Abstract
Massive rotator cuff tears constitute approximately 20% of all rotator cuff tears. Poor tissue quality or significant retraction can lead to failure of the repair. The anterior rotator cuff cable is essential in transmitting force to the proximal humerus and serves as the main load-bearing structure within the supraspinatus. Utilizing the long head of the biceps tendon (LHBT) for anterior cable reconstruction in the setting of rotator cuff tears, known as biceps augmentation, has the potential for improved biomechanical and healing properties. Importantly, the proximal LHBT remains attached to the superior glenoid labrum, serving as a viable collagen scaffold, a structural scaffold for the cable, and potentially as a conduit for living tenocytes to migrate into the hypovascular region of the rotator cuff, promoting repair healing. Similar methods utilize the transfer of the intact LHBT into the rotator cuff without a biceps tenodesis. While this accomplishes the aforementioned goals, it may create a source of biceps pain in these patients, and it changes the length–tension relationship of the LHBT distal to the transfer site. In this technical note, we detail an anterior cable reconstruction employing an autologous LHBT to reinforce a repaired massive rotator cuff tear with concurrent subpectoral tenodesis of the LHBT to achieve goals of 1) rotator cuff augmentation and grafting and, importantly and 2) securing the LHBT in a subpectoral position to mitigate pain and maintain supination strength while maintaining the anatomic length–tension relationship of the biceps. We feel this approach is superior in ensuring sufficient tendon is retained for an effective transfer and allows for a subpectoral tenodesis to prevent biceps symptoms.
Keywords: Anterior cable reconstruction, Biceps transfer, Subpectoral tenodesis, Rotator cuff tear, Biceps augmentation, Surgical technique
Massive rotator cuff tears (MRCTs) represent a significant portion of rotator cuff tears, accounting for approximately 20% of all tears.3 The rotator cuff anterior cable, which is the most anterior 8 to 12 mm of the supraspinatus tendon located immediately posterior to the bicipital groove, serves as the primary load-bearing structure within the supraspinatus and is responsible for transmitting force to the proximal humerus.7 Tears involving the anterior cable have been shown to result in greater tear gap distance, increased tendon stiffness, and increased tendon strain compared to tears involving the rotator crescent.7 Due to these findings, successful anterior cable repair or reconstruction remains a particular area of focus during rotator cuff repair.
Biceps autograft augmentation, performed by incorporating the long head of the biceps tendon (LHBT) into the rotator cuff repair construct, aims to leverage the LHBT’s biological profile for enhanced biomechanical properties. Studies have demonstrated repair of the anterior cable using the autologous biceps tendon, with some surgeons opting to detach both proximal and distal ends, and some choosing to leave the proximal portion intact while tenodesing just distal to the greater tuberosity footprint.5,9,11
In this technical note, we propose utilizing the LHBT to restore the anterior cable in the setting of a rotator cuff tear. However, unlike prior methods, we perform a tenodesis proximal to the myotendinous junction in a subpectoral position and simultaneously preserve the attachment of the proximal LHBT at its origin on the superior glenoid tubercle. This technique provides sufficient tendon for transfer while effectively eliminating biceps symptoms by maintaining the anatomic length and tension of the biceps distal to the rotator cuff augmentation.
Surgical technique
Indications and contraindications
This surgical technique is indicated for patients who have a rotator cuff tear with a proximally intact biceps tendon and have not responded to conservative treatments such as injections or physiotherapy. It is also suitable for individuals over the age of 65 presenting with severe rotator cuff tendinopathy in combination with a full-thickness rotator cuff tear or in elderly individuals with tears involving the anterior cable. The biceps attachment to the supraglenoid tubercle and superior glenoid labrum must be intact as a requisite for this transfer. However, unlike techniques in which the biceps is transferred without distal tenodesis, we have found that neither the biceps anchor nor tendon need to be completely without disease for successful transfer. For greatest success, there should be remaining cuff into which the biceps can be directly transferred. Advanced glenohumeral arthritis, infection, absent LHBT proximal attachment, and catastrophic deficiencies of the superior rotator cuff are contraindications.
Step 1: surgical preparation and surgical equipment
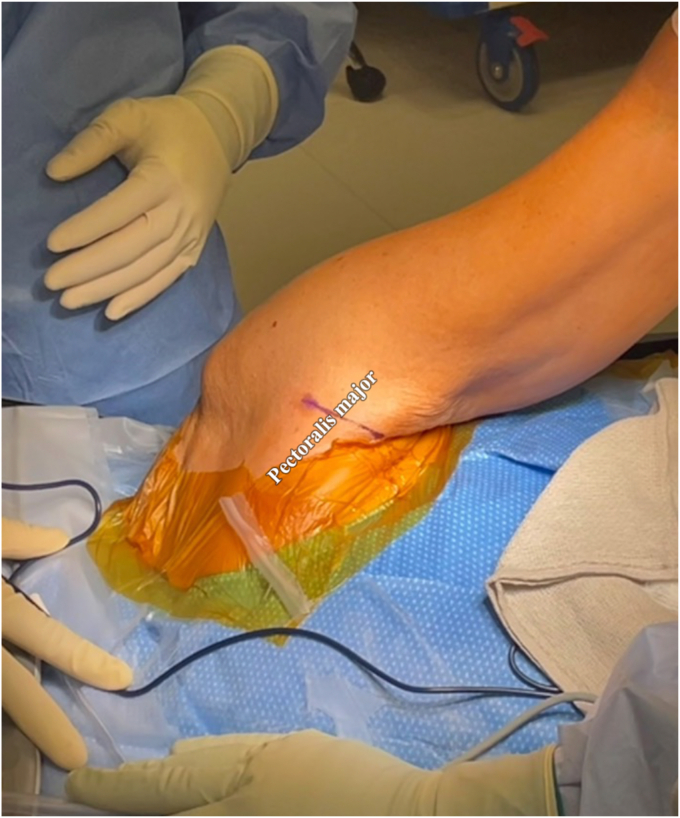
After administration of general anesthesia, interscalene block, and intravenous antibiotics, a range of motion assessment is performed. The patient is then positioned in the lateral decubitus position on a bean bag with all bony prominences well-padded. This surgery may also be performed in the beach chair position, depending on the surgeon's preference. The arm and shoulder are prepped and draped in a sterile fashion (Fig. 1). Local anesthetic is administered into the anterior axillary fold, or supplemental blocks may be employed, as an interscalene block alone will not reach this region innervated by the pectoral nerve branches.
Figure 1.
Sufficient abduction of the shoulder allows for an incision to be made over the pectoralis major tendon. In females, we prefer an axillary incision in Langer’s lines (purple ink line) as it is more cosmetically pleasing.
The patient’s arm is suspended with 10-15 lbs of weight for balanced traction. We prefer a traction device that allows for 2 positions of abduction: 1 of greater abduction for the subpectoral tenodesis, which affords easier retraction of the pectoralis major and 1 of less abduction for the arthroscopic portion of the case. The high abduction portion facilitates proper exposure, allowing the surgeon to dissect under the pectoralis major more easily and to adequately retract the short head of the biceps brachii.
Step 2: incision and dissection
In women, a 2–3-cm incision can be made in the anterior axillary fold to allow the patients to wear strapless dresses or other apparel without the incisional scar being visible. In male patients, a standard 3-cm vertical subpectoral or axillary incision may be made. Once the incision is made, a Weitlaner self-retaining retractor is used to maintain exposure as blunt dissection is performed down to the pectoralis major tendon using a curved hemostat.
Step 3: LHBT identification, release, & tenodesis
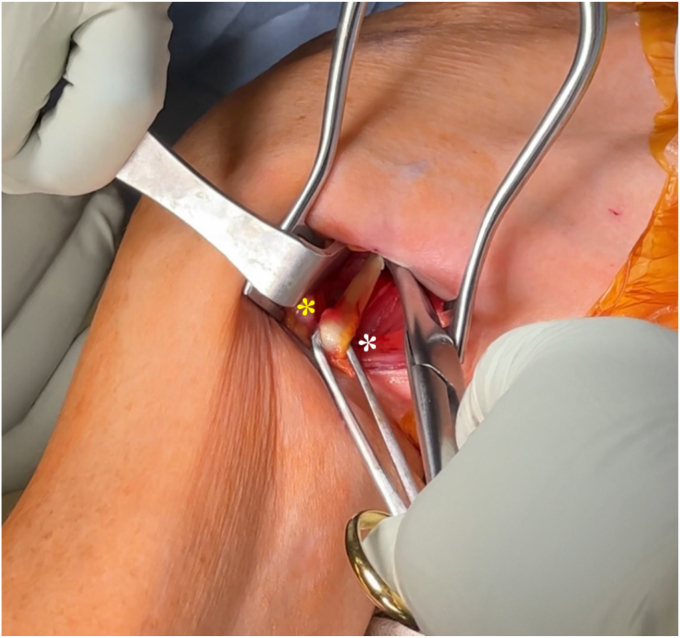
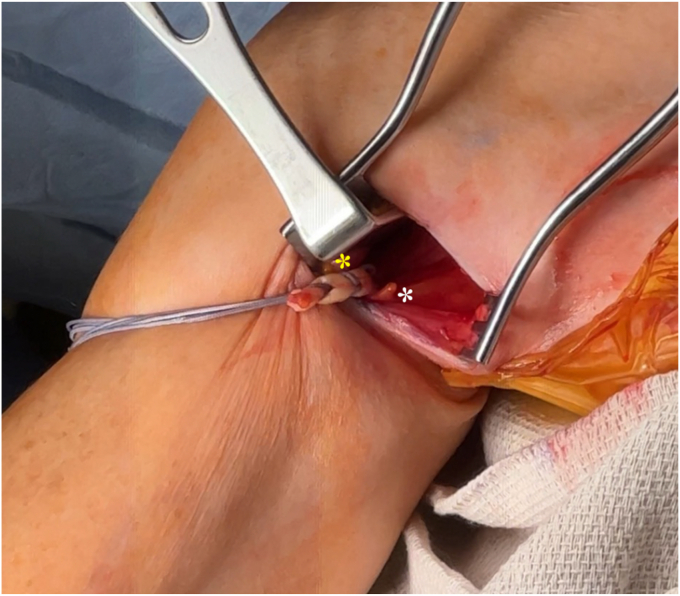
When the pectoralis major is identified and adequately retracted using an Army-Navy or similar retractor, the short head of the biceps is retracted posteriorly while exposing the LHBT, which may be palpated and traced into the bicipital groove. An Allis clamp is used to retrieve the long head of the biceps brachii. Curved Mayo scissors are then slid around the tendon at the myotendinous junction, tracing the tendon up into the bicipital groove until it is released (Fig. 2). During this release, a portion or all of the transverse humeral ligament may be released; however, the senior surgeon prefers to leave the proximal portion of this ligament intact. A running locking whipstitch is performed at the myotendinous junction to create a construct with a biceps cortical button (Arthrex, Naples, FL) (Fig. 3). Next, a guidewire is drilled bicortically and a 6.0-mm reamer unicortically to create a humeral tunnel for placement of the LHBT. A 6.5-mm reamer is used unicortically in larger men, while a 6.0-mm reamer is used in women. The button is flipped on the far cortex, and a tension slide is used to introduce the long head of the biceps brachii tendon into the prepared tunnel. In this fashion, the natural tension of the LHBT is able to be calibrated to the patient’s normal anatomy.
Figure 2.
Image showing the cutting of the long head of the biceps proximal to the myotendinous junction. The pectoralis major and short head of the biceps brachii are retracted to allow visualization of the long head. Yellow asterisk, pectoralis major tendon; white asterisk, short head of biceps brachii.
Figure 3.
Image depicting the long head of the biceps brachii cut proximal to the myotendinous junction. A running locking whipstitch is performed, preparing for the creation of a construct with a biceps cortical button. Yellow asterisk, pectoralis major tendon; white asterisk, short head of biceps brachii.
Step 4: synovectomy, débridement, & subacromial decompression
A complete synovectomy is performed with resection of inflamed tissue. Next, a standard posterior arthroscopy portal is established in a subacromial position. Typically, 2 accessory lateral portals are also established. The subacromial space is then débrided of adhesions, and an acromioplasty is performed for clear impingement, but not routinely.
Step 5: anterior cable reconstruction and rotator cuff repair
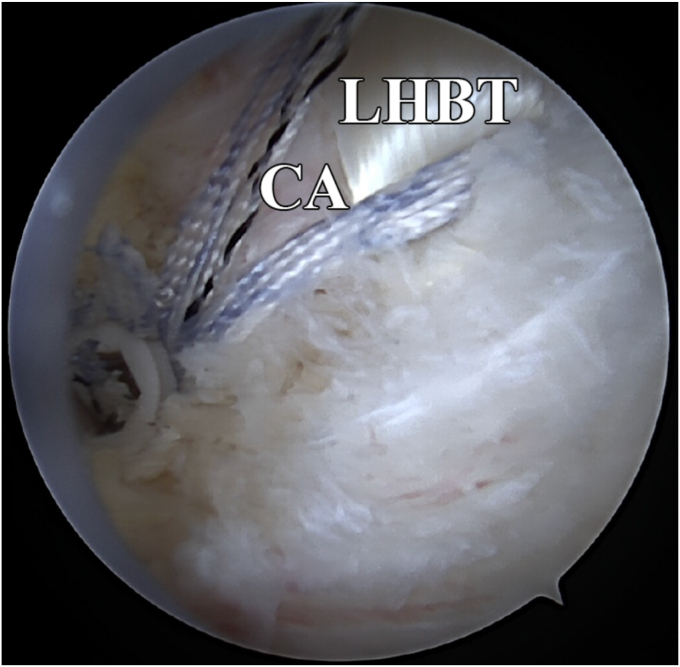
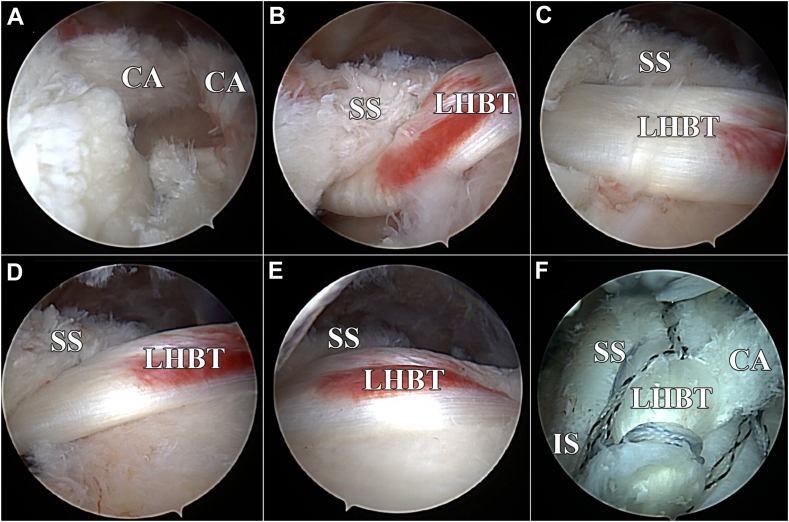
A simple suture retriever or similar device is used to pass a locking luggage tag suture around the LHBT in a simple closed-loop fashion. In smaller cuff tears, this can be done while looking intra-articularly and passing the device through the rotator cuff defect. The tendon is then brought through the defect in the rotator cuff to reconstruct the anterior cable. A retrograde suture passer, in this technique a Scorpion (Arthrex, Naples, FL, USA), is then used to sew the LHBT to the anterior cable of the supraspinatus. After the coracohumeral ligament is released, the biceps/anterior cable construct is placed into a lateral anchor just posterior to the bicipital groove (Fig. 4). In patients with soft bone, this anchor may be placed directly into the bicipital groove. Once the anterior cable is restored, the remaining rotator cuff mobilization and repair are performed in a tension-free manner. In cases where a margin convergence is utilized, the LHBT-anterior cable construct is also sutured to corresponding regions of the posterior rotator cuff (Fig. 5). It is important to ensure that the reconstruction is tension-free, that it restores the anterior cable, and that the transferred biceps fills the collagen void created by the acute or chronic rotator cuff tendon tear.
Figure 4.
Image depicting the biceps/anterior cable construct placed into a lateral anchor posterior to the biceps groove and lateral to the RC footprint. CA, anterior cable; LHBT, long head of biceps tendon; RC, rotator cuff.
Figure 5.
Images depicting the reconstruction of the anterior cable of the supraspinatus. (A) Traumatic tear with remnant anterior cable remaining on the greater tuberosity footprint. (B-E) Biceps being pulled through the rotator cuff defect to reconstruct the anterior cable. (F) Biceps imbricated into the rotator cuff repair. SS, supraspinatus; IS, infraspinatus; CA, anterior cable; LHBT, long head of biceps tendon.
Step 6: wound closure
Once the repair is completed, all arthroscopic equipment is removed, and a suture is placed in each portal. The original subpectoral incision is closed in a multilayered fashion using a 3-0 deep dermal layer, a subcutaneous 4-0 monofilament, and surgical glue. Supplemental anesthetic is administered in the case of axillary incisions. Sterile dressings are applied.
Step 7: postoperative management
Postoperatively, the patient should expect to follow rehabilitation protocols similar to a standard rotator cuff repair. In the initial 6 weeks, the patient should maintain sling use. Starting on postoperative day 1, patients can initiate pendulum hangs, hand squeezes, and active range of motion exercises for the elbow. By week 2, passive external rotation up to 30° can be incorporated. The patient should not lift anything until 4 weeks postop. From weeks 6 to 8, patients can start using their arm for activities of daily living, with weight restrictions to the weight of a coffee cup. At week 8, patients can begin biceps and triceps strengthening, and at week 16, begin total arm strengthening. Between weeks 20 and 24, patients can work towards a full return to their previous activities, supplemented by a work-hardening program.
Discussion
In this technical note, we demonstrate a surgical technique for anterior cable reconstruction with the use of an autologous LHBT in the setting of a MRCT (Video 1). In addition to existing techniques, we feel it is critical to perform a subpectoral tenodesis to complete the transfer, ensure adequate tendon length for the transfer, and ensure length–tension preservation on the myotendinous junction of the remaining LHBT to mitigate biceps symptoms in these patients (Table I). To date, we have successfully performed this technique on approximately 30 patients with no complaints of biceps symptoms and successful repairs in all but 1 patient despite the challenging population: large to massive tears, elderly patients with full-thickness tears, and full-thickness tears with severe tendinosis.
Table I.
Pearls & pitfalls.
| Pearls | Pitfalls |
|---|---|
|
|
In 1971, Neviaser first described using the LHBT as an autologous augmentation in MRCTs.8 His technique involved severing the LHBT proximally near the supraglenoid tuberosity and distally at the upper level of the bicipital groove, then longitudinally splitting it to cover the humeral head. The use of LHBT augmentation in rotator cuff repair procedures has become more widespread in recent years, driven by emerging evidence of its biological advantages and relative simplicity of transferring without an adjuvant tenodesis, as we describe.2
Several augmentation surgical techniques similar to ours to reconstruct the anterior cable in large or MRCTs have been reported. Veen et al released the LHBT proximally adjacent to the superior glenoid tubercle, as well as distally 2 cm into the muscle, and harvested the entire tendon as an autograft.11 The graft was anchored from the posterior aspect of the greater tubercle to the superior part of the lesser tubercle. De Giacomo et al and Park et al left the proximal portion of the LHBT attached to its origin on the superior glenoid tubercle.5,9 Subsequently, they performed a tenodesis 10 mm distal from the lateral edge of the greater tuberosity footprint as a graft to the anterior cable without addressing the biceps distal to the rotator cuff repair site.
Our approach differs from previous techniques in that we initially performed a tenodesis proximal to the myotendinous junction while also keeping the proximal LHBT attached to its origin on the superior glenoid tubercle. We do this for 2 reasons: 1) to ensure the adequate length of LHBT for transfer by allowing the entire length of the tendon for transfer and 2) to prevent biceps symptomatology distally with a biomechanically secure subpectoral tenodesis that maintains the length–tension relationship of the LHBT distally (Table II).
Table II.
Advantages & disadvantages.
| Advantages | Disadvantages |
|---|---|
|
|
LHBT, long head of the biceps tendon.
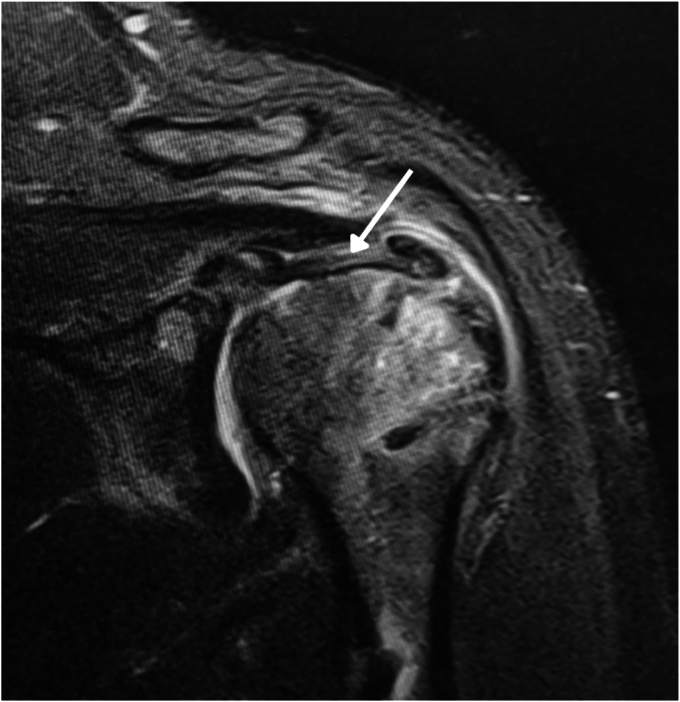
One study showed that autologous tenocytes of the LHBT scaffold can release growth factors and aid in the healing capabilities of the repair.4 Another study demonstrated that cells from surgical debris of meniscectomy procedures, when taken from autologous donors, even donors in the 80s, exhibit strong growth potential at the implantation site, suggesting a promising therapeutic avenue using autologous cells from tissue otherwise considered surgical waste.1 Thus, transferring the ample tendon proximal to the myotendinous junction allows one to take advantage of the inherent properties of autologous tissue. The preserved attachment of the LHBT at the superior glenoid tubercle also acts as a conduit for blood supply to the hypovascular region of the rotator cuff, further increasing the rate of tissue repair.13 Additionally, by leaving the biceps tendon attached to the supraglenoid tuberosity, the tendon acts as a humeral head depressor that may prevent superior migration of the humeral head while maintaining full range of motion, thus providing further biomechanical benefits (Fig. 6).9 Finally, using an autograft to repair the natural rotator cable also eliminates the risk of potential reactions to synthetic or allograft tissues.10,12 and Kim et al have recently shown clinical equivalency between autologous biceps and human dermal allograft augmentation of large anterior rotator cuff tears.6
Figure 6.
MRI of the left shoulder shows the long head of the biceps (arrow) incorporated into the rotator cuff repair. MRI, magnetic resonance imaging.
One notable limitation of our technical note is that the technique requires the proximal and distal biceps tendons to be intact and functional, which may not be the case in some patients. Additionally, while we have detailed the surgical method, we have not yet analyzed the long-term results in patients who have undergone this technique or compared the outcomes with other established methods.
Conclusion
We present a technique for the repair of the anterior cable in a rotator cuff tear using the LHBT for autologous augmentation. Future studies should consider the long-term outcomes of this technique and assess the comparison of outcomes with similar surgical methods.
Disclaimers:
Funding: No funding was disclosed by the authors.
Conflicts of interest: The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this technical note.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.xrrt.2024.07.004.
Supplementary Data
Supplementary Figure S1.

References
- 1.Baker B.M., Nathan A.S., Huffman G.R., Mauck R.L. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage. 2009;17:336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boileau P., Krishnan S.G., Coste J.S., Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18:1002–1012. doi: 10.1053/jars.2002.36488. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart S.S., Danaceau S.M., Pearce C.E. Arthroscopic rotator cuff repair: analysis of results by tear size and by repair technique—margin convergence versus direct tendon-to-bone repair. Arthrosc J Arthrosc Relat Surg. 2001;17:905–912. doi: 10.1053/jars.2001.26821. [DOI] [PubMed] [Google Scholar]
- 4.Colbath G., Murray A., Siatkowski S., Pate T., Krussig M., Pill S., et al. Autograft long head biceps tendon can be used as a scaffold for biologically augmenting rotator cuff repairs. Arthroscopy. 2022;38:38–48. doi: 10.1016/j.arthro.2021.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Giacomo A.F., Park M.C., Lee T.Q. Anterior cable reconstruction using the proximal biceps tendon for large rotator cuff defects. Arthrosc Tech. 2021;10:e807–e813. doi: 10.1016/j.eats.2020.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S.H., Shin S.-J. No difference in clinical outcomes following repair of large retracted anterior rotator cuff tears using patch augmentation with human dermal allograft versus anterior cable reconstruction with biceps tendon autograft. Arthroscopy. 2024;40:294–302. doi: 10.1016/j.arthro.2023.08.077. [DOI] [PubMed] [Google Scholar]
- 7.Mesiha M.M., Derwin K.A., Sibole S.C., Erdemir A., McCarron J.A. The biomechanical relevance of anterior rotator cuff cable tears in a cadaveric shoulder model. J Bone Joint Surg Am. 2013;95:1817–1824. doi: 10.2106/jbjs.L.00784. [DOI] [PubMed] [Google Scholar]
- 8.Neviaser J.S. Ruptures of the rotator cuff of the shoulder. New concepts in the diagnosis and operative treatment of chronic ruptures. Arch Surg. 1971;102:483–485. doi: 10.1001/archsurg.1971.01350050049015. [DOI] [PubMed] [Google Scholar]
- 9.Park M.C., Itami Y., Lin C.C., Kantor A., McGarry M.H., Park C.J., et al. Anterior cable reconstruction using the proximal biceps tendon for large rotator cuff defects limits superior migration and subacromial contact without inhibiting range of motion: a biomechanical analysis. Arthroscopy. 2018;34:2590–2600. doi: 10.1016/j.arthro.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Valentin J.E., Badylak J.S., McCabe G.P., Badylak S.F. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/jbjs.E.01008. [DOI] [PubMed] [Google Scholar]
- 11.Veen E.J.D., Koorevaar C.T., Diercks R.L. Using the long head of biceps tendon autograft as an anatomical reconstruction of the rotator cable: an arthroscopic technique for patients with massive rotator cuff tears. Arthrosc Tech. 2018;7:e699–e703. doi: 10.1016/j.eats.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wietlisbach L.E., Cheema A.N., Huang J.H., Luo X., Huffman G.R. Revision arthroscopic surgery after rotator cuff repair with a collagen graft: histologic evaluation of biopsy specimens from two patients. JSES Rev Rep Tech. 2022;2:412–418. doi: 10.1016/j.xrrt.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfgang G.L. Surgical repair of tears of the rotator cuff of the shoulder. Factors influencing the result. J Bone Joint Surg Am. 1974;56:14–26. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.