Abstract
Currently, evidence concerning the link between maximal aortic diameter and in-hospital mortality in cases of acute type B aortic dissection (ATBAD) is insufficient. Thus, this study aimed to explore the relationship between the maximal aortic diameter at the time of admission and the early prognosis of patients diagnosed with ATBAD. A total of 678 patients with ATBAD were included between January 2016 and December 2018, during which their clinical data was gathered. The independent variable analyzed was the maximal diameter of the aorta, while the dependent variable was mortality during hospitalization. Factors considered in this analysis included the patients’ age, gender, body mass index (BMI), medical history of hypertension, stroke, diabetes, atherosclerosis, smoking habits, chronic kidney insufficiency, time until presentation, systolic and diastolic blood pressures, ejection fraction, presence of aortic regurgitation, symptoms, involvement of abdominal vessels, laboratory findings, and treatment approaches. Of these patients collected, the mean age was 56.03 ± 12.22 years, and approximately 82.45% of them were male. After analysis, it was found that the maximal aortic diameter of patients with ATBAD was positively correlated with in-hospital mortality (OR = 1.06, 95% CI 1.03 to 1.10). Surprisingly, a J curve relationship was detected between maximal aortic diameter (point 31 mm) and in-hospital death for patients with ATBAD. The effect sizes and confidence intervals of the right (maximal aortic diameter > 31 mm) and left (maximal aortic diameter ≤ 31 mm) aspects of the inflection point were 1.06 (1.02–1.11) and 1.03 (0.83–1.28), respectively. In addition, the stratified analysis showed a stable relationship between maximal aortic diameter and in-hospital mortality, while there was no significant difference in the interaction between different subgroups. In patients with ATBAD, a J-curve relationship was identified between the maximal aortic diameter and in-hospital mortality. Specifically, when the maximal aortic diameter exceeds 31 mm, a positive correlation with in-hospital death was observed.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77649-3.
Keywords: Acute type B aortic dissection, Maximal aortic diameter, In-hospital mortality
Subject terms: Risk factors, Cardiovascular biology, Interventional cardiology
Introduction
Acute type B aortic dissection (ATBAD) is a severe cardiovascular condition with an alarming incidence rate of approximately 4.4 per 100,000 individuals1. Despite advancements in thoracic endovascular aortic repair (TEVAR) as the standard treatment, the in-hospital mortality rate remains troublingly high at 14%2. The variability in clinical presentations of ATBAD complicates the prediction of patient outcomes at the time of hospital admission3. Therefore, it is imperative to deepen our understanding of the early prognosis of ATBAD. This is essential for optimizing therapeutic strategies and providing informed guidance to patients and their families, tailored to the specific characteristics of each case.
In the context of acute type A aortic dissection, aortic diameter stands as a crucial prognostic factor, closely tied to the critical decision-making process concerning prophylactic surgical replacement of the ascending aorta4. Current clinical guidelines recommend aortic aneurysm resection when the maximal aortic diameter exceeds 55 mm, recognizing this measurement as the primary anatomical predictor of dissection and rupture5. However, in patients with ATBAD, the decision for surgery is not dictated solely by maximal aortic diameter. Instead, it involves a more nuanced assessment that prioritizes symptoms and the specifics of the dissected anatomy, reflecting the complexity of clinical judgment in this group. Recent studies have highlighted the importance of aortic diameter as a prognostic marker for long-term survival in ascending aortic dissection6. Nonetheless, the precise impact of maximal aortic diameter on ATBAD-related mortality remains uncertain, warranting further investigation to clarify its role in clinical outcomes.
In the realm of ATBAD, the relationship between maximal aortic diameter and in-hospital mortality has unfortunately been underexplored. This gap has motivated our investigation, which is specifically designed to examine the association between aortic diameter and in-hospital mortality rates among ATBAD patients. Our study carefully considers potential confounding factors, ensuring a thorough analysis that addresses this critical oversight in the existing literature.
Methods
Study design
This investigation employs a retrospective observational approach, carried out at a renowned university-affiliated hospital in Changsha, China. In our analytical framework, the baseline maximal aortic diameter was designated as the independent variable of interest, while the dependent variable, central to our study’s focus, was in-hospital mortality.
Patient selection
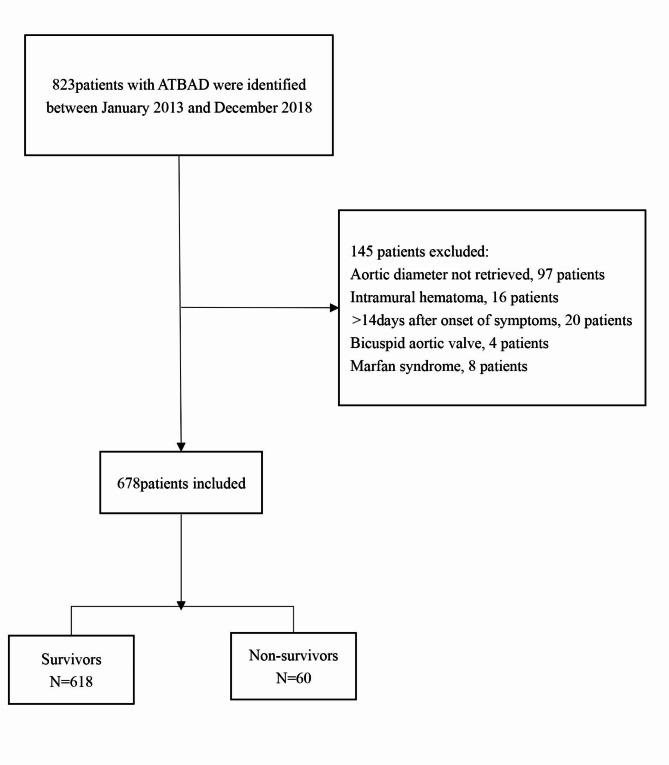
Data were collected from consecutive patients diagnosed with ATBAD at the Second Xiangya Hospital of Central South University, located in Hunan, China, on a non-selective basis. The ethics committee provided permission for this study, and we reviewed the electronic medical records (EMRs) of ATBAD patients to gather the necessary information. In total, 678 patients who were hospitalized and treated in the facility between January 2016 and December 2018 were included in our study. An ATBAD diagnosis was characterized as any dissection excluding the ascending aorta that occurred within 14 days following the onset of symptoms. The diagnosis was confirmed through standard imaging methods, specifically computed tomography angiography. Inclusion criteria consisted of cases diagnosed with ATBAD. Exclusions were made for patients with intramural hematoma, symptoms persisting beyond 14 days, Marfan syndrome, a bicuspid aortic valve, and those without a recorded aortic diameter.
Ethics declarations
The informed consent was waived by the Ethics Committee of the Second Xiangya Hospital, Central South University (Changsha, China) due to the retrospective nature of the study. This research was carried out in line with the principles set forth in the Declaration of Helsinki. Prior to the start of the study, ethical approval was secured from the Ethics Committee of the Second Xiangya Hospital, Central South University (Changsha, China, No. 2022-096). All procedures complied with recognized guidelines and regulations.
Variables
Present data on aortic diameters in ATBAD patients rely on computed tomography angiography and obtained in the first diagnostic CT study. Aortic cross-sections perpendicular to the long axis of the aorta were used to measure the maximum diameter. The maximal aortic diameter including the area affected by the aortic dissection. The aortic diameter measurement method used in this study was previously published by Sun et al.7,8. The computed tomography angiography (CTA) images, with a slice thickness of 1.5 mm, were meticulously post-processed using the 3mensio Vascular software suite, version 10.0, developed in Maastricht, Netherlands. Arterial structures often display an irregularly round configuration. The largest diameter is used as the reference measure when the difference between the maximum and minimum diameters, expressed as a percentage of the minimum diameter, is less than 5%. However, if this ratio exceeds 5%, the average diameter is adopted as the reference metric9. Finally, we chose the largest value as the maximum aortic diameter. CTA scans performed for all subjects in this study were performed using Siemens Healthcare Somatom Definition Flash, a second-generation dual-source computed tomography scanner located in Erlangen, Germany. In-hospital mortality was defined as death due to various causes during hospitalization. Covariates included patient biochemical characteristics, demographics, imaging studies, and treatment factors, which might affect maximum aortic diameter or in-hospital death. a perfectly fitted model would contain the following continuous variables at baseline based on this list including patient age, time to presentation, body mass index (BMI), diastolic and systolic blood pressure (DBP, SBP), white blood cell (WBC), platelet (PLT), neutrophil (NE), hemoglobin (Hb), aspartate alanine transaminase (ALT), aminotransferase (AST), albumin (ALB), direct bilirubin (DB), total bilirubin (TB), blood urea nitrogen (BUN), uric acid (UA), creatinine (Cr), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), D-dimer, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ejection fraction (EF). Regarding the categorical variables of the model were sex, hypertension, diabetes, stroke, atherosclerosis (means that the patient had history of coronary atherosclerotic heart disease and/or carotid plaque), chronic renal insufficiency (CRI), smoking status, aortic regurgitation, symptom, abdominal vessel involvement, and management. Multiple imputation was used as modern statistical approaches for handling missing data following Rubin’s rules. We dropped variables that have more than 5% missingness based on possible analytical bias10.
Statistical analysis
In this study, we expressed percentages for categorical variables, while mean ± SD or upper and lower quartiles (25th, 75th) were used for continuous variables. Statistical tests included analysis of variance (ANOVA), Kruskal Wallis H test, or chi-square test. We divided the patients into three groups (T1, T2 and T3) according to the value of maximal aortic diameter (Tertile). This grouping is automatically generated by statistical software, and the different number of people in each group may be due to the different fineness and dispersion of the data. Univariate and multivariate regression (linear) models were used to detect the correlation between maximal aortic diameter and in-hospital death. To capture non-linearities in maximal aortic diameter values and in-hospital mortality, a penalized nonlinear term is proposed for the generalized additive model (GAM). To identify the point of inflection, a detailed calculation was conducted, followed by the development of a linear two-piece regression model using an advanced recursive algorithm. The model’s optimal fit was rigorously assessed through the likelihood log-ratio test to ensure predictive accuracy. Additionally, hierarchical linear regression models were carefully employed for subgroup analyses. Survival curves were generated using the Kaplan-Meier method, and their equivalence was thoroughly compared with the log-rank test. For these statistical analyses, we utilized EmpowerStats by X&Y Inc Solutions, Boston, MA, alongside the R programming environment. Statistical significance was stringently determined, with significance set at a threshold of p < 0.05, based on a two-tailed test.
Results
Characteristics data of all patients
In accordance with the predefined inclusion and exclusion criteria, our study enrolled a total of 678 participants, as depicted in Fig. 1. Table 1 provides an overview of the baseline tertile maximal aortic diameter values for this patient cohort.82.45% of the sample were male participants and the average participant age was 56.03 ± 12.22 years. Relatively higher baseline values of age, BUN, aortic regurgitation, and mortality were found in participants from the uppermost group of admission maximal aortic diameter (T3). This was also noted for sex (male) in the T2 group. Statistical significance was not found for BMI, hypertension, diabetes, stroke, atherosclerosis, CRI, smoking status, time to presentation, SBP, DPB, WBC, NE, PLT, Hb, ALT, ALB, AST, TB, DB, UA, Cr, TG, TC, LDL, HDL, D-Dimer, CRP, ESR, EF value, abdominal vessel involvement, symptom, and management among the aortic diameter groups (p = > 0.05). The number of deaths between different treatment groups were showed in sTable 1.
Fig. 1.
Flow chart of patient enrollment.
Table 1.
Basline characteristics of the patients (N = 678).
| Characteristic | Maximal aortic diameter(mm) (Tertile) | P-value | ||
|---|---|---|---|---|
| T1 (22–31) | T2 (32–35) | T3 (36–83) | ||
| No. of patients | 221 | 191 | 266 | |
| Age (years) | 55.95 ± 13.14 | 54.39 ± 11.22 | 57.29 ± 12.01 | 0.043 |
| Gender (male) | 172 (77.83%) | 168 (87.96%) | 219 (82.33%) | 0.026 |
| BMI (kg/m2) | 24.53 ± 3.97 | 25.14 ± 4.15 | 24.34 ± 4.23 | 0.110 |
| Hypertension | 146 (66.06%) | 139 (72.77%) | 195 (73.31%) | 0.168 |
| Diabetes | 10 (4.52%) | 6 (3.14%) | 13 (4.89%) | 0.645 |
| Stroke | 11 (4.98%) | 10 (5.24%) | 13 (4.89%) | 0.985 |
| Atherosclerosis | 21 (9.50%) | 14 (7.33%) | 35 (13.16%) | 0.115 |
| CRI | 6 (2.71%) | 6 (3.14%) | 5 (1.88%) | 0.676 |
| Smoking | 79 (35.75%) | 72 (37.70%) | 101 (37.97%) | 0.866 |
| Time to presentation (h, median (Q1–Q3)) | 27.00 (15.00-168.00) | 24.00 (14.50–144.00) | 48.00 (14.00-168.00) | 0.087 |
| SBP (mmHg) | 148.25 ± 27.24 | 147.74 ± 24.25 | 145.15 ± 27.20 | 0.380 |
| DBP (mmHg) | 84.24 ± 15.74 | 84.97 ± 15.56 | 83.90 ± 15.65 | 0.768 |
| WBC(×109/L) | 10.21 ± 3.66 | 10.34 ± 3.85 | 9.80 ± 3.34 | 0.242 |
| NE (%) | 79.45 ± 9.91 | 78.31 ± 11.24 | 78.10 ± 9.59 | 0.313 |
| PLT(×109/L) | 197.91 ± 65.74 | 196.89 ± 75.19 | 203.11 ± 90.52 | 0.653 |
| Hb (g/L) | 125.59 ± 21.50 | 129.64 ± 19.22 | 125.18 ± 20.17 | 0.073 |
| ALT (u/L) (median (Q1–Q3)) | 19.90 (13.40–33.70) | 18.90 (12.90–31.10) | 18.35 (13.50-31.65) | 0.056 |
| AST(u/L) (median (Q1–Q3)) | 19.60 (15.60–27.30) | 19.60 (15.40–26.40) | 20.00 (15.80–28.60) | 0.238 |
| ALB (g/L) | 35.91 ± 5.08 | 36.55 ± 5.25 | 35.14 ± 5.44 | 0.052 |
| TB (μmol/L) | 15.37 ± 10.32 | 15.41 ± 9.03 | 16.06 ± 12.69 | 0.740 |
| DB (μmol/L) | 5.86 ± 6.48 | 5.77 ± 4.48 | 6.34 ± 6.69 | 0.542 |
| Cr (μmol/L) (median (Q1–Q3)) | 76.20 (61.20-102.20) | 80.60 (65.45–97.70) | 79.00 (62.65–96.33) | 0.408 |
| BUN (mmol/L) (median (Q1–Q3)) | 5.90 (4.70–7.64) | 6.03 (4.95–7.57) | 6.95 (5.21–9.24) | 0.011 |
| UA (μmol/L) | 309.94 ± 114.10 | 329.92 ± 114.46 | 311.48 ± 111.33 | 0.140 |
| TG (mmol/L) | 1.59 ± 1.18 | 1.89 ± 2.19 | 1.53 ± 0.86 | 0.589 |
| TC (mmol/L) | 4.22 ± 1.02 | 4.26 ± 1.15 | 4.09 ± 0.94 | 0.169 |
| HDL (mmol/L) | 1.17 ± 0.30 | 1.16 ± 0.33 | 1.21 ± 1.30 | 0.828 |
| LDL (mmol/L) | 2.35 ± 0.90 | 2.31 ± 0.95 | 2.23 ± 0.98 | 0.378 |
| D-dimer (ug/mL) (median(Q1–Q3)) | 2.94 (1.33–5.76) | 3.13 (1.78–6.82) | 3.20 (1.82–7.20) | 0.098 |
| CRP (mg/L) (median (Q1–Q3)) | 49.80 (9.44–112.00) | 33.90 (9.72–111.50) | 41.50 (9.57-109.75) | 0.854 |
| ESR (mm/h) | 38.19 ± 27.58 | 37.16 ± 32.85 | 38.29 ± 29.99 | 0.914 |
| EF value (%) | 63.62 ± 5.52 | 61.88 ± 8.48 | 62.87 ± 8.34 | 0.068 |
| Aortic regurgitation | 44 (19.91%) | 63 (32.98%) | 106 (39.85%) | < 0.001 |
| Abdominal vessel involvement | 77 (34.84%) | 62 (32.46%) | 88 (33.08%) | 0.864 |
| Symptom | 0.216 | |||
| Chest pain | 160 (72.40%) | 137 (71.73%) | 172 (64.66%) | |
| Back pain | 16 (7.24%) | 11 (5.76%) | 22 (8.27%) | |
| Abdominal pain | 22 (9.95%) | 14 (7.33%) | 20 (7.52%) | |
| Syncope | 2 (0.90%) | 2 (1.05%) | 2 (0.75%) | |
| Others | 21 (9.50%) | 27 (14.14%) | 50 (18.80%) | |
| Management | 0.158 | |||
| Medical | 39 (17.65%) | 40 (20.94%) | 71 (26.69%) | |
| Endovascular | 174 (78.73%) | 146 (76.44%) | 185 (69.55%) | |
| Surgical | 8 (3.62%) | 5 (2.62%) | 10 (3.76%) | |
| Mortality | 0.003 | |||
| Survivor | 211 (95.48%) | 176 (92.15%) | 231 (86.84%) | |
| Non-survivor | 10 (4.52%) | 15 (7.85%) | 35 (13.16%) | |
BMI body mass index, CRI chronic renal insufficiency, SBP systolic blood pressure, DBP diastole blood pressure, WBC white blood cell, NE neutrophil, PLT platelet, Hb hemoglobin, ALT alanine transaminase, AST aspartate aminotransferase, ALB albumin, TB total bilirubin, DB direct bilirubin, Cr creatinine, BUN blood urea nitrogen, UA uric acid, TG triglyceride, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, CRP C-reactive protein, ESR erythrocyte sedimentation rate, EF ejection fraction.
Univariate predictors of in-hospital mortality
The univariate analyses in Table 2 revealed that age, BMI, gender, hypertension, stroke, diabetes, atherosclerosis, CRI, smoking, time to presentation, DBP, SBP, WBC, NE, PLT, AST, ALB, DB, BUN, UA, HDL, TC, TG, CRP, D-Dimer, ESR, EF value, aortic regurgitation, and abdominal vessel involvement did not contribute to the outcome variable. Nevertheless, the analysis revealed significant correlations between several variables and the outcome variable. Specifically, ALT, Cr, aortic diameter, and symptom presence demonstrated a positive correlation with the outcome variable. Conversely, Hb, TB, LDL, and endovascular management were found to exhibit a negative correlation.
Table 2.
Univariate analysis for in-hospital mortality.
| Statistics | OR(95%CI) | P-value | |
|---|---|---|---|
| Age (years) | 56.03 ± 12.22 | 0.98 (0.96, 1.01) | 0.157 |
| Gender (male) | 559 (82.45%) | 2.49 (0.97, 6.35) | 0.057 |
| BMI | 24.63 ± 4.13 | 0.96 (0.90, 1.03) | 0.257 |
| Hypertension | 480 (70.80%) | 0.69 (0.40, 1.20) | 0.185 |
| Diabetes | 29 (4.28%) | 0.36 (0.05, 2.67) | 0.316 |
| Stroke | 34 (5.01%) | 2.34 (0.93, 5.90) | 0.071 |
| Atherosclerosis | 70 (10.32%) | 0.96 (0.40, 2.32) | 0.931 |
| CRI | 17 (2.51%) | 2.27 (0.63, 8.14) | 0.208 |
| Smoking | 252 (37.17%) | 1.14 (0.66, 1.96) | 0.635 |
| Time to presentation (h, median (Q1–Q3)) | 26.50 (15.00-168.00) | 1.00 (1.00, 1.00) | 0.706 |
| SBP (mmHg) | 146.89 ± 26.41 | 1.00 (0.99, 1.01) | 0.900 |
| DBP (mmHg) | 84.32 ± 15.63 | 1.00 (0.98, 1.02) | 0.938 |
| WBC (×109/L) | 10.08 ± 3.59 | 1.01 (0.94, 1.09) | 0.787 |
| NE (%) | 78.60 ± 10.18 | 1.01 (0.98, 1.03) | 0.711 |
| PLT(×109/L) | 199.66 ± 78.78 | 1.00 (1.00, 1.00) | 0.515 |
| Hb (g/L) | 126.57 ± 20.42 | 0.98 (0.97, 1.00) | 0.008 |
| ALT (u/L) (median (Q1–Q3)) | 18.80 (13.30-32.38) | 1.00 (1.00, 1.00) | 0.017 |
| AST (u/L) (median (Q1–Q3)) | 19.70 (15.50–27.70) | 1.00 (1.00, 1.00) | 0.102 |
| ALB (g/L) | 35.79 ± 5.30 | 0.99 (0.94, 1.04) | 0.619 |
| TB (μmol/L) | 15.65 ± 10.98 | 0.96 (0.92, 1.00) | 0.037 |
| DB (μmol/L) | 6.02 ± 6.07 | 0.93 (0.84, 1.02) | 0.101 |
| Cr (μmol/L) (median (Q1–Q3)) | 78.00 (62.80–98.90) | 1.00 (1.00, 1.00) | 0.005 |
| BUN (mmol/L) (median (Q1–Q3)) | 6.29 (4.92–8.23) | 1.01 (0.99, 1.02) | 0.328 |
| UA (μmol/L) | 316.17 ± 113.29 | 1.00 (1.00, 1.00) | 0.434 |
| TG (mmol/L) | 1.65 ± 1.45 | 1.11 (0.97, 1.27) | 0.120 |
| TC (mmol/L) | 4.18 ± 1.03 | 0.82 (0.62, 1.07) | 0.149 |
| HDL (mmol/L) | 1.18 ± 0.85 | 0.45 (0.18, 1.12) | 0.086 |
| LDL (mmol/L) | 2.29 ± 0.94 | 0.62 (0.45, 0.86) | 0.004 |
| D-dimer (ug/mL) (median (Q1–Q3)) | 3.10 (1.66–6.46) | 1.02 (0.99, 1.05) | 0.139 |
| CRP (mg/L) (median (Q1–Q3)) | 41.50 (9.57–111.00) | 1.00 (0.99, 1.00) | 0.165 |
| ESR (mm/h) | 37.94 ± 30.04 | 1.00 (1.00, 1.01) | 0.421 |
| EF value (%) | 62.83 ± 7.60 | 1.01 (0.97, 1.04) | 0.777 |
| Aortic regurgitation | 213 (31.42%) | 0.71 (0.38, 1.30) | 0.264 |
| Abdominal vessel involvement | 227 (33.48%) | 1.47 (0.86, 2.52) | 0.161 |
| Maximal aortic diameter(mm) | 35.40 ± 7.90 | 1.04 (1.02, 1.07) | 0.001 |
| Symptom | |||
| Chest pain | 469 (69.17%) | 1.0 | |
| Back pain | 49 (7.23%) | 2.44 (1.01, 5.89) | 0.047 |
| Abdominal pain | 56 (8.26%) | 2.44 (1.06, 5.62) | 0.036 |
| Syncope | 6 (0.88%) | 7.32 (1.29, 41.57) | 0.025 |
| Others | 98 (14.45%) | 2.24 (1.12, 4.47) | 0.022 |
| Management | |||
| Medical | 150 (22.12%) | 1.0 | |
| Endovascular | 505 (74.48%) | 0.12 (0.06, 0.21) | < 0.001 |
| Surgical | 23 (3.39%) | 0.44 (0.12, 1.57) | 0.207 |
BMI body mass index, CRI chronic renal insufficiency, SBP systolic blood pressure, DBP diastole blood pressure, WBC, white blood cell, NE neutrophil, PLT platelet, Hb hemoglobin, ALT alanine transaminase, AST aspartate aminotransferase, ALB albumin, TB total bilirubin, DB direct bilirubin, Cr creatinine, BUN blood urea nitrogen, UA uric acid, TG triglyceride, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, CRP C-reactive protein, ESR erythrocyte sedimentation rate, EF ejection fraction.
Unadjusted and adjusted model results
The effect of maximal aortic diameter on mortality rates during hospitalization was inferred using three distinct models after accounting for potential confounders. Table 3 displays the relevant effect sizes (odds ratios) and their corresponding 95% confidence intervals. In both the unadjusted model (crude model) and Model I (which considered age, gender, and BMI as adjusted covariates), each 1 mm increase in maximal aortic diameter was associated with a 4% rise in in-hospital mortality, with odds ratios and 95% confidence intervals recorded as (1.04, 95% CI 1.02–1.07) and (1.04, 95% CI 1.01–1.07), respectively. Model II, the fully adjusted model, indicated that a similar 1 mm increase in maximal aortic diameter led to a 6% increase in the likelihood of in-hospital death (1.06, 95% CI 1.03–1.10). Furthermore, upon categorizing the maximum aortic diameter from a continuous metric to a categorical one (tertile), diverse trends in effect values among the various groups were observed, implying a potentially non-linear association between maximal aortic diameter and in-hospital death.
Table 3.
Relationship between maximal aortic diameter and in-hospital mortality in different models.
| Exposure | Crude model (OR, 95% CI, P) | Model I (OR, 95% CI, P) | Model II (OR, 95% CI, P) |
|---|---|---|---|
| Maximal aortic diameter (mm) | 1.04 (1.02, 1.07) 0.001 | 1.04 (1.01, 1.07) 0.005 | 1.06 (1.03, 1.10) 0.001 |
| Maximal aortic diameter (mm) (tertile) | |||
| T1 | Ref | Ref | Ref |
| T2 | 1.80 (0.79, 4.10) 0.163 | 1.68 (0.73, 3.86) 0.219 | 2.39 (0.83, 6.89) 0.108 |
| T3 | 3.20 (1.55, 6.61) 0.002 | 3.14 (1.51, 6.54) 0.002 | 4.44 (1.71, 11.55) 0.002 |
| P for trend | 0.001 | 0.001 | 0.002 |
Crude Model adjusted for none.
Model I adjusted for age, gender, BMI.
Model II adjusted for Model I and hypertension, diabetes, stroke, atherosclerosis, CRI, smoking, time to presentation, SBP, DBP, WBC, NE, PLT, Hb, ALT, AST, ALB, TB, DB, Cr, BUN, UA, TG, TC, HDL, LDL, D-Dimer, CRP, ESR, EF value, aortic regurgitation, abdominal vessel involvement, symptom, and management.
Nonlinearity results between maximal aortic diameter and in-hospital mortality
Based on smoothed curves adjusted for covariates, a J-curve association of maximum aortic diameter with in-hospital mortality was determined (Table 4; Fig. 2). The results were obtained using a linear regression model or a binomial linear regression model, p value less than 0.05 was used for the log-likelihood ratio test. Consequently, a bipartite linear regression was deemed the most suitable approach for determining the possible relationship between maximal aortic diameter and mortality during hospitalization. Utilizing the findings from the recursive algorithm along with the two-segment linear regression analysis, the predicted inflection point was determined to be 31 mm. The effect size and 95% confidence interval (CI) were found to be 1.03 and range from 0.83 to 1.28, respectively, for cases where the maximal aortic diameter was ≤ 31 mm. Conversely, for instances where the maximal aortic diameter exceeded 31 mm, the effect size and 95% CI were recorded as 1.06 and between 1.02 and 1.11, respectively.
Table 4.
The results of the two-piecewise linear model.
| Mortality (OR, 95% CI) | P-value | |
|---|---|---|
| Fitting model by standard linear regression | 1.06 (1.03, 1.10) | 0.001 |
| Fitting model by two-piecewise linear regression | ||
| Inflection point of maximal aortic diameter (mm) | 31 | |
| ≤ 31 | 1.03 (0.83, 1.28) | 0.793 |
| > 31 | 1.06 (1.02, 1.11) | 0.001 |
| P for log-likelihood ratio test | 0.047 | |
Adjusted: age, gender, BMI, hypertension, diabetes, stroke, atherosclerosis, CRI, smoking, time to presentation, SBP, DBP, WBC, NE, PLT, Hb, ALT, AST, ALB, TB, DB, Cr, BUN, UA, TG, TC, HDL, LDL, D-Dimer, CRP, ESR, EF value, aortic regurgitation, abdominal vessel involvement, symptom, and management.
Fig. 2.
Association maximal aortic diameter and in-hospital mortality. A non-linear association between aortic diameter and in-hospital mortality was found (P < 0.001) in a generalized additive model (GAM). The red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. All adjusted for age, gender, BMI, hypertension, diabetes, stroke, atherosclerosis, CRI, smoking, time to presentation, SBP, DBP, WBC, NE, PLT, Hb, ALT, AST, ALB, TB, DB, Cr, BUN, UA, TG, TC, HDL, LDL, D-Dimer, CRP, ESR, EF value, aortic regurgitation, abdominal vessel involvement, symptom, and management.
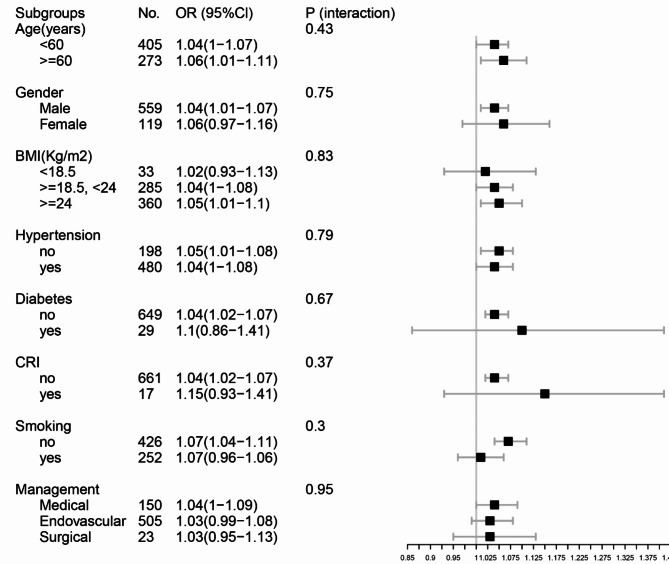
Subgroup analysis
Participant data such as age, gender, BMI, hypertension, diabetes, CRI, smoking status, and treatment status were stratification variables used to determine the evolution of the corresponding effect size (Fig. 3). The stability of the relationship between maximal aortic diameter and in-hospital mortality among patients with ATBAD was affirmed through subgroup analysis. Our findings revealed no statistically significant differences in this relationship across the various subgroups examined. In addition, considering whether surgery had a strong influence on outcome variables, we performed smooth curve fitting in the surgical and non-surgical groups and found that the relationship between the maximum aortic diameter and in-hospital mortality tended to be consistent across groups (sFigure 1).
Fig. 3.
Results of subgroup analysis and interaction analysis. BMI body mass index, CRI chronic renal insufficiency.
Survival curve analysis
Figure 4 displays a Kaplan-Meier survival curve, demonstrating the cumulative in-hospital survival rates among ATBAD patients, categorized by maximal aortic diameter. Significantly, the curve reveals that patients with a maximal aortic diameter of 31 mm or less experience a markedly better prognosis, with a p-value of less than 0.05.
Fig. 4.
Survival curve analysis in the chronological trend in mortality after ATBAD.
Discussion
Our examination of the data reveals that in the completely adjusted model, a rise in maximal aortic diameter exceeding 31 mm correlated with a heightened risk of in-hospital mortality. Specifically, the model-based effect sizes suggest that a 1 mm increase in maximal aortic diameter resulted in a 6% rise in in-hospital mortality. Conversely, when the maximal aortic diameter was 31 mm or less, this association was not observed [1.03 (95% CI 0.83–1.28), p = 0.793]. Additionally, the relationship between maximal aortic diameter and in-hospital death was found to resemble a J-shaped curve.
Despite broad data evidence of this correlation signal, the mechanisms leading to its formation remain unclear. As stated in the Stanford classification, ATBAD refers to the tearing of the aorta’s inner layer, which permits blood to infiltrate the aortic wall, consequently splitting the layers into true and false lumens, while excluding the ascending aorta11. Previous studies demonstrated that aortic diameter in AD patients is usually significantly widened compared with the normal population, which may be associated with decreased elastin and elastic fibers, abnormal collagen content, increased inflammatory factors, and oxidative stress in the aortic media12. Moreover, heightened pressures within the aortic false lumen may induce stress in the vessel wall, thereby raising the likelihood of aneurysm expansion and rupture13. In our research, we discovered that among patients diagnosed with ATBAD, there was a direct correlation between aortic diameter and in-hospital mortality, particularly when the maximum aortic diameter exceeded 31 mm upon admission, aligning with findings from prior investigations. Existing literature indicates that the size of the aortic aneurysm is directly linked to mortality risk associated with aortic rupture and dissection, especially when the maximum aortic diameter reaches 40 mm or more14.
Research conducted by Wen et al.15. indicated that in patients suffering from acute aortic dissection, the size of the aorta was a significant risk factor and was independently linked to in-hospital mortality. Their findings revealed that an aortic diameter of ≥ 47 mm provided a higher specificity for predicting in-hospital death among AD patients. Nevertheless, information on the type of aortic dissection and the treatments utilized, such as whether surgery was performed, was not available, these factors are critical in assessing the prognosis of aortic dissection. In a separate study focusing on patients with Type A aortic dissection, Trimarchi et al.16. reported that the in-hospital mortality rates for individuals with an aortic diameter less than 55 mm were 6.6%, while those with a diameter of 55 mm or greater experienced a mortality rate of 23.0% (P < 0.001). Furthermore, Akihito Matsushita et al.17. proposed that an initial aortic diameter exceeding 40 mm was a primary predictor of adverse outcomes following uncomplicated Type A aortic dissection, suggesting that these patients might benefit from prompt endovascular intervention. However, it should be noted that these studies did not incorporate nonlinear or stratified analysis, and the sample size of ATBAD patients was relatively small. The findings from this research demonstrate a J-shaped curve and a threshold effect concerning the relationship between maximum aortic diameter and in-hospital mortality in this particular patients’ demographic.
To the best of our knowledge, these findings can be viewed as the first observation of a J-curve relationship between maximal aortic diameter and in-hospital death in patients with ATBAD. At the conclusion of this research, we expect to refine predictive models that will improve the accuracy of estimating in-hospital mortality rates among patients with ATBAD. This advancement is set to make a substantial contribution to future medical prognostication. Our work has the following advantages over the previous studies. Initially, this study was designed with a substantial sample size, allowing for a thorough evaluation of outcomes in comparison to previous research. A total of 678 consecutive patients diagnosed with ATBAD were enrolled, enhancing the generalizability of our findings. Furthermore, we introduced an innovative nonlinear model, offering a deeper exploration of the subject. The relationship between maximal aortic diameter and in-hospital mortality was represented by a J-shaped curve, providing a more nuanced understanding of this clinical phenomenon. Additionally, the maximal aortic diameter was analyzed both as a categorical and continuous variable, a methodological approach that strengthens the robustness of our conclusions and reduces the impact of random variability in subsequent data analyses. Finally, we performed a subgroup analysis by different study characteristics and population features. It markedly increased the reliability and accuracy of the conclusion.
Although this work provided valuable information, it still suffered from some limitations. First, our study’s conclusions are derived from a Chinese population and may not be directly applicable to other populations with different genetic backgrounds or anthropometric characteristics. It is important to consider indexing the aortic diameter to metrics such as body surface area (BSA) or height to account for inter-individual variations. Future studies should explore the applicability of these findings across diverse populations. Second, false lumen thrombosis is a critical prognostic factor in patients with ATBAD, however, our study did not assess the status of false lumen thrombosis. Prospective studies with detailed and consistent data are needed. One additional limitation of this study is the unavailability of data on blood pressure management prior to the event, which has been shown to impact aortic diameter measurements. As a result, it is possible that the highest recorded aortic diameter levels were affected by antihypertensive medications, potentially influencing the conclusions of the research.
Conclusions
Altogether, our results pointed out that a J-curve relationship exists between maximal aortic diameter and in-hospital death. In addition, a correlation between maximal aortic diameter and in-hospital mortality is positive when the maximal aortic diameter exceeds 31 mm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Natural Science Foundation of Hunan Province (No. 2023JJ40867), Hunan Health and Family Planning Commission Project (No. B202310007057 and No. 202214053517), and Natural Science Foundation of Changsha (No. kq2208330). The key Research and Development Program of Hunan Province (2019SK2022) supports this manuscript.
Author contributions
The collection of data was carried out by XT and SQ. Data analysis was performed by XS. The manuscript was drafted by GY and LS, who also gathered the patient information. The final manuscript received approval from all authors.
Data availability
In the present study, the datasets utilized and/or examined can be obtained from the corresponding author upon a reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The informed consent was waived by the Ethics Committee of the Second Xiangya Hospital, Central South University (Changsha, China) due to the retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu, Y. et al. Type a aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J. Am. Coll. Cardiol. 76, 1703–1713 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Evangelista, A. et al. Insights from the International Registry of Acute Aortic dissection: a 20-Year experience of collaborative clinical research. Circulation. 137, 1846–1860 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Tolenaar, J. L. et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 130, S45–S50 (2014). [DOI] [PubMed]
- 4.Bossone, E., LaBounty, T. M. & Eagle, K. A. Acute aortic syndromes: diagnosis and management, an update. Eur. Heart J. 39, 739–749 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Mussa, F. F. et al. Acute aortic dissection and intramural hematoma: a systematic review. JAMA-J. Am. Med. Assoc. 316, 754–763 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Wu, Y. et al. Analysis of ascending aortic diameter and long-term prognosis in patients with ascending aortic dissection. Echocardiogr.-J. Card. 38, 531–539 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Sun, L. et al. Aortic arch type, a novel morphological indicator and the risk for acute type B aortic dissection. Interact. Cardiovasc. Ther. 34, 446–452 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun, L. et al. Aortic geometric alteration associated with acute type B aortic dissection: angulation, tortuosity, and arch type. Front. Physiol. 12, 708651 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, L. et al. Impact of oversizing on the risk of retrograde dissection after TEVAR for acute and chronic type B dissection. J. Endovasc. Ther. 23, 620–625 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Bernhardt, P. W. Model validation and influence diagnostics for regression models with missing covariates. Stat. Med. 37, 1325–1342 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Erbel, R. et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2873–2926 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wu, D., Shen, Y. H., Russell, L., Coselli, J. S. & LeMaire, S. A. Molecular mechanisms of thoracic aortic dissection. J. Surg. Res. 184, 907–924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Munoz, A. et al. False lumen rotational flow and aortic stiffness are associated with aortic growth rate in patients with chronic aortic dissection of the descending aorta: a 4D flow cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Res. 24, 20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aortic Wall Inflammation Predicts. Abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation. 136, 787–797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen, D., Jia, P., Du, X., Dong, J. Z. & Ma, C. S. Value of N-terminal pro-brain natriuretic peptide and aortic diameter in predicting in-hospital mortality in acute aortic dissection. Cytokine. 119, 90–94 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Trimarchi, S. et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J. Thorac. Cardiovasc. Surg. 142, e101–e107 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Matsushita, A., Hattori, T., Tsunoda, Y., Sato, Y. & Mihara, W. Impact of initial aortic diameter and false-lumen area ratio on type B aortic dissection prognosis. Interact. Cardiovasc. Ther. 26, 176–182 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In the present study, the datasets utilized and/or examined can be obtained from the corresponding author upon a reasonable request.