directions. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121699. [DOI] [PubMed] [Google Scholar]
- 45.Wijnands P.E., Wood J.S., Reedijk J., Maaskant W.J. Disordered CuN(6) jahn-teller centers in hexakis(1-methyltetrazole)copper(II) tetrafluoroborate: a temperature-dependent X-ray diffraction and EPR study. Inorg. Chem. 1996;35:1214–1222. doi: 10.1021/ic950315t. [DOI] [PubMed] [Google Scholar]
- 46.Gao Z., Zhao Z.H., Wang H., Bai Y., Zhang X., Zhang Z., Mei H., Yuan M., Zhang G. Jahn-teller distortions induced by in situ Li migration in lambda-MnO(2) for boosting electrocatalytic nitrogen fixation. Angew Chem. Int. Ed. Engl. 2024;63 doi: 10.1002/anie.202318967. [DOI] [PubMed] [Google Scholar]
- 47.Zeng A., Jiao J., Zhang H., Zhao E., He T., Xu Z., Xiao X. Slow-released cationic redox activity promoted stable anionic redox and suppressed jahn-teller distortion in layered sodium manganese oxides. ACS Appl. Mater. Interfaces. 2024;16:7119–7129. doi: 10.1021/acsami.3c16320. [DOI] [PubMed] [Google Scholar]
- 48.Eberl C. Programmable materials. Programmable Materials. 2023;1:e3. doi: 10.1017/pma.2023.3. [DOI] [Google Scholar]
- 49.Xu W., Deng R., Wang L., Li J. Multiresponsive rolling circle amplification for DNA logic gates mediated by endonuclease. Anal. Chem. 2014;86:7813–7818. doi: 10.1021/ac501726s. [DOI] [PubMed] [Google Scholar]
- 50.Yao D., Li H., Guo Y., Zhou X., Xiao S., Liang H. A pH-responsive DNA nanomachine-controlled catalytic assembly of gold nanoparticles. Chem Commun. 2016;52:7556–7559. doi: 10.1039/c6cc03089k. [DOI] [PubMed] [Google Scholar]
- 51.Goodman R.P., Heilemann M., Doose S., Erben C.M., Kapanidis A.N., Turberfield A.J. Reconfigurable, braced, three-dimensional DNA nanostructures. Nat. Nanotechnol. 2008;3:93–96. doi: 10.1038/nnano.2008.3. [DOI] [PubMed] [Google Scholar]
- 52.Yu Z., Li N., Zheng P., Pan W., Tang B. Temperature-responsive DNA-gated nanocarriers for intracellular controlled release. Chem Commun. 2014;50:3494–3497. doi: 10.1039/c3cc49183h. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y., Li Q., Wang L., Guo Q., Liu S., Zhu S., Sun Y., Fan Y., Sun Y., Li H., Tian X., Luo D., Shi S. Advances in regenerative medicine applications of tetrahedral framework nucleic acid-based nanomaterials: an expert consensus recommendation. Int. J. Oral Sci. 2022;14:51. doi: 10.1038/s41368-022-00199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang L., Li J., Li Q., Huang Q., Shi J., Yan H., Fan C. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew Chem Int Ed Engl. 2014;53:7745–7750. doi: 10.1002/anie.201403236. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y., Macfarlane R.J., Jones M.R., Mirkin C.A. Transmutable nanoparticles with reconfigurable surface ligands. Science. 2016;351:579–582. doi: 10.1126/science.aad2212. [DOI] [PubMed] [Google Scholar]
- 56.Li D., Yang Z., Luo Y., Zhao X., Tian M., Kang P. Delivery of MiR335-5p-pendant tetrahedron DNA nanostructures using an injectable heparin lithium hydrogel for challenging bone defects in steroid-associated osteonecrosis. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202101412. [DOI] [PubMed] [Google Scholar]
- 57.Bai L., Feng M., Li Q., Zhao Y., Zhang G., Cai Z., Xiao J., Lin Y. Curcumin delivery using tetrahedral framework nucleic acids enhances bone regeneration in osteoporotic rats. Chem Eng J. 2023;472 doi: 10.1016/j.cej.2023.144978. [DOI] [Google Scholar]
- 58.Li S.H., Liu Y.H., Tian T.R., Zhang T., Lin S.Y., Zhou M., Zhang X.L., Lin Y.F., Cai X.X. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small. 2021;17 doi: 10.1002/smll.202104359. [DOI] [PubMed] [Google Scholar]
- 59.Zhou M., Gao S., Zhang X., Zhang T., Zhang T., Tian T., Li S., Lin Y., Cai X. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact. Mater. 2021;6:1676–1688. doi: 10.1016/j.bioactmat.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Y., Shi R., Lu W., Shi S., Chen Y. Framework nucleic acids as promising reactive oxygen species scavengers for anti-inflammatory therapy. Nanoscale. 2024;16:7363–7377. doi: 10.1039/d3nr05844a. [DOI] [PubMed] [Google Scholar]
- 61.Fu L., Li P., Zhu J., Liao Z., Gao C., Li H., Yang Z., Zhao T., Chen W., Peng Y., Cao F., Ning C., Sui X., Guo Q., Lin Y., Liu S. Tetrahedral framework nucleic acids promote the biological functions and related mechanism of synovium-derived mesenchymal stem cells and show improved articular cartilage regeneration activity in situ. Bioact. Mater. 2022;9:411–427. doi: 10.1016/j.bioactmat.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu L., Li P., Wu J., Zheng Y., Ning C., Liao Z., Yuan X., Ding Z., Zhang Z., Sui X., Shi S., Liu S., Guo Q. Tetrahedral framework nucleic acids enhance the chondrogenic potential of human umbilical cord mesenchymal stem cells via the PI3K/AKT axis. Regen Biomater. 2023;10 doi: 10.1093/rb/rbad085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahabipour F., Oskuee R.K., Dehghani H., Shokrgozar M.A., Aninwene G.E., 2nd, Bonakdar S. Cell-cell interaction in a coculture system consisting of CRISPR/Cas9 mediated GFP knock-in HUVECs and MG-63 cells in alginate-GelMA based nanocomposites hydrogel as a 3D scaffold. J. Biomed. Mater. Res. 2020;108:1596–1606. doi: 10.1002/jbm.a.36928. [DOI] [PubMed] [Google Scholar]
- 64.Leng Q., Chen L., Lv Y. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10:3190–3205. doi: 10.7150/thno.42640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sidharthan D.S., Abhinandan R., Balagangadharan K., Selvamurugan N. Advancements in nucleic acids-based techniques for bone regeneration. Biotechnol. J. 2022;17 doi: 10.1002/biot.202100570. [DOI] [PubMed] [Google Scholar]
- 66.Shin J., Cho J.H., Jin Y., Yang K., Lee J.S., Park H.J., Han H.S., Lee J., Jeon H., Shin H., Cho S.W. Mussel adhesion-inspired reverse transfection platform enhances osteogenic differentiation and bone formation of human adipose-derived stem cells. Small. 2016;12:6266–6278. doi: 10.1002/smll.201601868. [DOI] [PubMed] [Google Scholar]
- 67.Jia S., Yang X., Song W., Wang L., Fang K., Hu Z., Yang Z., Shan C., Lei D., Lu B. Incorporation of osteogenic and angiogenic small interfering RNAs into chitosan sponge for bone tissue engineering. Int J Nanomedicine. 2014;9:5307–5316. doi: 10.2147/IJN.S70457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin H., Tang Y., Lozito T.P., Oyster N., Wang B., Tuan R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019;10:254. doi: 10.1186/s13287-019-1350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rady A.A.M., Hamdy S.M., Abdel-Hamid M.A., Hegazy M.G.A., Fathy S.A., Mostafa A.A. The role of VEGF and BMP-2 in stimulation of bone healing with using hybrid bio-composite scaffolds coated implants in animal model. Bull. Natl. Res. Cent. 2020;44:131. doi: 10.1186/s42269-020-00369-x. [DOI] [Google Scholar]
- 70.Shao X.R., Lin S.Y., Peng Q., Shi S.R., Li X.L., Zhang T., Lin Y.F. Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/beta-catenin signaling pathway. Nanomedicine. 2017;13:1809–1819. doi: 10.1016/j.nano.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Zhou M., Liu N.X., Shi S.R., Li Y., Zhang Q., Ma Q.Q., Tian T.R., Ma W.J., Cai X.X., Lin Y.F. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14:1227–1236. doi: 10.1016/j.nano.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Zhou M., Liu N., Zhang Q., Tian T., Ma Q., Zhang T., Cai X. Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2019;52 doi: 10.1111/cpr.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui W., Chen X., Zhu J., Zhang M., Xiao D., Qin X., Zhang T., Lin Y. Preventive effect of tetrahedral framework nucleic acids on bisphosphonate-related osteonecrosis of the jaw. Nanoscale. 2020;12:17196–17202. doi: 10.1039/d0nr03731a. [DOI] [PubMed] [Google Scholar]
- 74.Shao X., Lin S., Peng Q., Shi S., Wei X., Zhang T., Lin Y. Tetrahedral DNA nanostructure: a potential promoter for cartilage tissue regeneration via regulating chondrocyte phenotype and proliferation. Small. 2017;13 doi: 10.1002/smll.201602770. [DOI] [PubMed] [Google Scholar]
- 75.Li P., Fu L., Liao Z., Peng Y., Ning C., Gao C., Zhang D., Sui X., Lin Y., Liu S., Hao C., Guo Q. Chitosan hydrogel/3D-printed poly(epsilon-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121131. [DOI] [PubMed] [Google Scholar]
- 76.Shi S., Lin S., Shao X., Li Q., Tao Z., Lin Y. Modulation of chondrocyte motility by tetrahedral DNA nanostructures. Cell Prolif. 2017;50 doi: 10.1111/cpr.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi S., Peng Q., Shao X., Xie J., Lin S., Zhang T., Li Q., Li X., Lin Y. Self-assembled tetrahedral DNA nanostructures promote adipose-derived stem cell migration via lncRNA XLOC 010623 and RHOA/ROCK2 signal pathway. ACS Appl. Mater. Interfaces. 2016;8:19353–19363. doi: 10.1021/acsami.6b06528. [DOI] [PubMed] [Google Scholar]
- 78.Li J., Lai Y., Li M., Chen X., Zhou M., Wang W. Repair of infected bone defect with Clindamycin-Tetrahedral DNA nanostructure Complex-loaded 3D bioprinted hybrid scaffold. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134855. [DOI] [Google Scholar]
- 79.Zhang Y., Wei L., Miron R.J., Shi B., Bian Z. Bone scaffolds loaded with siRNA-Semaphorin4d for the treatment of osteoporosis related bone defects. Sci. Rep. 2016;6 doi: 10.1038/srep26925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Malcolm D.W., Benoit D.S.W. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials. 2017;139:127–138. doi: 10.1016/j.biomaterials.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manaka T., Suzuki A., Takayama K., Imai Y., Nakamura H., Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32:9642–9648. doi: 10.1016/j.biomaterials.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 82.Cui Z.K., Fan J., Kim S., Bezouglaia O., Fartash A., Wu B.M., Aghaloo T., Lee M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J Control Release. 2015;217:42–52. doi: 10.1016/j.jconrel.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X., Wang T., Zhang Z., Liu H., Li L., Wang A., Ouyang J., Xie T., Zhang L., Xue J., Tao W. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today. 2023;68:177–203. doi: 10.1016/j.mattod.2023.06.011. [DOI] [Google Scholar]
- 84.Balint R., Cassidy N.J., Cartmell S.H. Electrical stimulation: a novel tool for tissue engineering. Tissue Engineering Part B-Reviews. 2013;19:48–57. doi: 10.1089/ten.teb.2012.0183. [DOI] [PubMed] [Google Scholar]
- 85.Arambula-Maldonado R., Mequanint K. Carbon-based electrically conductive materials for bone repair and regeneration. Materials Advances. 2022;3:5186–5206. doi: 10.1039/d2ma00001f. [DOI] [Google Scholar]
- 86.Yu C., Ying X., Shahbazi M.A., Yang L., Ma Z., Ye L., Yang W., Sun R., Gu T., Tang R., Fan S., Yao S. A nano-conductive osteogenic hydrogel to locally promote calcium influx for electro-inspired bone defect regeneration. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122266. [DOI] [PubMed] [Google Scholar]
- 87.Jia F., Lin S., He X., Zhang J., Shen S., Wang Z., Tang B., Li C., Wu Y., Dong L., Cheng K., Weng W. Comprehensive evaluation of surface potential characteristics on mesenchymal stem cells' osteogenic differentiation. ACS Appl. Mater. Interfaces. 2019;11:22218–22227. doi: 10.1021/acsami.9b07161. [DOI] [PubMed] [Google Scholar]
- 88.Beyenbach K.W., Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 89.Kravtsova V.V., Petrov A.M., Matchkov V.V., Bouzinova E.V., Vasiliev A.N., Benziane B., Zefirov A.L., Chibalin A.V., Heiny J.A., Krivoi I.I. Distinct alpha2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016;147:175–188. doi: 10.1085/jgp.201511494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou T., Yan L., Xie C., Li P., Jiang L., Fang J., Zhao C., Ren F., Wang K., Wang Y., Zhang H., Guo T., Lu X. A mussel-inspired persistent ROS-scavenging, electroactive, and osteoinductive scaffold based on electrochemical-driven in situ nanoassembly. Small. 2019;15 doi: 10.1002/smll.201805440. [DOI] [PubMed] [Google Scholar]
- 91.Orapiriyakul W., Tsimbouri M.P., Childs P., Campsie P., Wells J., Fernandez-Yague M.A., Burgess K., Tanner K.E., Tassieri M., Meek D., Vassalli M., Biggs M.J.P., Salmeron-Sanchez M., Oreffo R.O.C., Reid S., Dalby M.J. Nanovibrational stimulation of mesenchymal stem cells induces therapeutic reactive oxygen species and inflammation for three-dimensional bone tissue engineering. ACS Nano. 2020;14:10027–10044. doi: 10.1021/acsnano.0c03130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cui L., Zhang J., Zou J., Yang X., Guo H., Tian H., Zhang P., Wang Y., Zhang N., Zhuang X., Li Z., Ding J., Chen X. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119617. [DOI] [PubMed] [Google Scholar]
- 93.Dixon D.T., Gomillion C.T. Conductive scaffolds for bone tissue engineering: current state and future outlook. J. Funct. Biomater. 2022;13:1. doi: 10.3390/jfb13010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun J., Zhao D., Wang Y., Chen P., Xu C., Lei H., Wo K., Zhang J., Wang J., Yang C., Su B., Jin Z., Luo Z., Chen L. Temporal immunomodulation via wireless programmed electric cues achieves optimized diabetic bone regeneration. ACS Nano. 2023;17:22830–22843. doi: 10.1021/acsnano.3c07607. [DOI] [PubMed] [Google Scholar]
- 95.Castro N., Fernandes M.M., Ribeiro C., Correia V., Minguez R., Lanceros-Mendez S. Magnetic bioreactor for magneto-, mechano- and electroactive tissue engineering strategies. Sensors. 2020;20:3340. doi: 10.3390/s20123340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panda A.K., Sitaramgupta V.S.N.t., Pandya H.J., Basu B. Electrical stimulation waveform-dependent osteogenesis on PVDF/BaTiO(3) composite using a customized and programmable cell stimulator. Biotechnol. Bioeng. 2022;119:1578–1597. doi: 10.1002/bit.28076. [DOI] [PubMed] [Google Scholar]
- 97.Liu X., George M.N., Li L., Gamble D., Miller Ii A.L., Gaihre B., Waletzki B.E., Lu L. Injectable electrical conductive and phosphate releasing gel with two-dimensional black phosphorus and carbon nanotubes for bone tissue engineering. ACS Biomater. Sci. Eng. 2020;6:4653–4665. doi: 10.1021/acsbiomaterials.0c00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X., Zhuang Y., Cao Y., Cai F., Lv Y., Zheng Y., Yang J., Shi X. Electrospun biomimetic periosteum capable of controlled release of multiple agents for programmed promoting bone regeneration. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202303134. [DOI] [PubMed] [Google Scholar]
- 99.Su Y., Zeng L., Deng R., Ye B., Tang S., Xiong Z., Sun T., Ding Q., Su W., Jing X., Gao Q., Wang X., Qiu Z., Chen K., Quan D., Guo X. Endogenous electric field-coupled PD@BP biomimetic periosteum promotes bone regeneration through sensory nerve via Fanconi anemia signaling pathway. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202203027. [DOI] [PubMed] [Google Scholar]
- 100.Wang L., Zeng X., Yan G., Chen X., Luo K., Zhou S., Zhang P., Li J., Wong T.W. Biomimetic scaffolds with programmable pore structures for minimum invasive bone repair. Nanoscale. 2021;13:16680–16689. doi: 10.1039/d1nr04124j. [DOI] [PubMed] [Google Scholar]
- 101.Go G., Yoo A., Song H.W., Min H.K., Zheng S., Nguyen K.T., Kim S., Kang B., Hong A., Kim C.S., Park J.O., Choi E. Multifunctional biodegradable microrobot with programmable morphology for biomedical applications. ACS Nano. 2021;15:1059–1076. doi: 10.1021/acsnano.0c07954. [DOI] [PubMed] [Google Scholar]
- 102.Omidian H., Chowdhury S.D. Advancements and applications of injectable hydrogel composites in biomedical research and therapy. Gels. 2023;9:533. doi: 10.3390/gels9070533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L., Fu L., Zhang X., Chen L., Cai Q., Yang X. Hierarchical and heterogeneous hydrogel system as a promising strategy for diversified interfacial tissue regeneration. Biomater. Sci. 2021;9:1547–1573. doi: 10.1039/d0bm01595d. [DOI] [PubMed] [Google Scholar]
- 104.Wang H., Hansen M.B., Lowik D.W., van Hest J.C., Li Y., Jansen J.A., Leeuwenburgh S.C. Oppositely charged gelatin nanospheres as building blocks for injectable and biodegradable gels. Adv Mater. 2011;23:H119–H124. doi: 10.1002/adma.201003908. [DOI] [PubMed] [Google Scholar]
- 105.Li D., Chen K., Tang H., Hu S., Xin L., Jing X., He Q., Wang S., Song J., Mei L., Cannon R.D., Ji P., Wang H., Chen T. A logic-based diagnostic and therapeutic hydrogel with multistimuli responsiveness to orchestrate diabetic bone regeneration. Adv Mater. 2022;34 doi: 10.1002/adma.202108430. [DOI] [PubMed] [Google Scholar]
- 106.Dong Q., Wan Z., Li Q., Liu Y., Zhang P., Zhang X., Niu Y., Liu H., Lv L., Zhou Y. 3D-printed near-infrared-light-responsive ondemand drug-delivery scaffold for bone regeneration. Biomater. Adv. 2023;159 doi: 10.21203/rs.3.rs-2702534/v1. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y., Li C., Zhang W., Deng J., Nie Y., Du X., Qin L., Lai Y. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact. Mater. 2022;16:218–231. doi: 10.1016/j.bioactmat.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L., Zeng X., Chen X., Zeng X., Luo K. Programmable, biodegradable composite scaffolds with variable pore morphology for minimal invasive bone repair. Compos Part A-Appl S. 2022;162 doi: 10.1016/j.compositesa.2022.107130. [DOI] [Google Scholar]
- 109.Xiang Z., Guan X., Ma Z., Shi Q., Panteleev M., Ataullakhanov F.I. Bioactive fibrous scaffolds with programmable release of polypeptides regulate inflammation and extracellular matrix remodeling. Regen Biomater. 2023;10 doi: 10.1093/rb/rbad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bayer E.A., Jordan J., Roy A., Gottardi R., Fedorchak M.V., Kumta P.N., Little S.R. Programmed platelet-derived growth factor-BB and bone morphogenetic protein-2 delivery from a hybrid calcium phosphate/alginate scaffold. Tissue Eng Part A. 2017;23:1382–1393. doi: 10.1089/ten.TEA.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Timin A.S., Muslimov A.R., Zyuzin M.V., Peltek O.O., Karpov T.E., Sergeev I.S., Dotsenko A.I., Goncharenko A.A., Yolshin N.D., Sinelnik A., Krause B., Baumbach T., Surmeneva M.A., Chernozem R.V., Sukhorukov G.B., Surmenev R.A. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS Appl. Mater. Interfaces. 2018;10:34849–34868. doi: 10.1021/acsami.8b09810. [DOI] [PubMed] [Google Scholar]
- 112.Kalogeropoulou M., Diaz Payno P.J., Mirzaali M.J., van Osch G., Fratila-Apachitei L.E., Zadpoor A.A. 4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications. Biofabrication. 2024 doi: 10.1088/1758-5090/ad1e6f. [DOI] [PubMed] [Google Scholar]
- 113.Lai J., Liu Y., Lu G., Yung P., Wang X., Tuan R.S., Li Z.A. 4D bioprinting of programmed dynamic tissues. Bioact. Mater. 2024;37:348–377. doi: 10.1016/j.bioactmat.2024.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen X., Huang Z., Yang Q., Zeng X., Bai R., Wang L. 3D biodegradable shape changing composite scaffold with programmable porous structures for bone engineering. Biomed Mater. 2022;17 doi: 10.1088/1748-605X/aca133. [DOI] [PubMed] [Google Scholar]
- 115.Choudhury S., Joshi A., Agrawal A., Nain A., Bagde A., Patel A., Syed Z.Q., Asthana S., Chatterjee K. NIR-responsive deployable and self-fitting 4D-printed bone tissue scaffold. ACS Appl. Mater. Interfaces. 2024;16:49135–49147. doi: 10.1021/acsami.4c10385. [DOI] [PubMed] [Google Scholar]
- 116.Bicho D., Canadas R.F., Goncalves C., Pina S., Reis R.L., Oliveira J.M. Porous aligned ZnSr-doped beta-TCP/silk fibroin scaffolds using ice-templating method for bone tissue engineering applications. J Biomater Sci Polym. 2021:1966–1982. doi: 10.1080/09205063.2021.1952382. Ed. 32. [DOI] [PubMed] [Google Scholar]
- 117.Zhou X., Jin Y., Du J. Functionally graded scaffolds with programmable pore size distribution based on triply periodic minimal surface fabricated by selective laser melting. Materials. 2020;13 doi: 10.3390/ma13215046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ji H., Wang Y., Liu H., Liu Y., Zhang X., Xu J., Li Z., Luo E. Programmed core-shell electrospun nanofibers to sequentially regulate osteogenesis-osteoclastogenesis balance for promoting immediate implant osseointegration. Acta Biomater. 2021;135:274–288. doi: 10.1016/j.actbio.2021.08.050. [DOI] [PubMed] [Google Scholar]
- 119.Xia Y., Sun J., Zhao L., Zhang F., Liang X.-J., Guo Y., Weir M.D., Reynolds M.A., Gu N., Xu H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151–170. doi: 10.1016/j.biomaterials.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 120.Sun T.W., Yu W.L., Zhu Y.J., Yang R.L., Shen Y.Q., Chen D.Y., He Y.H., Chen F. Hydroxyapatite Nanowire@Magnesium silicate core-shell hierarchical nanocomposite: synthesis and application in bone regeneration. ACS Appl. Mater. Interfaces. 2017;9:16435–16447. doi: 10.1021/acsami.7b03532. [DOI] [PubMed] [Google Scholar]
- 121.He Z., Liu S., Li Z., Xu J., Liu Y., Luo E. Coaxial TP/APR electrospun nanofibers for programmed controlling inflammation and promoting bone regeneration in periodontitis-related alveolar bone defect models. Mater Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou X., Qian Y., Chen L., Li T., Sun X., Ma X., Wang J., He C. Flowerbed-inspired biomimetic scaffold with rapid internal tissue infiltration and vascularization capacity for bone repair. ACS Nano. 2023;17:5140–5156. doi: 10.1021/acsnano.3c00598. [DOI] [PubMed] [Google Scholar]
- 123.Yin C., Zhao Q., Li W., Zhao Z., Wang J., Deng T., Zhang P., Shen K., Li Z., Zhang Y. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020;102:416–426. doi: 10.1016/j.actbio.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 124.Liao C., Li Y., Tjong S.C. Polyetheretherketone and its composites for bone replacement and regeneration. Polymers. 2020;12:2858. doi: 10.3390/polym12122858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He M., Huang Y., Xu H., Feng G., Liu L., Li Y., Sun D., Zhang L. Modification of polyetheretherketone implants: from enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021;129:18–32. doi: 10.1016/j.actbio.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 126.Xie L., Wang G., Wu Y., Liao Q., Mo S., Ren X., Tong L., Zhang W., Guan M., Pan H., Chu P.K., Wang H. Programmed surface on poly(aryl-ether-ether-ketone) initiating immune mediation and fulfilling bone regeneration sequentially. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng Y., Gao A., Bai J., Liao Q., Wu Y., Zhang W., Guan M., Tong L., Geng D., Zhao X., Chu P.K., Wang H. A programmed surface on polyetheretherketone for sequentially dictating osteoimmunomodulation and bone regeneration to achieve ameliorative osseointegration under osteoporotic conditions. Bioact. Mater. 2022;14:364–376. doi: 10.1016/j.bioactmat.2022.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei X., Zhou W., Tang Z., Wu H., Liu Y., Dong H., Wang N., Huang H., Bao S., Shi L., Li X., Zheng Y., Guo Z. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis. Bioact. Mater. 2023;20:16–28. doi: 10.1016/j.bioactmat.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park S.Y., Kim K.H., Kim S., Lee Y.M., Seol Y.J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics. 2019;11:393. doi: 10.3390/pharmaceutics11080393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding Z., Fan Z., Huang X., Lu Q., Xu W., Kaplan D.L. Silk-hydroxyapatite nanoscale scaffolds with programmable growth factor delivery for bone repair. ACS Appl. Mater. Interfaces. 2016;8:24463–24470. doi: 10.1021/acsami.6b08180. [DOI] [PubMed] [Google Scholar]
- 131.Han S., Paeng K.W., Park S., Jung U.W., Cha J.K., Hong J. Programmed BMP-2 release from biphasic calcium phosphates for optimal bone regeneration. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120785. [DOI] [PubMed] [Google Scholar]
- 132.Cai Z., Jiang H., Lin T., Wang C., Ma J., Gao R., Jiang Y., Zhou X. Microspheres in bone regeneration: fabrication, properties and applications. Mater Today Adv. 2022;16 doi: 10.1016/j.mtadv.2022.100315. [DOI] [Google Scholar]
- 133.Wang Q., Zhang Y., Li B., Chen L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin-nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B. 2017;5:6963–6972. doi: 10.1039/c7tb00949f. [DOI] [PubMed] [Google Scholar]
- 134.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E., Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xin T., Mao J., Liu L., Tang J., Wu L., Yu X., Gu Y., Cui W., Chen L. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum. ACS Appl. Mater. Interfaces. 2020;12:6840–6851. doi: 10.1021/acsami.9b18496. [DOI] [PubMed] [Google Scholar]
- 136.Cui J., Yu X., Yu B., Yang X., Fu Z., Wan J., Zhu M., Wang X., Lin K. Coaxially fabricated dual-drug loading electrospinning fibrous mat with programmed releasing behavior to boost vascularized bone regeneration. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202200571. [DOI] [PubMed] [Google Scholar]
- 137.Hu Z., Ma C., Rong X., Zou S., Liu X. Immunomodulatory ECM-like microspheres for accelerated bone regeneration in Diabetes mellitus. ACS Appl. Mater. Interfaces. 2018;10:2377–2390. doi: 10.1021/acsami.7b18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Long J., Wang Y., Lu M., Etxeberria A.E., Zhou Y., Gu P., Song P., Min L., Luo Y., Nand A.V., Ray S., Sun Y., Fan Y., Tu C., Zhang X. Dual-cross-linked magnetic hydrogel with programmed release of parathyroid hormone promotes bone healing. ACS Appl. Mater. Interfaces. 2023;15:35815–35831. doi: 10.1021/acsami.3c03047. [DOI] [PubMed] [Google Scholar]
- 139.Su P., Tian Y., Yang C., Ma X., Wang X., Pei J., Qian A. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 2018;19:2343. doi: 10.3390/ijms19082343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vanden Berg-Foels W.S. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng Part B Rev. 2014;20:28–39. doi: 10.1089/ten.TEB.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Q., Ren H., Baker G.L. Synthesis and click chemistry of a new class of biodegradable polylactide towards tunable thermo-responsive biomaterials. Polym. Chem. 2015;6:1275–1285. doi: 10.1039/C4PY01425A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malik Q.U.A., Iftikhar S., Zahid S., Safi S.Z., Khan A.F., Nawshad M., Ghafoor S., Khan A.S., Tufail Shah A. Smart injectable self-setting bioceramics for dental applications. Mater Sci Eng C Mater Biol Appl. 2020;113 doi: 10.1016/j.msec.2020.110956. [DOI] [PubMed] [Google Scholar]
- 143.Wu S., Liu X., Yeung K.W.K., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. J. Mater. Sci. Technol. 2014;80:1–36. doi: 10.1016/j.mser.2014.04.001. [DOI] [Google Scholar]
- 144.Tan L., Yu X., Wan P., Yang K. Biodegradable materials for bone repairs: a review. J. Mater. Sci. Technol. 2013;29:503–513. doi: 10.1016/j.jmst.2013.03.002. [DOI] [Google Scholar]
- 145.Barrère F., Mahmood T.A., de Groot K., van Blitterswijk C.A. Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. Mat Sci Eng R. 2008;59:38–71. doi: 10.1016/j.mser.2007.12.001. [DOI] [Google Scholar]
- 146.Prasadh S., Wong R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Science International. 2018;15:48–55. doi: 10.1016/s1348-8643(18)30005-3. [DOI] [Google Scholar]
- 147.Bhat S., Kumar A. Biomaterials and bioengineering tomorrow's healthcare. Biomatter. 2013;3 doi: 10.4161/biom.24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fan X., Chung J.Y., Lim Y.X., Li Z., Loh X.J. Review of adaptive programmable materials and their bioapplications. ACS Appl. Mater. Interfaces. 2016;8:33351–33370. doi: 10.1021/acsami.6b09110. [DOI] [PubMed] [Google Scholar]
- 149.Jeon S.J., Hauser A.W., Hayward R.C. Shape-morphing materials from stimuli-responsive hydrogel hybrids. Acc. Chem. Res. 2017;50:161–169. doi: 10.1021/acs.accounts.6b00570. [DOI] [PubMed] [Google Scholar]
- 150.Lendlein A., Balk M., Tarazona N.A., Gould O.E.C. Bioperspectives for shape-memory polymers as shape programmable, active materials. Biomacromolecules. 2019;20:3627–3640. doi: 10.1021/acs.biomac.9b01074. [DOI] [PubMed] [Google Scholar]
- 151.Dong J., Qian J., Yu K., Huang S., Cheng X., Chen F., Jiang H., Zeng W. Rational design of organelle-targeted fluorescent probes: insights from artificial intelligence. Research. 2023;6:75. doi: 10.34133/research.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hayat H., Nukala A., Nyamira A., Fan J., Wang P. A concise review: the synergy between artificial intelligence and biomedical nanomaterials that empowers nanomedicine. Biomed Mater. 2021;16 doi: 10.1088/1748-605X/ac15b2. [DOI] [PubMed] [Google Scholar]
- 153.Dai X., Chen Y. Computational biomaterials: computational simulations for biomedicine. Adv Mater. 2023;35 doi: 10.1002/adma.202204798. [DOI] [PubMed] [Google Scholar]
- 154.Zhang S., Mu W., Dong D., Wei J., Fang M., Shao L., Zhou Y., He B., Zhang S., Liu Z., Liu J., Tian J. The applications of artificial intelligence in digestive system neoplasms: a review. Health Data Sci. 2023;3:5. doi: 10.34133/hds.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mackay B.S., Marshall K., Grant-Jacob J.A., Kanczler J., Eason R.W., Oreffo R.O.C., Mills B. The future of bone regeneration: integrating AI into tissue engineering. Biomed Phys Eng Express. 2021;7 doi: 10.1088/2057-1976/ac154f. [DOI] [PubMed] [Google Scholar]
- 156.Chen H., Liu Y., Balabani S., Hirayama R., Huang J. Machine learning in predicting printable biomaterial formulations for direct ink writing. Research. 2023;6:197. doi: 10.34133/research.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma L., Yu S., Xu X., Moses Amadi S., Zhang J., Wang Z. Application of artificial intelligence in 3D printing physical organ models. Mater Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cao Y., Sun L., Liu Z., Shen Z., Jia W., Hou P., Sang S. 3D printed-electrospun PCL/hydroxyapatite/MWCNTs scaffolds for the repair of subchondral bone. Regen Biomater. 2023;10:rbac104. doi: 10.1093/rb/rbac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meng M., Wang J., Huang H., Liu X., Zhang J., Li Z. 3D printing metal implants in orthopedic surgery: methods, applications and future prospects. J Orthop Translat. 2023;42:94–112. doi: 10.1016/j.jot.2023.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ahmed N., Abbasi M.S., Zuberi F., Qamar W., Halim M.S.B., Maqsood A., Alam M.K. Artificial intelligence techniques: analysis, application, and outcome in dentistry-A systematic review. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/9751564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gandelman J.S., Byrne M.T., Mistry A.M., Polikowsky H.G., Diggins K.E., Chen H., Lee S.J., Arora M., Cutler C., Flowers M., Pidala J., Irish J.M., Jagasia M.H. Machine learning reveals chronic graft-versus-host disease phenotypes and stratifies survival after stem cell transplant for hematologic malignancies. Haematologica. 2019;104:189–196. doi: 10.3324/haematol.2018.193441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weygers I., Kok M., Seel T., Shah D., Taylan O., Scheys L., Hallez H., Claeys K. In-vitro validation of inertial-sensor-to-bone alignment. J. Biomech. 2021;128 doi: 10.1016/j.jbiomech.2021.110781. [DOI] [PubMed] [Google Scholar]
- 163.Matijevich E.S., Scott L.R., Volgyesi P., Derry K.H., Zelik K.E. Combining wearable sensor signals, machine learning and biomechanics to estimate tibial bone force and damage during running. Hum. Mov. Sci. 2020;74 doi: 10.1016/j.humov.2020.102690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiong Y., Han J., Wang Y., Wang Z.L., Sun Q. Emerging iontronic sensing: materials, mechanisms, and applications. Research. 2022;2022 doi: 10.34133/2022/9867378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yuan J., Zhang Y., Liu S., Zhu R. Wearable leg movement monitoring system for high-precision real-time metabolic energy estimation and motion recognition. Research. 2023;6 doi: 10.34133/research.0214. [DOI] [Google Scholar]
- 166.Zhou J., See C.W., Sreenivasamurthy S., Zhu D. Customized additive manufacturing in bone scaffolds-the gateway to precise bone defect treatment. Research. 2023;6:239. doi: 10.34133/research.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W., Yin Y., Zhou H., Fan Y., Yang Y., Gao Q., Li P., Gao G., Li J. Recent advances in electrospinning techniques for precise medicine. Cyborg Bionic Syst. 2024;5:101. doi: 10.34133/cbsystems.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guzzi E.A., Tibbitt M.W. Additive manufacturing of precision biomaterials. Adv Mater. 2020;32 doi: 10.1002/adma.201901994. [DOI] [PubMed] [Google Scholar]
- 169.Barrs R.W., Jia J., Silver S.E., Yost M., Mei Y. Biomaterials for bioprinting microvasculature. Chem Rev. 2020;120:10887–10949. doi: 10.1021/acs.chemrev.0c00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wan Z., Zhang P., Liu Y., Lv L., Zhou Y. Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26–42. doi: 10.1016/j.actbio.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 171.Tavafoghi M., Darabi M.A., Mahmoodi M., Tutar R., Xu C., Mirjafari A., Billi F., Swieszkowski W., Nasrollahi F., Ahadian S., Hosseini V., Khademhosseini A., Ashammakhi N. Multimaterial bioprinting and combination of processing techniques towards the fabrication of biomimetic tissues and organs. Biofabrication. 2021;13 doi: 10.1088/1758-5090/ac0b9a. [DOI] [PubMed] [Google Scholar]
- 172.Farahani M., Carthew J., Bhowmik S., Shard C., Nunez-Nescolarde A., Gomez G.A., Cadarso V.J., Combes A.N., Frith J.E. Emerging biomaterials and technologies to control stem cell fate and patterning in engineered 3D tissues and organoids. Biointerphases. 2022;17 doi: 10.1116/6.0002034. [DOI] [PubMed] [Google Scholar]
- 173.Hoang P., Ma Z. Biomaterial-guided stem cell organoid engineering for modeling development and diseases. Acta Biomater. 2021;132:23–36. doi: 10.1016/j.actbio.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bai L., Wu Y., Li G., Zhang W., Zhang H., Su J. AI-enabled organoids: construction, analysis, and application. Bioact. Mater. 2024;31:525–548. doi: 10.1016/j.bioactmat.2023.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nosrati H., Nosrati M. Artificial intelligence in regenerative medicine: applications and implications. Biomimetics. 2023;8:442. doi: 10.3390/biomimetics8050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun W., Lee J., Zhang S., Benyshek C., Dokmeci M.R., Khademhosseini A. Engineering precision medicine. Adv. Sci. 2019;6 doi: 10.1002/advs.201801039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.