Summary
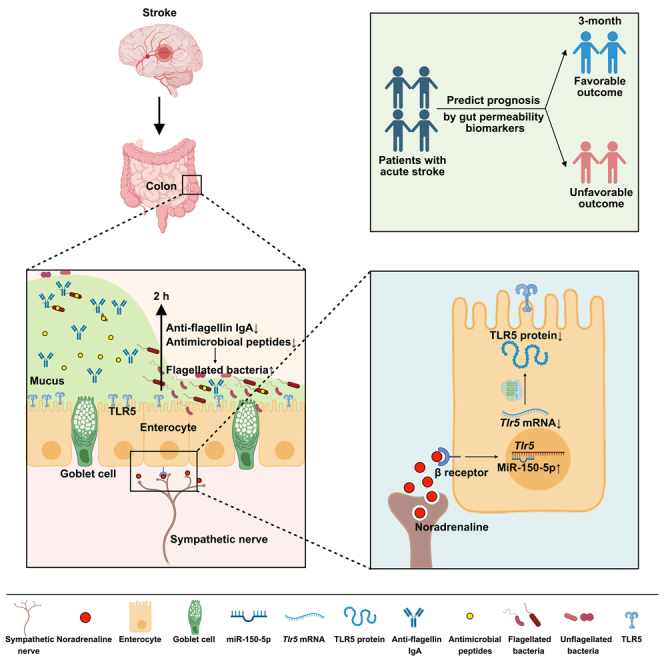
The gut permeability significantly increases after ischemic stroke, partly due to disrupted mucosal barrier, but the mechanism remains elusive. Here, we found that the mucus disruption starts at 2 h post stroke, whereas goblet cell functions remain intact. Meanwhile, the flagellated bacteria Helicobacter thrives and penetrates in the mucus layer. Elimination of the mucosal microbiota or transplantation of Helicobacter in germ-free mice reveals an important role of the mucosal microbiota in mucus disruption. The bacterial invasion is due to downregulated Toll-like receptor 5 (TLR5) and its downstream products flagellin-specific IgA and antimicrobial peptides. Knockdown of intestinal TLR5 increases the abundance of flagellated bacteria and exacerbates mucus injury. Intestinal TLR5 is downregulated by the activation of sympathetic nerve. Serum noradrenaline level is positively associated with flagellin level in patients with stroke and patients’ prognosis. These findings reveal a neural pathway in which the sympathetic nerve disrupts the mucosal barrier, providing potential therapeutic targets for stroke injury.
Keywords: ischemic stroke, intestinal barrier, Toll-like receptor 5, flagellated bacteria, sympathetic nerve, mucus layer
Graphical abstract
Highlights
-
•
The gut mucosal barrier is impaired with flagellated bacteria invasion at 2 h post stroke
-
•
The bacterial invasion is due to reduced downstream products of TLR5
-
•
Activated sympathetic nerve decreases intestinal TLR5 expression
-
•
Biomarkers for gut permeability can predict prognosis in patients with stroke
Wang et al. demonstrate that downregulation of intestinal TLR5 by sympathetic activation leads to reduced production of antimicrobial substances and further promotes the invasion of flagellated bacteria and disruption of the mucus layer. Serum levels of flagellin and noradrenaline are associated with prognosis in patients with stroke.
Introduction
Stroke is the second leading cause of death and disability globally. The absolute number of patients with stroke, including those who die or survive with disabilities, has doubled in the past 3 decades.1 From 2020 to 2050, it is estimated that stroke mortality will continue to increase by 50% and disability-adjusted life-years will increase by 31%, causing a great burden to families and society.2 Ischemic stroke represents approximately 87% of total strokes and is currently one of the main focuses of neurology research.3 Recanalization is the primary therapeutic strategy for ischemic stroke, with addition of neuroprotective treatments.4 However, almost all of these neuroprotective treatments attempting to protect ischemic penumbra have failed in clinical trials.5 Therefore, researchers are looking for alternative therapeutic targets from peripheral organs other than the brain.
One of the important research directions is to look in the gut, as it is increasingly evident that stroke increases gut permeability, which facilitates the translocation of toxic gut contents into systemic circulation, leading to exacerbation of stroke injury.6 Our previous study also revealed that stroke induces excessive production of nitrate in the intestine, promoting the growth of Enterobacteriaceae, which accelerated systemic inflammation through translocation of lipopolysaccharide (LPS) from the gut to the bloodstream and further aggravated brain infarction.7 These studies emphasize the importance of the gut barrier in stroke injury, but how the barrier is disrupted after stroke remains elusive.
The physical barrier in the intestine comprises two constituents, i.e., the epithelial barrier connected by tight junctions including zonula occludens (ZO), occludins, and claudins, and the mucosal barrier formed by a mucus layer, whose primary function is to prevent luminal components from penetrating into underlying intestinal tissues.8,9 Mucus layer is a reservoir for the proteins secreted by epithelial cells, such as secretory immunoglobulin A and antimicrobial peptides.10,11 Therefore, the mucus layer functions as the first line of host defense against invading pathogens. However, in previous investigations of stroke, most attention has been paid to intestinal tight junctions, while the mucus barrier has been neglected.12 It is natural to link the mucus barrier to goblet cells as mucin is biosynthesized by goblet cells.13 Indeed, reduced goblet cell number has been observed at 72 h after stroke.14 However, studies investigating the dynamic changes in the mucus layer during stroke and the mechanism underlying an impaired mucus layer, other than goblet cell dysfunction, have remained scarce.
In this study, we found that the thickness of the mucus layer was significantly decreased starting at 2 h post stroke, but the goblet cell remained intact at the same time, suggesting alternative explanations for the disruption of the mucus layer. Meanwhile, the expression of Toll-like receptor 5 (TLR5), a receptor that senses bacterial flagella, was significantly downregulated post stroke, suggesting that mucosal microbiota might be involved in the mucus disruption. We further revealed a sympathetic nerve-mediated downregulation of intestinal TLR5, which led to reduced production of flagellin-specific IgA and antimicrobial substances, which facilitated the invasion of flagellated bacteria and disruption of the mucus layer. Our study may provide therapeutic potential of targeting the sympathetic nervous system for the protection of the gut barrier and stroke injury.
Results
The disruption of the mucosal barrier contributes to increased gut permeability after stroke
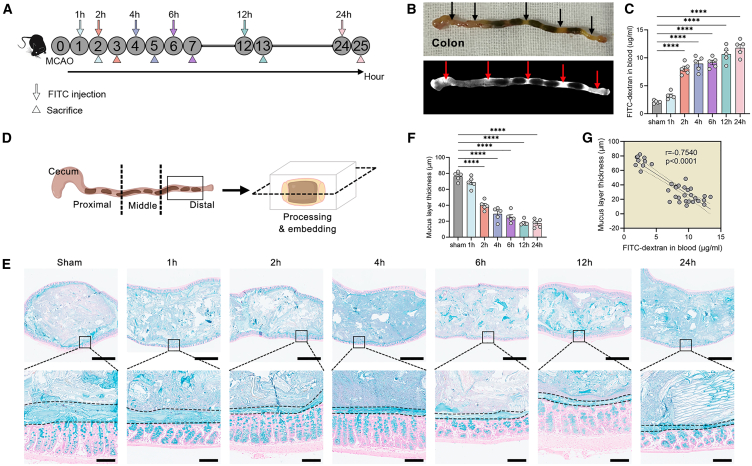
To examine the gut permeability after stroke, we utilized a mouse model of cerebral ischemia/reperfusion injury by middle cerebral artery occlusion (MCAO). Mice were orally gavaged with fluorescein isothiocyanate (FITC)-labeled dextran at different time points, i.e., 1, 2, 4, 6, 12, and 24 h after stroke (Figure S1A), and the FITC-dextran in the blood was measured 1 h after administration. The sham-operated mice were sacrificed at 2 h after operation. We observed impaired gastrointestinal motility in mice after MCAO (Figures S1B and S1C); large proportion of the fluorescent bolus reached the upper gastrointestinal (GI) tract (Figures S1D and S1E). Interestingly, the concentration of FITC-dextran in the blood peaked at 3 h (the 2-h stroke group) after stroke and declined afterward (Figure S1F), suggesting a recovery of permeability in the small intestine. As the colon harbors the densest microbiota of the GI tract, it is more important to investigate the permeability of the colon after stroke. Therefore, we injected FITC-dextran in the proximal colon of mice at different time points after stroke, and mice were sacrificed 1 h after administration (Figures 1A and 1B). The permeability of the colon increased dramatically in the 2-h group and gradually increased afterward (Figure 1C). There were no differences in FITC-dextran concentrations between sham mice sacrificed at different time points after operation (Figure S1G), suggesting that the changes in permeability were not due to effects of surgery. Next, we investigated the integrity of the mucosal barrier. Distal colon with fecal pellet was harvested and longitudinally sectioned (Figure 1D). We measured the mucus layer with Alcian blue staining and found that the thickness of the mucus layer decreased significantly in the 2-h group (Figures 1E and 1F), and there was a significantly negative association between FITC-dextran in the blood and mucus layer thickness (Figure 2G). There was no difference in mucus layer thickness between sham mice sacrificed at different time points after operation (Figure S1H). To investigate the contribution of intestinal tight junction, we used transmission electron microscope to observe epithelial ultrastructure; we found that the tight junctions remained intact until 6 h after stroke when dilatations were observed (Figure S2A). Similarly, the expressions of tight junction proteins ZO-1, occludin, and claudin-4 were not significantly decreased until 6 or 12 h after stroke (Figures S2B and S2C). Moreover, we detected the translocation of microbiota to the Peyer’s patch and spleen in the 2-h group and groups afterward (Figures S3A–S3D). More importantly, selective depletion of colonic bacteria led to a significant reduction of bacterial load that was detectable in the spleen and Peyer’s patch at 2 h after stroke (Figures S3E and S3F), suggesting that the majority of translocating bacteria originated from the colon. We also evaluated the brain infarction by 2,3,5-triphenyltetrazolium chloride staining. Brain infarction (white color indicates infarction zone and red color indicates non-infarction zone) could not be seen in the 1-h or 2-h groups (Figures S4A and S4B). There were apparent boundaries between the white and red regions in brain slices of the 24-h group. When we shorten the time of staining, we observed a pink area in brain slices of 2-h group mice (Figure S4C), implicating a salvageable penumbra region. Although brain infarction could not be seen in gross specimen in the 1-h or 2-h groups, apoptotic neurons in the CA1 region of the hippocampus (a brain region that is selectively vulnerable to ischemic insults15) could be detected, and the numbers of apoptotic neurons continued to increase over time (Figures S4D and S4E).
Figure 1.
The disruption of the mucosal barrier contributes to the increased gut permeability after stroke
(A) Experimental design. Mice were subjected to cerebral ischemia/reperfusion injury by middle cerebral artery occlusion (MCAO). Fluorescein isothiocyanate (FITC)-labeled dextran was injected to proximal colon of mice at different time points after stroke and its concentration in the blood was measured 1 h after administration.
(B) Gross picture of colon and fluorescence image.
(C) Concentrations of FITC-dextran in the blood (n = 5).
(D) Schematic diagram of tissue sampling, processing, and slicing.
(E) Alcian blue-stained sections of the colon with pellet (the mucus layer is defined by the black dashed lines). Scale bars, 1 mm (top); 100 μm (bottom).
(F) Thickness of the mucus layer (n = 5).
(G) Spearman’s correlations between thickness of the mucus layer and FITC-dextran in the blood. Data were represented as mean ± SEM. Statistical comparison was performed by one-way ANOVA with Dunnett’s multiple comparisons test (C and F). ns, not significant, ∗p < 0.05, ∗∗∗∗p < 0.0001.
Please see also Figures S1–S5.
Figure 2.
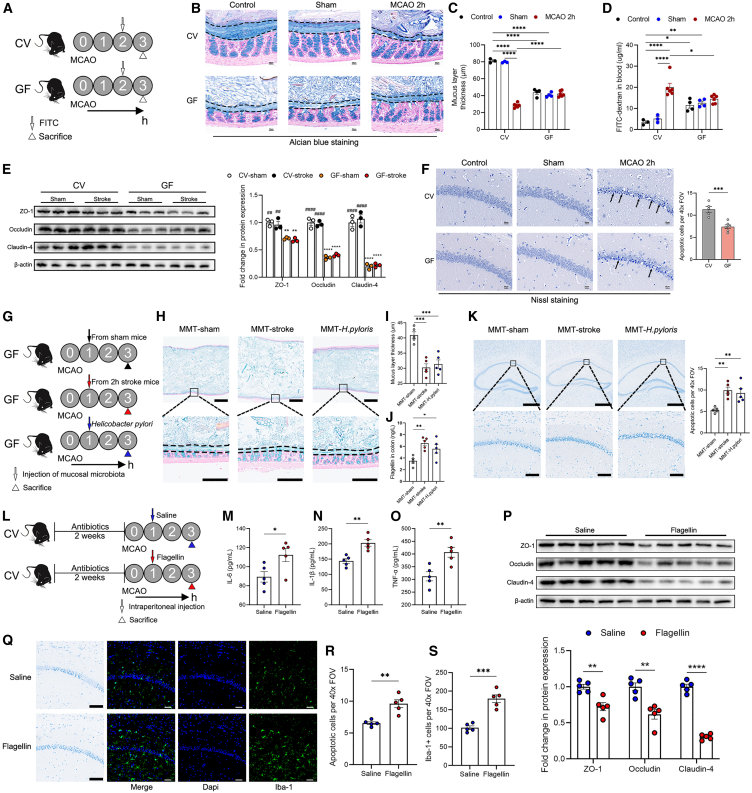
The mucosal microbiota contributes to the disruption of the mucus layer and exacerbation of brain injury
(A) Experimental design. Conventional (CV) and germ-free (GF) mice were subjected to MCAO and administrated with FITC at 2 h after cerebral reperfusion, and were sacrificed 1 h after administration.
(B and C) Alcian blue-stained sections of the colon with pellet (the mucus layer is defined by the black dashed lines) and measurement of the thickness of the mucus layer (n = 3–6). Scale bars, 30 μm.
(D) Concentrations of FITC-dextran in the blood (n = 3–6).
(E) Expressions of tight junction proteins in the colonic tissue of mice (n = 3).
(F) Nissl staining of apoptotic neurons in the CA1 region of the hippocampus and measurement (n = 5). The black arrows indicate apoptotic neurons with condensed nucleus, concentrated cytoplasm, and disarrangement of cells. Scale bars, 20 μm.
(G) Experimental design. Mucosal microbiota samples were collected from sham and 2-h mice. GF mice were subjected to mucosal microbiota transplantation (MMT) by injecting the mucosal microbiota to the proximal colon at 1 h after MCAO, with a group of mice receiving Helicobacter pylori. Mice were sacrificed at 3 h after MCAO.
(H and I) Alcian blue-stained sections of the colon with pellet (the mucus layer is defined by the black dashed lines) and measurement of the thickness of the mucus layer (n = 5). Scale bars, 1 mm (top); 250 μm (bottom).
(J) Concentrations of flagellin in the colon (n = 5).
(K) Nissl staining of apoptotic neurons in the CA1 region of the hippocampus and measurement (n = 5). Scale bars, 500 μm (top); 100 μm (bottom).
(L) Experimental design. After antibiotics treatment for 2 weeks, mice were intraperitoneally injected with saline or flagellin at 1 h after cerebral reperfusion, and were sacrificed at 3 h after MCAO.
(M–O) Levels of IL-6, IL-1β, and TNF-α in the blood (n = 5).
(P) Expressions of tight junction proteins in the brain (n = 5).
(Q–S) Nissl staining of apoptotic neurons and immunostaining of Iba-1+ microglia in the CA1 region of the hippocampus and measurement (n = 5). Scale bars, 100 μm (Nissl staining); 50 μm (immunostaining). Data were represented as mean ± SEM. Statistical comparison was performed by two-tailed unpaired Student’s t test (F, M–P, R, and S), two-way ANOVA with Turkey’s multiple comparisons test (C and D) or one-way ANOVA with Dunnett’s multiple comparisons test (E, I–K). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ##p < 0.01, ####p < 0.0001 versus GF-sham.
Please see also Figures S6–S9.
Goblet cells are the resource of mucus.13 Thus, we next determined whether goblet cells were responsible for the decreased thickness of mucus layer. Using Alcian blue staining that stains acidic mucins and periodic acid Schiff staining that stains neutral mucins to identify mucin-containing goblet cells, we found that goblet cell number did not decrease in the 2-h group (Figures S5A and S5B). Additionally, we tested the expressions of goblet cell markers including Muc2, Trefoil factor 3 (regulates viscosity of the mucosal layer and facilitates epithelial repair),16 Krüppel-like factor 4 (involves in stem cell maintenance and differentiation of goblet cells),17 Resistin-like molecule beta (promotes secretion of mucin),18 and several glycosyltransferases such as β-1,3-galactosyltransferase 1 (C1galt1), glucosaminyl (N-acetyl) transferase 1 (C2gnt), β-1,3-N-acetylglucosaminyltransferase 6 (B3gnt6), ST6N-acetylgalactosaminide α-2,6-sialyltransferase (St6gal1), fucosyltransferase 1, and fucosyltransferase 3. However, we did not find a significant decrease in the expressions of these genes except for St6gal1 (Figure S5C). These findings suggest that the mucosal microbiota might be responsible for the disruption of the mucus layer.
The mucosal microbiota contributes to the disruption of the mucus layer and exacerbation of brain injury
Next, we measured the mucosal microbiota composition of the sham, 1-h, and 2-h groups by 16S rRNA gene sequencing. We found that the Chao1 index and Observed species were significantly increased in the 2-h group (Figures S6A and S6B). In addition, the mucosal microbiota composition shifted significantly in the 2-h group, as evidenced by separation of plots in the principal coordinate analysis (PCoA) (Figure S6C). The abundances of Proteobacteria at the phylum level and Helicobacter at the genus level significantly increased in the 2-h group (Figures S6D and S6E), which was confirmed by linear discriminant analysis effect size analysis (Figure S6F). Proteobacteria and Helicobacter are typically flagellated bacteria.19 We found that the predicted gene functions including flagellar assembly, bacterial chemotaxis, bacterial motility proteins, and epithelial cell signaling in Helicobacter pylori infection were significantly increased in the 2-h group (Figures S6G–S6J). We also tested the fecal microbiota composition and found that there was a significant difference between the fecal microbiota and mucosal microbiota (Figure S6K). Furthermore, the abundances of Proteobacteria and Helicobacter did not evidently increase in the fecal microbiota of the 2-h group (Figures S6L–S6O), suggesting that the mucosal microbiota are more quickly affected by the changes of host’s physiology due to their closer contact with the host. To further identify the key species, we sequenced the metagenome of mucosal microbiota of sham and 2-h group. As is expected, there was a clear separation in the PCoA plots between the 2 groups (Figure S7A). Moreover, the abundances of Proteobacteria (phylum), Helicobacter (genus), and Helicobacter ganmani (species) were higher in the 2-h group than those in the sham group (Figures S7B–S7H).
We then investigate whether the removal of microbiota would mitigate mucosal barrier injury and further protect against stroke injury. Conventional (CV) and germ-free (GF) mice were subjected to MCAO and administrated with FITC at 2 h after cerebral reperfusion, and were sacrificed 1 h after administration (Figure 2A). Although the mucus layer thickness, goblet cell number, gut permeability, and the expressions of tight junction proteins in the colon were already significantly disrupted in GF mice compared to CV mice before stroke, these phenotypes were not significantly affected by stroke in GF mice (Figures 2B–2E and S8). Importantly, the absence of gut microbiota protected against ischemic damage in the brain, as evidenced by decreased apoptotic neurons in GF mice (Figure 2F). Next, we used broad-spectrum antibiotics (Abx) to eliminate the gut microbiota. Mice were subjected to Abx treatment for 14 consecutive days before MCAO (Figure S9A). The α- and β-diversity of fecal and mucosal microbiota changed significantly in the Abx groups (Figures S9B–S9D). Although Abx treatment led to increased relative abundances of Proteobacteria and Helicobacter in both fecal and mucosal microbiota prior to stroke, their absolute abundances were very low (Figures S9D–S9K). Similarly, the decreased mucosal microbiota load exerted beneficial effects on the mucus layer and brain injury in Abx mice (Figure S10).
Next, we further determine whether the presence of dysbiotic mucosal microbiota disrupts the mucus. We collected the mucosal microbiota from sham and 2-h group mice, then we performed mucosal microbiota transplantation (MMT) to GF mice by injecting the mucosal microbiota to the proximal colon at 1 h after cerebral reperfusion, with a group of mice receiving H. pylori (Figure 2G). We found that mice receiving MMT from the 2-h group mice or H. pylori had a significantly lower mucus layer thickness, higher flagellin level in the colon, and more apoptotic neurons than those receiving MMT from sham mice (Figures 2H–2K), emphasizing an important role of mucosal microbiota in the mucus layer and brain injury after stroke. To further substantiate the role of flagellin in the exacerbation of brain injury after stroke, Abx-treated mice were intraperitoneally injected with saline or flagellin at 1 h after cerebral reperfusion, and were sacrificed at 3 h after MCAO (Figure 2L). Compared to the saline group, the flagellin group exhibited significantly higher levels of serum proinflammatory cytokines, including IL-6, IL-1β, and TNF-α (Figures 2M–2O), lower expressions of tight junction proteins of the blood-brain barrier (Figure 2P), and more prominent microglial activation, resulting in more apoptotic neurons in the brain (Figures 2Q–2S).
TLR5 is downregulated in the intestine with decreased levels of flagellin-specific IgA and antimicrobial peptides
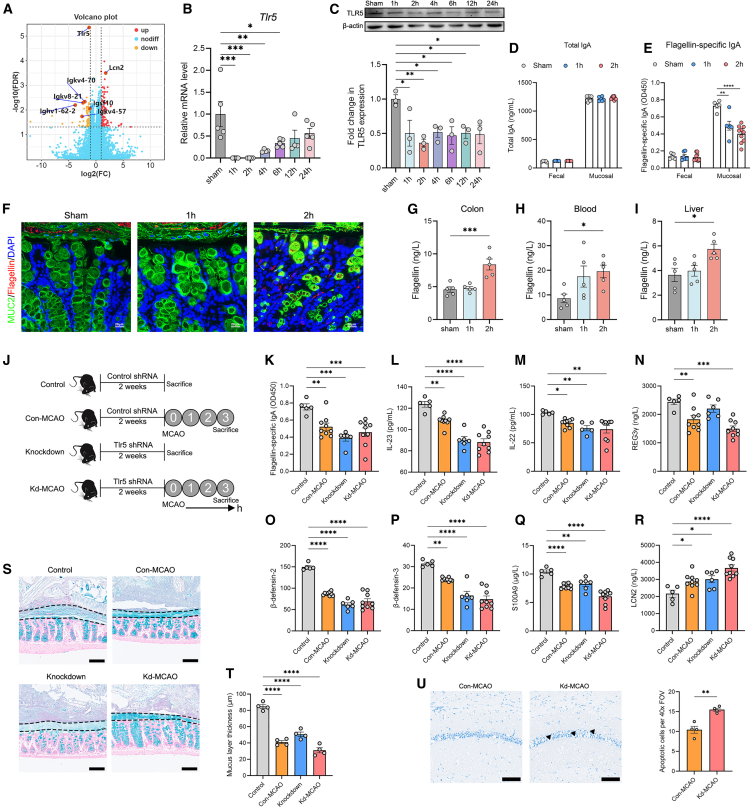
Next, we sought to investigate the mechanism underlying the changes of mucosal microbiota. We performed RNA sequencing of the colon of sham versus 2-h group mice and found that the expression of Tlr5, a receptor that recognizes bacterial flagella, exhibited the most significant change (Figure 3A). We verified the downregulation of TLR5 by PCR and western blotting (Figures 3B and 3C). There were no differences in the expressions of intestinal TLR5 between sham mice sacrificed at different time points after operation (Figure S1I). In addition to TLR5, transcriptome analysis also revealed downregulation of immunoglobulin (Ig)-related genes (Figure 3A). Therefore, we measure the levels of total IgA and flagellin-specific IgA and found that the level of IgA was significantly higher in mucosal layer than in luminal feces (Figures 3D and 3E). There was no significant difference in the level of total IgA between the sham, 1-h, and 2-h groups, whereas the mucosal level of flagellin-specific IgA was significantly lower in the 1-h and 2-h groups compared to the sham group (Figures 3D and 3E). Labeling of flagellin revealed that flagellated bacteria had breached the mucosal barrier and reached the epithelium in the 2-h group mice (Figure 3F). Moreover, the levels of flagellin in the colon, blood, and liver were significantly higher in the 2-h group than those in the sham group (Figures 3G–3I).
Figure 3.
TLR5 is downregulated in the intestine with decreased levels of flagellin-specific IgA and antimicrobial peptides
(A) Volcano plot of transcripts from RNA sequencing of the colon of sham versus 2-h group mice. Orange dots were downregulated genes and red dots were upregulated genes in the 2-h group (n = 6) compared with the sham group (n = 6).
(B) The gene expression of Tlr5 in the colon (n = 4–5).
(C) The protein expression of TLR5 in the colon (n = 3).
(D and E) The levels of total IgA and flagellin-specific IgA in the luminal content and mucosal layer of the colon (n = 5–10).
(F) Double immunostaining of MUC2 and flagellin in the colon. Red arrows indicate the flagellated bacteria. Scale bars, 20 μm.
(G–I) Measurements of flagellin concentrations in the colon, blood, and liver (n = 5).
(J) Experimental design. Mice were injected with AAV2/9-U6-tlr5 shRNA-CMV-EGFP-WPRES (knockdown group) to knock down TLR5; control mice were injected with AAV2/9-U6-NC shRNA-CMV-EGFP-WPRES (control group) once a week for 2 weeks, after which mice were subjected to MCAO and sacrificed.
(K–R) The levels of (L) IL-23 and (M) IL-22 in the colon and levels of (K) flagellin-specific IgA, (N) regenerating islet-derived protein 3γ (REG3γ), (O) β-defensin-2, (P) β-defensin-3, (Q) S100A9, and (R) lipocalin-2 (LCN2) in the mucus.
(S and T) Alcian blue-stained sections of the colon with pellet (the mucus layer is defined by the black dashed lines) and measurement of the thickness of the mucus layer (n = 4). Scale bars, 100 μm.
(U) Nissl staining of apoptotic neurons in the CA1 region of the hippocampus and measurement (n = 4). The triangles indicate apoptotic neurons with condensed nucleus, concentrated cytoplasm, and disarrangement of cells. Scale bars, 100 μm. Data were represented as mean ± SEM. Statistical comparison was performed by two-tailed unpaired Student’s t test (U), or one-way ANOVA with Dunnett’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Please see also Figures S11–S13.
To further investigate the connection between TLR5 and mucosal microbiota, we performed a TLR5 knockdown experiment. Mice were injected with AAV2/9-U6-tlr5 shRNA-CMV-EGFP-WPRES (knockdown group) to knock down TLR5; control mice were injected with AAV2/9-U6-NC shRNA-CMV-EGFP-WPRES (control group) once a week for 2 weeks, after which mice were subjected to MCAO and sacrificed (Figure 3J). The expression of TLR5 was significantly decreased after the knockdown (Figures S11A and S11B). We found that the levels of IL-23 and IL-22, which are important cytokines to induce the expressions of antimicrobial peptides,20 were significantly decreased after Tlr5 knockdown (Figures 3L and 3M). Additionally, the levels of flagellin-specific IgA, and antimicrobial peptides including β-defensin-2, β-defensin-3, and S100A9, were significantly decreased in the knockdown group, except for regenerating islet-derived protein 3γ and lipocalin-2 (Figures 3K–3R). These findings revealed multiple downstream products of TLR5 signaling pathway, in which the decreased level of flagellin-specific IgA might contribute to the increased abundance of flagellated bacteria. Indeed, the abundances of Proteobacteria and Helicobacter were higher in the knockdown group than in the control group (Figures S11C–S11H). The knockdown group exhibited significantly lower mucus layer thickness (Figures 3S and 3T) and more apoptotic neurons in the brain after stroke, compared to the control group (Figure 3U).
We also performed TLR5 upregulation experiment to further substantiate our findings. Mice were injected with AAV2/9-CAG-tlr5-3flag-WPRE (AAV-TLR5 group) to upregulate TLR5; control mice were injected with AAV2/9-CAG-MCS-WPRE (AAV-NC) once a week for 3 weeks, after which mice were subjected to MCAO (AAV-NC-MCAO group and AAV-TLR5-MCAO group) (Figure S12A). The expression of TLR5 was significantly increased after treatment (Figures S12B and S12C). The α- and β-diversity were significantly different between the AAV-NC-MCAO group and AAV-TLR5-MCAO group (Figures S13A and S13B). Moreover, the abundances of Proteobacteria and Helicobacter were lower in AAV-TLR5-MCAO group than in the AAV-NC-MCAO group (Figures S13C–S13F). The mucus layer thickness was significantly increased in the AAV-TLR5-MCAO group, and the goblet cell number was not affected (Figures S12D and S12E), whereas the magnitude of mucosal amelioration was not evident compared to that of the knockdown group (Figure 3T). There were significantly reduced levels of flagellin in the colon, blood, and liver of the AAV-TLR5-MCAO group (Figures S12F–S12H), with decreased apoptotic neurons and reduced infarction volume (Figures S12I and S12J).
Noradrenaline released by intestinal sympathetic nerve downregulates the expression of TLR5
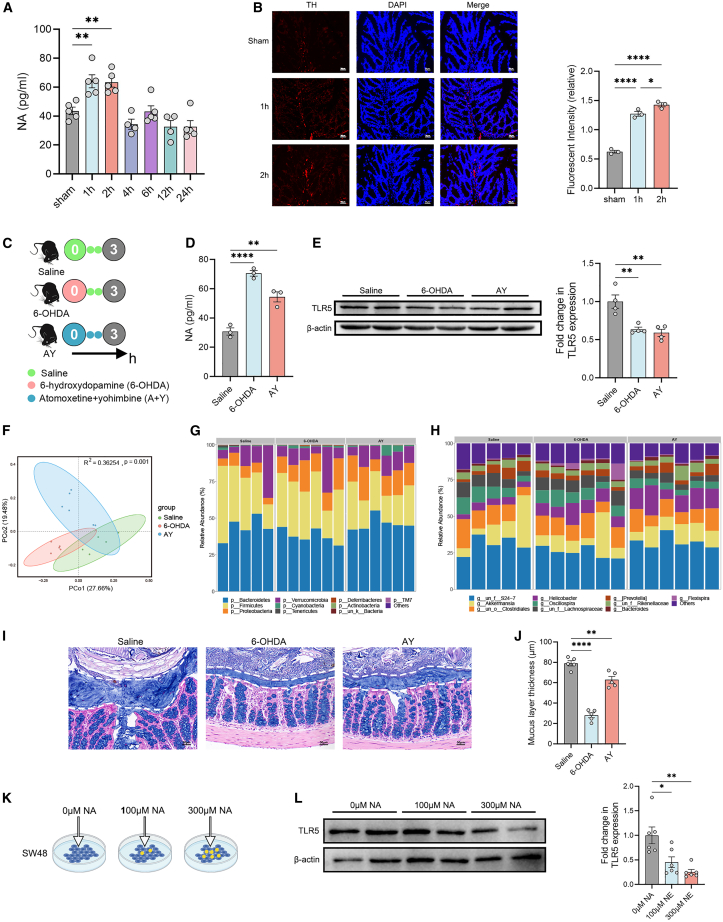
Next, we sought to investigate the mechanism underlying downregulation of TLR5 during acute stage of stroke. Given that the expression of TLR5 was downregulated in the first few hours after stroke, which indicates a swift response from the brain to the gut, we speculated that the neural pathway might be involved in the process. First, we measured the concentrations of several neurotransmitters in the colon and found that the levels of noradrenaline (NA) were significantly elevated in the 1-h and 2-h groups (Figure 4A), whereas the levels of epinephrine (EPI), 5-hydroxytryptamine (5-HT), and substance P (SP) did not exhibit significant changes (Figures S14A–S14C). Immunostaining displayed significantly increased tyrosine hydroxylase (TH)+ sympathetic NA fibers in the colon of the 1-h and 2-h groups (Figure 4B), which is further supported by the reduced blood flow in the proximal colon (Figure S14D).
Figure 4.
Noradrenaline released by intestinal sympathetic nerve downregulates the expression of TLR5
(A) Measurements of noradrenaline (NA) concentration in the colon tissue of mice (n = 4–5).
(B) Immunostaining of tyrosine hydroxylase (TH)+ sympathetic NA fibers in the colon of mice (n = 3). Scale bars, 30 μm.
(C) Experimental design. Mice were intraperitoneal injected with 6-hydroxydopamine (6-OHDA) followed by two consecutive injection of saline every hour. Another group of mice was intraperitoneally injected with atomoxetine and yohimbine (A + Y) every hour for three consecutive times. The control mice were intraperitoneally injected with saline.
(D) Measurement of NA concentration in the colon tissue of mice (n = 3).
(E) Expression of TLR5 in the colon of mice after treatment (n = 3).
(F) PCoA of the mucosal microbiota composition based on Bray-Curtis distance in mice (n = 5–6).
(G and H) Relative abundance of mucosal microbiota from individual samples at the (G) phylum level and (H) genus level.
(I and J) Alcian blue staining of colonic tissue with pellet and measurement of the thickness of the mucus layer (n = 5). Scale bar, 30 μm.
(K) Experimental design. SW48 cells that originated from the colon were supplemented with different concentrations of NA, and the expressions of TLR5 were analyzed after 2 h of treatment.
(L) Expression of TLR5 in SW48 cell cultures (n = 6). Data were represented as mean ± SEM. Statistical comparison was performed by one-way ANOVA with Dunnett’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Please see also Figures S14–S17.
To test the hypothesis that the activation of sympathetic nerve downregulates the expression of TLR5, we employed two pharmacological strategies to activate the sympathetic tone in vivo. Mice were intraperitoneally injected with 6-hydroxydopamine (6-OHDA, a neurotoxin that causes sudden release of NA stored in sympathetic nerves),21 followed by two consecutive injections of saline every hour. Another group of mice were intraperitoneally injected with atomoxetine and yohimbine (A + Y, induces a moderate sympathetic tone) every hour for three consecutive times. The control mice were intraperitoneally injected with saline (Figure 4C). We found that the level of NA in the colon was significantly increased after sympathetic activation (Figure 4D). Treatment of 6-OHDA also significantly increased the level of EPI (Figure S14E). Neither 6-OHDA nor A + Y treatment changed the levels of 5-HT or SP in the colon (Figures S14F and S14G). Notably, we found that the activation of sympathetic tone significantly decreased the expression of TLR5 in the colon (Figure 4E). Furthermore, sympathetic activation significantly altered the mucosal microbiota composition (Figure 4F), leading to increased abundances of Proteobacteria and Helicobacter in the mucus layer (Figures 4G, 4H, S13I, and 13J). Treatment of 6-OHDA or A + Y resulted in decreased thickness of the mucus layer, especially by 6-OHDA (Figures 4I and 4J). Of note, activation of sympathetic tone did not affect the goblet cell number (Figure S14H). Moreover, we performed in vitro study to investigate the effect of NA on the expression of TLR5 in intestinal cells. We used SW48 cells that originated from the colonic epithelium. Different concentrations of NA were supplemented into SW48 cell cultures, and the expressions of TLR5 were analyzed after 2 h (Figure 4K). We found that the expression of TLR5 was downregulated by NA in a dose-dependent manner (Figure 4L).
Next, we sought to explore how the expression of Tlr5 is inhibited by sympathetic activation. As is predicted by the TargetScan, there are binding sites between Tlr5 and miR-150-5p (Figure S15A). Dual-luciferase reporter gene assay revealed that miR-150-5p could specifically target Tlr5 mRNA, leading to its inhibition (Figures S15B and S15C). The half-life of Tlr5 mRNA and the protein turnover of TLR5 require further investigation. We measured the expression of miR-150-5p by in situ hybridization and found that treatment of 6-OHDA and A + Y significantly increased the expression of miR-150-5p (Figure S15D). To investigate whether reducing sympathetic tone would exert amelioration on mucosal barrier disruption and stroke injury, we employed two pharmacological strategies that block the β-adrenergic receptors. Mice were intraperitoneally injected with propranolol or metoprolol at cerebral reperfusion. The control group was intraperitoneally injected with saline. Mice were sacrificed at 3 h after cerebral reperfusion (Figure S16A). We found that the thickness of the mucus layer was increased by propranolol or metoprolol treatment, and the goblet cell number was not influenced (Figures S16B–S16E). As expected, both treatments relieved ischemic stroke injury, as evidenced by decreased number of apoptotic neurons (Figures S16F and S16G).
To better understand the kinetics of the pathway in the intestine after stroke, we measured the changes in the levels of the key elements of the study at different time points within 3 h after stroke (Figure S17A). The levels of NA and miR-150-5p exhibited a significant increase at 15 min after stroke (Figures S17B and S17C). The expressions of Tlr5 mRNA and TLR5 protein exhibited a significant decrease at 30 min and 1 h after stroke, respectively (Figures S17D–17F). The level of flagellin-specific IgA significantly decreased at 1 h, and gene copies of 16s rRNA in the mucus layer significantly increased at the same time point (Figures S17G and S17H). The levels of IL-6 and IL-1β were significantly increased at 2.5 h and the level of TNF-α was significantly increased at 3 h post stroke (Figures S17I–S17K).
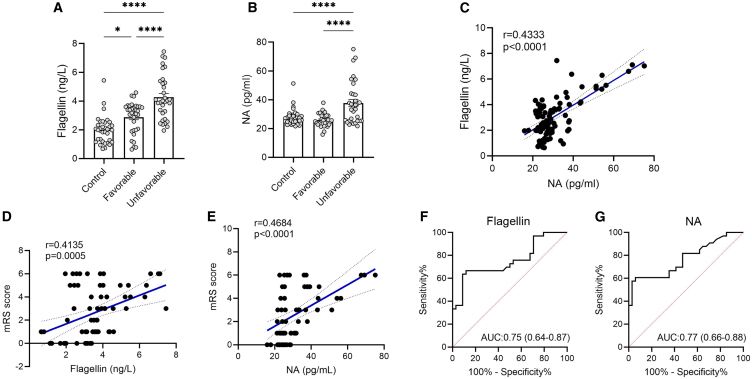
Serum biomarkers for gut permeability are associated with prognosis in patients
Finally, based on the findings from animal studies, we tested whether serum flagellin level is correlated with NA level in patients with stroke. Sixty-seven patients with acute ischemic stroke were recruited in our study. We collected the serum samples from patients at admission. Functional outcome was evaluated at 3 months after stroke onset using the modified Rankin Scale (mRS). Patients with a mRS <3 were considered to have a favorable outcome (favorable group) and patients with a mRS ≥3 were considered to have an unfavorable outcome (unfavorable group).22 We recruited 34 age- and gender-matched controls without a history of stroke. We found that flagellin level was significantly increased in patients with stroke especially in those with an unfavorable outcome (Figure 5A). Although there was no significant difference in levels of NA between the control and favorable groups, the unfavorable group had higher serum NA than the other groups (Figure 5B). Of note, the flagellin level was significantly associated with the NA level (r = 0.4333, p < 0.0001) (Figure 5C). Additionally, both flagellin and NA levels were positively associated with mRS score (Figures 5D and 5E). To investigate whether these biomarkers at acute stage of stroke could predict functional outcome at 3 months, the receiver operating characteristic (ROC) curve was used to evaluate the performance of the prediction model. We found that the value of the area under the curve (AUC) of flagellin and NA was 0.75 (95% confidence interval: 0.64–0.87) and 0.77 (95%: 0.66–0.88), respectively (Figures 5F and 5G). Additionally, we found that the unfavorable group had higher levels of serum biomarkers for gut permeability, including LPS, LPS-binding protein, and D-lactate at the acute stage of stroke (Figures S18A–18C). Furthermore, these serum biomarkers were also positively associated with mRS score (Figures S18D–S18F), with an AUC of 0.88, 0.79, and 0.81, respectively (Figures S18G–S18I). Thus, serum biomarkers for gut permeability have important prognostic value in stroke outcome.
Figure 5.
Serum biomarkers for gut permeability are associated with prognosis in patients
(A and B) Measurements of serum flagellin and NA concentrations in controls (N = 34), patients with ischemic stroke with a favorable outcome (N = 34), and those with an unfavorable outcome (N = 33).
(C) Spearman’s correlations between serum flagellin level and NA concentrations.
(D and E) Spearman’s correlations between serum flagellin, NA concentrations, and modified Rankin Scale (mRS) score.
(F and G) The ROC curve of serum flagellin and NA at acute stage of stroke as predictors for prognosis at 3 months. Data were represented as mean ± SEM. Statistical comparison was performed by one-way ANOVA with Dunnett’s multiple comparisons test. ∗p < 0.05, ∗∗∗∗p < 0.0001.
Please see also Figures S18 and S19.
Moreover, anal swab samples were collected from patients with stroke during the acute stage of stroke, and fecal samples were collected from controls. Although the α- and β-diversity were comparable between the favorable and unfavorable groups, they were significantly different from the control group (Figures S19A and S19B). Although we did not find a high abundance of Helicobacter in anal swab samples of patients with stroke, we did observe higher abundances of flagellated bacteria including Proteobacteria, Enterobacteriaceae, and Escherichia-Shigella in the unfavorable group than in the favorable group (Figures S19C and S19E). Importantly, the gene function of epithelial cell signaling in H. pylori infection (PATH: ko05120) and bacterial invasion of epithelial cells (PATH: ko05100) were significantly augmented in the unfavorable group (Figures S19F and S19G).
Discussion
Emerging evidence has revealed an impaired intestinal barrier after stroke, which in turn exacerbates stroke injury.6 Yet, how the mucosal barrier is disrupted remains unclear. It is reasonable to link an impaired mucus layer to goblet cell malfunction; however, we found that goblet cells remained intact post stroke when the mucus layer had been disrupted significantly. Here, we proposed a neural pathway that is responsible for the disruption of mucus layer. As ischemic stroke induces acute stress with autonomic hyperstimulation,23 sympathetic tone is elevated in the intestine. These pathological changes happen in a matter of hours after stroke. Activated sympathetic nerve downregulated the expression of intestinal TLR5. We found that knockdown of TLR5 led to decreased levels of flagellin-specific IgA and several antimicrobial peptides, leading to the proliferation of flagellated bacteria and an impaired mucosal barrier. Furthermore, the proinflammatory substance flagellin produced by flagellated bacteria caused neuroinflammation that exacerbated brain injury after stroke.
The gut barrier plays a crucial role in secondary stroke injury. The intestine has several lines of defense to prevent the invasion of microorganisms, and the mucus layer is the first line of defense.24 This may explain why the disruption of the mucosal barrier is prior to the destruction of tight junctions. Consistent with previous findings, we found the gut permeability peaks at the first few hours and then declines within the first 24 h after stroke via oral gavage of FITC-dextran.25 However, we observed reduced GI motility after stroke, so the result might reflect the permeability of the upper GI. As the colon harbors the densest microbiota in the GI tract, it is more important to investigate the permeability of the colon after stroke. We further discovered that the colonic permeability was significantly associated with the thickness of the mucus layer, suggesting that the mucus layer is the major contributor to the integrity of the gut barrier during the acute stage of stroke. In the meantime, the infarction volume and apoptotic neurons continued to increase during the first 24 h of stroke, emphasizing the importance of protecting the gut barrier to ameliorate secondary stroke injury. Indeed, we found that biomarkers for gut permeability are associated with prognosis in patients with stroke.
Our findings suggest that the mucosal bacteria were responsible for the decreased thickness of the mucus layer. This hypothesis is supported by the findings that stroke did not decrease the thickness of the mucus layer in GF mice and Abx-treated mice, and further substantiated by the MMT experiment. GF mice have been widely used as a powerful tool to investigate the effects of different gut microbiota composition on stroke injury via fecal microbiota transplantation.26,27,28 These studies help to strengthen the notion that a perturbed gut microbiota leads to worse stoke injury. However, the conclusions from studies using GF animals should be interpreted with caution. In the current study, we further revealed the importance of the mucosal bacteria. The luminal microbiota has been considered a passenger of the intestinal microecology, whereas the mucosal microbiota plays a more important role. Interestingly, we found that MMT of H. pylori could disrupt the mucus layer. Of note, it has been reported that flagellin of H. pylori FlaA has different amino acids in its TLR5 epitope and therefore does not stimulate TLR5.29 H. pylori colonizes in the mucus layer of gastric epithelium, where it exerts lytic activity by producing proteases and lipases that disintegrate mucin.30 However, its colonization and effects in the mucus of the colon remain largely unknown. A recent study demonstrated that inoculation of H. pylori induces a mucus-degrading microbiota signature and accelerates colorectal carcinogenesis.31 The role of H. pylori in the pathogenesis of colonic diseases and the correlation between H. pylori infection and stroke prognosis require further investigation.
The effects of Abx on stroke injury have been reported to yield controversial results. One study conducted by Winek et al. found that Abx increases mortality rate in mice after stroke,32 while other studies found that Abx reduces infarct volume and improves neurological outcome in mice.33,34,35 In the current study, we found that 2 weeks of Abx treatment protects against stroke injury. Short-term Abx treatment cannot ameliorate stroke injury, and a reduced bacterial density is sufficient to induce neuroprotection.34 Based on our findings and those of others, we speculated that long-term and continuous Abx treatment is necessary to eliminate the mucosal bacteria.
In our study, we found that the activation of sympathetic nerve after stroke initiates a series of events, i.e., miR-150-5p/TLR5/IgA axis, leading to microbiota dysbiosis. Given that the activation of sympathetic nervous tone could be a common trait in various diseases complicated with acute or chronic stress, one may speculate that this could be the common cause of dysbiosis in these diseases. Indeed, the sympathetic tone is increased in patients with inflammatory bowel disease (IBD).36 Interestingly, a lower expression of TLR5 has been found in the colonic epithelial cells of patients with IBD.37 Moreover, the observations that TLR5 knockout mice are prone to colitis and have an increased abundance of flagellated bacteria further support this hypothesis.20,38,39 It should be noted that the mechanisms underlying the expression of TLR5 and the activation of TLR5 are different. The knockdown of TLR5 would lead to less activation of its downstream signaling whereas the inhibition of TLR5 does not necessarily lead to downregulation of its expression. This is demonstrated in our study that both stroke- and AAV-driven downregulation of TLR5 expression led to impaired mucus layer, which was ameliorated by AAV-driven TLR5 upregulation. Moreover, in the context of MCAO-driven downregulation of TLR5 expression, injection of flagellin, despite the activation of TLR5 signaling, could not improve the impaired mucus layer.
NA is secreted from sympathetic nerve fibers, but it can also be synthesized by bacteria. Previous study has demonstrated that the source of NA in the intestine after stroke is derived from the host.14 In our study, we found that sympathetic activation led to changed mucosal microbiota composition. However, it should be noted that norepinephrine (NE) secreted into the intestinal lumen can have direct effect on microbiota. For example, enteric pathogen Citrobacter rodentium can exploit NE to activate its virulence via a QseC/QseE-dependent pathway.40 Although we did not find Citrobacter rodentium in the mucus of our study, whether Helicobacter could be affected by NE or other neurotransmitters warrants further studies. Clinical studies have found an association between pharmacological blockade of the β-receptor and reduced stroke mortality as well as poststroke infection.41 Animal research has provided evidence for adrenergic-induced systemic immunodeficiency following stroke.42 Further evidence indicates that it is the sympathetic nerve but not the hypothalamic-pituitary-adrenal axis that contributes to stroke-related infections.43 Our study extended these findings and provided an angle to illustrate the causal relationship between activation of sympathetic nerve and stroke-related infection.
Limitations of the study
We acknowledge several limitations to this study. First, the sample size of the cohort in this study is relatively small, and we did not measure the mucus layer, intestinal expression of TLR5, or mucosal microbiota in patients with stroke. Future studies with a larger cohort, and ideally, with biopsy samples of the colonic tissue would help strengthen our findings. Second, although we found an increased abundance of H. ganmani, and we did observe a decreased thickness of the mucus layer after MMT of H. pylori, the lytic properties of Helicobacter warrant further investigation. Additionally, the specificity or necessity of flagellin to impair the mucus barrier was not fully investigated in the current study. Finally, we have revealed that NA could upregulate miR-150-5p, but the exact mechanism needs further investigations.
Conclusion
In conclusion, we identified a pathway in which the activation of sympathetic nerve downregulates the expression of TLR5; the loss of TLR5 leads to insufficient flagellin-specific IgA and antimicrobial peptides, leading to an impaired mucus barrier with the invasion of flagellated bacteria. Our study may provide therapeutic potential of targeting the sympathetic nervous system for the protection of the gut barrier and stroke injury.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yan He (yanhe@i.smu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The transcriptome data (NCBI: PRJNA1154613), metagenome sequencing data (NCBI: PRJNA1154791), and 16S rRNA gene sequencing data (NCBI: PRJNA1154919) have been deposited at NCBI database.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by National Key R&D Program of China, China (2019YFA0802300, SQ2022YFA090032) and National Natural Science Foundation of China (NSFC 82022044, 82272391, 81870936, and 82171317).
Author contributions
Y.H., J.Y., K.X., and H.Z. were responsible for the study design and supervision; H.W., J. Li, and G.W. were responsible for the animal experiments; G.W. was responsible for the cell experiments; X. Lin, J.C., J. Liang, and J.X. were responsible for clinical studies; J.Z., X. Luo, H.M., and Z.L. were responsible for bioinformatics analysis; and H.W. and J. Li drafted the manuscript and Y.H. revised the manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-ZO1 | Abcam | Cat# ab216880 RRID: AB_2909434 |
| Anti-Occludin | Abcam | Cat# ab216327 RRID: AB_2737295 |
| Anti-Claudin4 | Abcam | Cat# ab53156 RRID: AB_869176 |
| Anti-β-actin | Abcam | Cat# ab8226 RRID: AB_306371 |
| Anti-MUC2 | Abcam | Cat# ab272692 RRID: AB_2888616 |
| Anti-TLR5 | Abcam | Cat# ab13876 RRID: AB_2240729 |
| Anti-Flagellin | Abcam | Cat# ab93713 RRID: AB_10563522 |
| Anti-Tyrosine Hydroxylase | Abcam | Cat# ab137869 RRID: AB_2801410 |
| Anti-IgA alpha chain (HRP) | Abcam | Cat# ab97235 RRID: AB_10681186 |
| Biological samples | ||
| Human anal swab | Nanfang hospital of Southern Medical University | N/A |
| Human serum | Nanfang hospital of Southern Medical University | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Flagellin | Glpbio | Cat# GP23503 |
| 2,3,5-triphenyltetrazolium chloride | Sigma | Cat# T8877-100G |
| Fluorescein isothiocyanate dextran | Sigma | Cat# FD4-1G |
| Ampicillin sodium salt | Sigma | Cat# A9518-25G-9 |
| Neomycin trisulfate salt hydrate | Sigma | Cat# N6386-100G |
| Metronidazole | Sigma | Cat# M1547-25G |
| Vancomycin | Macklin | Cat# V820413 |
| Glycine | Sigma | Cat# V900144-500g |
| 30%Acr-Bis | Leagene | Cat# PE0103 |
| TEMED | FUDE | Cat# FD2100 |
| DMEM | Gibco | Cat# 8123445 |
| FBS (cell culture) | Corning | Cat# 17323002 |
| Penicillin Streptomycin | Gibco | Cat# 2585600 |
| Trypsin-EDTA | Gibco | Cat# 25200-056 |
| Tween 20 | Aladdin | Cat# T104863-100mL |
| Glutaraldehyde | Aladdin | Cat# G105907-50mL |
| Propranolol | MCE | Cat# HY-B0573B |
| Metoprolol | Macklin | Cat# M875830-25mg |
| 6-hydroxydopamine | Sigma | Cat# H4381-100mg |
| TMB ELISA Substrate | Abcam | Cat# ab171523 |
| 450 nm Stop Solution for TMB Substrate | Abcam | Cat# ab171529 |
| L-noradrenaline | Aladdin | Cat# N109573 |
| Atomoxetine hydrochloride | Sigma | Cat# PHR1679-500MG |
| Yohimbine hydrochloride | Sigma | Cat# Y3125-5G |
| ECL Western Blotting Substrate | Biosharp | Cat# BL520B |
| Multicolor Pre-stained Protein Ladder | Epizyme | Cat# WJ102 |
| Bovine Serum Albumin | Sigma | Cat# V900933 |
| TRIzol reagent | Invitrogen | Cat# 15596018 |
| RIPA lysis buffer | Thermo Scientific | Cat# 89901 |
| Protease and phosphatase inhibitors | Thermo Scientific | Cat# 78442 |
| Bacteria EUB338 probe | Focobio | Cat# Gut001 |
| MicroRNA-150-5p | Focobio | Cat# 007.231070 |
| Critical commercial assays | ||
| Mouse IgA ELISA kit | Abclonal | Cat# RK00162 |
| Mouse REG3β ELISA kit | MEIMIAN | Cat# MM-44727M1 |
| Mouse REG3γ ELISA kit | MEIMIAN | Cat# MM-1114M1 |
| Mouse flagellin ELISA kit | MEIMIAN | Cat# MM-0761M1 |
| Mouse noradrenaline ELISA kit | MEIMIAN | Cat# MM-0876M |
| Mouse epinephrine ELISA kit | MEIMIAN | Cat# MM-0351M1 |
| Mouse 5-hydroxytryptamine ELISA kit | MEIMIAN | Cat# MM-0443M1 |
| Mouse substance P ELISA kit | MEIMIAN | Cat# MM-0445M1 |
| Human D-lactate ELISA kit | Bioswamp | Cat# HM11235 |
| Human lipopolysaccharide ELISA kit | Bioswamp | Cat# HM11067 |
| Human LBP ELISA kit | Bioswamp | Cat# HM11071 |
| Human flagellin ELISA kit | MEIMIAN | Cat# MM-1094H1 |
| Human noradrenaline ELISA kit | MEIMIAN | Cat# MM-0995H1 |
| DNA extraction kit | Qiagen | Cat# 51804 |
| BCA kit | Thermo Scientific | Cat# 23227 |
| SYBR Green Master Mix | Vazyme | Cat# Q111-02 |
| PAGE Gel Fast Preparation Kit (10%) | Epizyme | Cat# PG112 |
| PAGE Gel Fast Preparation Kit (12.5%) | Epizyme | Cat# PG113 |
| Deposited data | ||
| Transcriptome data | This paper | PRJNA1154613 |
| Metagenome sequencing data | This paper | PRJNA1154791 |
| 16S rRNA data | This paper | PRJNA1154919 |
| Experimental models: Cell lines | ||
| Homo: SW48 | MeisenCTCC | CTCC-ZHYC-0044 |
| Experimental models: Organisms/strains | ||
| Specific pathogen free C57BL/6J | Guangdong Medical Laboratory Animal Center | N/A |
| Germ free C57BL/6J | Jingtuo Biotechnology Co., Ltd. | N/A |
| Oligonucleotides | ||
| Primers for qPCR | This study | See Table S1 |
| Software and algorithms | ||
| Graphpad Prism 8 software | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ software | NIH | https://imagej.nih.gov/ij/ |
| Adobe illustrator 2021 | Adobe | https://www.adobe.com/ |
Experimental model and subject details
Study cohort and sample collection
The ischemic stroke patients were recruited from the Department of Neurology of Nanfang Hospital of Southern Medical University (Guangzhou, China). The inclusion criteria are as follows: (1) an age >18 years; (2) a diagnosis of acute ischemic stroke according to established guidelines44; (3) patients underwent interventional operation. The exclusion criteria are as follows: (1) patients administered antibiotics or probiotics within 3 months before admission; (2) patients with advanced cancer, serious internal or neurological diseases, or gastrointestinal symptoms in the past 3 months; (3) patients for whom serum or anal swap samples could not be obtained with 3 days of admission; (4) patients without a 3-month follow-up. The healthy control subjects were recruited from the community without a history of stroke. The absence of a history of stroke was confirmed by inquiring about past medical history and checking the medical systems if the participants had been admitted to the hospital. The exclusion criteria are as follows: (1) individuals administered antibiotics or probiotics within 3 months at sample collection; (2) patients with advanced cancer, serious internal or neurological diseases, or gastrointestinal symptoms in the past 3 months; (3) patients for whom serum samples could not be obtained. All participants provided written informed consent in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2020-169) and registered at http://clinicaltrials.gov (NCT04688138). Blood samples from stroke patients were collected at admission. Most of the anal swab samples were collected within 6 h after interventional therapy, except for seven patients. The blood samples were isolated by centrifugation at 3,000 rpm for 10 min and stored at −80°C until testing. Anal swab samples were snap frozen and stored at −80°C until testing.
Mice and MCAO-induced mouse stroke model
The experiments were approved by the Ethics Committee for Animal Care and Research of Zhujiang Hospital of Southern Medical University (Guangdong, China) and were performed according to the ARRIVE guidelines. As female hormone estrogens significantly affect experimental stroke injury,45 male mice were used in this study. Adult male C57BL/6J mice (8–10 weeks, 22–25 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). GF mice were purchased from Jingtuo Biotechnology Co., Ltd. (Shenzhen, China). Upon arrival to the facility, mice were randomly allocated to the cages, with 3–5 mice per cage. Each timepoint/group of the experiment includes more than 2 cages of mice. Focal cerebral ischemia was induced by transient (60 min) middle cerebral artery occlusion (MCAO) using an intraluminal filament. Briefly, Surgical anesthesia was induced by intraperitoneal injection of 1.25% tribromoethanol (0.02 mL/g of body weight). Body temperature was maintained throughout the procedure with a feedback-controlled heating blanket. A filament was introduced into the external carotid artery and gently advanced into the internal carotid artery until it reached the middle cerebral artery. After 60 min of cerebral ischemia, the filament was withdrawn to establish reperfusion. No antibiotics nor anti-inflammatory drugs were used for this surgery. Sham mice were subjected to the initial anesthetic and neck incision only. In the experiments that include sham control, the time point for post-sham surgery was the same as the 2h stroke group. The MCAO modeling was performed following the same procedure in any animals of our study, and the MCAO modeling of GF mice were performed in a germ-free facility with sterile surgical instruments. The swap samples of skin and peritoneal fluid of GF mice were collected before tissue harvest and cultured in blood agar plates under aerobic and anaerobic conditions to exclusion the possibility of contamination. All mice underwent MCAO were tested by 2,3,5-triphenyltetrazolium chloride (TTC) staining or Nissl staining to confirm brain ischemic injury.
SW48 cell culture and treatment
Human colorectal adenocarcinoma cell line SW48 (CTCC (the Chinese Tissue Culture Collections)-ZHYC-0044) were obtained from MeisenCTCC (Zhejiang Meisen Cell Technology Co., Ltd). SW48 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco #8123445), 10% Fetal Bovine Serum (FBS) (Corning #17323002), with 1% Penicillin Streptomycin (Gibco #2585600) in a humidified incubator at 37°C and 5% CO2. Cells were digested by 0.25% Trypsin-EDTA (Gibco, #25200-056), and passaged every 2–3 days in T75 flasks according to CTCC recommendations. About 80% confluent T75 flasks were diluted, and approximately 3×105 cells seeded onto 6-well plates. SW48 cell incubation solution containing 0 μM L-noradrenaline (Aladdin #N109573) was used as the control group. SW48 cells were treated with different concentration of L-noradrenaline (100 μM, 300 μM) as the treatment group. The cells were collected 2 h after NA treatment.
Method details
Isolation and collection of the mucus layer
Colonic mucus isolation and collection was performed by following procedures that have been previously described.11,46,47 Briefly, after mice were sacrificed, the colon was removed immediately, then the colon was longitudinal dissected and the luminal contents were removed to expose the lumen surface. The tissue was washed 3 times with Dulbecco’s phosphate-buffered saline (DPBS), after which the mucus was gently scraped off with a cover glass slide and collected into a tube and put into liquid nitrogen.
TTC staining and infarct volume measurement
At different timepoints after MCAO surgery, mice were subjected to anesthesia by intraperitoneal injection of 1.25% tribromoethanol (0.02 mL/g of body weight). The mice were then transcardially perfused with phosphate-buffered saline (PBS), followed by perfusion with 4% paraformaldehyde (PFA). The intact brain was harvested and stored in −30°C refrigerator for 10 min, then the brain was dissected, cut into 2-mm tissue slices, and stained with 1% TTC for 15 min at 37°C. After TTC staining, the brain was immersed in 4% PFA for fixation. The brain slices were arranged in order and photographed after fixation for 24 h. The cerebral infarct area was calculated using Image-Pro Plus software 6.0 (red areas indicate no infarction, pink areas indicate ischemic penumbra, and white areas indicate infarction). We used an edema correction formula for the infarct volume. The infarct area was calculated as the area of the nonischemic hemisphere minus the non-infarcted area of the ischemic hemisphere. The total cerebral infarct volume was calculated by integrating the measured volumes of different sections in one mouse brain tissue specimen.
Nissl staining
The brain tissue was removed carefully, postfixed with 4% PFA for 24 h, and then cryoprotected with 30% sucrose for 48 h. Serial frozen coronal sections (4 mm thick) were cut using a cryostat (Leica CM1950) at −20°C, and all sections containing a portion of the hippocampus were stored at −20°C. Then, the sections were dried in air, washed twice with distilled water (2 min/time), and stained with 1% toluidine blue for 5 min. The sections were washed 3 times with distilled water (3 min/time), placed in 70% ethanol for 2 min, washed twice with 95% ethanol (2 min/time), and then washed with xylene for 5 min. Sections were observed with a microscope (DM2500 microscope; Leica).
Immunofluorescence staining
After sacrifice, one centimeter of the colon tissue containing a fecal pallet or without contents (for goblet cell number measurement) was dissected and removed. Colon tissues were immediately immersed in Carnoy’s fixative liquid (60% dry ethanol, 30% chloroform, and 10% glacial acetic acid) to preserve the mucus layer for 24–48 h at room temperature, and then processed. Samples were paraffin-embedded without exposure to water to maintain mucus layer structure and integrity. 5 mm tissue cross-sections were heated at 60°C for 10 min prior to incubation in xylene twice for 10 min, and then rehydrated with decreasing concentrations of ethanol (100%, 95%, 70%, 50% and 30%) for 5 min each at room temperature. Antigen retrieval was performed by steaming samples in antigen retrieval solution for 15min and leaving samples in warm solution for an additional 15min. Samples were washed in PBS and blocked with 5% FBS in PBS for 30 min at 4°C. The tissues were stained with an anti-mucin 2 (MUC2, Abcam, ab272692) primary antibody for the mucus layer, anti-Tyrosine Hydroxylase (TH, Abcam, ab137869) primary antibody for sympathetic nerve, anti-flagellin primary antibody for flagellated bacteria, anti-Iba1 (Abcam, ab178846) primary antibody and fluorescent secondary antibody. Slides were imaged on a microscope and mucus layer thickness was measured using CaseViewer. 20 measurements were performed for each section and the average calculated for each mouse.
Alcian blue (AB), periodic acid-Schiff (PAS), and fluorescence in situ hybridization (FISH) staining
Histological slides of colonic samples were deparaffinized and stained with Alcian blue solution (Sigma-Aldrich) and Periodic Acid-Schiff (PAS) staining kit (Sigma-Aldrich) according to the manufacturer’s instructions. For FISH staining, slides with paraffin sections were placed in 100% xylene for 5 min, 3 times, and then rehydrated for 5 min each in 100% ethanol, 90% ethanol, and 70% ethanol. Slides were placed in PBS for 5 min, 3 times and then incubated with 0.2N HCl for 30 min at room temperature and washed in PBS for 5 min twice. Proteinase K was added to the slides and placed at 37°C for 30 min, followed by PBS wash for 3 times and 0.2 × SSC wash for 3 min. Sufficient 4% PFA was dropped to each slide and incubated for 20 min at room temperature. RNA hybridization buffer was dropped to each slide at 55°C for 2 h. Pre-hybridization buffer was wiped off and denatured probed was applied and covered by a coverslip and sealed with rubber cement. The slide was kept in a moist chamber at 37°C overnight. The coverslip was removed the slides were washed with PBS for 5 min. BSA solution was dropped to the slides and incubated at 37°C for 60 min. Anti-Digoxin was diluted with BSA and second antibody was dropped to each slide and incubated at 37°C for 60 min. The slides were washed with PBS for 5 min twice and DAPI was dropped to each slide and covered with a coverslip and incubated at room temperature for 5 min in the dark.
Gut permeability assessment
Fluorescein isothiocyanate (FITC)-labeled dextran were purchased from Sigma (MO, USA). FITC-labeled dextran was prepared 1 day prior to experiment by dissolving into saline to form a concentration of 120 mg/mL. Mice were orally administrated with FITC-labeled dextran at different timepoints after stroke at a dose of 600 mg/kg. Mice were sacrificed 1 h later and blood was collected. To assess the colonic permeability, FITC-dextran were injected to the proximal colon of mice, and blood samples were collected after 1 h of administration. Serum concentrations of FITC-labeled dextran were determined by a microplate fluorescence reader with an excitation wavelength of 485 nm and an emission wavelength of 528 nm.
Gastrointestinal (GI) motility analysis
The measurement of GI motility has been previously described.28 Briefly, 1 h after administration of FITC-labeled dextran, mice were sacrificed and the whole GI tract from the stomach to the colon was dissected and photographed. To quantify intestinal motility, the GI tract was divided into 15 segments, the segments were flushed with 1 mL PBS, the fluorescence of the flushing solution were measured by a microplate fluorescence reader.
Enzyme linked immunosorbent assay (ELISA)
The blood samples were drawn from portal vein of mice and the serum was isolated by a 20 min incubation of blood at room temperature followed by centrifugation at 3,000 rpm for 15 min at 4°C. The colon and liver samples were homogenized in PBS. ELSA was performed according to manufacturer’s protocols for IgA (RK00162, Abclonal), REG3β (MM-44727M2, MeiMian), REG3γ (MM-1114M2, MeiMian), flagellin (MM-0761M1, MeiMian), NA (MM-0876M1, MeiMian), EPI (MM-0351M1, MeiMian), 5-HT (MM-0443M1, MeiMian), SP (MM-0445M1), IL-23 (ABclonal, RK00102), IL-22 (ABclonal, RK00108), DEFB2 (ELK Biotechnology, ELK2863), DEFB3 (lmmunoClone, IC-DEFb103A-Mu), S100A9 (ABclonal, RK03168), LCN2 (Abmart, AB-5861A), IL-6 (Invitrogen, 88–7064), IL-1β (Invitrogen, 99-8013A), TNF-α (Invitrogen, 88–7324), IL-10 (88–7105). Level of flagellin-specific IgA was measured as previously described.19,48 The fecal and mucosal samples were homogenized in PBS and centrifuged at 8,000 g for 2 min. The supernatant was collected for further analysis. For flagellin-specific IgA measurement, ELISA plates were coated overnight at 4°C with 100μL of 1 μg/mL of purified flagellin from Salmonella typhimurium that is produced in Escherichia coli. The next day, the plate was washed 3 times with PBS containing 0.1% Tween 20 (PBST). Then the wells were blocked with 0.5% of BSA dissolved in PBS and incubated for 1 h at room temperature. Then, 100 μL of samples were added to the wells and incubated at 37°C for 1 h, followed by 3 times of washing with PBST. The IgA alpha chain (HRP) antibody (Abcam) secondary antibody was diluted 1 : 5,000 in PBST and 100μL of antibody was applied to the wells and incubated for 1 h at 37°C, followed by 3 times of washing with PBST. Then, 100 μL of TMB peroxidase substrate solution was applied to each well, followed by 100 μL of H2SO4 to stop the reaction. Absorbance was read at 450 nm with a Synergy H1 plate reader.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted using a TRIzol reagent (Invitrogen, CA, USA). Then, the cDNA was synthesized using a reverse transcription reagent kit (R211-01, Vazyme). After mixing the cDNA with SYBR Green Master Mix (Q111-02, Vazyme) and specific primers, the qRT-PCR program was performed on a 7500 Real-Time PCR System (Applied Biosystems, MA, USA). For miR-150-5p, total RNA was reverse transcribed to cDNA using the Mir-XTM miRNA First-Strand Synthesis Kit (Takara Bio Inc., CA, USA) according to the instructions of the manufacturer. The miRNA was quantitatively analyzed using SYBR Green PCR Master Mix (Q111-02, Vazyme), small nuclear U6 was used as an internal control for miR-150-5p and GAPDH was used as internal control for mRNA.
Protein extraction and western blotting
Colon tissues were lysed radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology, China) containing the protease inhibitor phenylmethylsulfonyl fluoride (PMSF; Beyotime Biotechnology, China) using a homogenizer and incubated on ice for 30 min. After centrifugation at 12,000 rpm for 30 min at 4°C, the supernatants were collected. Protein concentration was measured by BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA), and a total of 40 μg protein was separated by 10% SDS–PAGE and subsequently electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore, Temecula, CA, USA). The membrane was blocked with 5% nonfat milk at room temperature for 1 h and then incubated with primary antibodies against ZO-1 (1:1000; Abcam, ab216880), Occludin (1:1000; Abcam, ab216327), Claudin-4 (1: 1000; Abcam, ab53156), Toll-like receptor 5 (1:1000; Abmart, T56757) and β-actin (1:1000; Abcam, ab8226) at 4°C overnight. The membrane was washed with PBST incubated with a horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (1:10,000; Thermo Scientific). The membranes were then visualized with an enhanced chemiluminescence system (Thermo Scientific, Rockford, IL). The band intensity was assessed using ImageJ software.
Transmission electron microscopy (TEM)
To visualize tight junctions in the intestine, tissue fragments of 1 mm3 were cut with a sharp razor blade, well-chosen areas of the paraffin block and reprocessed as previously described.49 Briefly, the paraffin-embedding tissues were dewaxed and hydrated, fixed in aqueous 1% osmium tetroxide, followed by dehydration with increasing concentration of ethanol and propylene oxide. Samples were then embedded with Epon 812, trimmed into 50 nm sections, and stained with 3% uranyl acetate and lead citrate in the standard manner. Images were acquired with a transmission electron microscope (EM 90; Zeiss, Oberkochen, Germany).
RNA sequencing
RNA-seq was performed by Genedenovo (Genedenovo, Guangzhou, China). Briefly, total RNA was extracted using Trizol reagent kit (15596026, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase free agarose gel electrophoresis. After total RNA was extracted, eukaryotic mRNA was enriched by Oligo(dT) beads. Then, the enriched mRNA was fragmented into short fragments using fragmentation buffer and reversely transcribed into cDNA by using NEBNext Ultra RNA Library Prep Kit for Illumina (7530, New England Biolabs, Ipswich, MA, USA). The purified double-stranded cDNA fragments were ligated to Illumina sequencing adapters. The ligation reaction was purified with the AMPure XP Beads (1.0X). Ligated fragments were subjected to size selection by agarose gel electrophoresis and PCR amplified. The resulting cDNA library was sequenced using Illumina Novaseq6000 by Gene Denovo Biotechnology Co.
Metagenome sequencing
Genomic DNA was extracted using HiPure Bacterial DNA Kits (Magen, Guangzhou, China) according to the manufacturer’s instructions. The DNA quality was detected usingQubit (Thermo Fisher Scientific, Waltham, MA) and Nanodrop (Thermo Fisher Scientific, Waltham, MA) accordingly. Qualified genomic DNA was firstly fragmented by sonication to a size of 350bp, and then end-repaired, A-tailed, and adaptor ligated using NEBNext ΜLtraDNALibraryPrep Kit for Illumina (NEB, USA) according to the preparation protocol. DNA fragments with length of 300–400 bp were enriched by PCR. At last, PCR products were purified using AMPure XP system (Beckman Coulter, Brea, CA, USA) and libraries were analyzed for size distribution by 2100 Bioanalyzer (Agilent, Santa Clara, CA) and quantified using real-time PCR. Genome sequencing was performed on the Illumina Novaseq 6000sequencer using the pair-end technology (PE 150). Raw data from Illumina platform were filtered using FASTP (version 0.18.0) by the following standards,1) removing reads with ≥10% unidentified nucleotides (N); 2) removing reads with ≥50% bases having phred quality scores ≤20; 3) removing reads aligned to the barcode adapter. After filtering, resulted clean reads were used for genome assembly. Clean reads of each sample were assembled individually using MEGAHIT (version1.1.2) stepping over a k-mer range of 21–141 (or 27 to 127) to generate sample (or group)-derived assembly. Genes were predicted based on the final assembly contigs (>500bp) using MetaGeneMark (version 3.38). The predicted genes ≥300 bp in length from all samples were pooled and combined based on ≥ 95% identity and 90%readscoverage using CD-HIT (version 4.6) in order to reduce the number of redundant genes for the downstream assembly step. The reads were re-aligned to predicted gene using Bowtie (version 2.2.5) to count reads numbers. Final gene catalog was obtained from non-redundant genes with gene reads count >2. For taxonomic annotation, the predicted genes were mapped to NCBI non-redundant genome databases using Diamond (version 0.9.24). The alignment result was then submitted to MEGAN (version 6.19.9) to estimate the taxonomic compositions with weighted LCA algorithm.
16S rRNA gene sequencing and analysis
Bacterial genomic DNA was extracted using a QIAamp PowerFecal Pro DNA Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. The barcoded primers V4F (GTGYCAGCMGCCGCGGTAA) and V4R (GGACTACNVGGGTWTCTAAT) were used to amplify the V4 variable region of the 16S rRNA gene. The full-length (V1V9) bacterial 16S rRNA gene sequences were amplified using barcoded 27F (AGRGTTYGATYMTGGCTCAG) and 1492R (RGYTA CCTTGTTACGACTT) primers. PCR was performed according to a previously described method.7 All PCR amplicons were mixed and sequenced using the Illumina iSeq 100 platform. The sequences of V4 region were against to the Greengenes1 reference database, and the sequences of full-length (V1V9) were against to a combination of rrnDB v5.6 and NCBI 16S RefSeq from 17 September, 2020 reference database. The Chao1 index, Observed species, Shannon index, and Simpson index were determined to assess α-diversity. Bray-Curtis was used to analyze the β-diversity by illustrating the phylogenetic dissimilarity among samples. As a dimensionality reduction method, principal-coordinate analysis (PCoA) was used to describe the relationships among samples based on the distance matrix and visualize the unsupervised grouping pattern of the complex dataset. Linear discriminant analysis effect size (LEfSe) was used to compare the discriminative data between groups.
Broad-spectrum antibiotics treatment
For antibiotic treatment, after acclimatization for 1 week, mice were treated with broad-spectrum antibiotics. Four kinds of antibiotics (1 g ampicillin, 1 g neomycin sulfate, 1 g metronidazole, and 1 g vancomycin, Sigma-Aldrich, CA, USA) were dissolved in 1 L drinking water (replace every 3 days) were provided ad libitum to the mice for 14 consecutive days.
Selective depletion of colonic bacteria and bacteriological analysis
To selectively deplete colonic microbiota, mice were subjected to enema prior to MCAO modeling to empty the colon, and 100 μL of broad-spectrum antibiotics was injected to proximal colon at the time of ischemia/reperfusion induction. Control mice were injected with 100 μL of PBS at the time of ischemia/reperfusion induction without enema. To determined bacterial load, mice were sacrificed and spleen and Peyer’s patches were removed, weighted and homogenized with a beads beater, 10 μL of homogenate was serially diluted and plated on brain heart infusion (BHI) agar plates. Plates were incubated for 24 h at 37°C and bacteria were quantified by counting the number of colony-forming units (CFUs).
AAV administration
The expression of TLR5 was regulated by a colonic AAV injection method, as previously described.50 Briefly, mice were fasted overnight and a concentration of 20 mM N-acetyl-L-cysteine (NAC) was prepared. Then, mice were anesthesia induced by intraperitoneal injection of 1.25% tribromoethanol. The colon was washed with an intrarectal injection of 600 μL of 20 mM NAC using a stainless-steel straight, round-tipped microsyringe and allowed to drain for 15 min, and this step was repeated twice. Then, 5 × 1010 physical particles of AAV in 100 μL PBS were administered by enema. The viruses were generated by Shanghai SunBio biomedical technology Co. (Shanghai, China). For the knockdown of Tlr5, mice were injected with AAV2/9-U6-tlr5 shRNA-CMV-EGFP-WPRES, control mice were injected with AAV2/9-U6-NC shRNA-CMV-EGFP-WPRES. For the upregulation of Tlr5, mice were injected with AAV2/9-CAG-tlr5-3flag-WPRE, control mice were injected with AAV2/9-CAG-MCS-WPRE. The control and knockdown/upregulation group mice were raised in separated cages, there were 5–8 cages of mice per group. In the knockdown experiment, AAV was administered once a week for 2 consecutive weeks. In the upregulation experiment, AAV was administered once a week for 3 consecutive weeks.
Luciferase reporter assay
To verify the specific binding site of miR-150-5p to the 3′-UTR of Tlr5 mRNA, the sequence of the putative binding site was mutated. The pmirGLO, pmirGLO-h-Tlr5-WT or pmirGLO-h-Tlr5-mut was mixed with miR-150-5p mimics or mimic negative control (NC), respectively, which were then mixed with Lipofectamine 2000 reagent. The mixture was transfected into HEK 293T cells. Luciferase activities were assessed after 24 h after transfection using Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
Mucosal microbiota transplantation
The mucus samples from sham-operated mice and 2h stroke mice were collected as described above, then 5 mucus samples of each group were mixed and homogenized in sterilized PBS and were centrifuged at 200 g for 1 min and 100 μL of supernatant was administered to mice by injection into the proximal colon. For MMT of H.pylori, 100 μL of 1∗109 cfu/mL H.pylori (ATCC 43504) was administered to mice by injection into the proximal colon.
Injection of flagellin
For the method of flagellin injection, 20 μg of purified flagellin from Salmonella typhimurium that is produced in Escherichia coli was dissolved in 100 μL saline, and was intraperitoneally injected at 1 h after cerebral reperfusion, mice were sacrificed at 3 h after MCAO.
Pharmacological manipulation of sympathetic tone
The pharmacological method to activate sympathetic nervous system has been previously described.14 Mice were intraperitoneal injected with 6-hydroxydopamine (6-OHDA, Sigma), 100 mg/kg in 0.1 mL saline, followed by two consecutive injection of 0.1 mL saline every hour. Another group of mice were intraperitoneal injected with atomoxetine (Sigma), 0.1 mg/kg, and yohimbine (Sigma), 1 mg/kg, in 0.1 mL saline every hour for three consecutive times. The control mice were intraperitoneal injected with 0.1 mL saline every hour for three consecutive time. Mice were sacrificed 1 h after injection.
Inhibition of sympathetic tone
The pharmacological method to inhibit sympathetic nervous system has been previously described.25 A group of mice were intraperitoneally injected with propranolol (Sigma) 30 mg/kg, at cerebral reperfusion. Another group of mice were intraperitoneally injected with metoprolol (Sigma) 3 mg/kg at cerebral reperfusion. The control group were intraperitoneally injected with saline. Mice were sacrificed at 2 h after cerebral reperfusion.
Laser speckle imaging
The blood flow of colon of mice was measured by an RFLSI Pro+ laser speckle system and review software (RWD) as previously described.7 Briefly, mice were anesthetized and placed on a warming plate maintained at 37°C, and the abdominal hair was removed. Then, a small incision was made and the colon was exposed and kept moist with warm saline. Data were acquired from a 1.6 cm × 1.6 cm field of view using a 785 nm, 90 mW laser with a sampling rate of 60 Hz at a working distance of 10 cm. Flux over time was analyzed by LSCI review software (RWD). In each visible colonic branching vessel, regions of interest (ROIs) were measured based on the intensification of blood flow.
Quantification and statistical analysis
Statistical analysis was performed using GraphPad PRISM as indicated in figure legends.
Published: October 8, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101754.
Contributor Information
Kaiyu Xu, Email: xukaiyu09@smu.edu.cn.
Jia Yin, Email: yinj@smu.edu.cn.
Yan He, Email: yanhe@i.smu.edu.cn.
Supplemental information
References
- 1.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/s1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin V.L., Owolabi M.O., World Stroke Organization–Lancet Neurology Commission Stroke Collaboration Group Pragmatic solutions to reduce the global burden of stroke: a World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023;22:1160–1206. doi: 10.1016/s1474-4422(23)00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saini V., Guada L., Yavagal D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology. 2021;97 doi: 10.1212/wnl.0000000000012781. S6–s16. [DOI] [PubMed] [Google Scholar]
- 4.Hankey G.J. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/s0140-6736(16)30962-x. [DOI] [PubMed] [Google Scholar]
- 5.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/s1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 6.Gwak M.G., Chang S.Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021;21 doi: 10.4110/in.2021.21.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu K., Gao X., Xia G., Chen M., Zeng N., Wang S., You C., Tian X., Di H., Tang W., et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021 doi: 10.1136/gutjnl-2020-323263. [DOI] [PubMed] [Google Scholar]
- 8.Seo K., Seo J., Yeun J., Choi H., Kim Y.I., Chang S.Y. The role of mucosal barriers in human gut health. Arch Pharm. Res. (Seoul) 2021;44:325–341. doi: 10.1007/s12272-021-01327-5. [DOI] [PubMed] [Google Scholar]
- 9.Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50:1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pabst O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y., Kim G., Shafer S., Chen Z., Kubo S., Ji Y., Luo J., Yang W., Perner S.P., Kanellopoulou C., et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185:1172–1188.e28. doi: 10.1016/j.cell.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye D., Hu Y., Zhu N., Gu W., Long G., Tao E., Fang M., Jiang M. Exploratory Investigation of Intestinal Structure and Function after Stroke in Mice. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/1315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luis A.S., Hansson G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe. 2023;31:1087–1100. doi: 10.1016/j.chom.2023.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houlden A., Goldrick M., Brough D., Vizi E.S., Lénárt N., Martinecz B., Roberts I.S., Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikonenko A.G., Radenovic L., Andjus P.R., Skibo G.G. Structural features of ischemic damage in the hippocampus. Anat. Rec. 2009;292:1914–1921. doi: 10.1002/ar.20969. [DOI] [PubMed] [Google Scholar]
- 16.Madsen J., Nielsen O., Tornøe I., Thim L., Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007;55:505–513. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 17.Di Giammartino D.C., Kloetgen A., Polyzos A., Liu Y., Kim D., Murphy D., Abuhashem A., Cavaliere P., Aronson B., Shah V., et al. KLF4 is involved in the organization and regulation of pluripotency-associated three-dimensional enhancer networks. Nat. Cell Biol. 2019;21:1179–1190. doi: 10.1038/s41556-019-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbert D.R., Yang J.Q., Hogan S.P., Groschwitz K., Khodoun M., Munitz A., Orekov T., Perkins C., Wang Q., Brombacher F., et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J. Exp. Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullender T.C., Chassaing B., Janzon A., Kumar K., Muller C.E., Werner J.J., Angenent L.T., Bell M.E., Hay A.G., Peterson D.A., et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–581. doi: 10.1016/j.chom.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassaing B., Ley R.E., Gewirtz A.T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–1377.e17. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microb. 2014;5:381–389. doi: 10.4161/gmic.28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broderick J.P., Adeoye O., Elm J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke. 2017;48:2007–2012. doi: 10.1161/strokeaha.117.017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balch M.H.H., Nimjee S.M., Rink C., Hannawi Y. Beyond the Brain: The Systemic Pathophysiological Response to Acute Ischemic Stroke. J. Stroke. 2020;22:159–172. doi: 10.5853/jos.2019.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson M.E.V., Sjövall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley D., Mason L.J., Mackin K.E., Srikhanta Y.N., Lyras D., Prakash M.D., Nurgali K., Venegas A., Hill M.D., Moore R.J., Wong C.H.Y. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016;22:1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W., Romano K.A., Li L., Buffa J.A., Sangwan N., Prakash P., Tittle A.N., Li X.S., Fu X., Androjna C., et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe. 2021;29:1199–1208.e5. doi: 10.1016/j.chom.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh V., Sadler R., Heindl S., Llovera G., Roth S., Benakis C., Liesz A. The gut microbiome primes a cerebroprotective immune response after stroke. J. Cerebr. Blood Flow Metabol. 2018;38:1293–1298. doi: 10.1177/0271678x18780130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh V., Roth S., Llovera G., Sadler R., Garzetti D., Stecher B., Dichgans M., Liesz A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016;36:7428–7440. doi: 10.1523/jneurosci.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clasen S.J., Bell M.E.W., Borbón A., Lee D.H., Henseler Z.M., de la Cuesta-Zuluaga J., Parys K., Zou J., Wang Y., Altmannova V., et al. Silent recognition of flagellins from human gut commensal bacteria by Toll-like receptor 5. Sci. Immunol. 2023;8 doi: 10.1126/sciimmunol.abq7001. [DOI] [PubMed] [Google Scholar]
- 30.Slomiany B.L., Slomiany A. Mechanism of Helicobacter pylori pathogenesis: focus on mucus. J. Clin. Gastroenterol. 1992;14:S114–S121. [PubMed] [Google Scholar]
- 31.Ralser A., Dietl A., Jarosch S., Engelsberger V., Wanisch A., Janssen K.P., Middelhoff M., Vieth M., Quante M., Haller D., et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut. 2023;72:1258–1270. doi: 10.1136/gutjnl-2022-328075. [DOI] [PubMed] [Google Scholar]
- 32.Winek K., Engel O., Koduah P., Heimesaat M.M., Fischer A., Bereswill S., Dames C., Kershaw O., Gruber A.D., Curato C., et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke. 2016;47:1354–1363. doi: 10.1161/strokeaha.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., Sita G., Racchumi G., Ling L., Pamer E.G., et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benakis C., Poon C., Lane D., Brea D., Sita G., Moore J., Murphy M., Racchumi G., Iadecola C., Anrather J. Distinct Commensal Bacterial Signature in the Gut Is Associated With Acute and Long-Term Protection From Ischemic Stroke. Stroke. 2020;51:1844–1854. doi: 10.1161/strokeaha.120.029262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisel C., Prass K., Braun J., Victorov I., Wolf T., Megow D., Halle E., Volk H.D., Dirnagl U., Meisel A. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.str.0000109041.89959.4c. [DOI] [PubMed] [Google Scholar]
- 36.Straub R.H., Herfarth H., Falk W., Andus T., Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J. Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 37.Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 38.Vijay-Kumar M., Sanders C.J., Taylor R.T., Kumar A., Aitken J.D., Sitaraman S.V., Neish A.S., Uematsu S., Akira S., Williams I.R., Gewirtz A.T. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 2007;117:3909–3921. doi: 10.1172/jci33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho F.A., Koren O., Goodrich J.K., Johansson M.E.V., Nalbantoglu I., Aitken J.D., Su Y., Chassaing B., Walters W.A., González A., et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira C.G., Russell R., Mishra A.A., Narayanan S., Ritchie J.M., Waldor M.K., Curtis M.M., Winter S.E., Weinshenker D., Sperandio V. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio. 2016;7 doi: 10.1128/mBio.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sykora M., Siarnik P., Diedler J., VISTA Acute Collaborators β-Blockers, Pneumonia, and Outcome After Ischemic Stroke: Evidence From Virtual International Stroke Trials Archive. Stroke. 2015;46:1269–1274. doi: 10.1161/strokeaha.114.008260. [DOI] [PubMed] [Google Scholar]
- 42.Wong C.H.Y., Jenne C.N., Lee W.Y., Léger C., Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 43.Prass K., Meisel C., Höflich C., Braun J., Halle E., Wolf T., Ruscher K., Victorov I.V., Priller J., Dirnagl U., et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers W.J., Derdeyn C.P., Biller J., Coffey C.S., Hoh B.L., Jauch E.C., Johnston K.C., Johnston S.C., Khalessi A.A., Kidwell C.S., et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/str.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 45.Gibson C.L., Gray L.J., Murphy S.P., Bath P.M.W. Estrogens and experimental ischemic stroke: a systematic review. J. Cerebr. Blood Flow Metabol. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 46.Johansson M.E.V., Hansson G.C. Analysis of assembly of secreted mucins. Methods Mol. Biol. 2012;842:109–121. doi: 10.1007/978-1-61779-513-8_6. [DOI] [PubMed] [Google Scholar]
- 47.Shan M., Gentile M., Yeiser J.R., Walland A.C., Bornstein V.U., Chen K., He B., Cassis L., Bigas A., Cols M., et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanidad K.Z., Amir M., Ananthanarayanan A., Singaraju A., Shiland N.B., Hong H.S., Kamada N., Inohara N., Núñez G., Zeng M.Y. Maternal gut microbiome-induced IgG regulates neonatal gut microbiome and immunity. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abh3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Song W., Wu Q., Gao X., Li J., Tan C., Zhou H., Zhu J., He Y., Yin J. Fecal Transplantation from db/db Mice Treated with Sodium Butyrate Attenuates Ischemic Stroke Injury. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00042-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J., Song Y., Li G., Xiao P., Liu Y., Xue Y., Cao Q., Tu X., Pan T., Jiang Z., et al. Fbxw7 increases CCL2/7 in CX3CR1hi macrophages to promote intestinal inflammation. J. Clin. Invest. 2019;129:3877–3893. doi: 10.1172/jci123374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The transcriptome data (NCBI: PRJNA1154613), metagenome sequencing data (NCBI: PRJNA1154791), and 16S rRNA gene sequencing data (NCBI: PRJNA1154919) have been deposited at NCBI database.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.