Abstract
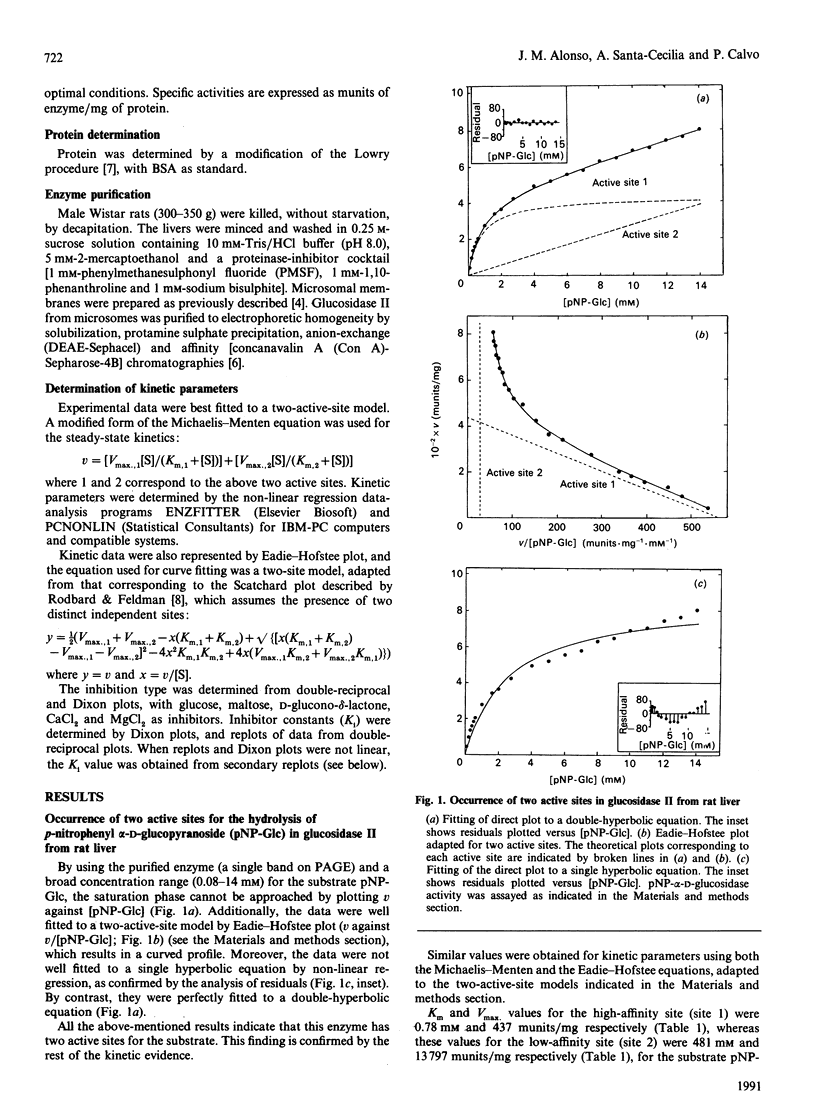
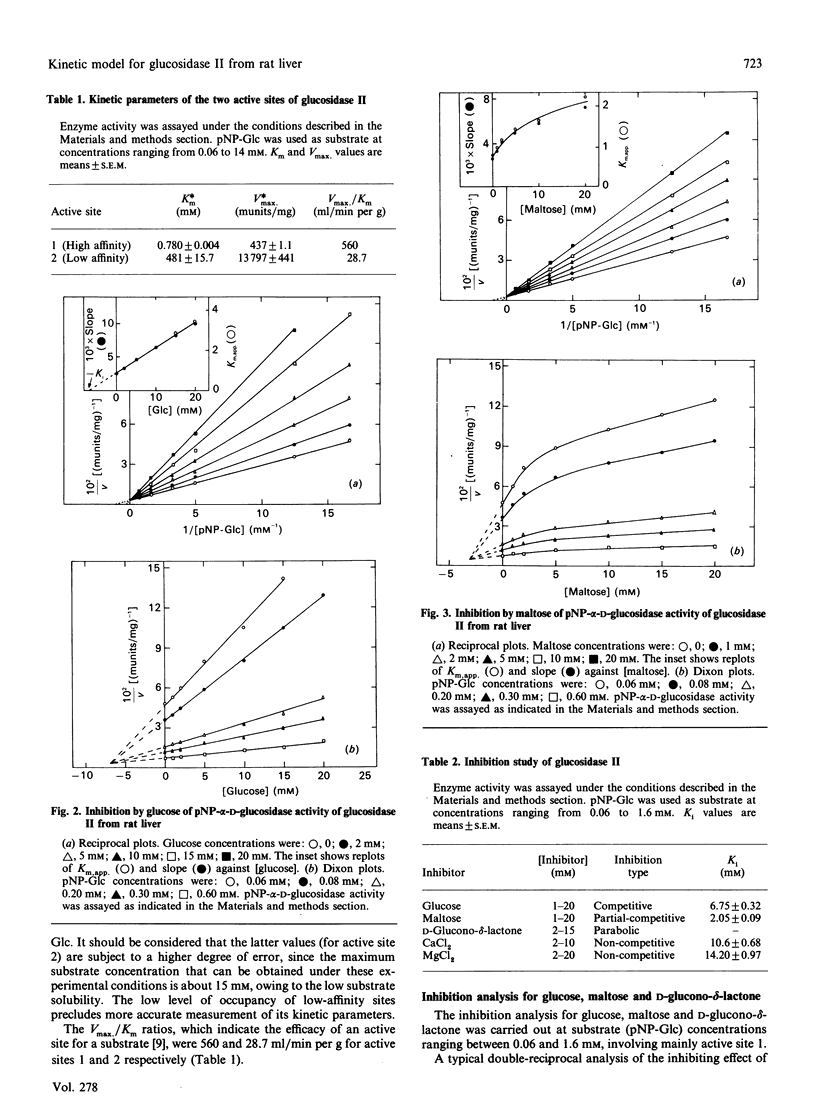
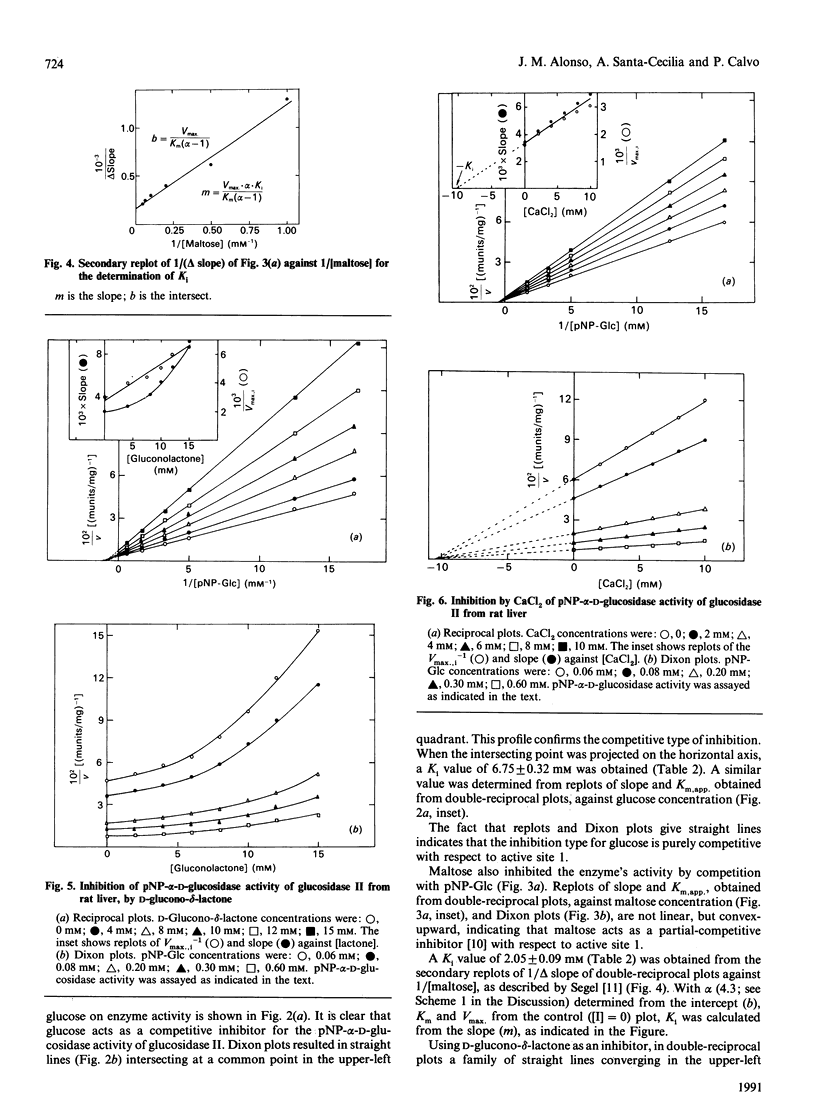
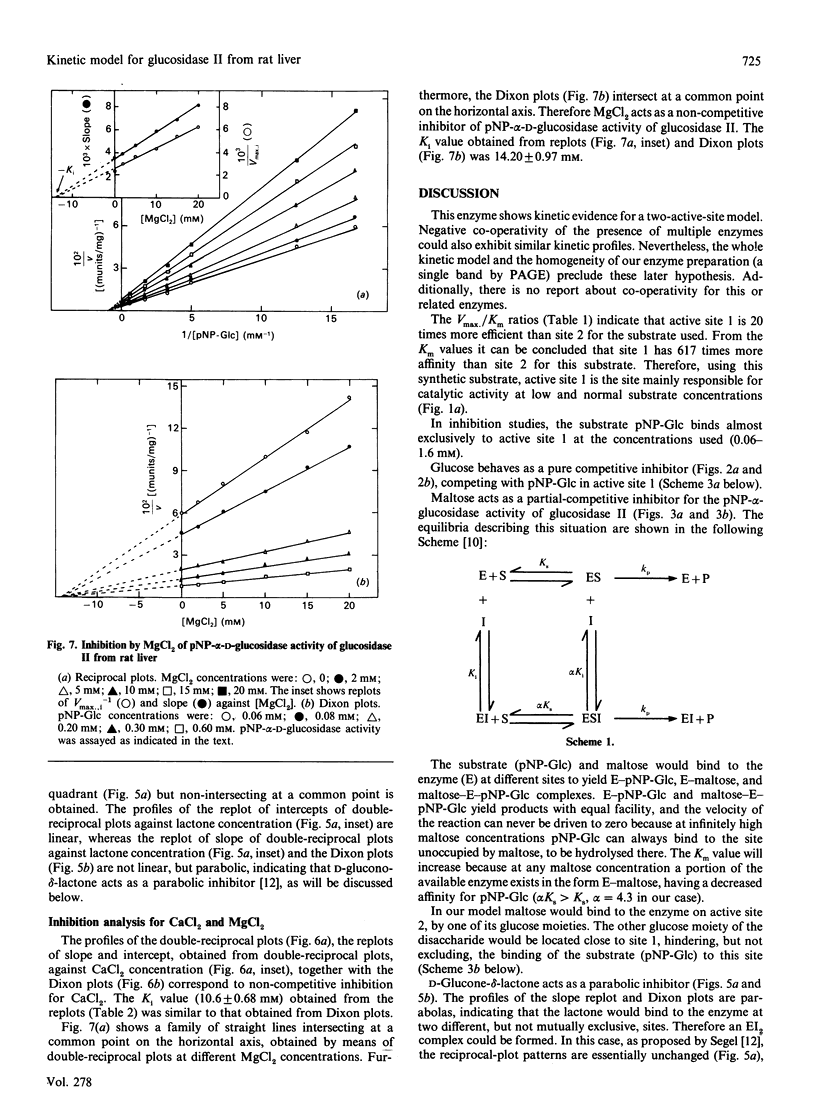
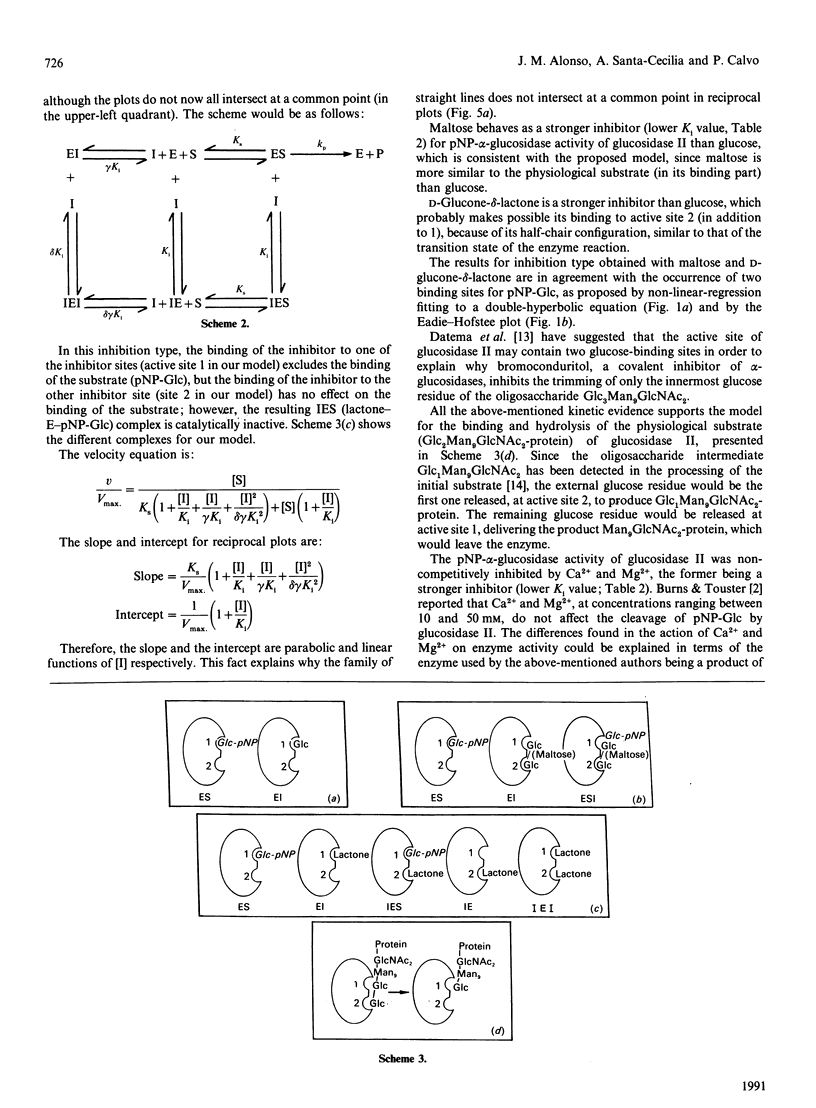
Glucosidase II is an enzyme involved in glycoprotein biosynthesis, releasing both alpha-1,3-linked glucose residues from the protein-linked oligosaccharide Glc2Man9GlcNAc2-R in the processing of N-glycans. We studied the kinetic properties of the enzyme, purified to homogeneity and, for the first time, we have been able to demonstrate the occurrence of two active sites in this enzyme and to establish the mechanisms of binding and hydrolysis of the physiological substrate at its active site(s). The analyses of data fitting to single and double hyperbolic equations and the Eadie-Hofstee profile, together with the inhibition kinetics, demonstrate that the enzyme has two different active sites. The Km and Vmax. values for the high-affinity site (site 1) were 0.78 mM and 437 munits/mg respectively, whereas the values for the low-affinity site (site 2) were 481 mM and 13797 munits/mg respectively, for the p-nitrophenyl alpha-D-glucopyranoside substrate. The Vmax./Km ratios, which indicate the efficacy of an active site for a substrate, were 560 and 28.7 ml/min per g for active sites 1 and 2, respectively. The inhibition type, with respect to site 1, for glucose, maltose, D-glucone-delta-lactone, CaCl2 and MgCl2 was pure-competitive, partial-competitive, parabolic, non-competitive and non-competitive respectively. Ki values for glucose, maltose, CaCl2 and MgCl2 were 6.75, 2.05, 10.60 and 14.20 mM respectively. Thus glucose would bind to active site 1, maltose to site 2 (and near to site 1) and D-glucone-delta-lactone to either site 1 or 2. The following hydrolysis mechanism for the physiological substrate (Glc2Man9GlcNAc2-protein) of glucosidase II may be concluded from all the foregoing kinetic evidence: the external glucose would be the first released residue, at active site 2, thereafter producing Glc1Man9GlcNAc2-protein; the remaining glucose would be released at active site 1, delivering the Man9GlcNAc2-protein product, which would leave the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns D. M., Touster O. Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J Biol Chem. 1982 Sep 10;257(17):9990–10000. [PubMed] [Google Scholar]
- Datema R., Romero P. A., Legler G., Schwarz R. T. Inhibition of formation of complex oligosaccharides by the glucosidase inhibitor bromoconduritol. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6787–6791. doi: 10.1073/pnas.79.22.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting J. J., Lennarz W. J. N-asparagine-linked oligosaccharides: glucosidase-1 from hen oviduct. Methods Enzymol. 1982;83:429–432. doi: 10.1016/0076-6879(82)83040-1. [DOI] [PubMed] [Google Scholar]
- Grinna L. S., Robbins P. W. Glycoprotein biosynthesis. Rat liver microsomal glucosidases which process oligosaccharides. J Biol Chem. 1979 Sep 25;254(18):8814–8818. [PubMed] [Google Scholar]
- Hino Y., Rothman J. E. Glucosidase II, a glycoprotein of the endoplasmic reticulum membrane. Proteolytic cleavage into enzymatically active fragments. Biochemistry. 1985 Jan 29;24(3):800–805. doi: 10.1021/bi00324a040. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Michael J. M., Kornfeld S. Partial purification and characterization of the glucosidases involved in the processing of asparagine-linked oligosaccharides. Arch Biochem Biophys. 1980 Jan;199(1):249–258. doi: 10.1016/0003-9861(80)90278-7. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Feldman H. A. Theory of protein-ligand interaction. Methods Enzymol. 1975;36:3–16. doi: 10.1016/s0076-6879(75)36003-5. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]


