Abstract
Background:
Chlamydia trachomatis and Trichomonas vaginalis, two prevalent sexually transmitted infections, are known to increase HIV risk in women and could potentially diminish preexposure prophylaxis efficacy, particularly for topical interventions that rely on local protection. We investigated in macaques whether coinfection with Chlamydia trachomatis/Trichomonas vaginalis reduces protection by vaginal tenofovir (TFV) gel.
Methods:
Vaginal TFV gel dosing previously shown to provide 100 or 74% protection when applied either 30 min or 3 days before simian HIV(SHIV) challenge was assessed in pigtailed macaques coinfected with Chlamydia trachomatis/Trichomonas vaginalis and challenged twice weekly with SHIV162p3 for up to 10 weeks (two menstrual cycles). Three groups of six macaques received either placebo or 1% TFV gel 30 min or 3 days before each SHIV challenge. We additionally assessed TFV and TFV diphosphate concentrations in plasma and vaginal tissues in Chlamydia trachomatis/Trichomonas vaginalis coinfected (n=4) and uninfected (n=4) macaques.
Results:
Chlamydia trachomatis/Trichomonas vaginalis coinfections were maintained during the SHIV challenge period. All macaques that received placebo gel were SHIV infected after a median of seven challenges (one menstrual cycle). In contrast, no infections were observed in macaques treated with TFV gel 30 min before SHIV challenge (P< 0.001). Efficacy was reduced to 60% when TFV gel was applied 3 days before SHIV challenge (P=0.07). Plasma TFV and TFV diphosphate concentrations in tissues and vaginal lymphocytes were significantly higher in Chlamydia trachomatis/Trichomonas vaginalis coinfected compared with Chlamydia trachomatis/Trichomonas vaginalis uninfected macaques.
Conclusion:
Our findings in this model suggest that Chlamydia trachomatis/Trichomonas vaginalis coinfection may have little or no impact on the efficacy of highly effective topical TFV modalities and highlight a significant modulation of TFV pharmacokinetics.
Keywords: HIV/AIDS prevention, macaque model, microbicide gel, preexposure prophylaxis repeat challenge, sexually transmitted infections, simian HIV, tenofovir
Background
Preexposure prophylaxis (PrEP) with daily oral tenofovir disoproxil fumarate (TDF) and emtricitabine combination is a recommended strategy for HIV prevention [1]. Alternative PrEP modalities with topically delivered antiretroviral drugs to vaginal or rectal tissues are also under development. Such topical products include gels, inserts, or intravaginal rings that release tenofovir (TFV) or other antiretroviral drugs [2,3]. Recent data from two studies evaluated an intravaginal ring releasing dapivirine and demonstrated between 27 and 31% efficacy in women [3,4]. Previous results from the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 showed that a vaginal gel containing 1% TFV reduced HIV acquisition by 39% overall and 54% in highly adherent women [5]. However, a second phase III trial in South Africa [Follow-on African Consortium for Tenofovir Studies (FACTS) 001] failed to show protective efficacy because of poor product adherence [6]. Current efforts continue to focus on the development of topical products, including TFV-releasing vaginal inserts or intravaginal rings, which like TFV gels, show high efficacy in preclinical macaque models but may be more desired by women [7–10].
Although PrEP efficacy studies generally relate protection with drug concentrations [11], it is not known if efficacy will be diminished in subpopulations at higher risk of HIV infection due, for example, those with sexually transmitted infections (STIs). In a recent subanalysis of CAPRISA 004 participants, elevated systemic innate immune activation was found to be a significant risk factor for acquiring HIV (odds ratio of 11.27). Similarly, genital tract inflammation was associated with a higher risk (14-fold) for acquiring HIV infection [12]. Potential causes of heightened immune activation and genital tract inflammation were not fully defined although they may include infection with STIs such as Neisseria gonorrhoeae, herpes simplex virus type 2, Chlamydia trachomatis, or Trichomonas vaginalis [13–17].
Chlamydia trachomatis and Trichomonas vaginalis are among the most prevalent STIs and have been associated with a two to five-fold increase in the risk of HIV acquisition in women [18]. Both STIs can modulate local immune factors and cause an influx of immune cells to the vaginal mucosa, thus creating an environment that may facilitate HIV transmission. These biological changes likely contribute to the increased HIV risk and raise questions about their impact on the effectiveness of PrEP products that rely on topically applied drugs that act mainly by protecting HIV target cells in mucosal tissues. For instance, a high density of immune cells at the vaginal mucosa might require more drug to provide coverage to an increased population of susceptible target cells. Similarly, cellular activation, which increases deoxynucleotide triphosphate (dNTP) pools, can simultaneously increase HIV permissiveness and decrease antiviral activity of drugs like TFV by favoring the incorporation of, deoxyadenosine triphosphate (dATP) over its pharmacologically active TFV diphosphate (TFV-DP) analog [19].
The effect of Chlamydia trachomatis and Trichomonas vaginalis on topical TFV products in women can conceivably be assessed through subgroup analysis from participants from randomized PrEP trials. However, frequent testing and treatment of STIs often implemented during study protocols undermine the power of clinical trials to adequately address these questions [20]. Alternative approaches such as animal models can help better assess the effects of STI coinfections on PrEP efficacy under highly controlled experimental conditions. We previously developed a pigtailed macaque model of vaginal infection with a chimeric simian HIV (SHIV) that uses repeat, low-dose virus exposures to mimic populations at high risk of HIV infection [21]. This model predicted the efficacy of oral emtricitabine (FTC)/TDF in women [22,23], and demonstrated durable protection by a 1% TFV gel applied vaginally 30 min or 3 days prior to virus exposure [8,9]. We have also developed in pigtail macaques Chlamydia trachomatis/Trichomonas vaginalis coinfection model that recapitulates clinical signs of Chlamydia trachomatis and Trichomonas vaginalis infections in humans, including genital tract inflammation, upregulation of proinflammatory cytokines, and an increase (~2.5-fold) in susceptibility to vaginal SHIV infection [24,25]. Here we integrated the repeat low-dose SHIV challenge model with the Chlamydia trachomatis/Trichomonas vaginalis coinfection model to assess the potential effect of STIs on the efficacy of a vaginal 1% TFV gel.
Materials and methods
Macaques
Macaques were housed at the CDC under the care of CDC veterinarians in accordance with the Guide for the Care and Use of Laboratory Animals and all procedures approved by the CDC Institutional Animal Care and Use Committee.
Gel formulation and virus stock
TFV, kindly provided by Gilead Sciences, was formulated for investigational use at 1% (wt/wt) in 2% hydroxyethyl cellulose adjusted to pH 6.5 as previously described [9,26]. A 2% hydroxyethyl cellulose gel without TFV was used as a placebo control. The SHIV162p3 virus challenge stock was obtained from the NIH AIDS Research repository and expanded in pigtailed macaque peripheral blood mononuclear cells (PBMCs) prior to this study.
Chlamydia trachomatis/Trichomonas vaginalis coinfections in macaques
Macaques were coinfected with Chlamydia trachomatis and Trichomonas vaginalis, as previously described [24]. Because vaginal Chlamydia trachomatis/Trichomonas vaginalis coinfections are known to self-resolve, animals were reinoculated every 3–4 weeks to ensure STIs were maintained. APTIMA (Hologic, Bedford, Massachusetts, USA) vaginal swabs were collected twice weekly during efficacy and pharmacokinetics (PK) evaluations to monitor maintenance of Chlamydia trachomatis/Trichomonas vaginalis coinfections by nucleic acid amplification testing and confirmed with the Trichomonas In-Pouch culture system (BioMed Diagnostics, White City, Oregon, USA), as previously described [24].
Efficacy of vaginally applied tenofovir
Three groups of macaques (six per group) received placebo or 1% TFV gel vaginally 30 min or 3 days before vaginal SHIV162p3 [50 tissue culture infectious dose (TCID)] exposure. SHIV inoculations were done twice weekly for up to 10 weeks (two menstrual cycles) as previously described [9]. Blood was collected 30 min after gel dosing to measure plasma TFV levels and monitor for SHIV infection. Virus exposures were stopped when a macaque tested SHIV RNA positive in plasma from two consecutive blood draws. Infected macaques continued to receive placebo or TFV gel dosing for a median of 7 weeks (range=3–10) after confirmed infection. All experiments were done under highly controlled conditions by the same personnel using the same virus stock, inoculum dose, and procedures as described in previous studies [8].
Systemic and tissue tenofovir pharmacokinetics
The effect of Chlamydia trachomatis/Trichomonas vaginalis coinfection on systemic absorption of TFV from gels was evaluated longitudinally in six macaques following twice-weekly dosing during the 10-week challenge period. TFV in plasma samples (120 total) collected 30 min after administering TFV gel was measured by liquid chromatography–mass spectrometry as previously described [9]. Progesterone levels were measured in plasma (enzyme immunoassay; Wisconsin National Primate Research Center, Madison, Wisconsin, USA) and used to define the phase of the menstrual cycle as described previously [27]. Day 1 of each menstrual cycle was defined as the time point after the steepest decline in progesterone within a 32-day cycle. The follicular and luteal phase was defined as days 1–16 and 17–32 of the menstrual cycle, respectively.
In a separate study, we conducted an expanded PK evaluation of TFV-DP in vaginal tissues and purified vaginal lymphocytes collected at necropsy from SHIV-infected STI-negative (n=4) and STI-positive (n=4) macaques. Vaginal tissues were collected 2h or 3 days after dosing. Tissue homogenates and vaginal lymphocytes were prepared using enzyme cocktails and lymphocyte purification procedures, respectively, as previously described [9]. TFV-DP levels were quantified by HPLC (lower limit of quantitation=2.5 fmol/sample) and expressed as femtomoles (fmol) per milligram (mg) of tissue or million cells.
Simian HIV infection monitoring by polymerase chain reaction and serology
SHIV infection status was monitored in plasma by real-time PCR and serologic testing as previously described [8,9,21,28]. Assuming a 7-day eclipse period between infection and detection of SHIV RNA, the estimated time of infection was defined as two challenges (1 week) before first detection of SHIV RNA [25,29]. Animals were considered protected if seronegative and SHIV RNA negative during the challenge series and remained negative during the 10-week drug washout period. Monitoring for drug resistance emergence in breakthrough infections was done by genotypic screening (sensitive real-time PCR assay) for the TFV-associated K65R mutation as described previously [30].
Statistical analysis of efficacy and pharmacokinetics
The cumulative probability of remaining uninfected after repeated low-dose viral exposures was computed and graphically displayed using the product limit (Kaplan–Meier) estimator, and the log-rank test statistic was used to nonparametrically compare survival curves for the control and treatment groups. Uninfected macaques were right censored after two complete menstrual cycles. Hazard risk based on infections per month or menstrual cycle considers that cycling pigtailed macaques are more susceptible to SHIV infection during the luteal phase of the menstrual cycle as previously demonstrated in this model [9,31]. Intervention efficacy was calculated as 1–(p1/p0), where p1 and p0 denote the proportion of infections per menstrual cycle for the intervention and placebo control group, respectively. Exact binomial confidence limits were calculated for p1 and p2. Differences in drug absorption levels by Chlamydia trachomatis/Trichomonas vaginalis coinfection status were assessed using mixed effects models [32] that accounted for within-study participant correlation and controlled for menstrual cycle phase.
Results
Efficacy of tenofovir gel against simian HIVsf162P3 in the context of Chlamydia trachomatis/Trichomonas vaginalis coinfection
We designed this study to assess the effect of Chlamydia trachomatis/Trichomonas vaginalis coinfection under both optimal (™30 min) and suboptimal (™3 days) TFV gel dosing conditions previously shown to provide 100 and 74% protection to macaques against the same SHIV stock in the absence of STIs, respectively [8,9]. APTIMA testing confirmed the maintenance of Chlamydia trachomatis/Trichomonas vaginalis coinfections in all macaques throughout the challenge period with 176 of 230 (77%) and 191 of 230 (83%) samples testing positive for Chlamydia trachomatis and Trichomonas vaginalis, respectively. Figure 1 shows the survival curves for the placebo and TFV treatment arms. All macaques that received the placebo gel became infected after a median of seven challenges (1 month or menstrual cycle). In contrast, the six macaques treated with TFV gel 30 min before SHIV challenge remained completely protected after 20 challenges, resulting in an efficacy of 100% (P<0.001; log-rank test).
Fig. 1. Protection by vaginal TFV gel in macaques coinfected with Chlamydia trachomatis/Trichomonas vaginalis.
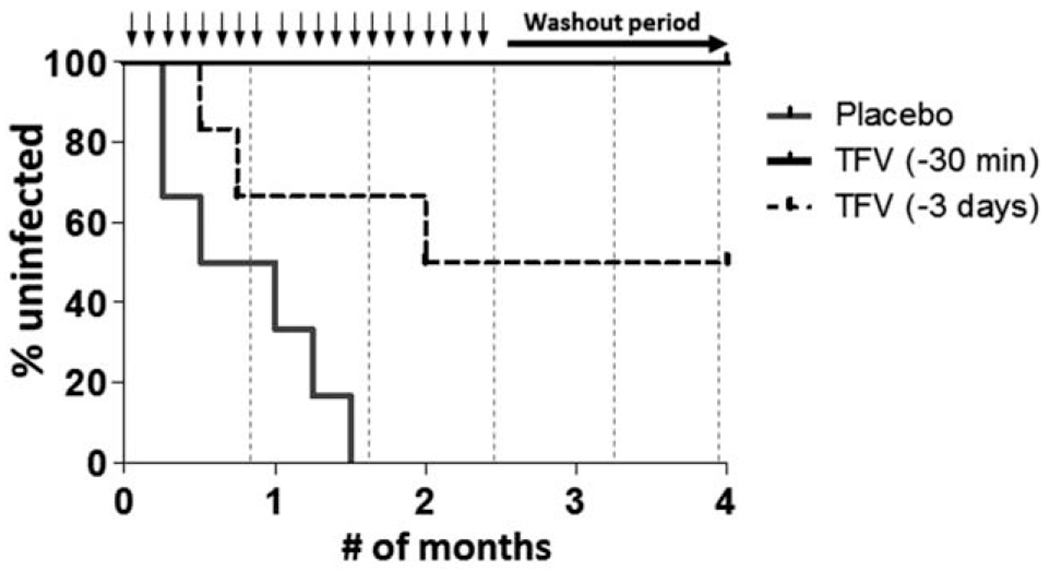
TFV, tenofovir. Kaplan–Meier plot representing the cumulative percentage of uninfected macaques as a function of the number of months or menstrual cycles. Chlamydia trachomatis/Trichomonas vaginalis coinfected macaques were challenged twice weekly with simian HIV (50 TCID) for up to 10 weeks followed by a 10-week washout period. Each survival curve represents the cumulative percentage of uninfected macaques treated with placebo gel (solid grey) or TFV gel 30 min (solid black) or 3 days (dotted black) before each challenge. Black arrows denote SHIV challenges and dotted vertical lines represent boost Chlamydia trachomatis/Trichomonas vaginalis inoculations.
In previous efficacy studies conducted in Chlamydia trachomatis/Trichomonas vaginalis uninfected macaques, TFV gel administered 3 days before SHIV challenge protected four of six macaques and resulted in an efficacy of 74% [9]. In the present study, the same TFV-dosing modality in Chlamydia trachomatis/Trichomonas vaginalis coinfected macaques protected three of six macaques, resulting in an calculated efficacy of 60% based upon the difference in number of infections per months at risk observed in macaques treated with TFV (−3 days) and placebo gel (P=0.07).
Acute virus dynamics, vaginal virus shedding, and drug resistance
TFV-treated macaques that became infected continued to receive TFV gel to monitor the effect on virologic outcome and emergence of drug resistance. Figure 2a shows that virus replication kinetics in plasma of the three breakthrough infections was similar to the placebo controls. Peak viremia in the breakthrough infections was 7.6, 7.3, and 6.7 log10 RNA copies/ml, and were within the range seen in placebo controls (median=8.0 log10 RNA, range=7.3–8.5 log10 RNA). We also measured vaginal virus shedding by quantitating SHIV RNA in vaginal fluids collected twice weekly up to 7 weeks from the first SHIV-positive test in plasma. Figure 2b shows a slight reduction in peak SHIV RNA detected in vaginal secretions in breakthrough infections (median=5.9 log10 RNA copies) compared with controls (median=7.2 log10 RNA copies) but no difference in the overall virologic outcome between the two groups (P=0.87 Mann–Whitney test).
Fig. 2. Plasma viral loads and vaginal viral shedding in STI-infected TFV-treated animals.
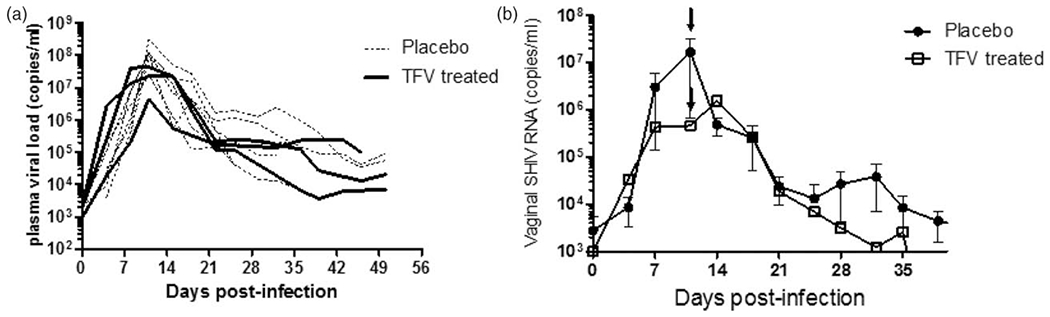
STI, sexually transmitted infection; TFV, tenofovir. (a) Individual plasma virus load kinetics of placebo (black dotted lines) and breakthrough infection (black solid line) under continued weekly gel dosing. Time zero indicates the time of first simian HIV RNA detection in plasma. (b) Mean virus shedding in controls (closed circles) and breakthrough infection (clear squares). Arrow indicates a time of peak plasma viral load.
We also monitored for the emergence of the TFV resistance K65R mutation in SHIV RNA isolated from plasma and vaginal secretions of breakthrough infections for up to 10 weeks following TFV gel dosing. None of the plasma (n=31) or vaginal secretions (n=20) samples tested had detectable K65R between peak plasma viral load and the end of study (data not shown).
Effect of Chlamydia trachomatis/Trichomonas vaginalis coinfection on plasma tenofovir concentrations
We previously showed that the phase of the menstrual cycle significantly influences vaginal antiretroviral absorption with higher drug concentrations observed in plasma during the progesterone-dominated luteal phase, likely reflecting the thinner and more permeable vaginal mucosa associated with this phase of the cycle [9,33]. As Chlamydia trachomatis/Trichomonas vaginalis coinfections have been associated with vaginal inflammation and changes in mucus composition, we investigated if the two coinfections affected the vaginal absorption of TFV following twice-weekly dosing over two complete menstrual cycles. Longitudinal assessment of plasma TFV levels measured 30min after TFV gel dosing in STI-infected macaques was compared with those previously seen among STI-negative animals dosed with the same TFV gel and dosing conditions. In Fig. 3a, the cumulative plasma TFV levels over two complete menstrual cycles revealed that detectable plasma TFV concentration in STI-infected macaques on average was 124.4 ng/ml higher than in STI-negative animals (P=0.02; mixed effects model). Further, higher TFV levels were consistently observed during both the luteal and follicular phase in STI-infected (median=80 and 13.5 ng/ml, respectively) compared with STI-negative (median = 8.5 ng/ml and undetectable, respectively) animals (Fig. 3b).
Fig. 3. Longitudinal pharmacokinetic assessment of plasma TFV concentrations in cycling macaques.
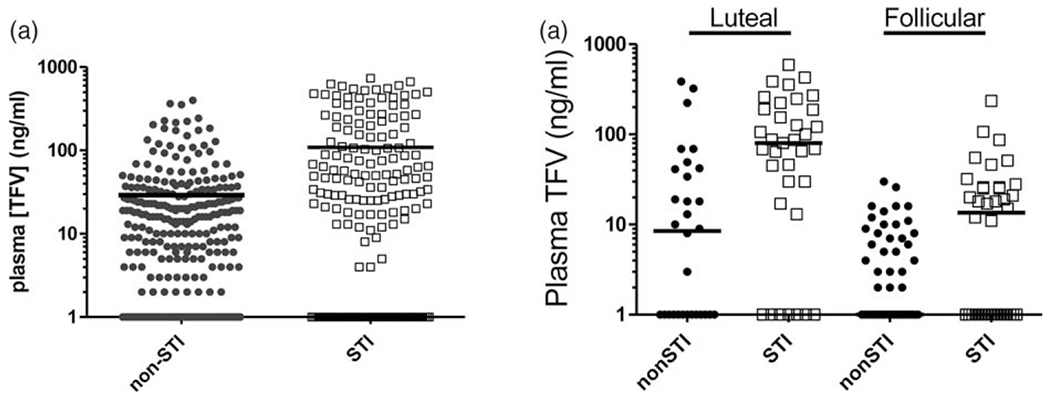
STI, sexually transmitted infection; TFV, tenofovir. (a) TFV concentrations in plasma of STI negative (closed circles) and STI positive (open squares) 30 min after vaginal administration of 1% TFV gel. (b) Plasma TFV concentrations in STI-negative (closed circles) and STI-positive (open squares) animals stratified by the luteal and follicular phase of the menstrual cycle. Solid black line indicates the median.
Effect of Chlamydia trachomatis/Trichomonas vaginalis coinfections on tenofovir concentrations in vaginal tissues
We next investigated the effect of Chlamydia trachomatis/Trichomonas vaginalis infection on TFV-DP levels in vaginal tissues and lymphocytes. For this analysis, vaginal biopsies were collected from SHIV-infected Chlamydia trachomatis/Trichomonas vaginalis-positive (n = 4) or Chlamydia trachomatis/Trichomonas vaginalis-negative (n = 4) macaques 2 h or 3 days after receiving a single dose of 1%TFV gel. Figure 4a shows that the median TFV-DP concentrations in tissue homogenates collected 2 h after dosing were 11 times higher in STI-infected [466 fmol/mg, interquartile range (IQR) = 45–1011 fmol/mg] compared with STI-uninfected (43 fmol/mg, IQR = 0–122 fmol/mg) animals, albeit the difference was not statistically significant (P = 0.24, mixed effects model). Consistent with previous studies, TFV-DP levels in tissues were reduced at 3 days in both groups but remained higher in STI-infected (162 fmol/mg, IQR = 55–445 fmol/mg) compared with STI-negative (60 fmol/mg, IQR 0–131 fmol/mg) animals (P=0.06). Figure 4b shows that median TFV-DP concentrations in vaginal lymphocytes collected at 3 days were also higher in STI-infected (1093 fmol/106 cells) than in STI-negative macaques (211 fmol/106 cells). Notably, the TFV-DP levels in lymphocytes collected from the STI-negative macaques in this study were consistent with those previously reported in STI-naive macaques [9].
Fig. 4. Tissue TFV-DP concentrations and median (bar) following vaginal dosing with 1% TFV gel.
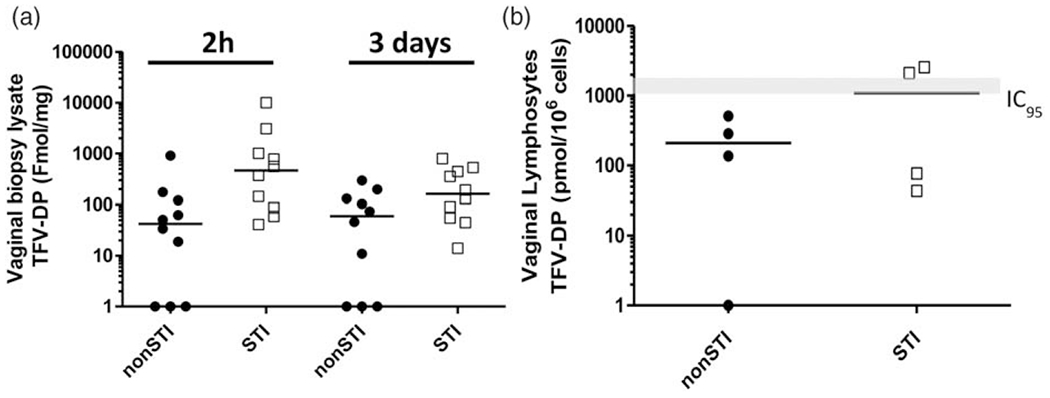
STI, sexually transmitted infection; TFV-DP, tenofovir diphosphate. (a) Vaginal tissue homogenates 2 h and 3 days after vaginal TFV gel dosing. (b) Vaginal lymphocytes from STI-negative (closed circles) and STI-infected (open squares) animals 3 days after vaginal TFV dosing. Solid black line indicates median TFV-DP concentrations. Grey line indicates TFV-DP concentrations that correspond to the in-vitro 95% inhibitory concentration (IC95) range [9].
Discussion
Vaginal Chlamydia trachomatis and Trichomonas vaginalis infections have been shown to increase the risk of HIV acquisition in women, thus raising concerns about their impact on topical PrEP products used for HIV prevention. Here, we show that TFV delivered by a vaginal gel was highly effective in preventing SHIV infection in macaques that were at increased risk of SHIV infection because of coinfection with Chlamydia trachomatis and Trichomonas vaginalis. The overall protective effect observed when TFV was applied 30 min (100% efficacy) or 3 days (60% efficacy) before SHIV challenge was comparable with that previously reported in Chlamydia trachomatis/Trichomonas vaginalis-naive macaques exposed to the same SHIV and TFV-dosing conditions (100 and 74% efficacy, respectively), suggesting little or no impact of Chlamydia trachomatis/Trichomonas vaginalis on efficacy in this model. We noted that Chlamydia trachomatis/Trichomonas vaginalis coinfection impacted the pharmacokinetics of TFV and resulted in higher drug absorption and tissue dosing which may have contributed to the observed protection. These observations are reassuring as they suggest that topical TFV delivered vaginally at protective concentrations maintains high efficacy under heightened susceptibility conditions.
Our results on topical TFV provides additional data to address questions on PrEP vulnerability to Chlamydia trachomatis/Trichomonas vaginalis coinfection as our recent work using the same model evaluated the impact of these STIs on the efficacy of oral TDF/FTC [34]. Like TFV gel, oral TDF/FTC administered pericoitally was also completely protective in the absence of Chlamydia trachomatis/Trichomonas vaginalis coinfections [22]. However, in contrast to the fully protective TFV gel, oral FTC/TDF protected four of six macaques coinfected with Chlamydia trachomatis/Trichomonas vaginalis, suggesting a modest loss of oral PrEP efficacy [34]. Although the protection with pericoital TDF/FTC was still significant, the breakthrough infections raise the possibility for a slightly higher vulnerability to Chlamydia trachomatis/Trichomonas vaginalis coinfection for oral TDF/FTC than for topical TFV gel. Possible explanations include the lower vaginal drug exposures with oral TDF/FTC, and thus higher susceptibility to a reduction in antiviral activity by Chlamydia trachomatis/Trichomonas vaginalis infection through mechanisms such as cell activation, target cell recruitment, or substrate competition. Notably, these findings highlight the importance of including relevant STI models as preclinical tools to better assess the efficacy of next-generation systemic and topical PrEP agents under conditions of increased susceptibility.
Our conclusions are supported by a study design that has several important advantages, including the assessment of two TFV dosing modalities with different efficacy measurements to determine if the effects of Chlamydia trachomatis/Trichomonas vaginalis coinfection are more evident or amplified under a lower efficacy dosing modality. Efficacy assessments were conducted under prolonged STI-induced inflammation conditions that were sustained by periodic Chlamydia trachomatis/Trichomonas vaginalis boosting. These design elements were successfully added to our established repeat SHIV challenge model to allow valid comparisons with previously generated data in the absence of STIs. However, we note that information attained from this model may not be applicable to all STIs, particularly those that are ulcerative and exhibit different inflammation patterns [35].
An important finding in our study was the documented pharmacological shift imposed by Chlamydia trachomatis/Trichomonas vaginalis coinfections. The twice-weekly sampling over 10 weeks revealed a significant increase in TFV absorption reflected by higher plasma TFV concentrations during both phases of the menstrual cycle. Moreover, the higher TFV absorption was associated with increased TFV-DP concentrations in vaginal tissues and lymphocytes. The increased TFV dosing may likely have played an important role in maintaining efficacy by counteracting the heightened susceptibility to SHIV infection. In particular, the increase in TFV dosing may have been more critical in conferring efficacy in animals exposed to SHIV under suboptimal dosing conditions with lower efficacy (−3 days) because TFV-DP levels in vaginal lymphocytes from animals in this modality have been shown to dip below the protective in-vitro IC95 threshold associated with full protection in STI-naive animals [9]. We noted that TFV-DP concentrations in vaginal lymphocytes 3 days after were indeed higher in the STI-infected animals with a median value approaching the fully protective IC95 threshold levels. However, despite achieving these levels, only three of six animals were protected, suggesting that the drug threshold needed for full protection is higher in the presence of Chlamydia trachomatis/Trichomonas vaginalis coinfections. Importantly, these data highlight the need of reevaluating the protective tissue drug targets for topical antiretrovirals to take into account shifts in the PK and pharmacodynamic relationships that may be associated with other STIs. The mechanism of the increased TFV absorption in Chlamydia trachomatis/Trichomonas vaginalis coinfected macaques is not clear but changes in pH, mucus composition, mucosal permeability, or drug transporter expression, could all enhance TFV uptake.
The PK findings in this macaque model raise the possibility that Chlamydia trachomatis/Trichomonas vaginalis coinfections may have a similar effect in women. Although we are not aware of data on Chlamydia trachomatis/Trichomonas vaginalis effects on TFV uptake in women, a recent analysis from CAPRISA 004 suggested that vaginal dysbiosis in women significantly impacts the effectiveness of TFV gel (Burgener et al. International AIDS Conference 2016). TFV gel was approximately three times more effective in women with healthy dominant vaginal Lactobacilli than in women with nondominant Lactobacilli and a high proportion of Gardnerella. Absorption and depletion of TFV by Gardnerella vaginalis was proposed as a plausible mechanism in modulating the effectiveness of TFV gel. We note that unlike women in CAPRISA 004, our Chlamydia trachomatis/Trichomonas vaginalis coinfection model does not reproduce the vaginal dysbiosis and nondominant Lactobacilli environment because of organisms such as Prevotella and Gardnerella. Thus, a different model is needed to better understand the impact of dysbiosis by these organisms on the PK and efficacy of topical TFV and novel PrEP products.
Drug resistance emergence when antiretroviral treatments fail to protect is a concern, particularly for drugs like TFV that are also widely used for treatment. Consistent with previous studies showing only wild-type virus in macaques infected with SHIV while treated with TFV gel, we found no evidence of the TFV-associated K65R mutation in SHIV from plasma or vaginal secretions in all three breakthrough infections despite the use of highly sensitive nucleic acid testing [30]. It is possible that both the duration of gel use and the once-weekly dosing are not sufficient to select drug resistance. Similarly, the inability to completely suppress virus shedding reflects the low mucosal antiviral activity from once-weekly gel dosing. This finding is in contrast with the reduction in vaginal SHIV shedding observed in a SHIV-infected macaque with a TDF ring, presumably because of higher and continuous antiviral activity released by the ring [36].
In summary, we show that TFV delivered vaginally by gel maintains prophylactic efficacy in macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. These results are reassuring as they suggest that these STIs may have little or no impact on PrEP with topical TFV in women. Pharmacokinetic data suggest that increased TFV dosing may have contributed to maintaining efficacy. Additionally, the model shows that the impact of Chlamydia trachomatis/Trichomonas vaginalis coinfection extends beyond mucosal inflammation and SHIV susceptibility to include modulation of TFV PK and efficacy thresholds. These findings may help inform the preclinical evaluation of next-generation PrEP products to better define efficacy and PK/pharmacodynamic relationships in STI settings.
Acknowledgements
We acknowledge the contributions of Frank Deyounks, Shanon Ellis, and Kristen Kelley for animal technical assistance and David Garber for programmatic support. We also thank Carol Farshy for APTIMA testing and Monica Morris for assistance with monitoring treatment and testing for clearance of Chlamydia trachomatis/Trichomonas vaginalis infections. We thank Jim Rooney at Gilead Sciences for providing tenofovir.
This work was partially supported by Interagency Agreement Y1-AI-0681-02 between Centers for Disease Control and Prevention and National Institute of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.FDA U. FDA approves first medication to reduce HIV risk. 2012 [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. MTN-020–ASPIRE Study Team. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solai LS, Seltzer H, Rossi L. Sister studies: the ring study and ASPIRE. Silver Spring, MD: International Partnership for Microbicides; 2016. [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees H. Facts 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. In: Conference of Retroviruses and Opportunistic Infection (CROI). Seattle, USA; 2015 [Google Scholar]
- 7.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A 2013; 110:16145–16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol 2009; 83:10358–10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik Z, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol 2012; 86:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira LE, Clark MR, Friend DR, Garber DA, McNicholl JM, Hendry RM, et al. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrob Agents Chemother 2014; 58:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med 2013; 64:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, et al. CAPRISA004 TRAPS team. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 2012; 206:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen CR, Plummer FA, Mugo N, Maclean I, Shen C, Bukusi EA, et al. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS 1999; 13:327–332. [DOI] [PubMed] [Google Scholar]
- 14.Ahn MH, Song HO, Ryu JS. Trichomonas vaginalis-induced neutrophil apoptosis causes anti-inflammatory cytokine production by human monocyte-derived macrophages. Parasite Immunol 2008; 30:410–416. [DOI] [PubMed] [Google Scholar]
- 15.Swygard H, Sena AC, Hobbs MM, Cohen MS. Trichomoniasis: clinical manifestations, diagnosis and management. Sex Transm Infect 2004; 80:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zariffard MR, Harwani S, Novak RM, Graham PJ, Ji X, Spear GT. Trichomonas vaginalis infection activates cells through toll-like receptor 4. Clin Immunol 2004; 111:103–107. [DOI] [PubMed] [Google Scholar]
- 17.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 2010; 201 Suppl 2:S114–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Lerma JG, Aung W, Cong ME, Zheng Q, Youngpairoj AS, Mitchell J, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011; 85:6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stillwaggon E, Sawers L. Rush to judgment: the STI-treatment trials and HIV in sub-Saharan Africa. J Int AIDS Soc 2015; 18:19844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis 2005; 191:164–173. [DOI] [PubMed] [Google Scholar]
- 22.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One 2012; 7:e50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins LT, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS 2014; 28:1431–1439. [DOI] [PubMed] [Google Scholar]
- 24.Henning T, Fakile Y, Phillips C, Sweeney E, Mitchell J, Patton D, et al. Development of a pigtail macaque model of sexually transmitted infection/HIV coinfection using Chlamydia trachomatis, Trichomonas vaginalis, and SHIV(SF162P3). J Med Primatol 2011; 40:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henning TR, Butler K, Hanson D, Sturdevant G, Ellis S, Sweeney EM, et al. Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis 2014; 210:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. HPTN 050 Protocol Team. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 2006; 20:543–551. [DOI] [PubMed] [Google Scholar]
- 27.Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav 1994; 56:801–810. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med 2008; 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol 2010; 84:10406–10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JA, Rompay KK, Delwart E, Heneine W. A rapid and sensitive real-time PCR assay for the K65R drug resistance mutation in SIV reverse transcriptase. AIDS Res Hum Retroviruses 2006; 22:912–916. [DOI] [PubMed] [Google Scholar]
- 31.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 2011; 57:261–264. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. [Google Scholar]
- 33.Dobard C, Sharma S, Parikh UM, West R, Taylor A, Martin A, et al. Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med 2014; 6:227ra235. [DOI] [PubMed] [Google Scholar]
- 34.Radzio J, Henning T, Jenkins L, Ellis S, Farshy C, Phillips C, et al. Combination emtricitabine and tenofovir disoproxil fumarate prevents vaginal simian/human immunodeficiency virus infection in macaques harboring Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis 2016; 213:1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol 2011; 65:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JM, Srinivasan P, Teller RS, Lo Y, Dinh CT, Kiser PF, Herold BC. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr 2015; 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


