Abstract
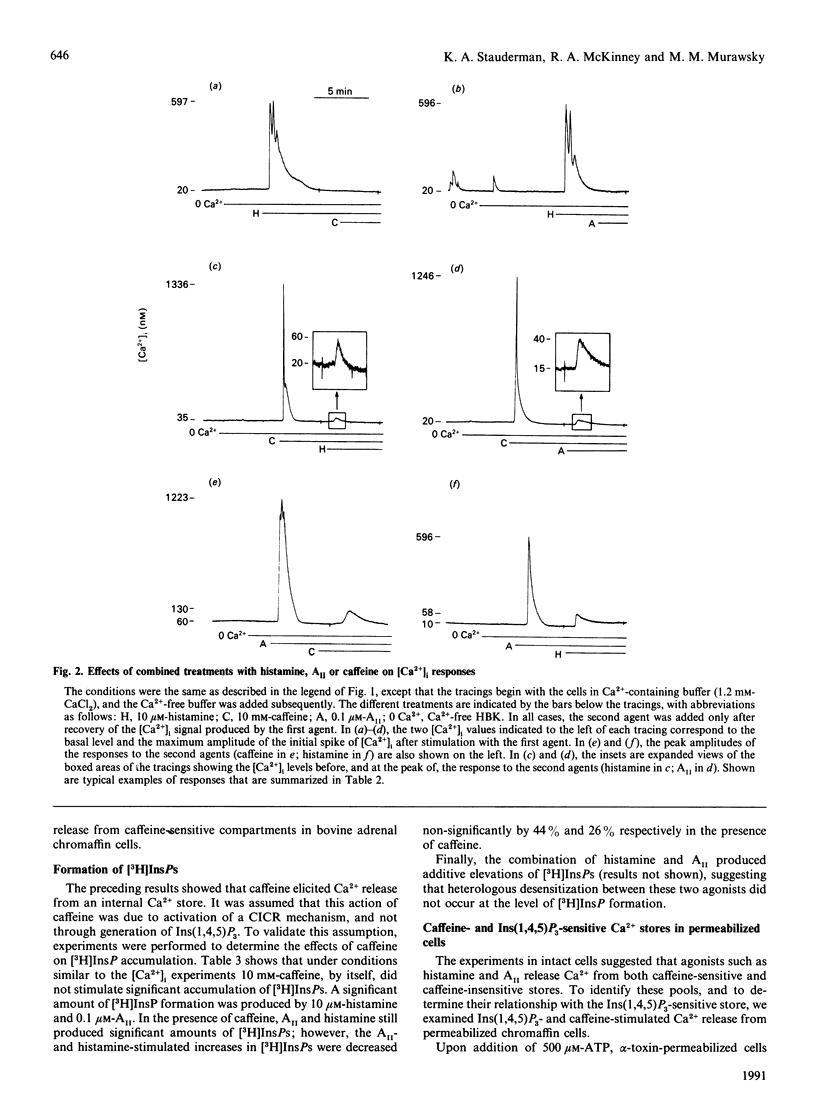
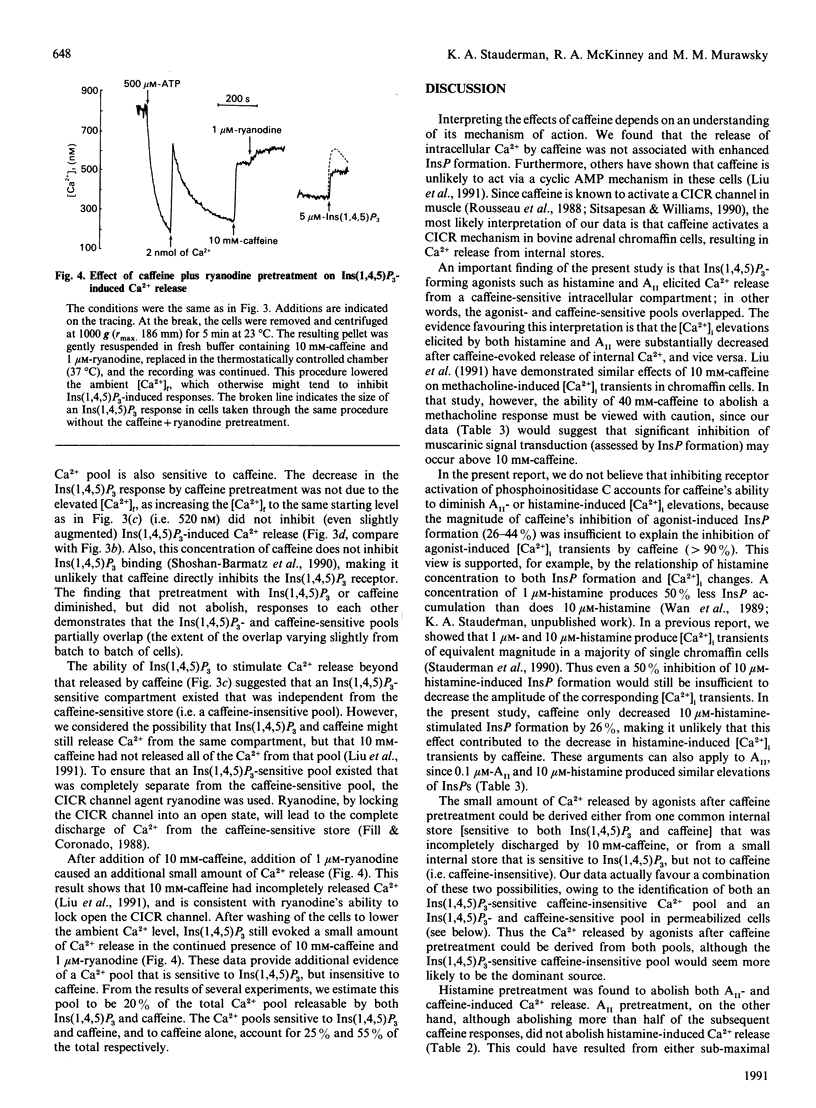
In single bovine adrenal chromaffin cells loaded with fura-2, histamine, angiotensin II (AII) and caffeine elicited large transient increases of intracellular free Ca2+ concentration [( Ca2+]i) in the absence of external Ca2+, with peak amplitudes averaging 726 +/- 138 (n = 14), 710 +/- 102 (n = 21) and 830 +/- 100 nM (n = 30) respectively. A substantial portion of the agonist-induced rise in [Ca2+]i depended on Ca2+ release from caffeine-sensitive stores, as pretreatment with caffeine diminished subsequent agonist responses by 90-95%. Conversely, pretreatment with histamine or AII decreased subsequent caffeine responses by 100% and 90% respectively. The effects of caffeine most likely resulted from activation of a Ca(2+)-induced Ca(2+)-release (CICR) process, whereas histamine and AII initially acted through generation of Ins(1,4,5)P3. The relationship of Ins(1,4,5)P3- and caffeine-sensitive Ca2+ pools was studied by using alpha-toxin-permeabilized chromaffin cells. Evidence was found for three non-mitochondrial, ATP-dependent, Ca2+ pools: one exclusively sensitive to Ins(1,4,5)P3 (pool 1), a second sensitive to both Ins(1,4,5)P3 and caffeine (pool 2), and a third exclusively sensitive to caffeine (pool 3). The existence of pools 1 and 3, and the ability of agonists such as histamine to discharge pool 3 completely, supports a two-pool model in which a caffeine-sensitive CICR mechanism plays a major role in the generation of agonist-induced Ca2+ spikes in bovine chromaffin cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. The caffeine-sensitive Ca2+ store in bovine adrenal chromaffin cells; an examination of its role in triggering secretion and Ca2+ homeostasis. FEBS Lett. 1990 Jun 18;266(1-2):91–95. doi: 10.1016/0014-5793(90)81514-o. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Iino M. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun. 1987 Jan 15;142(1):47–52. doi: 10.1016/0006-291x(87)90449-9. [DOI] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Kanaide H., Shogakiuchi Y., Nakamura M. The norepinephrine-sensitive Ca2+-storage site differs from the caffeine-sensitive site in vascular smooth muscle of the rat aorta. FEBS Lett. 1987 Apr 6;214(1):130–134. doi: 10.1016/0014-5793(87)80027-3. [DOI] [PubMed] [Google Scholar]
- Kao L. S. Calcium homeostasis in digitonin-permeabilized bovine chromaffin cells. J Neurochem. 1988 Jul;51(1):221–227. doi: 10.1111/j.1471-4159.1988.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Kostron H., Winkler H., Geissler D., König P. Uptake of calcium by chromaffin granules in vitro. J Neurochem. 1977 Mar;28(3):487–493. doi: 10.1111/j.1471-4159.1977.tb10419.x. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Thastrup O., Hanley M. R., Dannies P. S. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. S., Lin Y. J., Kao L. S. Caffeine-sensitive calcium stores in bovine adrenal chromaffin cells. J Neurochem. 1991 Jan;56(1):172–177. doi: 10.1111/j.1471-4159.1991.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Shogakiuchi Y., Nakamura M. Characteristics of the histamine-sensitive calcium store in vascular smooth muscle. Comparison with norepinephrine- or caffeine-sensitive stores. J Biol Chem. 1990 Apr 5;265(10):5610–5616. [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M. Caffeine-induced catecholamine secretion: similarity to caffeine-induced muscle contraction. Proc Soc Exp Biol Med. 1973 Jan;142(1):103–105. doi: 10.3181/00379727-142-36967. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Moskal J. R., Eiden L. E., Beinfeld M. C. Specific regulation of vasoactive intestinal polypeptide biosynthesis by phorbol ester in bovine chromaffin cells. Endocrinology. 1985 Sep;117(3):1020–1026. doi: 10.1210/endo-117-3-1020. [DOI] [PubMed] [Google Scholar]
- Rakow T. L., Shen S. S. Multiple stores of calcium are released in the sea urchin egg during fertilization. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9285–9289. doi: 10.1073/pnas.87.23.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V., Zhang G. H., Garretson L., Kraus-Friedmann N. Distinct ryanodine- and inositol 1,4,5-trisphosphate-binding sites in hepatic microsomes. Biochem J. 1990 Jun 15;268(3):699–705. doi: 10.1042/bj2680699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman K. A., Murawsky M. M., Pruss R. M. Agonist-dependent patterns of cytosolic Ca2+ changes in single bovine adrenal chromaffin cells: relationship to catecholamine release. Cell Regul. 1990 Aug;1(9):683–691. doi: 10.1091/mbc.1.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990 Mar;54(3):946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Stoehr S. J., Smolen J. E., Holz R. W., Agranoff B. W. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 1986 Feb;46(2):637–640. doi: 10.1111/j.1471-4159.1986.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Marsault R., Frelin C. Endothelin mobilizes Ca2+ from a caffeine- and ryanodine-insensitive intracellular pool in rat atrial cells. J Biol Chem. 1990 Apr 25;265(12):6782–6787. [PubMed] [Google Scholar]
- Vites A. M., Pappano A. Inositol 1,4,5-trisphosphate releases intracellular Ca2+ in permeabilized chick atria. Am J Physiol. 1990 Jun;258(6 Pt 2):H1745–H1752. doi: 10.1152/ajpheart.1990.258.6.H1745. [DOI] [PubMed] [Google Scholar]
- Wakade T. D., Bhave S. V., Bhave A., Przywara D. A., Wakade A. R. Ca2+ mobilized by caffeine from the inositol 1,4,5-trisphosphate-insensitive pool of Ca2+ in somatic regions of sympathetic neurons does not evoke [3H]norepinephrine release. J Neurochem. 1990 Nov;55(5):1806–1809. doi: 10.1111/j.1471-4159.1990.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Wan D. C., Bunn S. J., Livett B. G. Effects of phorbol esters and forskolin on basal and histamine-induced accumulation of inositol phosphates in cultured bovine adrenal chromaffin cells. J Neurochem. 1989 Oct;53(4):1219–1227. doi: 10.1111/j.1471-4159.1989.tb07418.x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakazato Y., Ohga A. The mode of action of caffeine on catecholamine release from perfused adrenal glands of cat. Br J Pharmacol. 1989 Oct;98(2):351–356. doi: 10.1111/j.1476-5381.1989.tb12603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. H., Albanesi J. P. Inositol 1,4,5-trisphosphate-triggered Ca2+ release from bovine adrenal medullary secretory vesicles. J Biol Chem. 1990 Aug 15;265(23):13446–13448. [PubMed] [Google Scholar]


