Abstract
Objective.
To determine the relationship between gout flare rate and self-categorization into remission, low disease activity (LDA), and patient acceptable symptom state (PASS).
Methods.
Patients with gout self-categorized as remission, LDA, and PASS, and reported number of flares over the preceding 6 and 12 months. Multinomial logistic regression was used to determine the association between being in each disease state (LDA and PASS were combined) and flare count, and self-reported current flare. A distribution-based approach and extended Youden index identified possible flare count thresholds for each state.
Results.
Investigators from 17 countries recruited 512 participants. Remission was associated with a median recalled flare count of zero over both 6 and 12 months. Each recalled flare reduced the likelihood of self-perceived remission compared with being in higher disease activity than LDA/PASS, by 52% for 6 months and 23% for 12 months, and the likelihood of self-perceived LDA/PASS by 15% and 5% for 6 and 12 months, respectively. A threshold of 0 flares in preceding 6 and 12 months was associated with correct classification of self-perceived remission in 58% and 56% of cases, respectively.
Conclusion.
Flares are significantly associated with perceptions of disease activity in gout, and no flares over the prior 6 or 12 months is necessary for most people to self-categorize as being in remission. However, recalled flare counts alone do not correctly classify all patients into self-categorized disease activity states, suggesting that other factors may also contribute to self-perceived gout disease activity.
Keywords: disease activity, gout, remission
In gout, a cardinal clinical manifestation of the disease is the gout attack or flare1. Typically, gout flares are intensely painful but relatively short-lived and recur to a variable extent. The purpose of therapy for gout is to greatly reduce or entirely prevent these episodes, usually achieved by lowering serum urate levels. Most gout treatment guidelines recommend targeting serum urate to a level below saturation levels2,3,4. Although clinical trials of urate-lowering therapy will often report flare as a secondary outcome of interest, it is a key outcome of interest to patients5 and physicians6. It seems plausible that a patient-centered expression of gout disease activity would be characterized mainly in terms of the frequency of gout flare.
However, one of the problems with using flare frequency as an outcome measure is that it is not known how infrequent flares need to be for patients with gout to consider their disease absent (in remission), at a low level of activity not requiring therapy, or at an acceptable level of activity. Without this information, it is difficult to interpret outcomes expressed in flare rates.
Further, when considering disease states in gout, momentary absence of signs and symptoms is not meaningful, since patients may be asymptomatic between gout flares despite active disease. Flare is considered by physicians to be an important indicator of remission, and there is some suggestion that physicians prefer 6 to 12 months of flare absence as an indicator of remission7. However, the minimum period of being flare-free that qualifies as being in remission is not well defined. It is necessary to determine patients’ opinion about this, in addition to the number of flares over the preferred time period that they perceive as acceptable or not.
Patient-oriented disease targets in other rheumatic diseases include the concepts of remission, low disease activity (LDA), and patient acceptable symptom state (PASS) as important disease states. Remission can be defined as the absence of all signs and symptoms of the disease, with the possibility of symptom recurrence7. LDA has been defined as “a useful target of treatment by both physician and patient, given current treatment possibilities and limitations”8. PASS has been defined as the “value beyond which the patient feels well”9; that is, a low, tolerable level of symptoms for the patient.
It is not yet known what frequency of gout flare would correspond to the disease activity states of remission, LDA, and PASS. Therefore, the objective of this study was to determine the experiences and opinions of patients with gout on the flare rate that corresponds to being in each of the described disease activity states.
MATERIALS AND METHODS
Patients with gout defined by the 2015 American College of Rheumatology (ACR)/European League Against Rheumatism classification criteria were recruited from 17 rheumatology clinics in the Asia Pacific, North America, South America, and Europe in order to validate criteria for the presence of a gout flare10. The study was approved by the coordinating center (University of Alabama at Birmingham, protocol number 151124003) and local institutional review boards at all study sites. All study participants gave signed informed consent and were enrolled regardless of presence or absence of gout flare during the study period. Participants were asked a series of questions about how many flares they had experienced in the previous 6 and 12 months, and whether or not they considered themselves to be currently experiencing a flare. In addition, participants were asked to consider the highest number of flares (over 6 and 12 months) that they felt could occur, while still considering themselves as being in each of the 3 disease activity states (remission, LDA, or PASS).
Participants answered questions that would classify them into none, one, or more, out of the 3 described disease activity states at the current time. A state of remission was defined as an affirmative response to the question, “Considering the number of attacks (flares) that you have had over the last [6 or 12] months, do you think your gout has gone away?” A state of LDA was defined as a negative response to the question, “Considering the number of attacks (flares) that you have had over the last [6 or 12] months, do you think you need more or stronger treatment?” A state of PASS was defined as an affirmative response to the question, “Considering the number of attacks (flares) that you have had over the last [6 or 12] months, would you say that your gout control is currently satisfactory?” Similar wording has been used to anchor this disease activity state in osteoarthritis11. Participants could self-categorize into none, any, or all 3 disease activity states, since each question was asked separately. Initial inspection of the flare count distributions for the LDA and PASS states showed that these appeared very similar. Further, of participants not in remission who self-categorized as being in LDA or PASS, 162/225 (72%) self-categorized as being in both disease states when considering the number of flares over the prior 6 months, and 154/219 (70%) for the prior 12 months. Therefore, the categories of LDA and PASS were combined for subsequent analysis. Participants were grouped into 3 mutually exclusive classes, defined according to self-categorizing as (1) all of remission/LDA/PASS (remission group), (2) LDA or PASS but not in remission (LDA/PASS group), and (3) none of the 3 disease states (high disease activity state). This grouping excluded participants who had self-categorized as being in remission but not in LDA or PASS, since such a combination was logically inconsistent.
SPSS V22 was used for analyses (IBM Corp.). To test the hypothesis that the different types of disease activity state were associated with different flare rates, a multinomial logistic regression model was used to model the influence of flare count and of currently experiencing gout flare upon inclusion in the disease activity class (remission, LDA/PASS, high disease activity). Separate models for considering the previous 6 months and 12 months were developed. The distribution of flare count corresponding to the highest number of flares that participants could have, while still believing that they were in each of 3 disease activity states, was compared using the Friedman 2-way ANOVA by ranks statistic.
Thresholds for the classification as remission, LDA/PASS, and no LDA were identified by inspection of the distribution of flare counts, choosing 0 flares to represent remission, and the 25th percentile of the flare count distribution in those classified as not being in any LDA state as the threshold between LDA/PASS and high disease activity. We also applied a 3-state analysis of the volume under a receiver-operating characteristic (ROC) surface (VUS) and extended Youden index implemented in R to identify the 2 thresholds between high disease activity and LDA/PASS, and between LDA/PASS and remission12. The VUS can be interpreted as the percent correctly classified in the corresponding 3-class structure13. Analogous to the area under a ROC curve for 2-class structures where a value of 0.5 indicates classification by chance, for a 3-class structure a VUS value of 0.33 indicates classification by chance.
RESULTS
Between January 4 and August 31, 2016, we recruited 512 patients with gout from rheumatology clinics in 17 countries. Table 1 shows the main demographic and disease characteristics. The sample was predominantly male, middle aged, and with a long disease duration. Seventy-five percent were on urate-lowering therapies. Not all participants answered every question, leading to some missing data (< 2% of cases excluded from any analysis).
Table 1.
Demographic and disease features.
| Values | |
|---|---|
|
| |
| Age, yrs, mean (SD) | 58 (14) |
| Sex, % male | 455 (89) |
| Ethnicity | |
| White | 281 (55) |
| East Asian | 51 (10) |
| Hispanic | 48 (10) |
| African/Black | 38 (8) |
| NZ Māori | 7 (1) |
| Pacific Islander | 13 (2) |
| South Asian | 7 (1) |
| Other | 64 (13) |
| Disease duration, yrs, mean (SD) | 12 (10) |
| Current gout flare | 157 (31) |
| No. participants self-categorized as remission | |
| 6 mos | 116 (23) |
| 12 mos | 110 (22) |
| No. participants self-categorized as LDA | |
| 6 mos | 224 (44) |
| 12 mos | 232 (46) |
| No. participants self-categorized as PASS | |
| 6 mos | 316 (62) |
| 12 mos | 299 (59) |
| Recalled no. flares, median (IQR) | |
| 6 mos | 2 (0–4) |
| 12 mos | 3 (1–8) |
Values are n (%) unless otherwise specified. LDA: low disease activity; NZ: New Zealand; PASS: patient acceptable symptom state.
Supplementary Figure 1 (available with the online version of this article) illustrates the distribution of recalled flare counts for each disease activity state, considering 6 months (Supplementary Figure 1A) and 12 months (Supplementary Figure 1B) of flares. The categories of LDA and PASS were combined for further analyses as described in the methodology. Thirteen (2.5%) participants self-categorized as in remission but not LDA and PASS when considering flares over the prior 6 months, and 15 (2.9%) over the prior 12 months; these participants were excluded from further analysis, as they provided inconsistent responses.
The recalled flare counts for each disease state group are shown in Table 2. Remission was associated with a median flare count of 0 flares over both 6 and 12 months, and this was different from LDA/PASS (median flare count 1 over 6 months, and 3 over 12 months). Results of the multivariable analysis, evaluating the independent effects of flare count and current flare status in relation to the self-categorized disease states, are shown in Table 3. For each flare over the previous 6 and 12 months, there was a 52% (1 minus the OR of being in remission: 0.48) and 23% (1 minus the OR of remission: 0.77) lower likelihood of self-categorizing as being in remission, respectively. This suggests that more recent flares had a much greater influence on self-categorization as being in remission. Each flare in the prior 6 months was associated with 15% lower odds of self-categorizing as being in LDA/PASS, and for the prior 12 months, with 5% lower odds. This suggests that more recent flares also had a stronger influence on self-categorization as LDA/PASS. In addition, we observed a very strong effect of current flare status, wherein not being in a flare increased the likelihood of a remission state 15-fold. This effect of current flare status was similar in magnitude for the 6- and 12-month time horizon models.
Table 2.
Recalled flare count by disease activity group, over the previous 6 and 12 months of flares.
| Flares in Previous 6 Months* | Flares in Previous 12 Months* | |
|---|---|---|
|
| ||
| Remission | 0 (0–1) | 0 (0–2) |
| LDA/PASS | 1 (0–3) | 3 (1–6) |
| Not in any LDA state | 4 (2–10) | 6 (3–15) |
Values are median (IQR).
Overall test of differences between groups Kruskal-Wallis, P < 0.001.
All posthoc between-group tests also significant at P < 0.001. LDA: low disease activity; PASS: patient acceptable symptom state.
Table 3.
Relation of number of recalled flares and current flare status to self-categorized disease state.
| Disease Activity Category* | OR (95% CI)‡ | P | ||
|---|---|---|---|---|
|
| ||||
| Over the previous 6 months | Remission | No. flares | 0.48 (0.38–0.60) | < 0.001 |
| Current flare absent† | 15.20 (5.58–41.37) | < 0.001 | ||
| LDA/PASS | No. flares | 0.85 (0.79–0.90) | < 0.001 | |
| Current flare absent | 5.74 (3.51–9.37) | < 0.001 | ||
| Over the previous 12 months | Remission | No. flares | 0.77 (0.70–0.85) | < 0.001 |
| Current flare absent† | 15.13 (5.68–40.34) | < 0.001 | ||
| LDA/PASS | No. flares | 0.95 (0.92–0.97) | < 0.001 | |
| Current flare absent | 5.35 (3.33–8.60) | < 0.001 | ||
Reference category: not in any of the 3 LDA states.
Reference category: current flare present.
OR are derived from a multinomial logistic regression model (separate models for 6 months and 12 months), where the dependent variable was disease activity category and the independent variables were flare count and presence/absence of current flare.
LDA: low disease activity; PASS: patient acceptable symptom state.
Recalled flare count alone was not sufficiently accurate to predict self-categorized disease activity class. In the regression models (Table 3), the Nagelkerke pseudo-R2 was 0.43 for 6 months of recalled flares and 0.42 for 12 months of recalled flares. Using a threshold of 0 flares for remission and the 25th percentile of the flare count distribution in those not in any LDA group (Figure 1), Table 4 shows the comparison between classification based on the distribution-based thresholds of flare count (0 for remission, 1–2 or 1–3 for LDA/PASS, > 2 or > 3 for higher disease activity) and self-categorization. This analysis showed that these thresholds correctly predicted self-categorized disease activity state in only 68 + 99 + 115 = 282 of 489 evaluable patients (58%) for the 6-month recalled flare question, and 48 + 93 + 127 = 268 of 481 (56%) for the 12-month recalled flare question.
Figure 1.
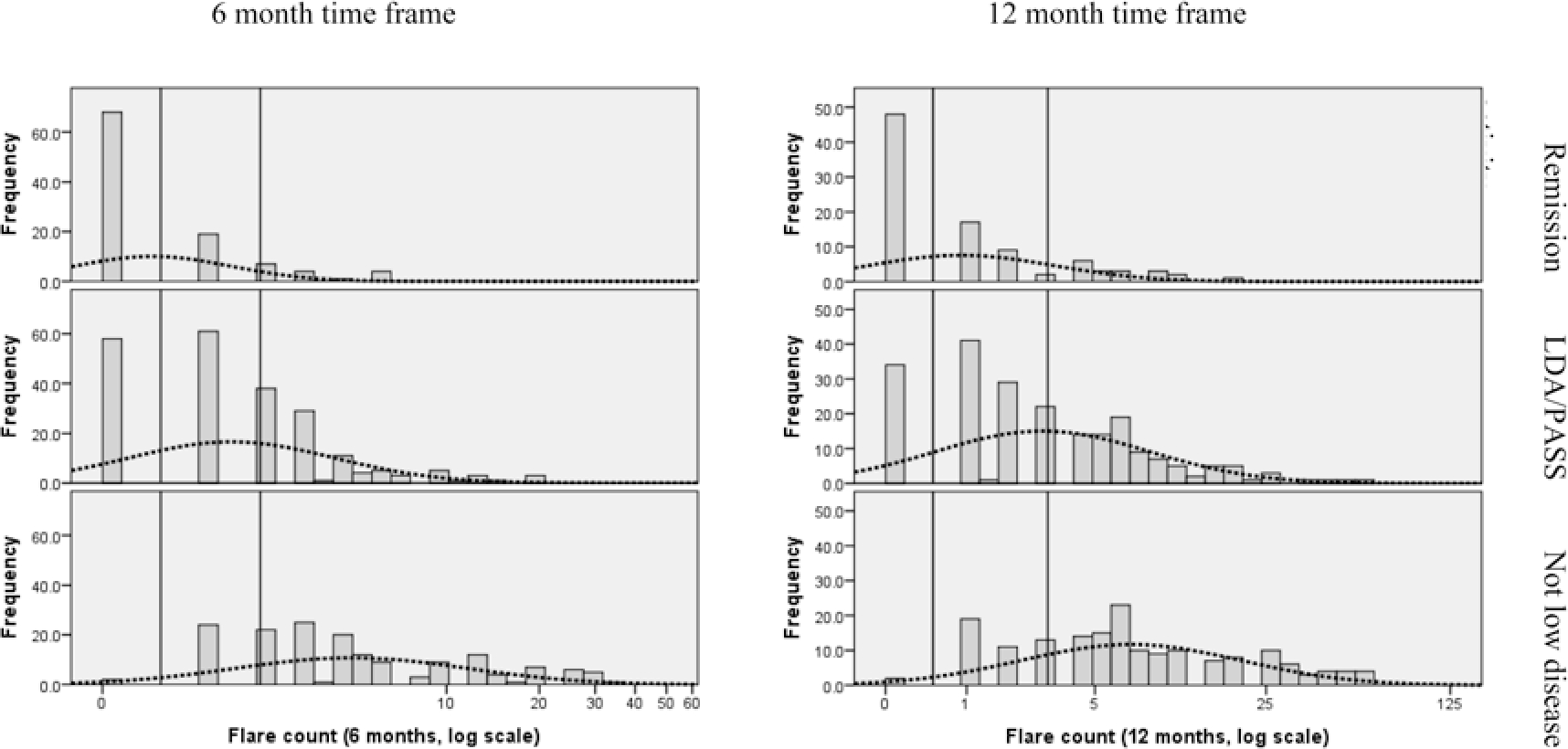
Distribution of flare counts by disease activity state. The dotted line represents the normal distribution of the log-transformed flare count. The vertical lines represent suggested thresholds for remission (0 flares for both 6 and 12 months) and LDA/PASS (1–2 flares for 6 months; 1–3 flares for 12 months). Two thresholds are required to divide the disease activity into 3 states: remission, LDA/PASS, and higher disease activity. LDA: low disease activity; PASS: patient acceptable symptom state.
Table 4.
Classification of disease activity state according to distribution-based thresholds versus observed self-categorization.
| Time Horizon | Classification Based on Distribution-based Thresholds | Remission | LDA/PASS | Not in Any LDA State |
|---|---|---|---|---|
|
| ||||
| 6 months | n = 103 | n = 223 | n = 163 | |
| Flare count = 0 (n = 128) | 68 (53%, 66%) a | 58 | 2 | |
| Flare count = 1–2 (n = 171) | 26 | 99 (58%, 44%) a | 46 | |
| Flare count > 2 (n = 190) | 9 | 66 | 115 (61%, 71%) a | |
| 12 months | n = 94 | n = 215 | n = 172 | |
| Flare count = 0 (n = 84) | 48 (57%, 51%) a | 34 | 2 | |
| Flare count = 1–3 (n = 164) | 28 | 93 (56%, 43%) a | 43 | |
| Flare count > 3 (n = 233) | 18 | 88 | 127 (54%, 74%) a | |
Values show row and then column percentages.
LDA: low disease activity; PASS: patient acceptable symptom state.
In an additional analysis, the VUS for 6-month flare count was 0.41 (95% CI 0.36–0.46), and the thresholds identified from the extended Youden index were 0 flares for the threshold between LDA/PASS and remission, and 3.5 flares for the threshold between LDA/PASS and not LDA/PASS; for 12-month flare count, the VUS was 0.38 (95% CI 0.33–0.43), and thresholds were 0 and 4.5 (data not shown).
In the final analysis, for each time horizon, participants reported the hypothetical maximum number of flares that they would be able to experience over 6 and 12 months and still consider themselves to be in the associated disease activity state. These results are shown in Table 5 and indicate that participants believed very few flares per year were necessary to achieve any of the LDA states. To be in the remission or PASS state, a median of no more than 0 flares over both 6 and 12 months was considered necessary by participants. To be in the LDA state, a median of no more than 1 flare over 6 and 12 months was considered necessary. There was a difference in the acceptable number of flares across the 3 disease states, with all pairwise comparisons statistically significant, suggesting that there was a hierarchy of disease activity: LDA > PASS > remission in this analysis, although the difference between PASS and remission was minimal and probably not clinically relevant.
Table 5.
Hypothetical maximal flare occurrence for each disease state.
| Time Horizon | Disease State | No. Flares, Median (IQR) | P * |
|---|---|---|---|
|
| |||
| No. flares over 6 months | Remission | 0 (0–0) | < 0.001 |
| LDA | 1 (0–2) | ||
| PASS | 0 (0–1) | ||
| No. flares over 12 months | Remission | 0 (0–0) | < 0.001 |
| LDA | 1 (0–2) | ||
| PASS | 0 (0–2) | ||
From related samples Friedman 2-way ANOVA by ranks (all pairwise comparisons were also statistically significant).
LDA: low disease activity; PASS: patient acceptable symptom state.
DISCUSSION
In this large, multinational study of patients with gout, recalled flare rates and especially current flare status were significantly associated with patient perception of disease activity. For each flare per year, the likelihood of self-categorizing as remission reduced by about one-quarter, but flares within the previous 6 months influenced self-perception of current disease activity approximately twice as strongly. Consistent with a prior physician-based study to define remission criteria in gout7, on average 0 flares were associated with self-categorization as being in remission. When patients were asked to describe how many flares would be consistent with a hypothetical remission state over 6 and 12 months, again 0 flares was the consistent answer, lending more support to the argument that this threshold is relevant for patients.
We observed similar classification thresholds using an extended Youden index and a simple inspection of the flare count distribution. However, neither approach led to correct classification in a substantial proportion of cases. The overall accuracy of flare count alone in predicting self-categorized disease activity state was less than 60%, and the VUS was only around 0.4, despite trying to frame the disease status question exclusively around flares, suggesting that other factors are relevant to patients in considering their disease status. These findings are consistent with a previous study of people with gout using a conjoint decision-making approach; the study showed that patients consider other factors such as activity limitations, serum urate, and pain between attacks as important disease outcome measures, in addition to flare frequency5. Similarly, qualitative interviews that informed development of the Outcome Measures in Rheumatology (OMERACT) domains for chronic gout studies identified a number of items in addition to flares including pain, activity limitation, tophus, and health-related quality of life14,15,16. These findings also support the inclusion of flares and additional variables (tophus, pain scores, serum urate, and patient global assessment) in the physician-generated provisional remission criteria7.
The accuracy of definitions using flares recalled over the previous 6 months in distinguishing between the disease activity states of remission, LDA/PASS, and no LDA appeared to be similar to the accuracy of definitions using flares recalled over the previous 12 months. The VUS is a 3-state equivalent to the area under the curve for a 2-state ROC analysis, and these VUS values were similar for the 6-month scenario and the 12-month scenario. If further work also demonstrated that residual disease activity (as measured with additional indicators) was similar for patients categorized as being in remission by the absence of flares over 6 months as over 12 months, there would seem to be no advantage in assessing remission status by the longer time frame. This has important practical implications, particularly when designing clinical trials.
There are some limitations to the strength of the conclusions that can be drawn. The flare count data were recalled retrospectively by patients, and flare occurrence was not standardized, so it is possible that recall bias could influence the analysis. However, it seems plausible that recall bias could occur randomly in both directions and thus dilute any effect of flare frequency that is present in the categorization as remission/LDA/PASS. Further, a similar relationship between flare counts and disease activity status was identified when asking patients about flares using a different approach that did not rely upon recall. Therefore, it seems unlikely that recall bias would materially affect the conclusions. In addition, we observed a likely “availability heuristic,” which is a well-described cognitive bias whereby more available information (current or more recent flare) more strongly influences perception of current disease status than less available information (more distant flares). Studies that document flares prospectively or in real time should be used in the future to confirm our findings. In particular, use of a standard flare definition or other “gold standard” to document flare occurrences would be of interest. It would also be of interest to ask patients about their expectation of future flare, since that could also be a factor in determining satisfaction with current gout control and treatment.
The other main limitation is that the patients were recruited from specialist clinics, whereas most people with gout are treated in primary care. It is likely that the patients in this study had more severe or treatment-resistant disease, which may limit the generalizability of the results. It is plausible that rheumatology clinic patients may have a higher flare rate threshold for thinking about disease activity status compared to primary care patients with gout. This would imply that even lower flare rates are necessary in primary care to achieve an LDA state and would tend to support the main conclusion that the target of care should be 0 flares. On the other hand, a survey of gout patients in primary care reported a significant discrepancy between satisfaction with current treatment (79% satisfied) and frequency of recent flares (71% had flare in previous 12 months)17. Further study in primary care patients is necessary to help understand this apparent discrepancy. This study did not collect information on serum urate levels or give instructions to local sites about sharing serum urate information with patients. It is plausible that patients who knew that their serum urate was at its target were more likely to consider themselves in remission. Finally, we did not collect information on self-management of flares with medications such as glucocorticoids, colchicine, or nonsteroidal anti-inflammatory drugs, which could have altered the perception of disease activity during the gout flare.
This study reports the first investigation into patient perceptions of disease activity states in gout. There is no available validated patient-reported instrument in gout for self-classification into remission, LDA, and PASS with known accuracy. Thus, there is also a potential for misclassification into each disease activity state. It is possible that a different frequency distribution of disease activity status and a different relationship between recalled flare rate and disease activity would be found with different definitions. However, as far as it was possible, we phrased questions in a manner consistent with the meaning of the disease activity state in other conditions. Further, it is also possible that misclassification could operate in both directions and thus be nondifferential, diluting observed effects. It is therefore uncertain as to whether our main conclusions would be greatly altered.
This work has identified possible flare thresholds that are meaningful for patients with gout, but the thresholds should be replicated, particularly for the states of LDA/PASS, in order to be useful for clinical assessment in practice and for clinical trial development. Other factors that could be related to the perception of remission in gout, such as tophaceous burden, quality of life, and functional scores should be investigated, with the goal of finding a meaningful and comprehensive index of treatment response and remission.
In summary, flares are significantly associated with perceptions of disease activity in gout, and 0 flares over prior 6 or 12 months are necessary for most people to self-categorize as being in remission. Clinical trials in the future should be designed to incorporate achievement of flare remission as an important endpoint, and clinicians in practice should consider flare remission a legitimate patient-centered target of therapy. In both settings, it is necessary to determine disease activity status over a standard time period and at a time during which the patient is not currently experiencing a flare. However, recalled flare counts alone do not accurately classify patients into self-categorized states, suggesting that other factors may also contribute to gout disease activity.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Eve Markovitz, Michael McEwan, and Joshua Melnick for their help entering and verifying patient data at the coordinating site (UAB). Mona Thorkildsen, nurses Gina Stenberg and Ingerid Müller (Diakonhjemmet Hospital, Oslo, Norway), and Dr. Michael Saddekni and nurse Stephanie Biggers (UAB) helped with participant enrollment. Drs. David Redden and Peng Li (UAB School of Public Health) gave advice on biostatistical questions. JAS contributed intellectually to the interpretation of data and analyses and participated in manuscript draft and revision, but was not involved in the conduct of study or data collection, nor received any support from the funding provided for this study. Data from this paper were presented at the 2016 [Gaffo, et al, Arthritis Rheumatol 2017; 68 (Suppl 10)] and 2017 [Taylor, et al, Arthritis Rheumatol 2017; 69 (Suppl 10)] ACR annual meetings.
Funding for central study coordination and data analysis was provided by Ironwood Pharmaceuticals, which had had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Ironwood Pharmaceuticals.
WT is a consultant for Pfizer, AstraZeneca, Abbvie, and Roche. ND receives grant/research support from AstraZeneca and Amgen; and is a consultant for Takeda, Pfizer, AstraZeneca, Horizon, and Kowa. KGS is a consultant for AstraZeneca, Horizon, Ironwood, SOBI, and Takeda. JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio Health, Medscape, WebMD, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Spherix, Practice Point Communications, the National Institutes of Health, and the American College of Rheumatology (ACR); owns stock options in Amarin pharmaceuticals and Viking therapeutics; is on the speaker’s bureau of Simply Speaking; is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies; serves on the FDA Arthritis Advisory Committee; is a member of the Veterans Affairs Rheumatology Field Advisory Committee; is the editor and the Director of the University of Alabama at Birmingham Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; and previously served as a member of the following committees: member, the ACR Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee, and the cochair of the ACR Criteria and Response Criteria subcommittee. LKS receives speaker fees from Amgen. AG is a consultant for SOBI and receives grant/research support from Amgen.
REFERENCES
- 1.Gout Neogi T.. Ann Intern Med 2016;165:ITC1–16. [DOI] [PubMed] [Google Scholar]
- 2.Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, et al. The British Society for Rheumatology guideline for the management of gout. Rheumatology 2017;56:e1–20. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WJ, Brown M, Aati O, Weatherall M, Dalbeth N. Do patient preferences for core outcome domains for chronic gout studies support the validity of composite response criteria? Arthritis Care Res 2013;65:1259–64. [DOI] [PubMed] [Google Scholar]
- 6.Taylor WJ, Schumacher HR Jr, Baraf HS, Chapman P, Stamp L, Doherty M, et al. A modified Delphi exercise to determine the extent of consensus with OMERACT outcome domains for studies of acute and chronic gout. Ann Rheum Dis 2008;67:888–91. [DOI] [PubMed] [Google Scholar]
- 7.de Lautour H, Taylor WJ, Adebajo A, Alten R, Burgos-Vargas R, Chapman P, et al. Development of preliminary remission criteria for gout using Delphi and 1000Minds consensus exercises. Arthritis Care Res 2016;68:667–72. [DOI] [PubMed] [Google Scholar]
- 8.Wells G, Boers M, Tugwell P, Group MW. Low disease activity state in rheumatoid arthritis: concepts and derivation of minimal disease activity. Clin Exp Rheumatol 2006;24:S52–9. [PubMed] [Google Scholar]
- 9.Tubach F, Wells GA, Ravaud P, Dougados M. Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: methodological issues. J Rheumatol 2005;32:2025–9. [PubMed] [Google Scholar]
- 10.Gaffo AL, Dalbeth N, Saag KG, Singh J, Rahn EJ, Mudano AS, et al. Validation of a definition of flare in patients with established gout. Arthritis Rheumatol 2018;70:462–7. [DOI] [PubMed] [Google Scholar]
- 11.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005;64:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Xiong C. Diagtest3grp: an R package for analyzing diagnostic tests with three ordinal groups. J Stat Softw 2012;51:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Frey EC. The meaning and use of the volume under a three-class ROC surface (VUS). IEEE Trans Med Imaging 2008;27:577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay K, Gow P, Vanderpyl J, Logo P, Dalbeth N. The experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach. J Clin Rheumatol 2011;17:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Diaz_Torne C, Pou MA, Castellvi I, Corominas H, Taylor WJ. Concerns of patients with gout are incompletely captured by OMERACT-endorsed domains of measurement for chronic gout studies. J Clin Rheumatol 2014;20:138–40. [DOI] [PubMed] [Google Scholar]
- 16.Tatlock S, Rüdell K, Panter C, Arbuckle R, Harrold LR, Taylor WJ, et al. What outcomes are important for gout patients? In-depth qualitative research into the gout patient experience to determine optimal endpoints for evaluating therapeutic interventions. Patient 2017;10:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Meulemeester M, Jansen T, Petersen G, Perez-Ruiz F. European patient voice in gout survey - subjective satisfaction in gout patients versus objective suboptimal gout care [abstract]. Ann Rheum Dis 2019;78:A153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


