Abstract
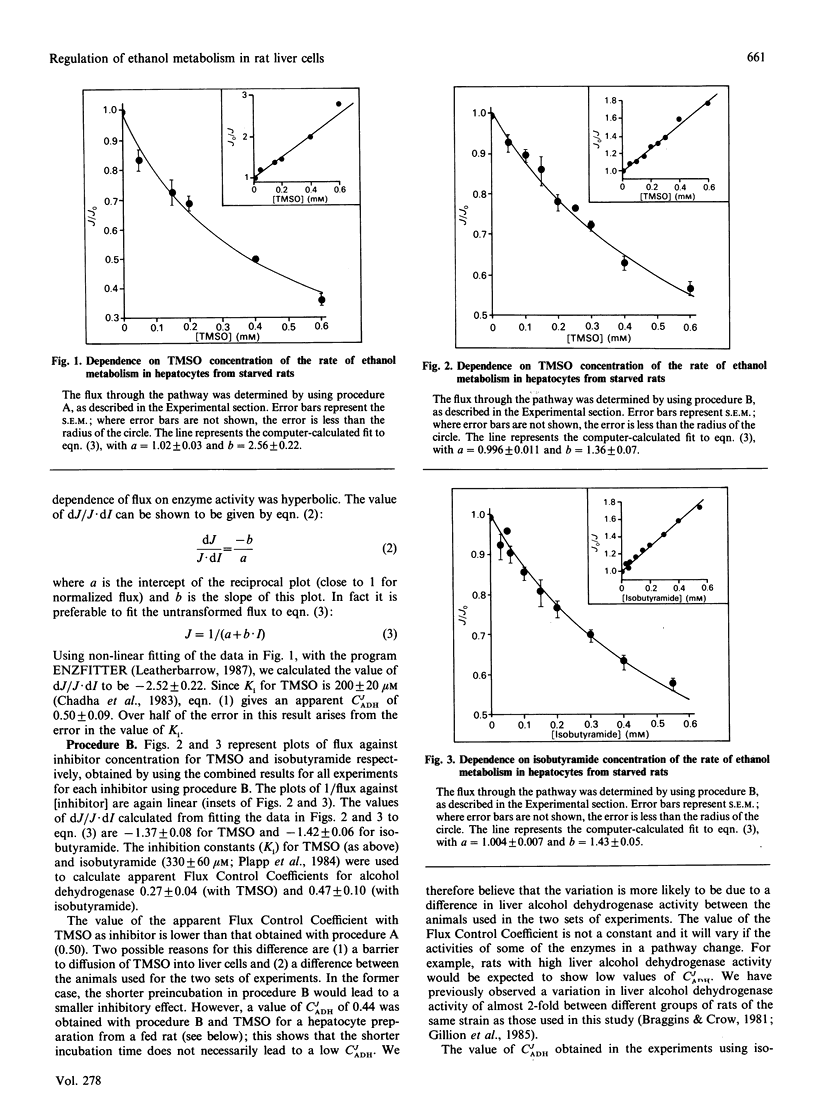
We used titration with the inhibitors tetramethylene sulphoxide and isobutyramide to assess quantitatively the importance of alcohol dehydrogenase in regulation of ethanol oxidation in rat hepatocytes. In hepatocytes isolated from starved rats the apparent Flux Control Coefficient (calculated assuming a single-substrate irreversible reaction with non-competitive inhibition) of alcohol dehydrogenase is 0.3-0.5. Adjustment of this coefficient to allow for alcohol dehydrogenase being a two-substrate reversible enzyme increases the value by 1.3-1.4-fold. The final value of the Flux Control Coefficient of 0.5-0.7 indicates that alcohol dehydrogenase is a major rate-determining enzyme, but that other factors also have a regulatory role. In hepatocytes from fed rats the Flux Control Coefficient for alcohol dehydrogenase decreases with increasing acetaldehyde concentration. This suggests that, as acetaldehyde concentrations rise, control of the pathway shifts from alcohol dehydrogenase to other enzymes, particularly aldehyde dehydrogenase. There is not a single rate-determining step for the ethanol metabolism pathway and control is shared among several steps.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braggins T. J., Crow K. E. The effects of high ethanol doses on rates of ethanol oxidation in rats. A reassessment of factors controlling rates of ethanol oxidation in vivo. Eur J Biochem. 1981 Oct;119(3):633–640. doi: 10.1111/j.1432-1033.1981.tb05654.x. [DOI] [PubMed] [Google Scholar]
- Bücher T., Brauser B., Conze A., Klein F., Langguth O., Sies H. State of oxidation-reduction and state of binding in the cytosolic NADH-system as disclosed by equilibration with extracellular lactate-pyruvate in hemoglobin-free perfused rat liver. Eur J Biochem. 1972 May 23;27(2):301–317. doi: 10.1111/j.1432-1033.1972.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Chadha V. K., Leidal K. G., Plapp B. V. Inhibition by carboxamides and sulfoxides of liver alcohol dehydrogenase and ethanol metabolism. J Med Chem. 1983 Jun;26(6):916–922. doi: 10.1021/jm00360a024. [DOI] [PubMed] [Google Scholar]
- Chadha V. K., Leidal K. G., Plapp B. V. Inhibition by carboxamides and sulfoxides of liver alcohol dehydrogenase and ethanol metabolism. J Med Chem. 1983 Jun;26(6):916–922. doi: 10.1021/jm00360a024. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Veech R. L. Enzymatic measurement of ethanol or NAD in acid extracts of biological samples. Anal Biochem. 1983 Jul 15;132(2):418–423. doi: 10.1016/0003-2697(83)90029-5. [DOI] [PubMed] [Google Scholar]
- Crow K. E., Braggins T. J., Batt R. D., Hardman M. J. Rat liver cytosolic malate dehydrogenase: purification, kinetic properties, role in control of free cytosolic NADH concentration. Analysis of control of ethanol metabolism using computer simulation. J Biol Chem. 1982 Dec 10;257(23):14217–14225. [PubMed] [Google Scholar]
- Crow K. E. Ethanol metabolism by the liver. Rev Drug Metab Drug Interact. 1985;5(2-3):113–158. doi: 10.1515/dmdi.1985.5.2-3.113. [DOI] [PubMed] [Google Scholar]
- Dawson A. G. Inhibitory effect of acetaldehyde on the oxidation of ethanol by a high-speed supernatant fraction of rat liver. Biochem Pharmacol. 1981 Aug 15;30(16):2349–2352. doi: 10.1016/0006-2952(81)90110-6. [DOI] [PubMed] [Google Scholar]
- Eriksson C. J., Marselos M., Koivula T. Role of cytosolic rat liver aldehyde dehydrogenase in the oxidation of acetaldehyde during ethanol metabolism in vivo. Biochem J. 1975 Dec;152(3):709–712. doi: 10.1042/bj1520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillion R. B., Crow K. E., Batt R. D., Hardman M. J. Effects of ethanol treatment and castration on liver alcohol dehydrogenase activity. Alcohol. 1985 Jan-Feb;2(1):39–41. doi: 10.1016/0741-8329(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Harrington M. C., Henehan G. T., Tipton K. F. The interrelationships of alcohol dehydrogenase and the aldehyde dehydrogenases in the metabolism of ethanol in liver. Biochem Soc Trans. 1988 Jun;16(3):239–241. doi: 10.1042/bst0160239. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Kalant H. The metabolism of ethanol and its metabolic effects. Pharmacol Rev. 1972 Mar;24(1):67–157. [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- Kalant H., Khanna J. M., Endrenyi L. Effect of pyrazole on ethanol metabolism in ethanol-tolerant rats. Can J Physiol Pharmacol. 1975 Jun;53(3):416–422. doi: 10.1139/y75-060. [DOI] [PubMed] [Google Scholar]
- Khanna J. M., Israel Y. Ethanol metabolism. Int Rev Physiol. 1980;21:275–315. [PubMed] [Google Scholar]
- Plapp B. V. Enhancement of the activity of horse liver alcohol dehydrogenase by modification of amino groups at the active sites. J Biol Chem. 1970 Apr 10;245(7):1727–1735. [PubMed] [Google Scholar]
- Plapp B. V., Leidal K. G., Smith R. K., Murch B. P. Kinetics of inhibition of ethanol metabolism in rats and the rate-limiting role of alcohol dehydrogenase. Arch Biochem Biophys. 1984 Apr;230(1):30–38. doi: 10.1016/0003-9861(84)90083-3. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Leidal K. G., Smith R. K., Murch B. P. Kinetics of inhibition of ethanol metabolism in rats and the rate-limiting role of alcohol dehydrogenase. Arch Biochem Biophys. 1984 Apr;230(1):30–38. doi: 10.1016/0003-9861(84)90083-3. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A., Heinrich R., Jacobasch G., Rapoport S. A linear steady-state treatment of enzymatic chains. A mathematical model of glycolysis of human erythrocytes. Eur J Biochem. 1974 Feb 15;42(1):107–120. doi: 10.1111/j.1432-1033.1974.tb03320.x. [DOI] [PubMed] [Google Scholar]
- Siew C., Deitrich R. A., Erwin V. G. Localization and characteristics of rat liver mitochondrial aldehyde dehydrogenases. Arch Biochem Biophys. 1976 Oct;176(2):638–649. doi: 10.1016/0003-9861(76)90208-3. [DOI] [PubMed] [Google Scholar]
- Stowell A. R., Crow K. E., Greenway R. M., Batt R. D. Determination of acetaldehyde in blood using automated distillation and fluorometry. Anal Biochem. 1978 Feb;84(2):384–392. doi: 10.1016/0003-2697(78)90055-6. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Guynn R., Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J. 1972 Apr;127(2):387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Groen A. K., Wanders R. J. Modern theories of metabolic control and their applications (review). Biosci Rep. 1984 Jan;4(1):1–22. doi: 10.1007/BF01120819. [DOI] [PubMed] [Google Scholar]



