Abstract
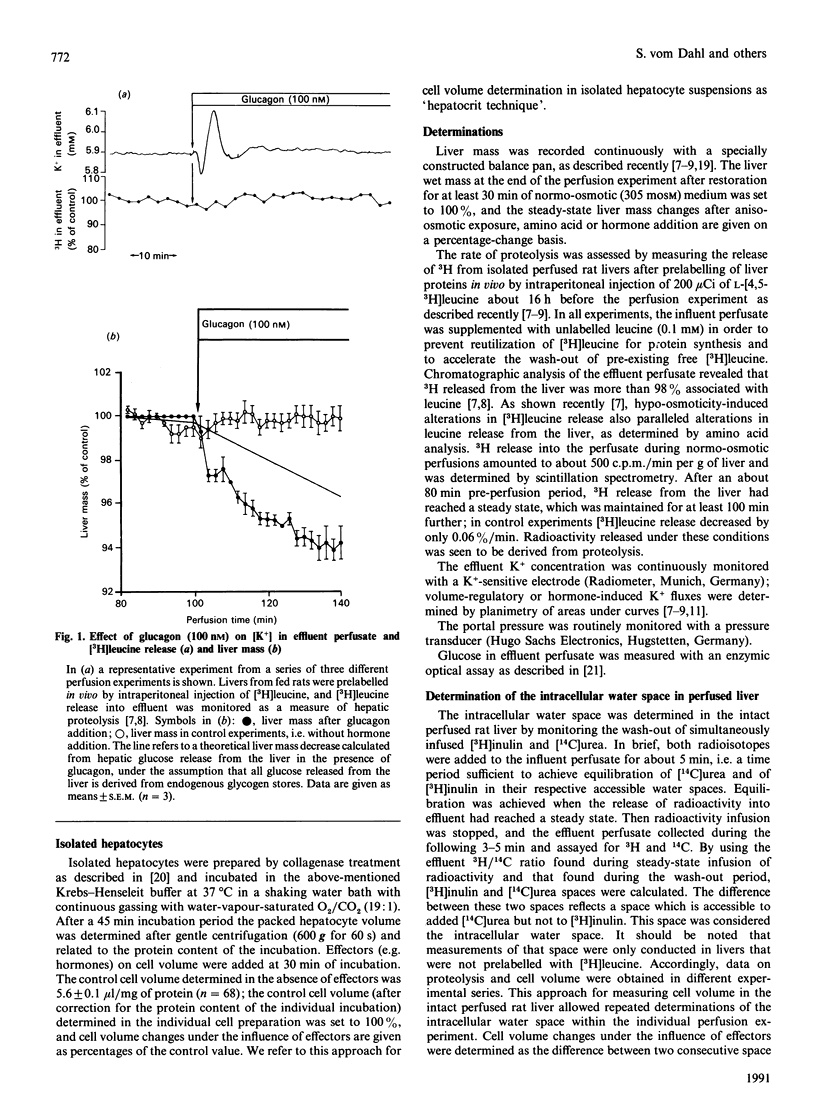
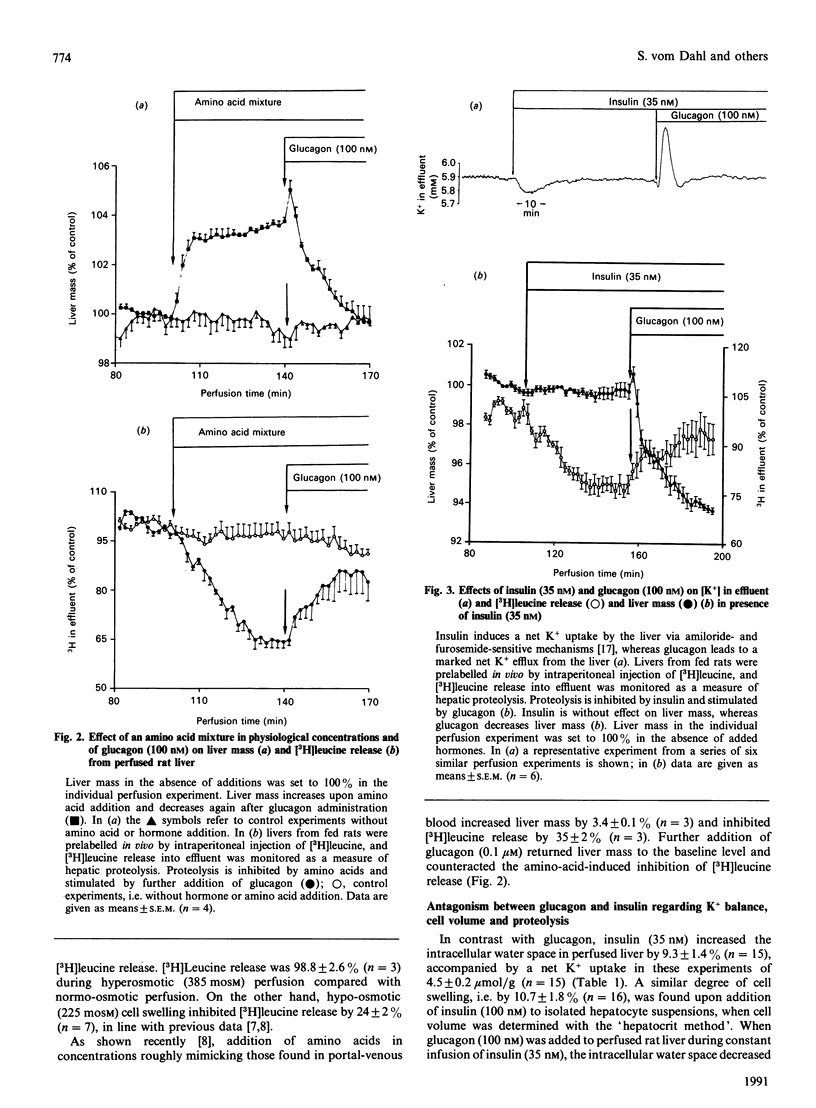
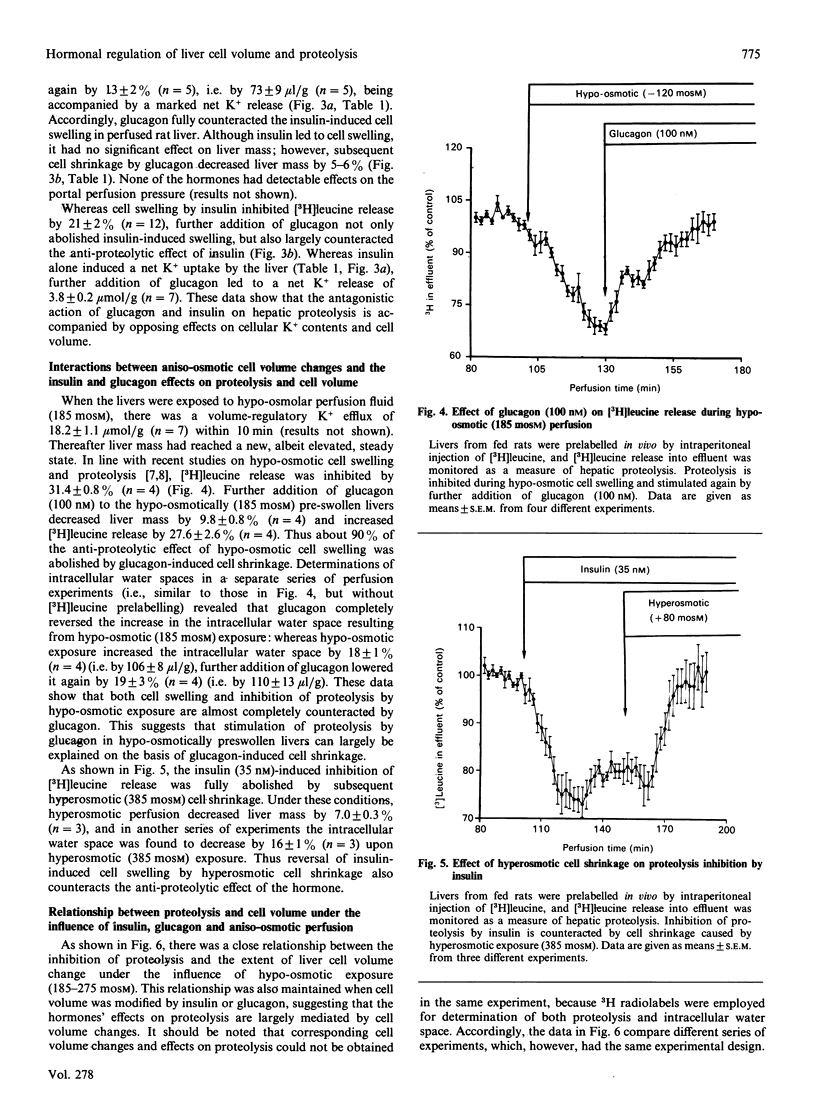
The effects of insulin and glucagon on liver cell volume and proteolysis were studied in isolated perfused rat liver. The rate of proteolysis was assessed as [3H]leucine release from single-pass-perfused livers from rats which had been prelabelled in vivo by intraperitoneal injection of [3H]leucine. The intracellular water space was determined from the wash-out profiles of simultaneously added [3H]inulin and [14C]urea. In normo-osmotic (305 mosM) control perfusions the intracellular water space was 548 +/- 10 microliters/g wet mass (n = 44) and was increased by 16.5 +/- 2.6% (n = 6), i.e. by 85 +/- 14 microliters/g, after hypoosmotic exposure (225 mosM). Glucagon (0.1 microM) decreased the intracellular water space by 17 +/- 4% (n = 4), whereas insulin (35 nM) increased the intracellular water space by 9.3 +/- 1.4% (n = 15). Also, in isolated rat hepatocyte suspensions insulin (100 nM) caused cell swelling by 10.7 +/- 1.8% (n = 16), which was fully reversed by glucagon. In perfused liver, insulin-induced cell swelling was accompanied by a hepatic net K+ uptake (4.5 +/- 0.2 mumol/g) and an inhibition of proteolysis by 21 +/- 2% (n = 12); further addition of glucagon led to a net K+ release of 3.8 +/- 0.2 mumol/g (n = 7) and fully reversed the insulin effects on both cell volume and proteolysis. Similarly, insulin-induced cell swelling and inhibition of proteolysis were completely antagonized by hyperosmotic (385 mosM) cell shrinkage. Furthermore, cell swelling and inhibition of proteolysis after hypo-osmotic exposure or amino acid addition were reversed by glucagon-induced cell shrinkage. There was a close relationship between the extent of cell swelling and the inhibition of proteolysis, regardless of whether cell volume was modified by insulin, glucagon or aniso-osmotic exposure. The data show that glucagon and insulin are potent modulators of liver cell volume, at least in part by alterations of cellular K+ balance, and that their opposing effects on hepatic proteolysis can largely be explained by opposing effects on cell volume. It is hypothesized that hormone-induced alterations of cell volume may represent an important, not yet recognized, mechanism mediating hormonal effects on metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Graf J., Haddad P., Haeussinger D., Lang F. Cell volume regulation in liver. Ren Physiol Biochem. 1988 May-Oct;11(3-5):202–220. doi: 10.1159/000173163. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Hallbrucker C., vom Dahl S., Lang F., Gerok W., Häussinger D. Inhibition of hepatic proteolysis by insulin. Role of hormone-induced alterations of the cellular K+ balance. Eur J Biochem. 1991 Jul 15;199(2):467–474. doi: 10.1111/j.1432-1033.1991.tb16145.x. [DOI] [PubMed] [Google Scholar]
- Hallbrucker C., vom Dahl S., Lang F., Häussinger D. Control of hepatic proteolysis by amino acids. The role of cell volume. Eur J Biochem. 1991 May 8;197(3):717–724. doi: 10.1111/j.1432-1033.1991.tb15963.x. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Protein degradation in hepatocyte monolayers. Effects of glucagon, adenosine 3':5'-cyclic monophosphate and insulin. Biochem J. 1980 Jan 15;186(1):71–79. doi: 10.1042/bj1860071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Hallbrucker C., vom Dahl S., Lang F., Gerok W. Cell swelling inhibits proteolysis in perfused rat liver. Biochem J. 1990 Nov 15;272(1):239–242. doi: 10.1042/bj2720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Bauers K., Gerok W. Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem. 1990 Mar 30;188(3):689–695. doi: 10.1111/j.1432-1033.1990.tb15451.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Exposure of perfused liver to hypotonic conditions modifies cellular nitrogen metabolism. J Cell Biochem. 1990 Aug;43(4):355–361. doi: 10.1002/jcb.240430407. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. The mutual interaction between cell volume and cell function: a new principle of metabolic regulation. Biochem Cell Biol. 1991 Jan;69(1):1–4. doi: 10.1139/o91-001. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Lang F. Volume regulation in liver: further characterization by inhibitors and ionic substitutions. Hepatology. 1990 Feb;11(2):243–254. doi: 10.1002/hep.1840110214. [DOI] [PubMed] [Google Scholar]
- Hüssinger D., Lang F., Bauers K., Gerok W. Control of hepatic nitrogen metabolism and glutathione release by cell volume regulatory mechanisms. Eur J Biochem. 1990 Nov 13;193(3):891–898. doi: 10.1111/j.1432-1033.1990.tb19414.x. [DOI] [PubMed] [Google Scholar]
- Jakubowski J., Jakob A. Vasopressin, insulin and peroxide(s) of vanadate (pervanadate) influence Na+ transport mediated by (Na+, K+)ATPase or Na+/H+ exchanger of rat liver plasma membrane vesicles. Eur J Biochem. 1990 Oct 24;193(2):541–549. doi: 10.1111/j.1432-1033.1990.tb19370.x. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., McGivan J. D. The effect of ammonium chloride and glucagon on the metabolism of glutamine in isolated liver cells from starved rats. Biochim Biophys Acta. 1978 Sep 21;543(1):16–28. doi: 10.1016/0304-4165(78)90450-6. [DOI] [PubMed] [Google Scholar]
- Lacey J. H., Bradford N. M., Joseph S. K., McGivan J. D. Increased activity of phosphate-dependent glutaminase in liver mitochondria as a result of glucagon treatment of rats. Biochem J. 1981 Jan 15;194(1):29–33. doi: 10.1042/bj1940029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F., Stehle T., Häussinger D. Water, K+, H+, lactate and glucose fluxes during cell volume regulation in perfused rat liver. Pflugers Arch. 1989 Jan;413(3):209–216. doi: 10.1007/BF00583532. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Wilson P. B., Blackmore P. F., Exton J. H. The hormone-sensitive hepatic Na+-pump. Evidence for regulation by diacylglycerol and tumor promoters. J Biol Chem. 1986 Nov 5;261(31):14551–14556. [PubMed] [Google Scholar]
- MILLER L. L. Glucagon: a protein catabolic hormone in the isolated perfused rat liver. Nature. 1960 Jan 23;185:248–248. doi: 10.1038/185248a0. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Mortimore G. E., Pösö A. R. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–564. doi: 10.1146/annurev.nu.07.070187.002543. [DOI] [PubMed] [Google Scholar]
- Schworer C. M., Mortimore G. E. Glucagon-induced autophagy and proteolysis in rat liver: mediation by selective deprivation of intracellular amino acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3169–3173. doi: 10.1073/pnas.76.7.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B. Amino acid control of autophagic sequestration and protein degradation in isolated rat hepatocytes. J Cell Biol. 1984 Aug;99(2):435–444. doi: 10.1083/jcb.99.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Wettstein M., vom Dahl S., Lang F., Gerok W., Häussinger D. Cell volume regulatory responses of isolated perfused rat liver. The effect of amino acids. Biol Chem Hoppe Seyler. 1990 Jun;371(6):493–501. doi: 10.1515/bchm3.1990.371.1.493. [DOI] [PubMed] [Google Scholar]
- vom Dahl S., Hallbrucker C., Lang F., Häussinger D. Role of eicosanoids, inositol phosphates and extracellular Ca2+ in cell-volume regulation of rat liver. Eur J Biochem. 1991 May 23;198(1):73–83. doi: 10.1111/j.1432-1033.1991.tb15988.x. [DOI] [PubMed] [Google Scholar]


