Abstract
Alphaherpesviruses express a heterodimeric glycoprotein, gE/gI, that facilitates cell-to-cell spread between epithelial cells and neurons. Herpes simplex virus (HSV) gE/gI accumulates at junctions formed between polarized epithelial cells at late times of infection. However, at earlier times after HSV infection, or when gE/gI is expressed using virus vectors, the glycoprotein localizes to the trans-Golgi network (TGN). The cytoplasmic (CT) domains of gE and gI contain numerous TGN and endosomal sorting motifs and are essential for epithelial cell-to-cell spread. Here, we swapped the CT domains of HSV gE and gI onto another HSV glycoprotein, gD. When the gD-gICT chimeric protein was expressed using a replication-defective adenovirus (Ad) vector, the protein was found on both the apical and basolateral surfaces of epithelial cells, as was gD. By contrast, the gD-gECT chimeric protein, gE/gI, and gE, when expressed by using Ad vectors, localized exclusively to the TGN. However, gD-gECT, gE/gI, and TGN46, a cellular TGN protein, became redistributed largely to lateral surfaces and cell junctions during intermediate to late stages of HSV infection. Strikingly, gE and TGN46 remained sequestered in the TGN when cells were infected with a gI−HSV mutant. The redistribution of gE/gI to lateral cell surfaces did not involve widespread HSV inhibition of endocytosis because the transferrin receptor and gE were both internalized from the cell surface. Thus, gE/gI accumulates in the TGN in early phases of HSV infection then moves to lateral surfaces, to cell junctions, at late stages of infection, coincident with the redistribution of a TGN marker. These results are related to recent observations that gE/gI participates in the envelopment of nucleocapsids into cytoplasmic vesicles (A. R. Brack, B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter, J. Virol. 74:4004–4016, 2000) and that gE/gI can sort nascent virions from cytoplasmic vesicles specifically to the lateral surfaces of epithelial cells (D. C. Johnson, M. Webb, T. W. Wisner, and C. Brunetti, J. Virol. 75:821–833, 2000). Therefore, gE/gI localizes to the TGN, through interactions between the CT domain of gE and cellular sorting machinery, and then participates in envelopment of cytosolic nucleocapsids there. Nascent virions are then sorted from the TGN to cell junctions.
The alphaherpesviruses herpes simplex virus (HSV) and varicella-zoster virus (VZV) and the swine pseudorabies virus (PrV) express a heterodimer composed of glycoproteins gE and gI that mediates cell-to-cell spread in certain cultured cells and in epithelial and neuronal tissues (12, 14–17, 25, 26, 29, 51, 59, 62). Both gE and gI are required to mediate efficient cell-to-cell spread; neither glycoprotein on its own can suffice. In vivo, cell-to-cell spread is an especially important parameter of alphaherpesvirus infection and pathogenesis. These viruses spread rapidly through mucosal or ocular epithelial tissues (in the case of HSV) or in the skin (in the case of VZV), as well as within synaptically connected neuronal networks. HSV and PrV gE− and gI− mutants are severely compromised in the ability to spread in animal models (4, 15, 16, 31, 44, 57). Cell-to-cell spread can be studied in the laboratory by using certain polarized epithelial cells that form extensive cell junctions; in these cells, gE− and gI− mutants are markedly reduced in the ability to spread from infected to uninfected cells. For example, plaques formed by an HSV gE− mutant on monolayers of HaCaT human keratinocytes were eightfold smaller than plaques produced by wild-type HSV (58). However, in other cells, e.g., highly transformed HeLa or HEp-2 cells, cells that do not form extensive junctions, these mutants do not show defects in cell-to-cell spread. Thus, the notion that gE/gI is a “nonessential” glycoprotein is a misnomer related to the use of cells that are not representative of those infected in vivo.
We previously reported that HSV gE/gI accumulated extensively along the lateral surfaces of epithelial cells, colocalizing with adherens junctions proteins but not with tight junctions (17, 58). These results suggested that gE/gI was sorted specifically to basolateral domains of polarized cells and then was retained at cell junctions, and not at nonjunctional surfaces, by binding to components of cell junctions. Moreover, we proposed that gE/gI might act by binding cellular receptors to promote movement of HSV virus particles from an infected cell to a neighboring uninfected cell across cell junctions (17, 58; M. Huber, T. McMillan, T. Wisner, and D. C. Johnson, Abstr. 25th Int. Herpesvirus Workshop, abstr. 7.06, 2000). This proposed property of gE/gI is by analogy to the well-characterized HSV receptor binding glycoprotein, gD (20, 27). gE and gI are encoded by genes adjacent to gD and show limited similarity to gD (34). Consistent with this hypothesis, PrV can facilitate cell-to-cell spread in the absence of gD in some cell types (37, 41, 45).
The extracellular domains of gE/gI are clearly important for cell-to-cell spread, but the cytoplasmic (CT) domains of gE/gI are also essential for this process. PrV and HSV mutants lacking the CT domains of gE/gI behave similarly to null mutants, as they are unable to spread well in cultured epithelial cells and are less neurovirulent (49, 51, 58). Moreover, we recently demonstrated that the gE CT domain is essential for directed transport of nascent HSV virions to epithelial cell junctions, a process that presumably enhances cell-to-cell spread (28).
The CT domains of alphaherpesvirus gE and gI contain tyrosine (YXXO/) and dileucine motifs and clusters of amino acids that are acidic and phosphorylated. These motifs act by binding components of the cellular sorting machinery to target membrane proteins to the trans-Golgi network (TGN), direct endocytosis of membrane proteins from the plasma membrane, and sort membrane proteins to basolateral surfaces of polarized cells (reviewed in references 8, 19, 35, and 36). Indeed, PrV gE/gI is rapidly endocytosed (49, 51), and VZV gE and gI when expressed by transfection are endocytosed and accumulate in the TGN (1, 2, 39, 40, 60). Tyrosine (YXXO/) motifs in gE clearly influence endocytosis and TGN localization (1, 2, 40, 49, 61), probably through interactions with the μ subunits of the AP-1, AP-2, AP-3, or AP-4 clathrin adapter complex (reviewed in references 19, 23, and 36). We recently reported a role for the μ1B/AP-1 complex in directed transport of PrV particles to cell junctions (28). There are also clusters of acidic residues adjacent to serine residues that are phosphorylated in the CT domain of gE (1, 58) that can potentially act in TGN sorting through interactions with PACS-1 (35, 53). gI contains dileucine motifs that can serve to cause endocytosis, TGN localization, and localization to the basolateral surfaces of polarized cells, through interactions with the β subunits of clathrin adapter complexes (reviewed in references 23, 32, and 36). Extensive phosphorylation of the CT tails of gE and gI may regulate the traffick of gE/gI (1, 18, 38, 58).
Given that gE and gI of HSV, PrV, and VZV contain several different sorting motifs, there has been intensive interest in the subcellular distribution of these glycoproteins (1, 2, 17, 39, 40, 41, 49, 51, 58, 60). Taken together, the results of these studies are often confusing, perhaps in part because these glycoproteins have been expressed by transfection in some experiments and by herpesvirus infection in other cases; moreover, in many cases gE has been expressed without gI, and vice versa. In HSV-infected cells, gE and gI are expressed primarily or exclusively as a complex and appear to function as a complex (7, 22, 26). In addition, there is a case to be made for expressing gE and gI in polarized epithelial cells or other cells that form extensive junctions where gE/gI functions, rather than in highly transformed cells where gE/gI does not function. There have been reports that VZV and HSV gE accumulate extensively in the TGN when expressed by transfection or infection of nonpolarized cells (1, 2, 60, 61). We found that HSV gE/gI accumulated primarily at epithelial cell junctions when expressed using recombinant adenovirus (Ad) vectors or by infection with HSV (17). One explanation for these differences could relate to inhibition of endocytosis during infection by HSV. Rapid endocytosis from the plasma membrane might cause accumulation of gE or gE/gI in the TGN, and inhibition of endocytosis by alphaherpesviruses could cause cell surface accumulation. Indeed, PrV inhibits endocytosis after 6 h of infection (50). Transfected cells might not show this effect or other effects of HSV, PrV, or VZV infection.
To characterize the subcellular distribution of gE/gI further and to examine the contributions of the CT in this, the gE and gI CT domains were substituted for the CT domain of gD. The CT domain of gE was sufficient to cause accumulation in the TGN, and gE/gI was also found in the TGN when expressed using recombinant Ad vectors. Moreover, gE/gI was found in the TGN of HSV-infected cells after 5 h of infection. However, after 11 h of infection with HSV, gE/gI was redistributed to lateral surfaces, accumulating at cell junctions. gI was essential for this redistribution. HSV infection did not grossly alter the endocytic machinery of cells. Therefore, while the CT domain of gE targets gE/gI to the TGN early in HSV infection, the complex moves to lateral surfaces of these polarized cells at intermediate times of infection by a process that does not involve inhibition of endocytosis.
MATERIALS AND METHODS
Cells and viruses.
All culture media were purchased from BioWhittaker Inc., Walkersville, Md. HEC-1A endometrial epithelial cells (5) were grown in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone). ARPE-19 cells (American Type Culture Collection) Dulbecco modified Eagle medium were grown in (DMEM)/F-12 medium (50:50) supplemented with 10% FBS. Madin-Darby bovine kidney (MDBK) cells (American Type Culture Collection) were grown in Eagle minimal essential medium supplemented with 10% FBS. 293 cells (Microbix, Toronto, Ontario, Canada) were grown in the same medium and passaged as described by Microbix. SV type 1 (HSV-1) strains F (wild type), F-US7kan (gI−), and F-gEβ (gE−) (15) were propagated and titered on Vero cells. All Ad vectors were replication-defective (E1−) viruses propagated and titered on 293 cells. Three Ad vectors expressing gD [AdgD1(E1−)], gE [Ad(E1−)gE], or gI [Ad(E1−)gI] have been described elsewhere (10, 17); in each case, the human cytomegalovirus immediate-early promoter drives glycoprotein expression. AdgD-gECT and AdgD-gICT, described below, were used to express gD-gECT and gD-gICT chimeric glycoproteins, respectively. A second Ad vector, Ad-Trans, expressing the tetracycline transactivator, was required to transactivate the tetracycline-regulated human cytomegalovirus promoters in AdgD-gECT and AdgD-gICT (47, 52). All infections with Ad vectors or HSV were done in the appropriate media supplemented with 2% FBS.
Antibodies.
Sheep anti-TGN46 immunopurified antibodies were obtained from Serotec. Rabbit anti-β-catenin polyclonal serum was obtained from Sigma. A mouse monoclonal antibody (MAb) specific for β-catenin was obtained from Transduction Laboratories (Lexington, Ky.). Rabbit anticalreticulin polyclonal antibodies were obtained from Affinity Bioreagents Inc. (Golden, Colo). The rabbit polyclonal serum specific for the 275-kDa mannose-6- phosphate receptor (MPR) was described previously (10). DL6, a MAb specific for HSV-1 gD, was a gift from Gary Cohen and Roselyn Eisenberg (University of Pennsylvania, Philadelphia). 3104, an HSV gI-specific MAb, and 3114, a gE-specific MAb (26), were gifts from Anne Cross and Nigel Stow (Institute of Virology, Glasgow, United Kingdom). 3114 was conjugated to Oregon Green, an analog of fluorescein, using a FluoReporter Oregon Green 488 protein labeling kit from Molecular Probes (Eugene, Oreg.). A rat antiserum specific for gE and gI was produced by immunizing rats with a soluble form of gE/gI (13). Secondary antibodies—Alexa 488-conjugated goat anti-rat immunoglobulin G (IgG), Alexa 488-conjugated goat anti-mouse IgG, Alexa 488-conjugated goat antifluorescein, Alexa 594-conjugated donkey anti-sheep IgG, and Alexa 594-conjugated goat anti-rabbit IgG—were all obtained from Molecular Probes. Cy3-conjugated goat anti-rabbit IgG was obtained from Jackson Immunoresearch Laboratories Inc. (West Grove, Pa.).
Construction of recombinant DNAs encoding chimeric proteins.
gD sequences were PCR amplified from pSS17, a plasmid containing a BamHI fragment derived from the US component of HSV-1(KOS) (33). gE and gI sequences were amplified from pUC-US7/8, a plasmid containing PCR-amplified DNA from the US region of HSV-1(F) (58), using Pfu DNA polymerase (Stratagene) and nucleotides from Promega. The DNA sequence encoding the gD extracellular and transmembrane domains, amino acids (aa) 1 to 364, was fused in frame to DNA encoding either the gE CT domain (aa 447 to 552) or the CT domain of gI (aa 297 to 390) by using a two-step PCR mutagenesis procedure as described elsewhere (58). Briefly, primers 1 and 2 (described below) were used to amplify the extracellular and transmembrane domains of gD. Primer 3 or 4 was used to amplify the cytosolic sequence of gE or gI. The PCR products from these two separate reactions were gel purified and mixed together with primers 1 and 4 in a second PCR to produce DNA encoding the gD-gECT chimera (1,467 bp) or the gD-gICT chimera (1,420 bp).
gD-gECT primers were primer 1 (CCTTGAATTCTCTTTTGTGTGGTGCGTTCCG), primer 2 (CCAGGCACGCCTCCTCATCCAGTACACAATTCC), primer 3 (GGAATTGTGTACTGGATGAGGAGGCGTGCCTGG), and primer 4 (GGGGCCTCTAGATGGGGCTCATTACCAGAAG). gD-gICT primers were primer 1 (CCTTGAATTCTCTTTTGTGTGGTGCGTTCCG), primer 2 (GCGGCGTTGACATCTGTGCATCCAGTACACAATTCC), primer 3 (GGAATTGTGTACTGGATGCACAGATGTCAACGCCGC), and primer 4 (TTAATCTAGACTATACCAACAGGGGAGGCGTTGG). In each case, boldface letters indicate gD sequences, underlining indicates gE or gI sequences, and italics indicate novel restriction sites (EcoRI and XbaI). The PCR products from the second reaction were inserted into pΔE1sp1BTet to create pΔE1sp1BTet gD-gECT and pΔE1sp1BTet gD-gICT. These plasmids were then entirely sequenced in both directions to verify their integrity.
Construction of recombinant Ad vectors.
293 cells were cotransfected with pJM17 (24), a plasmid containing the Ad serotype 5 genome, and either pΔE1sp1BTet gD-gECT or pΔE1sp1BTet gD-gICT as described elsewhere (24). Recombinant Ad vectors were screened by PCR and for expression of chimeric glycoproteins.
Immunoprecipitation of [35S]methionine/cysteine-labeled glycoproteins.
HEC-1A cells were infected with HSV-1(F) (25 PFU/cell), AdgD1(E1−) (100 PFU/cell), AdgD-gECT (10 PFU/cell), or AdgD-gICT (10 PFU/cell) in medium containing 2% FBS. Cells infected with AdgD-gECT and AdgD-gICT were coinfected with Ad-Trans using 10 PFU/cell. In each case, the titers for Ad vectors refer to those obtained on 293 cells that express E1 and allow plaque formation. At 6 h after infection with HSV-1(F) or 26 h after infection with Ad vectors, cells were washed three times with DMEM lacking cysteine and methionine and then labeled for 3 h in this medium supplemented with 50 μCi of [35S]methionine/cysteine (New England Nuclear, Boston, Mass.) per ml. Cell extracts were made using 50 mM Tris HCl (pH 7.5)–100 mM NaCl–1% NP-40–0.5% sodium deoxycholate containing 2 mg of bovine serum albumin (BSA) per ml and 1 mM phenylmethylsulfonyl fluoride. gD was immunoprecipitated with anti-gD MAb DL6 as described elsewhere (58). Proteins were analyzed on 10% polyacrylamide gels.
Confocal immunofluorescence microscopy.
HEC-1A cells, which were grown on Nunc Permanox 8 well slides until 70 to 80% confluent, were infected with HSV-1 strain F, F-gEβ, or F-US7kan at 25 PFU/cell. Similar cell monolayers were infected with AdgD1(E1−) or Ad(E1−)gE (100 PFU/cell), infected with both Ad(E1−)gE and Ad(E1−)gI (each at 100 PFU/cell), or coinfected with AdgD-gECT and Ad-Trans or AdgD-gICT and Ad-Trans (each at 10 PFU/cell). In experiments examining the modulation of gE/gI expressed by Ad vectors by subsequent HSV infection, cells were infected with Ad(E1−)gE and Ad(E1−)gI (each at 100 PFU/cell) for 24 h and then infected with F-gEβ (25 PFU/cell). For staining with anti-β-catenin, anti-HSV glycoprotein, and anti-TGN46 antibodies, infected cells were washed with phosphate-buffered saline (PBS) containing 1 mM MgCl2 and 1 mM CaCl2, fixed with 4% paraformaldehyde in PBS for 30 min, then washed three times with PBS. The cells were permeabilized using 0.2% Triton X-100 in PBS for 5 min, washed three times in PBS containing 0.02% Tween-20 (T-PBS), and then incubated with blocking buffer (T-PBS containing 2% FBS and 2% BSA) overnight. The cells were incubated with sheep anti-TGN46 for 1 h, washed three times in T-PBS, and then incubated with Alexa 594-conjugated donkey anti-sheep IgG for 1 h. The cells were washed three times in T-PBS and then incubated with blocking buffer containing 2% goat serum for 2 h. The cells were then incubated with either mouse anti-gE MAb 3114, rat anti gE/gI polyclonal antibody, or anti-gD MAb DL6 simultaneously with rabbit anti-β-catenin antibody for 1 h. Cells were then washed three times with T-PBS and incubated with Alexa 488-conjugated goat anti-mouse IgG or Alexa 488-conjugated goat anti-mouse IgG and Cy5-conjugated goat anti-rabbit IgG. For staining with calreticulin or MPR-specific antibody cells were first incubated with either rabbit anticalreticulin or rabbit anti-MPR antibody for 1 h, washed with T-PBS, and then incubated with Alexa 594-conjugated goat anti-rabbit IgG for 1 h. The cells were washed with T-PBS, then incubated with gD-specific MAb DL-6 or gE-specific MAb 3114 for 1 h, washed, and incubated with Alexa 488-conjugated goat anti-mouse IgG. Early endosomes and late endosomes/lysosomes were stained by incubating cells with Alexa 594-conjugated transferrin (Tf; Molecular Probes) for 15 min at 37°C. For early endosomes, the cells were then washed and immediately fixed. For late endosomes/lysosomes, the cells were washed and incubated for an additional 30 min at 37°C in medium. The cells were then fixed, permeabilized, and stained with anti-HSV glycoprotein antibodies. Cells were mounted on microscope slides using Prolong (Molecular Probes).
BFA and cycloheximide treatments.
Cells were treated with 5 μg of brefeldin A (BFA; Epicentre Technologies, Madison, Wis.) per ml for 30 min at 37°C or with 10 μg of cycloheximide (Sigma, St. Louis, Mo.) per ml for 2 h at 37°C beginning 24 h after infection with Ad vectors or after various times of HSV infection.
Confocal laser scanning immunofluorescence.
Images were captured using a Bio-Rad 1024 ES laser scanning confocal microscope on a Nikon Eclipse TE300 inverted fluorescence microscope using the 100× objective. In some cases confocal images were processed by Applied Precision Inc.'s Softworks deconvolution software running on a Silicon Graphics Octane computer running the IRIX 6.5 operating system. This processing was used to construct three-dimensional models of cells to visualize apical and basolateral surfaces.
Endocytosis of If and gE.
Semiconfluent monolayers of HEC-1A cells growing in 35-mm-diameter dishes were left uninfected or were infected with HSV-1(F) using 10 PFU/cell. After 4, 8, or 12 h of infection with HSV-1, the binding and uptake of 125I-labeled Tf were measured as described elsewhere (55). To quantify the numbers of specific and nonspecific Tf receptors, cells were washed three times with ice-cold serum-free medium and then incubated for 1 h with ice-cold uptake medium containing 1 mg of BSA per ml, 10 mM HEPES (pH 7.35), and 2 μg of of 125I-Tf (1.5 × 106 to 3.0 × 106 cpm/μg) per ml, with or without 2 mg of unlabeled Tf per ml. Cells were then washed in ice-cold medium and lysed in 0.5 ml lysis solution (0.1 N NaOH, 1% Triton-X 100). To measure specific and nonspecific Tf uptake, cells were washed three times with serum-free medium at 37°C and then incubated at 37°C with 125I-Tf (2 μg/ml) with or without unlabeled Tf (2 mg/ml) for 3, 6, 9, or 12 min. Cell surface Tf was removed by washing the cells once with ice-cold acid wash buffer (0.2 N acetic acid, 0.5 M NaCl) for 2 min and then three times in cold 150 mM NaCl–20 mM HEPES (pH 7.4)–1 mM CaCl2–5 mM KCl–1 mM MgCl2. Cells were lysed in lysis solution, and radioactivity was counted. Specific binding and uptake were calculated by subtracting nonspecific binding and uptake (observed in the presence excess unlabeled Tf) from total binding and uptake. Uptake of 125I-labeled anti-gE MAb 3114 was determined similarly except that nonspecific binding was not measured. Cells were incubated with 125I-labeled 3114 IgG (50 ng/ml; 7.7 × 106 cpm/μg) for 5, 10, 15, or 20 min at 37°C; then surface antibodies were removed with ice-cold acid wash buffer, the cells were solubilized in lysis solution, and radioactivity was counted.
RESULTS
Construction of chimeric proteins with the gE or gI CT domain replacing the CT domain of gD.
To examine the effects of the gE and gI CT domains on intracellular trafficking, these domains were swapped in place of the CT domain of HSV-1 gD. This was done by using a two-step PCR. DNA encoding aa 1 to 364 of gD was fused, in frame, to DNA encoding either aa 447 to 552 of gE (gD-gECT) or aa 297 to 390 of gI (gD-gICT) (Fig. 1). The products of these PCRs were ligated in shuttle plasmid pΔE1sp1BTet, containing the cytomegalovirus immediate-early core promoter coupled to the tetracycline resistance operon (Tet-responsive element) and used to construct replication-defective (E1−) Ad vectors (3, 47). Plasmids derived from pΔE1sp1BTet and encoding gD-gECT or gD-gICT were each cotransfected into 293 cells along with a second plasmid which contains the genome of Ad serotype 5. Transfected 293 cells yielded Ad vectors which were analyzed by PCR for the presence of the desired insert, and viruses containing chimeric HSV genes were plaque purified.
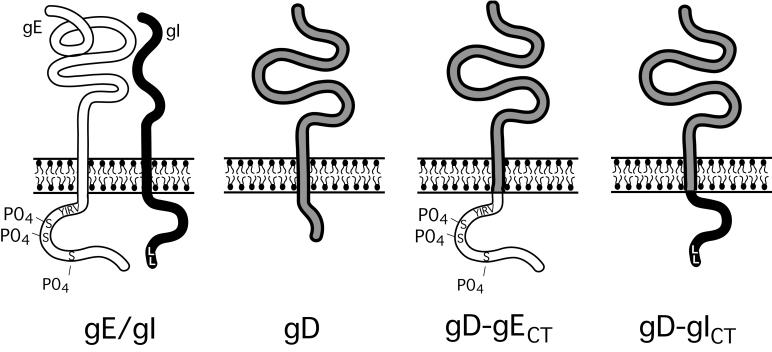
FIG. 1.
Schematic of HSV-1 glycoproteins gE/gI and gD and of glycoproteins chimeric gD-gECT and gD-gICT. The CT domain of gE is 106 residues in length (aa 447 to 552) and contains a tyrosine motif (YIRV), a cluster of acidic residues adjacent to several serine residues that are phosphorylated (58). The CT domain of gI is 94 residues in length (aa 297 to 390) and contains a dileucine motif at the C terminus. The gE and gI CT domains were transferred onto the extracellular and transmembrane domains of gD (residues 1 to 364), replacing the gD CT domain, creating gD-gECT and gD-gICT, respectively.
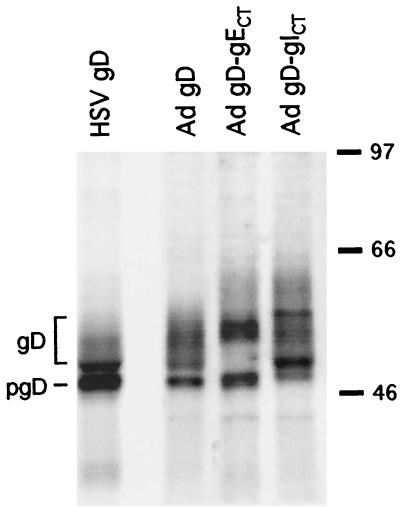
These Ad vectors were characterized for expression of HSV-1 glycoproteins by infecting HEC-1A cells and immunoprecipitating radiolabeled glycoproteins. Cells were infected with AdgD1(E1−), which expresses HSV-1 gD (10), with AdgD-gECT or AdgD-gICT, or with wild-type HSV-1 (strain F). The Tet-responsive elements in AdgD-gECT and AdgD-gICT were transactivated by coinfecting cells with a second Ad vector, Ad-Trans, which expresses the tetracycline transactivator protein (47, 52). The levels of wild-type gD, gD-gECT, and gD-gICT expressed by the Ad vectors were similar to levels observed in cells infected with HSV (Fig. 2). Chimeric glycoproteins gD-gECT and gD-gICT were larger than gD because the small CT domain of gD was replaced by the larger CT domain of gE or gI. In each case, there were several protein bands including various immature and mature for those of gD-gICT which may be related to phosphorylation (58) or other posttranslational modifications.
FIG. 2.
Expression of gD-gECT and gD-gICT by recombinant Ad vectors. HEC-1A cells were infected with HSV-1(F) or with Ad(E1−)gD1 or were coinfected with AdgD-gECT and Ad-Trans or with AdgD-gICT and Ad-Trans. Cells infect with HSV were labeled with [35S]methionine/cysteine from 6 to 9 h, while cells infected with Ad vectors were labeled from 26 to 29 h. Cell extracts were incubated with MAb DL6, which is specific for gD, and the antibody-antigen complexes were precipitated using protein-A agarose. Immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Positions of size markers in kilodaltons are shown on the right, and the approximate positions of mature gD and immature gD (pgD) are indicated on the left.
The cytosolic tail of gE, but not that of gI, mediates accumulation of gD in the TGN.
Previously, we found that gD was found on the apical as well as the basolateral surfaces of epithelial cells, and also throughout the cytoplasm, whereas gE/gI accumulated primarily at cell junctions (17). When gE was expressed without gI, the glycoprotein accumulated in cytoplasmic vesicles (17). To ascertain whether the CT domain of gE or gI could alter the subcellular distribution of gD, human epithelial HEC-1A cells were infected with Ad vectors expressing gD-gECT and gD-gICT for 24 h. The cells were fixed, permeabilized, and simultaneously stained with MAb specific for gD or gE and simultaneously with anti-TGN46 antibodies. TGN46, a type 1 transmembrane protein found predominantly in the TGN, rapidly cycles back to the TGN from endosomes and the basolateral surfaces of polarized cells (6, 21, 42, 43). Images of the cells were obtained using a laser scanning confocal microscope. The gD-gICT chimeric glycoprotein was found predominately on apical and lateral cell surfaces, as was wild-type gD (Fig. 3). The apical surface expression of these constructs was confirmed by processing confocal images taken every 0.2 μm through the field by deconvolution software, producing z-axis images (results not shown). Smaller fractions of gD and gD-gICT were also colocalized with TGN46 (yellow staining in Fig. 3).
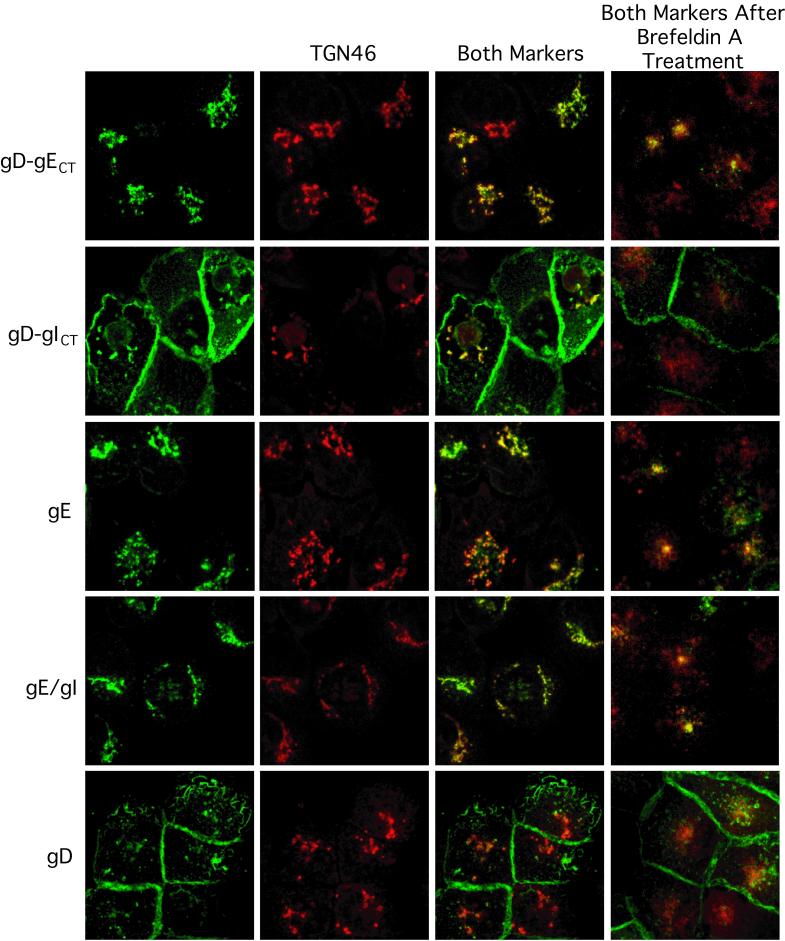
FIG. 3.
The gE CT domain mediates accumulation of glycoproteins in the TGN. The distributions of gD-gECT, gD-gICT, gE, gE/gI, and gD were compared in HEC-1A cells respectively infected with the following replication-defective Ad vectors: AdgD-gECT/Ad-Trans, AdgD-gICT/Ad-Trans, Ad(E1−)gE, Ad(E1−)gE and Ad(E1−)gI, and AdgD1(E1−). In some cases, cells were treated with BFA (5 μg/ml) for 30 min before fixing (rightmost panels). Cells were fixed, permeabilized with 0.2% Triton X-100, blocked, and then incubated sheep anti-TGN46 followed by Alexa 594-coupled donkey anti-sheep IgG. The cells were washed, blocked to eliminate cross-reactivity between donkey anti-sheep IgG and secondary goat antibodies, and then incubated with mouse MAb specific for gD, DL6 in the case of gD-gECT, gD-gICT, and gD, or a rat polyclonal serum specific for HSV-1 gE/gI. The mouse and rat antibodies were detected by using Alexa 488-conjugated goat anti-mouse IgG and goat anti-rat, respectively. The cells were viewed by laser scanning confocal microscopy. The green (HSV glycoproteins) and red (TGN46) channels were superimposed in the Both Markers column.
In contrast to the gD-gICT chimeric glycoprotein, gD-gECT, as well as gE and gE/gI, was predominantly or exclusively found in cytoplasmic vesicles, extensively colocalizing with TGN46 (Fig. 3). This distribution of gE and gE/gI was observed at 24, 48, and 72 h after infection with replication-defective Ad vectors at different multiplicities of infection. This was consistent with the conclusion that the CT domain of gE causes gE/gI to localize specifically to the TGN. However, it is frequently difficult to ascertain by immunofluorescence whether proteins are present in the TGN rather than in Golgi membranes (6). To examine this further, we used BFA to collapse the TGN and the Golgi apparatus. In cells treated with BFA, TGN membranes redistribute into a distinct perinuclear structure that colocalizes with microtubule-organizing centers, while other Golgi (cis- and medial-Golgi) membranes redistribute into the endoplasmic reticulum (46). After treatment of cells with BFA for 30 min, gE, gE/gI, and gD-gECT continued to be colocalized with TGN46 (Fig. 3, right panels), suggesting that these glycoproteins accumulated in the TGN rather than other Golgi membranes. BFA treatment of cells reduced colocalization between TGN46 and gD, and also between TGN46 and gD-gICT, but did not affect the accumulation of gD and gD-gICT at the apical and lateral cell surfaces. In addition, a 2-h cycloheximide treatment to block de novo protein synthesis beginning 24 h after infection with AdgD1(E1−) or AdgD-gICT reduced the small fraction of gD and gD-gICT that colocalized with TGN46 but did not affect accumulation of gE/gI or gD-gECT in the TGN (results not shown). This suggested that gD and gD-gICT move transiently through the TGN on their path to the cell surface, and when protein synthesis is blocked the glycoproteins are not found in the TGN.
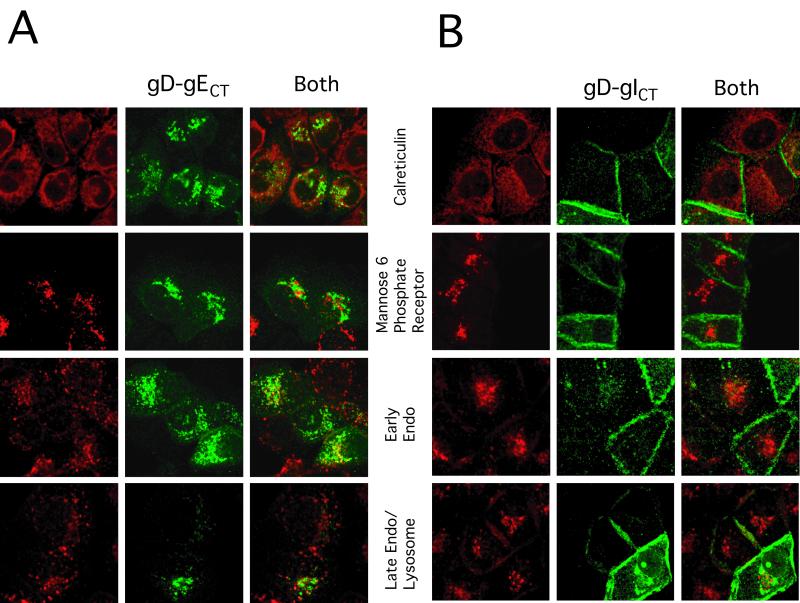
To further characterize the subcellular localization dictated by the gE CT domain, the distribution of gD-gECT was determined with respect to several other cellular membrane markers. gD-gECT did not colocalize with calreticulin, a marker for the endoplasmic reticulum (Fig. 4). There was some limited colocalization of gD-gECT with the 275-kDa MPR, a protein found in the cis-Golgi apparatus but also known to be present in the TGN, as well as in endosomes and on the plasma membrane (30). gD-gECT also showed limited colocalization with Tf receptors, early endosomes labeled by incubation with Alexa 594-conjugated Tf for 15 min and to a lesser extent with late endosomes/lysosomes, where the label was chased for 30 additional min (Fig. 4A). The presence of gD-gECT in endosomal compartments suggested that the glycoprotein might be cycling between the plasma membrane and the TGN in a manner analogous to TGN46. By contrast, the distribution of gD-gICT was very different; the glycoprotein did not colocalize with calreticulin, the MPR, or the Tf receptor and was found predominantly on the cell surfaces (Fig. 4B). Therefore, the CT domain of gE causes gD to accumulate in the TGN, whereas the CT domain of gI did not substantially alter the largely cell surface distribution of gD.
FIG. 4.
Subcellular localization of gD-gECT and gD-gICT compared with that of other cellular markers. HEC-1A cells were coinfected with either AdgD-gECT and Ad-Trans (A) or AdgD-gICT and Ad-Trans (B) as described in the legend to Fig. 3. The cells were stained with anti-gD MAb DL6 (green) and rabbit anticalreticulin antibodies or rabbit anti-MPR antibody (red) followed by Alexa 594-conjugated goat anti-rabbit IgG and Alexa 488-conjugated goat anti mouse IgG. Staining for early endosomes involved incubation with Alexa 594-conjugated Tf (red) for 15 min at 37°C, followed by washing and immediate fixation. Late endosomes/lysosomes were stained as for early endosomes, except that there was a further incubation at 37°C for 30 min.
Accumulation of gE/gI and gD-gECT in the TGN compared with previous results showing accumulation of gE/gI at cell junctions.
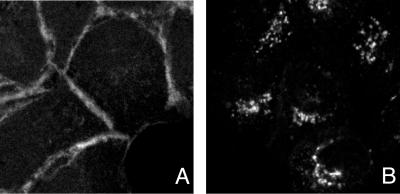
In an earlier study, gE/gI expressed by using Ad vectors accumulated primarily at cell junctions (17). Here, gD-gECT, gE, and gE/gI were all found predominantly in the cytoplasm, colocalizing extensively with TGN46. In these more recent experiments, accumulation of gE, gE/gI, and gD-gECT in the TGN was observed at early (24 h) and later (48 and 72 h) times after infection with these replication defective (E1−) Ad vectors, as well as with substantially different amounts of input virus (25 to 800 PFU/cell, as determined by titering on 293 cells) (data not shown). Thus, broadly different expression levels did not produce a different picture. Moreover, gE/gI, gE, and gD-gECT expressed using these Ad vectors in MDBK (bovine kidney epithelia) and ARPE-19 (human retinal epithelial) cells also accumulated in the TGN (data not shown). This discrepancy appears to relate to differences in the methods for staining of gE/gI in this study compared with the previous study. In the previous work (17), cells expressing gE/gI were stained with a mouse MAb specific for β-catenin, followed by a secondary goat anti-mouse conjugated to Texas Red; then the cells were washed and stained with mouse anti-gE MAb 3114 which had been modified with Oregon Green (a fluorescein analog), followed by BODIPY-conjugated goat antifluorescein antibodies. In reviewing this protocol, we realized that there was the potential for mouse anti-gE MAb used in the second round of staining to bind to anti-mouse IgG antibodies used in the first round of staining. To determine if this was the case, we repeated this protocol, adding an additional step in which the cells were incubated with mouse serum, to block any remaining sites on goat anti-mouse IgG antibodies, after the first step of staining and before addition of the anti-gE MAb. Using this blocking step, gE/gI was found in a perinuclear distribution (Fig. 5B), whereas without blocking with mouse serum, gE/gI appeared largely at cell junctions in a distribution identical to that of β-catenin (Fig. 5A). The perinuclear localization of gE/gI was also observed when cells were stained with anti-gE MAb 3114 alone (no anti-β-catenin antibodies), at 24, 48, and 72 h postinfection with Ad vectors, and using rat polyclonal anti-gE/gI antibodies (not shown). Therefore, it appears that the actual distribution of gE/gI expressed using these Ad vectors is in the TGN, rather than on the cell surface at cell junctions.
FIG. 5.
gE/gI accumulate in the TGN, rather than at cell junctions, in Ad-infected cells. HEC-1A cells were infected with Ad(E1−)gE and Ad(E1−)gI for 24 h and then stained by one of two methods. In panel A, the cells were stained essentially as previously described (17). The cells were incubated with a mouse MAb specific for β-catenin, followed by Alexa 594-conjugated goat anti-mouse IgG antibodies. The cells were washed and incubated with Oregon Green (a fluorescein analog) conjugated anti-gE MAb 3114, washed, and incubated with goat anti-fluorescein antibodies conjugated to Alexa 488. In panel B, the same protocol was followed except that following the Alexa 594-conjugated goat anti-mouse IgG antibodies, the cells were incubated with 2% mouse serum for 2 h, in order to prevent the anti-mouse antibodies from reacting with MAb 3114.
HSV-1 infection causes both gE/gI and TGN46 to move from the TGN to cell junctions, but infection with a gI- HSV mutant does not have this effect.
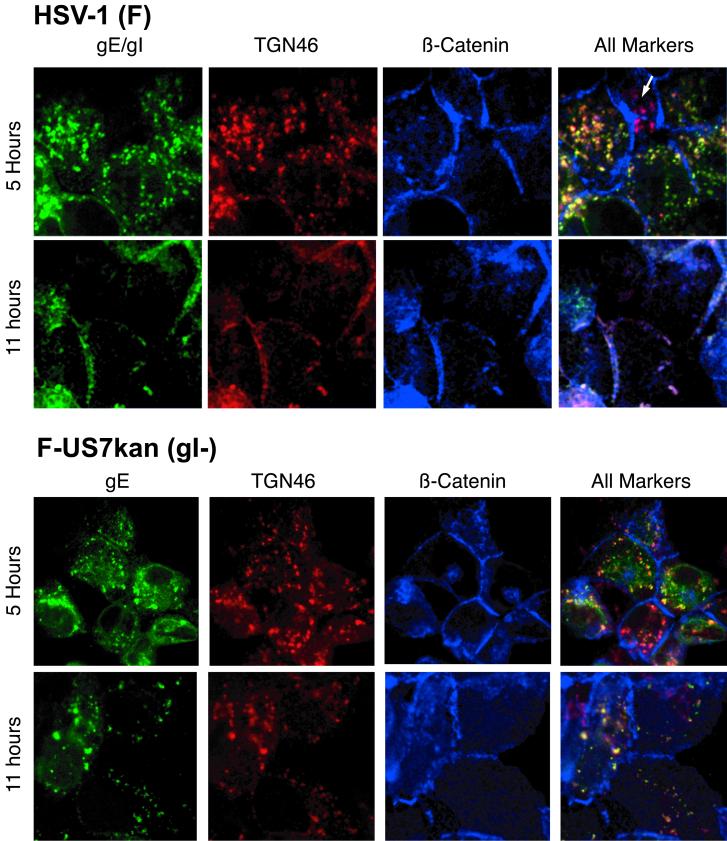
In previous studies, the distribution of gE/gI was characterized in HSV-infected cells, 12 h after infection, and gE/gI was found predominantly at cell junctions, although there was also some gE/gI observed in cytoplasmic vesicles (17, 58). In both of these studies, HSV-infected cells were stained with rabbit polyclonal antibodies specific for β-catenin and simultaneously with mouse anti-gE MAb 3114. Therefore, there was no possibility for cross-reactivity of secondary antibodies, and we expected that this staining represents the true distribution of anti-β-catenin and gE. To further characterize this, we infected HEC-1A cells with wild-type HSV-1 and compared the distribution of gE (green), TGN46 (red), and β-catenin (blue) simultaneously at early and later times after infection. After 5 h of infection, gE was exclusively in cytoplasmic vesicles colocalizing extensively with TGN46 and not with β-catenin (Fig. 6, row 1). Note that the cell in upper center of the upper panels, marked with a white arrow, was not infected and showed perinuclear TGN46 (red) but no gE staining, while other HSV-infected cells showed primarily yellow staining (red and green combined). After 11 h of infection with wild-type HSV, gE was seen more extensively on the cell surface, colocalizing with β-catenin (Fig. 6, row 2). Of particular interest, TGN46 also moved extensively to cell junctions by 11 h of infection with wild-type HSV (colocalization of all three fluorophores appears white). When cells were infected for 11 h and stained with anti-TGN antibody alone, i.e., without anti-gE and anti-β-catenin antibodies, TGN46 was also found at cell junctions (not shown). These results demonstrate that gE/gI produced in cells infected with wild-type HSV-1 gE/gI initially accumulates in the TGN, but progressively moves to lateral cell surfaces and specifically to cell junctions, at late times. Coincident with this movement, TGN46 was redistributed to lateral surfaces as well.
FIG. 6.
gE/gI accumulates in the TGN early after infection with wild-type HSV-1 but moves to cell junctions late in the infection. HEC-1A cells were infected with wild-type HSV-1 or with F-US7kan, a gI-negative HSV mutant. After 5 or 11 h, the cells were fixed, permeabilized, incubated with blocking buffer, and then incubated with sheep anti-TGN46 (red) for 1 h. The cells were then washed and incubated with donkey anti-sheep IgG coupled to Alexa 594. The cells were washed, blocked with 2% goat serum and 2% BSA, and then incubated simultaneously with a rat polyclonal serum specific for gE/gI (green) and rabbit serum specific for β-catenin (blue). The cells were washed and incubated with Alexa 488-conjugated goat anti-rat IgG antibodies and CY5-conjugated goat anti-rabbit IgG antibodies for 1 h. The cells were viewed by laser scanning confocal microscopy. The red, green, and blue channels were superimposed in the right panels (All markers).
After 5 h of infection of HEC-1A cells with F-US7kan, a mutant that does not express gI, gE was also predominantly in the TGN, colocalizing with TGN46 (Fig. 6, row 3). In some cells infected with F-US7kan, there was a fraction of gE that appeared to be in nonvesicular cytoplasmic structures, which did not stain with TGN46 antibodies. Importantly, and in contrast to the results with wild-type HSV-1, at 11 h of infection with F-US7kan, gE was found primarily sequestered in the TGN (Fig. 6, row 4). Moreover, TGN46 also did not move to lateral cell surfaces without gI. These are very striking observations, suggests that gE/gI, or at least gI, participates in, or is essential for, redistribution of gE/gI and TGN46 to lateral cell surfaces.
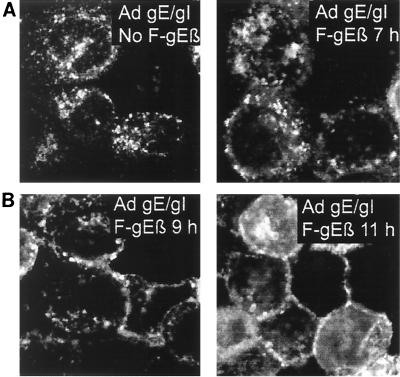
To determine whether gE/gI expressed by Ad vectors can be redistributed to the cell surface after superinfection of cells with HSV-1, HEC-1A cells were infected with Ad(E1−)gE and Ad(E1−)gI for 24 h and subsequently infected with an HSV gE− mutant, F-gEβ. In cells that were not infected with F-gEβ, and at early times after F-gEβ infection (7 h), the gE remained in the TGN (Fig. 7A). After 9 h of infection with the HSV gE− mutant, a small portion of the gE was observed at cell junctions, and by 11 h a large fraction of the gE was localized at cell junctions (Fig. 7B).
FIG. 7.
HSV infection causes redistribution of gE/gI expressed by Ad vectors to lateral cell surfaces. HEC-1A cells were coinfected with 1 Ad(E1−)gE and Ad(E1−)gI; after 24 h the cells were infected with F-gEβ (a gE− mutant) or were left uninfected. Cells were fixed at 7, 9, or 11 h after HSV infection and stained for TGN46, β-catenin, and gE as described in the legend to Fig. 6. Only the gE signal is presented here. The TGN46 stain extensively overlapped that of gE in cells left uninfected or HSV infected for 7 h, and the β-catenin stain overlapped extensively with the gE stain in cells infected with HSV for 11 h.
Endocytosis of the Tf receptor and gE/gI is not altered by HSV infection.
Cellular proteins that are resident in the TGN frequently recycle from endosomes and from the plasma membrane via endosomes. If endocytosis were to be inhibited, these membrane proteins could accumulate on the cell surface. Thus, inhibition of endocytosis could explain the movement of HSV gE/gI and TGN46 to the cell surface. It was reported that PrV inhibits the endocytosis of gE/gI, gB, and the Tf receptor after 6 h of infection (48, 50).
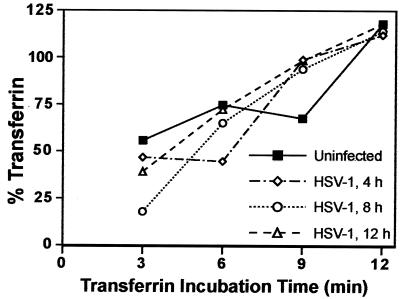
To examine the effects of HSV infection on endocytosis of a cellular protein, we characterized uptake of the Tf receptor. Uptake of 125I-labeled Tf was measured in uninfected HEC-1A cells and in cells infected with HSV-1 for 4, 8, and 12 h. Preceding the uptake experiments, we measured the number of Tf receptors present on cells, by binding labeled Tf at 4°C for 3 min. There was a steady increase in the number of available Tf receptors on the surfaces of cells during infection, apparently related to release of Tf receptors from intracellular sites to the plasma membrane (not shown). For uptake experiments, cells were incubated with labeled Tf at 37°C for 3, 6, 9, or 12 min and then washed with acid buffer to remove surface label. Control experiments at 4°C indicated that the acid buffer removed over 99% of the surface label. No measurement of uptake at time zero was made because binding had not occurred at this point. Measurements of Tf uptake after various times of HSV infection demonstrated that there was no change in the kinetics of Tf uptake until 12 h of HSV infection (Fig. 8). Given that gE/gI was largely redistributed to lateral surfaces by 11 h, endocytosis measurements were not made after 12 h.
FIG. 8.
HSV-1 does not inhibit endocytosis of Tf. HEC-1A cells were infected with wild-type HSV-1 for 4, 8, or 12 h or left uninfected. Specific binding of Tf was assessed by washing the cells in ice-cold uptake medium followed by a 1-h incubation with ice-cold uptake medium containing 125I-labeled Tf in either the presence or absence of a 1,000-fold excess of unlabeled Tf. Cells were washed and lysed with detergent, and cell-associated radioactivity was counted. Uptake of Tf was assessed by washing the cells with serum-free medium at 37°C and incubating cells for 3, 6, 9, or 12 min with 125I-Tf in either the presence or absence of excess unlabeled Tf at 37°C. Following these incubations, surface Tf was stripped from the cells by washing with 0.2 N acetic acid–0.5 M NaCl, the cells were washed, and internalized radioactivity was counted. Nonspecific binding or uptake (observed in the presence of excess unlabeled Tf) was subtracted from the total binding or uptake to give specific values. In all cases, nonspecific binding or uptake was less than 10% of the total binding. Percent Tf internalized was calculated by comparing the specific uptake at 37°C with the Tf that bound specifically at 4°C. Data points represent the mean of duplicate samples.
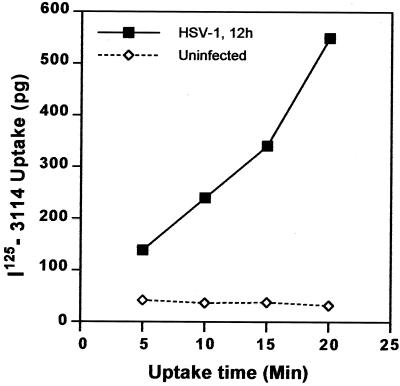
To examine internalization of gE/gI, HSV-infected cells were incubated with 125I-labeled anti-gE antibody 3114. Uptake of 3114 was observed at late times (12 h) of HSV infection (Fig. 9). Over 80% of the radiolabeled anti-gE antibody that was bound to cells was internalized within 20 min. This may or may not reflect the normal rates of internalization of gE/gI, because the antibody could increase the uptake. However, gE/gI appears to be free to be internalized, and there is no global inhibition of endocytosis in HSV-infected cells until 12 h of infection. Together these results suggest that HSV infection does not cause global disruption of the endocytic machinery, at least until after 12 h of infection, yet gE/gI relocalizes to lateral cell surfaces.
FIG. 9.
Internalization of radiolabeled anti-gE MAb 3114. HEC-1A cells that had been infected with wild-type HSV-1 for 12 h or left uninfected were incubated with 125I-labeled MAb 3114, which is specific for gE, for 5, 10, 15, or 20 min at 37°C. Surface antibodies were stripped by washing with 0.2 N acetic acid–0.5 M NaCl, the cells were washed further and lysed with detergent, and radioactivity was counted.
DISCUSSION
When HSV gE/gI was expressed in epithelial cells using replication-defective Ad vectors, the glycoprotein was found predominantly in the TGN colocalizing with TGN46. This was also the case during early stages of HSV infection, until 6 to 7 h. However, at intermediate to late stages of HSV infection, by 11 h, gE/gI moved to the lateral cell surfaces, to cell junctions, colocalizing with β-catenin.
It bears repeating that transport of gE/gI is preferentially to cell junctions; there is little or no glycoprotein on apical surfaces late in HSV infection of epithelial cells (17, 58; Huber et al., Abstr. 25th Int. Herpesvirus Workshop). This is not observed in other cells which form less extensive cell junctions or which do not become polarized. However, this said, the HEC-1A cell monolayers used in many of the experiments shown here were not fully confluent; there were free borders. Thus, a fraction of the cells were not in contact with other cells along a portion of their surfaces. With these cells, gE/gI accumulated extensively at cell junctions late after infection with HSV, and there was much less or no gE/gI at those lateral surfaces of cells that were not in contact with other cells, i.e., nonjunctional surfaces. This was previously documented by Wisner et al. (58) in experiments in which z-axis images were collected, and this demonstrated accumulation at cell junctions rather than on apical surfaces. It is important to note that β-catenin and E-cadherin, components of adherens junctions, can also distribute preferentially to cell junctions before cells are fully polarized (to the extent that tight junctions ring the entire subapical lateral surface and there is high transepithelial resistance). In fact, formation of adherens junctions precedes that of tight junctions and is a necessary prerequisite for tight junctions. Clearly, gE/gI can accumulate at cell junctions in epithelial cells, whether or not cells are fully polarized. However, it should also be noted that a large fraction or majority of cells in these monolayers were considered to be polarized, in that there was a ring of other cells surrounding them. With these cells, extensive contacts were formed between cells, including adherens junctions (see staining of β-catenin in Fig. 6, similar staining was observed with anti-E-cadherin antibodies) and tight junctions (staining with anti-ZO-1 antibodies). Whether or not these cells were “fully” polarized, as defined by transfer of small molecules across tight junctions, is not clear. Therefore, there is directed transport of gE/gI to epithelial cell junctions and/or retention there once the junctions are formed and regardless of whether cells are “fully” polarized.
The TGN localization of gE/gI seen early after HSV infection or in the absence of HSV proteins, in Ad-infected cells, appears to be largely due to the effects of the CT domain of gE, because a chimeric glycoprotein composed of the HSV gE CT domain fused to gD was also found exclusively in the TGN. Previous experiments demonstrated that the CT domain of gE was necessary for cell-to-cell spread (58), for accumulation at cell junctions in HSV-infected cells (a process that is intrinsically tied to TGN sorting) (58), and for specific sorting of nascent virions to cell junctions (28). We expect that the tyrosine motif (YIRV468) in the CT domain of gE is involved in TGN localization, linking gE to AP-1 clathrin adapter complexes (reviewed in references 8, 19, and 36). This hypothesis is supported by observations involving a similar tyrosine motif in VZV gE that participates in TGN localization (1, 61). Moreover, mutations affecting a tyrosine motif in the CT domain of PrV gE compromised cell-to-cell spread (49). However, it is also likely that the cluster of acidic residues in the CT domain of gE (D476 WSSDSEGERDQ) plays an important role in TGN localization. Similar acidic clusters in furin and in VZV gE, when phosphorylated, bind to PACS-1, which can then link to clathrin adapter complexes causing retrieval to the TGN (35, 53, 61).
By contrast to these results, a fusion protein including the gI CT domain was found largely on the apical and lateral cell surfaces, as was wild-type gD. Thus, the CT domain of HSV gI does not, on its own, cause TGN localization in these cells. Previous studies of cellular proteins have shown that dileucine motifs, similar to that in gI, can cause endocytosis and TGN localization, but this was not seen in these epithelial cells. The VZV gI was targeted to some extent to the TGN, as well as to the plasma membrane, when expressed without gE by transfection (54). However, there was little retrieval of VZV gI from the surface to the TGN, but coexpression with VZV gE promoted TGN localization (54). Thus, it appears that the CT domain of VZV and HSV gI is less effective than that of gE in causing TGN accumulation. Again, it is important to consider that the dileucine motif may well augment the effects of the gE CT. Consistent with the importance of the gI CT domain, PrV mutants lacking this domain were less neurovirulent and compromised in transynaptic spread (51). One of the most striking observations of the work described here is that gI was required for movement of gE/gI from the TGN to cell junctions during late stages of HSV infection. It seems likely that the CT of gI and its dileucine motif function in this process; this is under further study.
The extensive accumulation of gE and gE/gI in the TGN occurs during early phases of HSV infection or after expression of proteins using Ad vectors, but at intermediate to late times of infection, gE/gI moves to the lateral surfaces, to cell junctions. One obvious explanation for these observations was HSV-mediated inhibition of endocytosis or recycling from endosomes or the cell surface plasma. Consistent with this notion, Tirabassi and colleagues found that PrV uptake of gE- and gB-specific antibodies, and of the Tf receptors, was inhibited after 6 h of PrV infection (48, 50). However, we found that endocytosis of Tf receptors and of gE/gI was not inhibited by HSV infection before 12 h of infection. Our results may differ from that of Tirabassi et al. because the HSV may lack a viral function that blocks endocytosis or this difference may relate to our use of epithelial cells rather than nonpolarized PK15 cells. Nevertheless, HSV does not cause global inhibition of the host endocytic machinery, at least by 12 h; thus, this cannot explain the redistribution of gE/gI to lateral cell surfaces. However, it is possible that HSV inhibits intracellular recycling from endosomes back to TGN compartments (35, 53), and this could redirect gE/gI from these TGN compartments to endosomes and onward to the plasma membrane. This possibility is under further study.
Our results appear to be closely related to two other sets of observations. First, Brack et al. recently reported that PrV gE/gI functions in envelopment of nucleocapsids in the cytoplasm (9). PrV mutants lacking gE, or the CT domain of gE, as well as second glycoprotein, gM, accumulated large numbers of unenveloped capsids in the cytoplasm. This suggests that gE/gI and gM collaborate to drive envelopment of cytosolic capsids; i.e., budding occurs into cytoplasmic membranes. When viewed in the context of the results presented here and previous reports from Gershon and colleagues involving VZV gE/gI (54, 60) and Whealy et al. (56), it is likely that PrV, HSV, and VZV are all enveloped predominately into the TGN. Second, we recently reported that HSV virions were specifically delivered to the lateral surfaces, i.e., cell junctions of polarized epithelial cells, with 10- to 20-fold fewer particles delivered to apical surfaces (28). gE, and specifically the CT domain of gE, was required for this targeted delivery of nascent virions to cell junctions. In the absence of gE or its CT domain, there were 15- to 30-fold fewer virus particles at cell junctions, and increased numbers particles on apical surfaces, in the cell culture supernatants and accumulation of virions in cytoplasmic vesicles (28). Selective sorting of PrV to cell junctions was also observed, and this process was enhanced by expression of the μ1B subunit of AP-1 clathrin adapter complexes. Thus, in polarized cells, accumulation of gE/gI in the TGN appears to lead to envelopment there, followed by directed transport of nascent HSV and PrV virions to cell junctions. Without gE/gI, or just the CT domains, particles are misdirected and accumulate in the cytoplasm of epithelial cells.
The observation that TGN46 was also redistributed to lateral cell surfaces has important implications for understanding the movement of gE/gI from the TGN to lateral surfaces. TGN46 is a cellular protein that rapidly recycles from endosomes to the TGN, without residing extensively on the cell surface (21). Relocalization of both gE and TGN46 to lateral surfaces required expression of gI in cells. Since gI is extensively complexed with gE and is required for gE/gI to function, it appears that this process is connected to the movement of newly formed virions from intracellular membranes, i.e., the TGN, to cell epithelial junctions that occurs at intermediate to late times of HSV infection (28). The simplest conclusion is that the movement of gE/gI to cell junctions occurs as a consequence of, or in association with, movement of virions to cell junctions. gE is essential for movement of particles to cell junctions (28), and apparently gI is important as well. It is not clear why TGN46 also moves along with the virion during this process.
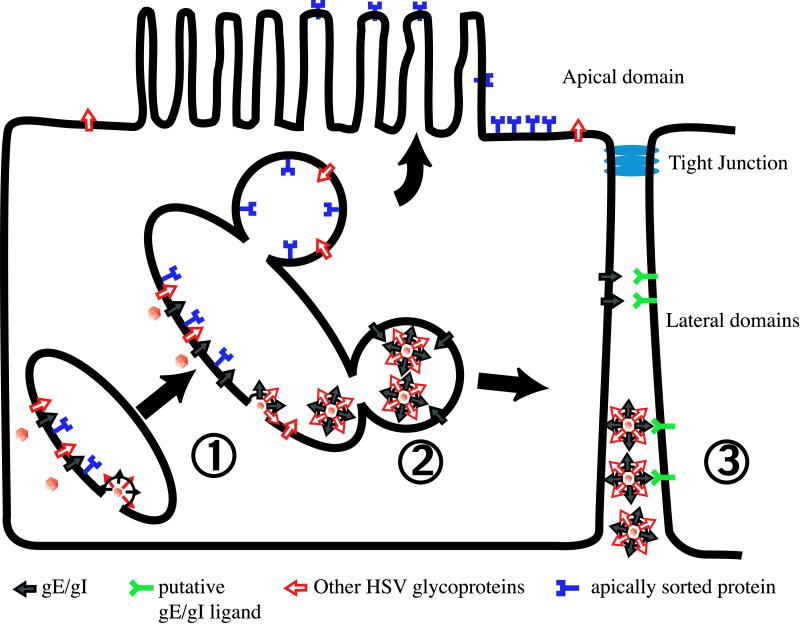
A model that attempts to describe the multiple effects of gE/gI in mediating cell-to-cell spread is depicted in Fig. 10. In step 1 (budding), gE/gI accumulates extensively in the TGN based on interactions with cellular sorting machinery, AP clathrin adapters, PACS-1, and perhaps other factors. This process likely involves recycling from endosomal compartments and the plasma membrane. Other alphaherpesvirus glycoproteins must also be targeted to this compartment through interactions with tegument proteins, gE/gI, other glycoproteins (gM, US9) or by mechanisms that have not yet been described. Envelopment of cytosolic nucleocapsids occurs, probably driven by gE/gI and gM, and enveloped virions are deposited within TGN vesicles. In step 2 (sorting), in epithelial cells, gE/gI apparently interacts with AP-1 clathrin adapters and perhaps other cellular sorting machinery, in part through the gE CT domain, so that transport vesicles containing nascent virions are sorted to lateral cell surfaces, rather than apical domains of the plasma membrane. This moves not only virions but also the contents of the TGN to cell junctions. It is also conceivable that the envelopment of capsids occurs in vesicles that have been previously sorted to lateral cell surfaces. In this latter scenario, gE/gI and other virions components assemble in a subset of TGN vesicles, those that have previously interacted with cellular sorting machinery, so that the contents of the vesicles are destined to lateral surfaces. Once virions reach cell junctions, gE/gI can mediate binding to cellular receptors there and entry into the apposing cell (step 3, receptor binding). This is based on evidence that gE/gI can accumulate extensively at cell junctions and may be able to substitute for gD to mediate cell-to-cell spread. Of course, other HSV glycoproteins (gD, gB, and gH/gL) are also necessary for receptor binding and fusion of the virion envelope with the recipient cell. Therefore, cell-to-cell spread relies on both the CT domains of gE/gI that sort virus to cell junctions and the extracellular domains that function to promote entry into other host cells.
FIG. 10.
A model summarizing the effects of gE/gI in sorting of virions to cell junctions and infection of neighboring cells. (1) gE/gI, other HSV glycoproteins, and tegument components accumulate in or at the TGN. Interactions between the CT domains of gE/gI and AP-1 clathrin adapter complexes are involved in the accumulation of gE/gI in the TGN. Cytoplasmic nucleocapsids bind to regions enriched in viral glycoproteins and envelopment occurs, delivering virions into the TGN vesicles. The CT domains of gE/gI are involved in this envelopment process, along with gM. (2) Vesicles containing nascent virions are sorted to lateral domains of the cell surface and away from the apical surface. This process requires the CT domains of gE/gI and involves AP-1 clathrin adapters. (3) Transport vesicles fuse with the plasma membrane, delivering virions into the space between two closely connected cells. gE/gI interacts with cellular receptors to mediate entry and infection of the apposing cell.
ACKNOWLEDGMENTS
We are very indebted to Aurelie Snyder for all her hard work and valuable expertise with the laser scanning confocal and deconvolution software. We thank Mary Huber for advice and for carefully reading the manuscript and Todd Wisner for excellent technical assistance, for advice on confocal experiments, and for disposing of all the radioactivity. We are also grateful to Gary Thomas for advice and antibodies, to the laboratory of Carolyn Enns for assistance with transferrin uptake assays, and to Andrew Townsend for help with the graphics. T.N.M. thanks Kim Goldsmith for watching his back throughout this work.
This work was supported by National Institutes of Health grant CA73996.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschuler Y, Barbas S M, Terlecky L J, Tang K, Hardy S, Mostov K E, Schmid S L. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 5.Ball J M, Moldoveaunu Z, Melsen L R, Kozlowski P A, Jackson S, Mulligan M J, Mestecky J F, Compans R W. A polarized human endometrial cell line that binds and transports polymeric IgA. In Vitro Cell Dev Biol. 1995;31:196–206. doi: 10.1007/BF02639434. [DOI] [PubMed] [Google Scholar]
- 6.Banting G, Ponnambalam S. TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim Biophys Acta. 1997;1355:209–217. doi: 10.1016/s0167-4889(96)00146-2. [DOI] [PubMed] [Google Scholar]
- 7.Bell S, Cranage M, Borysiewicz L, Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990;64:2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifacino J S, Dell'Angelica E C. Molecular bases for recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brack A R, Klupp B G, Granzow H, Tirabassi R, Enquist L W, Mettenleiter T C. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J Virol. 2000;74:4004–4016. doi: 10.1128/jvi.74.9.4004-4016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunetti C R, Dingwell K S, Wale C, Graham F L, Johnson D C. Herpes simplex virus gD and virions accumulate in endosomes by mannose-6-phosphate-dependent and -independent mechanisms. J Virol. 1998;72:3330–3339. doi: 10.1128/jvi.72.4.3330-3339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campadelli G, Brandimarti R, Di Lazzaro C, Ward P L, Roizman B, Torrisi M R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. . (Erratum, 9: preceding 311.) [PubMed] [Google Scholar]
- 13.Chapman T L, You I, Joseph I M, Bjorkman P J, Morrison S L, Raghavan M. Characterization of the interaction between the herpes simplex virus I Fc receptor and immunoglobulin G. J Biol Chem. 1999;274:6911–6919. doi: 10.1074/jbc.274.11.6911. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J I, Nguyen H. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingwell K S, Johnson D C. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edson C M. Phosphorylation of neurotropic alphaherpesvirus envelope glycoproteins: herpes simplex virus type 2 gE2 and pseudorabies virus gI. Virology. 1993;195:268–270. doi: 10.1006/viro.1993.1372. [DOI] [PubMed] [Google Scholar]
- 19.Folsch H, Ohno H, Bonifacino J S, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 20.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh R N, Mallet W G, Soe T T, McGraw T E, Maxfield F R. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanke T, Graham F L, Lulitanond V, Johnson D C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990;177:437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- 23.Hirst J, Robinson M S. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 24.Hitt M, Bett A J, Addison C L, Prevec L, Graham F L. Techniques for human adenovirus vector construction and characterization. Methods Mol Genet. 1995;78:13–30. [Google Scholar]
- 25.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson D C, Webb M, Wisner T W, Brunetti C. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J Virol. 2000;75:821–833. doi: 10.1128/JVI.75.2.821-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura H, Straus S E, Williams R K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 30.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 31.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 32.Le Borgne R, Hoflack B. Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr Opin Cell Biol. 1998;10:499–503. doi: 10.1016/s0955-0674(98)80065-3. [DOI] [PubMed] [Google Scholar]
- 33.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 35.Molloy S S, Anderson E D, Jean F, Thomas G. Bi-cycling the furin pathway:from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 36.Mostov K, ter Beest M B A, Chapin S J. Catch the u1B train to the basolateral surface. Cell. 1999;99:121–122. doi: 10.1016/s0092-8674(00)81643-8. [DOI] [PubMed] [Google Scholar]
- 37.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponnambalam S, Girotti M, Yaspo M, Owen C E, Perry A C F, Suganuma T, Nilsson T, Fried M, Banting G, Warren G. Primate homologues of rat TGN38: primary structure, expression and functional implications. J Cell Sci. 1996;109:675–685. doi: 10.1242/jcs.109.3.675. [DOI] [PubMed] [Google Scholar]
- 43.Rajasekaran A K, Humphrey J S, Wagner M, Miesenböck G, Le Bivic A, Bonifacino J S, Rodriguez-Boulan E. TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1994;5:1093–1103. doi: 10.1091/mbc.5.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajcani J, Herget U, Kaerner H C. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its glyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 1990;34:305–320. [PubMed] [Google Scholar]
- 45.Rauh I, Weiland F, Fehler F, Keil G M, Mettenleiter T C. Pseudorabies virus mutants lacking the essential glycoprotein gII can be complemented by glycoprotein gI of bovine herpesvirus 1. J Virol. 1991;65:621–631. doi: 10.1128/jvi.65.2.621-631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following brefedin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Streblow D M, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson J A. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- 48.Tirabassi R S. A mutational analysis of the glycoproteins E and I of pseudorabies virus: domains involved in trafficking, virulence, and direct cell-to-cell spread. Thesis. Princeton, N.J: Princeton University; 1999. [Google Scholar]
- 49.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tirabassi R S, Enquist L W. Role of the envelope protein gE in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tirabassi R S, Enquist L W. Role of the pseudorabies virus gI cytoplasmic tail in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J Virol. 2000;74:3505–3516. doi: 10.1128/jvi.74.8.3505-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomazin R, Boname J, Hegde N R, Lewinsohn D M, Altschuler Y, Jones T R, Cresswell P, Nelson J A, Riddell S R, Johnson D C. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cell. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 53.Wan L, Molloy S S, Thomas L, Liu G, Xiang Y, Rybak S L, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z-H, Gershon M D, Lungu O, Zhu Z, Gershon A. Trafficking of varicella-zoster virus glycoprotein gI: T388-dependent retention in the trans-Golgi network, secretion, and mannose 6-phosphate-inhibitable uptake of the ectodomain. J Virol. 2000;74:6600–6613. doi: 10.1128/jvi.74.14.6600-6613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warren R A, Green F A, Enns C A. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J Biol Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- 56.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisner T, Brunetti C, Dingwell K, Johnson D C. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J Virol. 2000;74:2278–2287. doi: 10.1128/jvi.74.5.2278-2287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao Z, Jackson W, Forghani B, Grose C. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J Virol. 1993;67:305–314. doi: 10.1128/jvi.67.1.305-314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuckermann F A, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]