Abstract
The effect of acute treatment with non-esterified fatty acids (NEFA) on transmembrane signalling has been investigated in three different cell lines. In EGFR T17 cells, pretreatment with cis-unsaturated (oleic and palmitoleic acids) NEFA, but not with saturated or trans-unsaturated NEFA, inhibited the epidermal-growth-factor (EGF)-induced increases in cytosolic [Ca2+], membrane potential and Ins(1,4,5)P3 generation. The blocking effect was found to be time- and dose-dependent and rapidly reversible after washout. However, oleic acid treatment did not block either binding of 125I-EGF to its receptor or EGF-induced autophosphorylation of the EGF receptor. The mechanism of action of NEFA could not be attributed to protein kinase C activation, since (i) down-regulation of the enzyme by long-term treatment with phorbol esters did not prevent blockade by oleic acid, and (ii) the effects of acutely administered phorbol ester and oleic acid were additive. In this cell line, signalling at bradykinin and bombesin receptors was also impaired by oleic acid. In A431 cells, oleic acid also blocked signal transduction at the EGF and B2 bradykinin receptors. Finally, in PC12 cells, oleic acid blocked the Ca2+ influx mediated by the activation of B2 bradykinin receptors. In conclusion: (1) NEFA block signal transduction by interfering with receptor-phospholipase C or phospholipase C-substrate interaction without preventing ligand binding; (2) NEFA do not act by a protein kinase C-mediated mechanism; (3) the effect of NEFA is dependent on their configuration rather than hydrophobicity or chain length; (4) this effect is evident in several different cell lines and receptor systems.
Full text
PDF

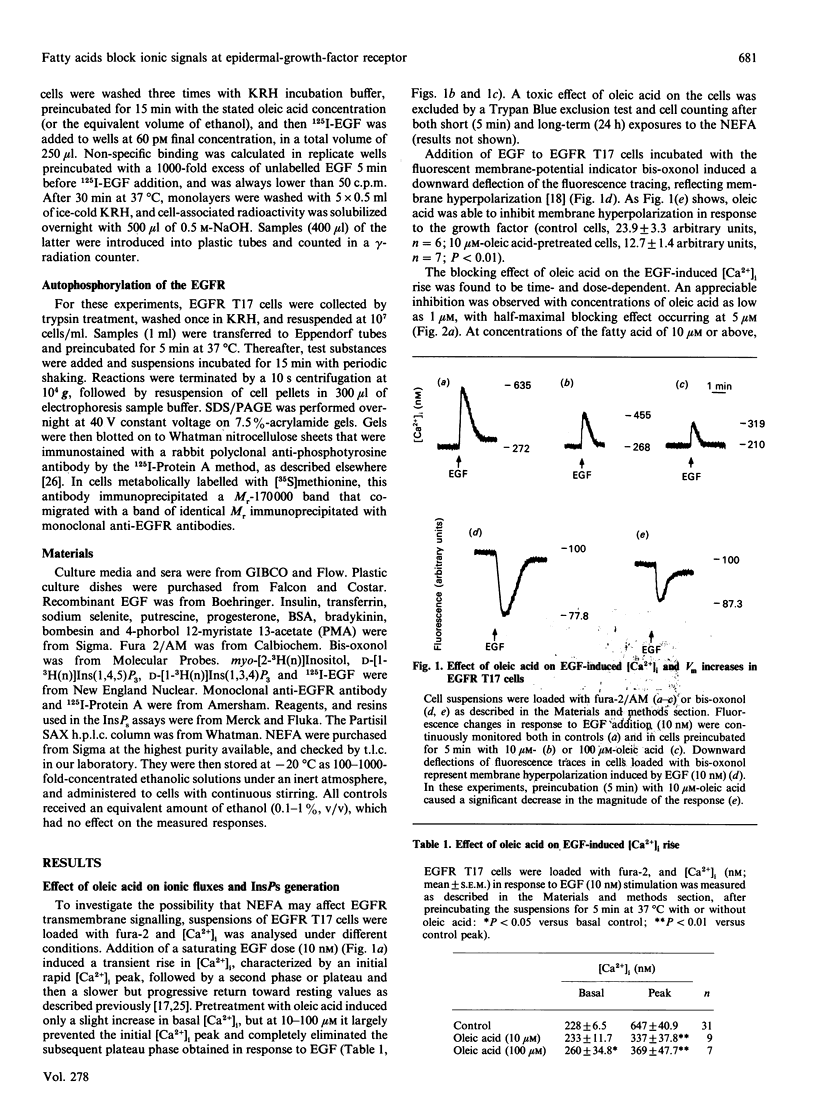
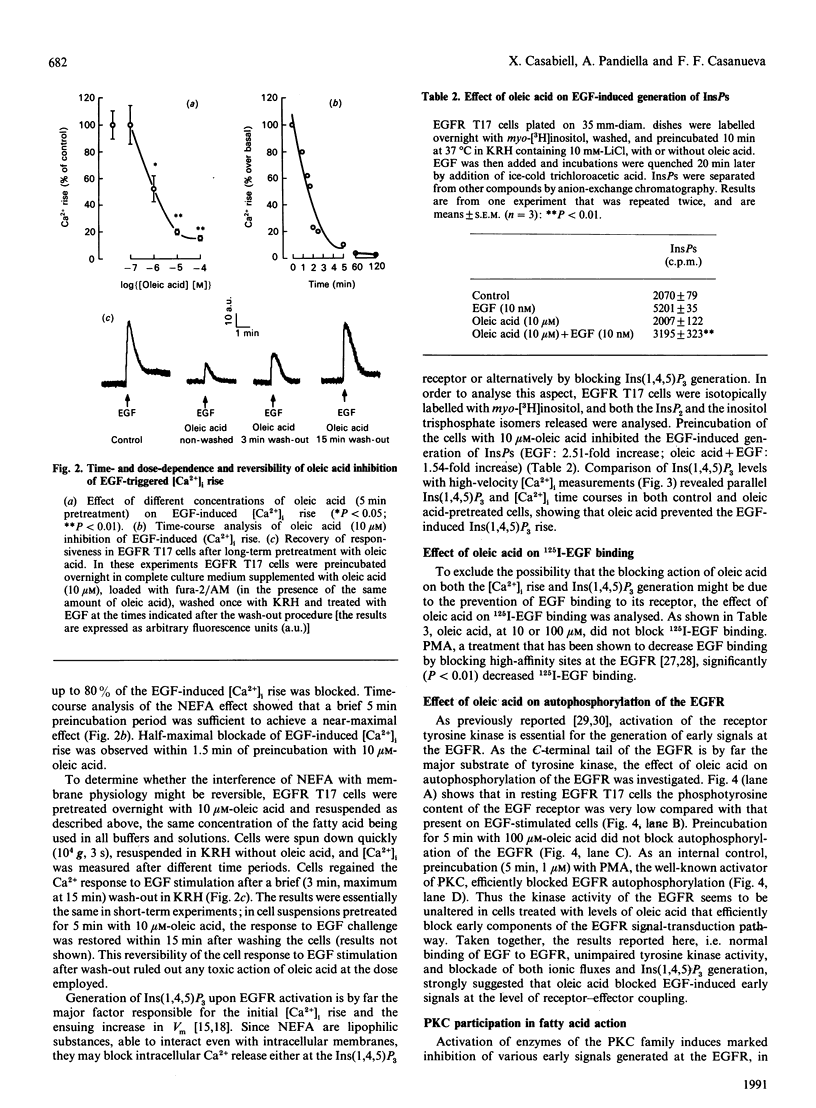
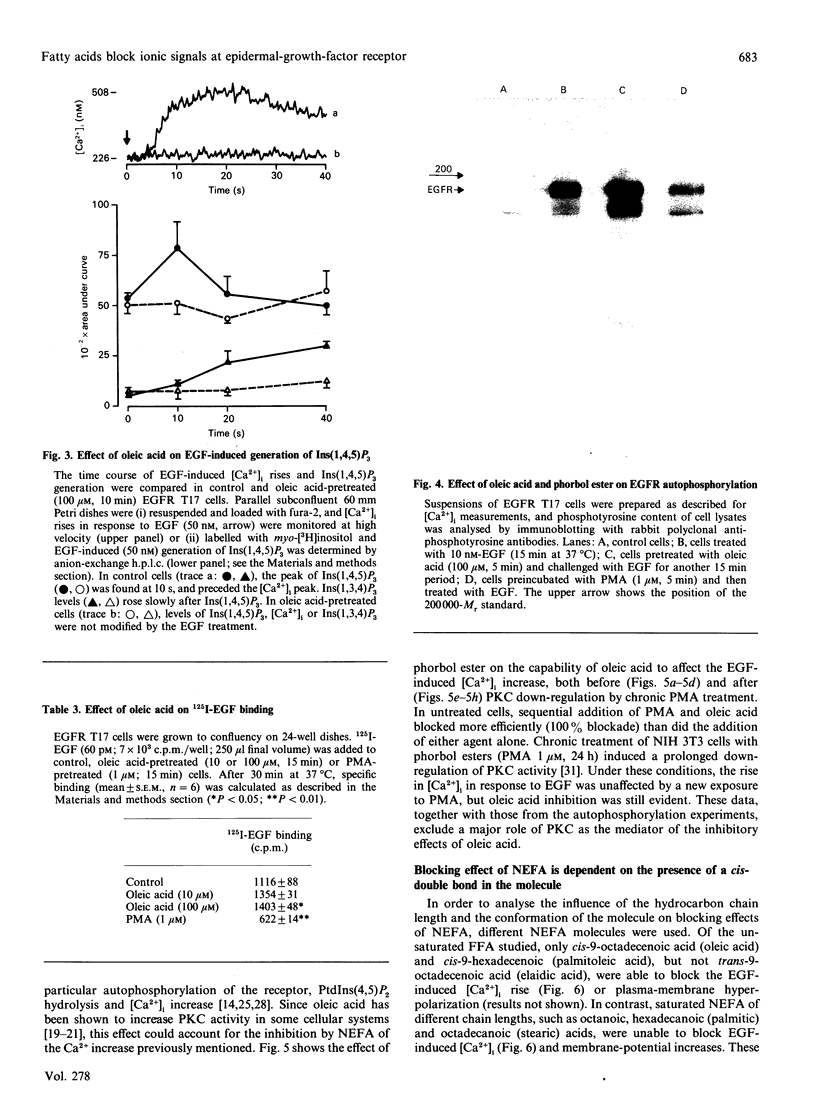



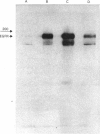
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Casanueva F. F., Villanueva L., Dieguez C., Diaz Y., Cabranes J. A., Szoke B., Scanlon M. F., Schally A. V., Fernandez-Cruz A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated GH secretion in man directly at the pituitary. J Clin Endocrinol Metab. 1987 Oct;65(4):634–642. doi: 10.1210/jcem-65-4-634. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Promotion of human T lymphocyte activation and proliferation by fatty acids in low density and high density lipoproteins. J Biol Chem. 1986 Mar 15;261(8):3620–3627. [PubMed] [Google Scholar]
- Farooqui A. A., Farooqui T., Yates A. J., Horrocks L. A. Regulation of protein kinase C activity by various lipids. Neurochem Res. 1988 Jun;13(6):499–511. doi: 10.1007/BF00973288. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Pandiella A., Meldolesi J., Pozzan T. Generation of inositol phosphates, cytosolic Ca2+, and ionic fluxes in PC12 cells treated with bradykinin. J Biol Chem. 1988 Nov 25;263(33):17350–17359. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gutknecht J. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J Membr Biol. 1988 Nov;106(1):83–93. doi: 10.1007/BF01871769. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Lynch R. D., Karnovsky M. J. Decrease in adhesion of cells cultured in polyunsaturated fatty acids. Cell. 1977 Sep;12(1):295–300. doi: 10.1016/0092-8674(77)90207-0. [DOI] [PubMed] [Google Scholar]
- Hovis J. G., Stumpo D. J., Halsey D. L., Blackshear P. J. Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem. 1986 Aug 5;261(22):10380–10386. [PubMed] [Google Scholar]
- Karnovsky M. J. Lipid domains in biological membranes: their structural and functional perturbation by free fatty acids and the regulation of receptor mobility. Co-presidential address. Am J Pathol. 1979 Nov;97(2):212–221. [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Bhalla D. K., Dragsten P., Hoover R. L., Karnovsky M. J. Model for capping derived from inhibition of surface receptor capping by free fatty acids. Proc Natl Acad Sci U S A. 1980 Jan;77(1):437–441. doi: 10.1073/pnas.77.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Koval M., Pagano R. E. Lipid recycling between the plasma membrane and intracellular compartments: transport and metabolism of fluorescent sphingomyelin analogues in cultured fibroblasts. J Cell Biol. 1989 Jun;108(6):2169–2181. doi: 10.1083/jcb.108.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Pozzan T. Pathways of Ca2+ influx at the plasma membrane: voltage-, receptor-, and second messenger-operated channels. Exp Cell Res. 1987 Aug;171(2):271–283. doi: 10.1016/0014-4827(87)90161-3. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Murphy M. G. Studies of the regulation of basal adenylate cyclase activity by membrane polyunsaturated fatty acids in cultured neuroblastoma. J Neurochem. 1986 Jul;47(1):245–253. doi: 10.1111/j.1471-4159.1986.tb02856.x. [DOI] [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Beguinot L., Velu T. J., Meldolesi J. Transmembrane signalling at epidermal growth factor receptors overexpressed in NIH 3T3 cells. Phosphoinositide hydrolysis, cytosolic Ca2+ increase and alkalinization correlate with epidermal-growth-factor-induced cell proliferation. Biochem J. 1988 Aug 15;254(1):223–228. doi: 10.1042/bj2540223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A., Magni M., Lovisolo D., Meldolesi J. The effect of epidermal growth factor on membrane potential. Rapid hyperpolarization followed by persistent fluctuations. J Biol Chem. 1989 Aug 5;264(22):12914–12921. [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Meldolesi J. Reinforcement of signal generation at B2 bradykinin receptors by insulin, epidermal growth factors, and other growth factors. J Biol Chem. 1989 Feb 25;264(6):3122–3130. [PubMed] [Google Scholar]
- Pjura W. J., Kleinfeld A. M., Karnovsky M. J. Partition of fatty acids and fluorescent fatty acids into membranes. Biochemistry. 1984 Apr 24;23(9):2039–2043. doi: 10.1021/bi00304a024. [DOI] [PubMed] [Google Scholar]
- Portilla D., Mordhorst M., Bertrand W., Morrison A. R. Protein kinase C modulates phospholipase C and increases arachidonic acid release in bradykinin stimulated MDCK cells. Biochem Biophys Res Commun. 1988 May 31;153(1):454–462. doi: 10.1016/s0006-291x(88)81246-4. [DOI] [PubMed] [Google Scholar]
- Renaud G., Bouma M. E., Foliot A., Infante R. Free fatty-acid uptake by isolated rat hepatocytes. Arch Int Physiol Biochim. 1985 Nov;93(4):313–319. doi: 10.3109/13813458509079612. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]
- Williamson J. R., Monck J. R. Hormone effects on cellular Ca2+ fluxes. Annu Rev Physiol. 1989;51:107–124. doi: 10.1146/annurev.ph.51.030189.000543. [DOI] [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Ikeda M., Busto R., Santiso M., Martinez E., Ginsberg M. D. Cerebral phosphoinositide, triacylglycerol, and energy metabolism in reversible ischemia: origin and fate of free fatty acids. J Neurochem. 1986 Sep;47(3):744–757. doi: 10.1111/j.1471-4159.1986.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Zippel R., Sturani E., Toschi L., Naldini L., Alberghina L., Comoglio P. M. In vivo phosphorylation and dephosphorylation of the platelet-derived growth factor receptor studied by immunoblot analysis with phosphotyrosine antibodies. Biochim Biophys Acta. 1986 Mar 19;881(1):54–61. doi: 10.1016/0304-4165(86)90096-6. [DOI] [PubMed] [Google Scholar]