Abstract
Triptolide (TP), one of the main active ingredients in Tripterygium wilfordii Hook F, is clinically used to treat immune diseases but is known to cause liver injury. The aim of this study was to investigate the biomarkers for TP-induced hepatotoxicity in mice and to determine potential mechanisms of its liver injury. LC/MS-based metabolomics was used to determine the metabolites that were changed in TP-induced liver injury. The accumulation of long-chain acylcarnitines in serum indicated that TP exposure disrupted endogenous peroxisome proliferator-activated receptor α (PPARα) signaling. Triptolide-induced liver injury could be alleviated by treatment of mice with the PPARα agonist fenofibrate, whereas the PPARα antagonist GW6471 increased hepatotoxicity. Furthermore, fenofibrate did not protect Ppara−/− mice from TP-induced liver injury, suggesting an essential role for the PPARα in the protective effect of fenofibrate. Elevated long-chain acylcarnitines may protect TP-induced liver injury through activation of the NOTCH-NRF2 pathway as revealed in primary mouse hepatocytes and in vivo. In agreement with these observations in mice, the increase in long-chain acylcarnitines was observed in the serum of patients with cholestatic liver injury compared with healthy volunteers. These data demonstrated the role of PPARα and long-chain acylcarnitines in TP-induced hepatotoxicity, and suggested that modulation of PPARα may protect against drug-induced liver injury.
Keywords: liver injury, metabolomics, PPARα, acylcarnitines
Tripterysium glycoside tablets derived from Tripterygium wilfordii Hook F have long been used for the treatment of autoimmune diseases in clinical practice. Triptolide (TP), one of the major bioactive diterpenes in tripterysium glycosides tablets, is most noted for its anti-inflammatory, immunosuppressive (Liu et al., 2005), anti-fertility (Matlin et al., 1993), anticancer (Yang et al., 2003), and antirheumatic (Tao et al., 2001). However, clinical use of TP is limited because of its toxicity to liver, kidney, spleen, and heart.
Liver injury in acute and chronic conditions may be induced by drugs, toxic xenobiotics, viral hepatitis, and multiple heritable gene mutations. Patients with suspected liver damage are initially subjected to liver function tests that include the assessment of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) levels. If the levels of these enzymes are abnormal, patients could be subjected to biopsy which is the gold standard used to diagnose liver injury (Soga et al., 2011). Therefore, development of novel diagnostic biomarker with greater sensitivity would enhance the efficiency in the diagnosis of liver damage. Previous studies found serum acylcarnitine levels can provide a better prediction to monitor the initiation of drug-induced hepatotoxicity compared with traditional diagnosis with serum ALT, AST, and ALP (Chen et al., 2009; McGill et al., 2014). Clinically, acylcarnitine levels were used as diagnostic markers of inherited diseases, including carnitine palmitoyltransferase 1 (CPT1) deficiency, medium-chain acyl-CoA dehydrogenase deficiency, very-long-chain acyl-CoA dehydrogenase deficiency, and CPT2 deficiency (Santra and Hendriksz, 2010). Interestingly, these defective genes were direct or indirect peroxisome proliferator-activated receptor α (PPARα) target genes (Mandard et al., 2004). A previous study demonstrated that acetaminophen (APAP) treatment can cause the accumulation of serum acylcarnitines through suppression of PPARα (Chen et al., 2009). In addition, activation of PPARα by fenofibrate can reduce the accumulation of acylcarnitines in mice administered cocaine and α-naphthylisothiocyanate (Shi et al., 2012; Zhao et al., 2017). Therefore, serum long-chain acylcarnitines may be a potential diagnostic biomarker for liver injury, and the activation of PPARα may be considered as a therapeutic target of liver injury.
In this study, the protective role of PPARα and long-chain acylcarnitines in hepatotoxicity was determined after TP exposure using ultra-performance chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOFMS)-based metabolomics. LC/MS-based metabolomics has been widely used for identifying the metabolic pathways associated with hepatotoxicity and renal toxicity (Chen et al., 2016, 2017; Li et al., 2012; Zhang et al., 2012; Zhao et al., 2018). This study revealed that TP could inhibit PPARα signaling and that PPARα activation by fenofibrate could significantly attenuate TP-induced liver injury. Long-chain acylcarnitines were found to be a liver injury biomarker and to protect liver injury through the NOTCH-NRF2 pathway. More importantly, cholestatic liver injury in patients also showed the accumulation of serum long-chain acylcarnitines. These findings provide important insights into the protective role of PPARα activation and long-chain acylcarnitines in TP-induced liver injury, and suggest that activation of PPARα may protect against clinical liver injury.
MATERIALS AND METHODS
Chemicals and reagents
Triptolide and fenofibrate were obtained from Chengdu Mansite Bio-technology Co Ltd (Chengdu, China). GW6471, formic acid, chlorpropamide, lauroylcarnitine (12:0-carnitine), myristoylcarnitine (14:0-carnitine), palmitoylcarnitine (16:0-carnitine), and stearoylcarnitine (18:0-carnitine) were purchased from Sigma-Aldrich (St. Louis, Missouri). N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) was obtained from Medchemexpress (Monmouth Junction, New Jersey). All organic reagents were of the highest grade commercially available.
Patients
A total of 36 serum samples were obtained from The Second Affiliated Hospital of Kunming Medical University. Eighteen patients were diagnosed with cholestatic liver injury and 18 healthy volunteers aged from 32 to 62 years were involved in this study. The health of all volunteers was assessed by clinicians before and during the study. Informed consent was obtained from all individual participants involved in the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee (The Second Affiliated Hospital of Kunming Medical University, registration number: PJ-2017-25). Clinical characteristics of patients, found in Supplementary Table 1, were measured using the VetScan VS2 Comprehensive Diagnostic Profile (Abaxis, Inc, Union City California).
Animals and treatment
Animal experiments were approved by the institutional ethical committee of Kunming Institute of Botany. Male C57BL/6J mice (6–8 weeks old) were purchased from Slaccas Laboratory Animal Co, Ltd (Hunan, China). Male wild-type (Ppara+/+) and Ppara-null (Ppara−/−) mice (6–8 weeks old) on the 129/Sv genetic background was previously described in Chen et al. (2009). The mice were maintained under a standard light/dark of 12 h/12 h cycle and humidity 50%–60% with water and standard rodent chow ad libitum.
Experiment 1: To find the biomarker of TP-induced liver injury, the C57BL/6J mice were given a single intraperitoneal injection of TP (1.0 mg/kg dissolved in 1% DMSO water solution, n = 4). All mice were killed after TP administration for 18 h. Serum and liver samples were collected and stored at −80°C until analysis.
Experiment 2: To investigate the protective effect of PPARα on TP-induced liver injury, the Ppara+/+ and Ppara−/− mice in 129/Sv genetic background were randomly assigned into 3 groups, respectively (n = 5): (1) control; (2) TP; (3) Fenofibrate+TP. Fenofibrate+TP group was orally treated with fenofibrate (200 mg/kg dissolved in 0.5% sodium carboxymethylcellulose) for 3 consecutive days before TP treatment (Zhao et al., 2017). Triptolide and Fenofibrate+TP groups were given a single dose of TP. All mice were killed 18 h after TP administration. Serum and liver samples were collected and stored at −80°C until analysis.
Experiment 3: To determine the role of PPARα on metabolism of TP, Ppara+/+ and Ppara−/− mice on the 129/Sv genetic background and fenofibrate treatment were used (n = 5): (1) TP (Ppara+/+); (2) TP (Ppara−/−); (3) Fenofibrate+TP (Ppara+/+). Fenofibrate+TP group (Ppara+/+) was orally treated with 200 mg/kg fenofibrate for 3 consecutive days before 1 mg/kg TP treatment. Triptolide (Ppara+/+), TP (Ppara−/−), and Fenofibrate+TP (Ppara+/+) groups were given a single IP dose of TP. All the experimental mice were housed separated in metabolic cages for 18 h. Urine and feces were collected for a period of 1–18 h, and serum samples were harvested at 15 min and 18 h after TP administration. All samples were stored at −80°C until analysis.
Experiment 4: To investigate the role of PPARα antagonist, GW6471, in TP-induced liver injury, the C57BL/6J mice were randomly assigned into 3 groups (n = 5): (1) control; (2) TP; (3) GW6471+TP. GW6471+TP group was treated with GW6471 (10 mg/kg dissolved in 4% Tween 80 saline solution, IP) for 3 consecutive days before TP treatment (Zhao et al., 2017). The TP and GW6471+TP groups were given a single dose of TP. All mice were killed after TP administration for 18 h. Serum and liver samples were collected and stored at −80°C until analysis.
Experiment 5: For study with incremental doses of TP, the C57BL/6J mice were treated with 0.5, 0.8, and 1 mg/kg TP. All mice were killed after TP administration for 18 h. Serum sample was collected and stored at −80°C until analysis.
Experiment 6: For study with time-effect and liver regeneration of TP, the C57BL/6J mice were randomly assigned into 2 groups (n = 40): (1) TP; (2) Fenofibrate+TP. Triptolide group was intraperitoneally treated with 1 mg/kg TP, and sacrificed at 0, 3, 6, 12, 18, 24, 48, 72 h after TP treatment. Fenofibrate+TP group was orally treated with 200 mg/kg fenofibrate for 3 consecutive days before 1 mg/kg TP treatment, and sacrificed at 0, 3, 6, 12, 18, 24, 48, 72 h after TP treatment.
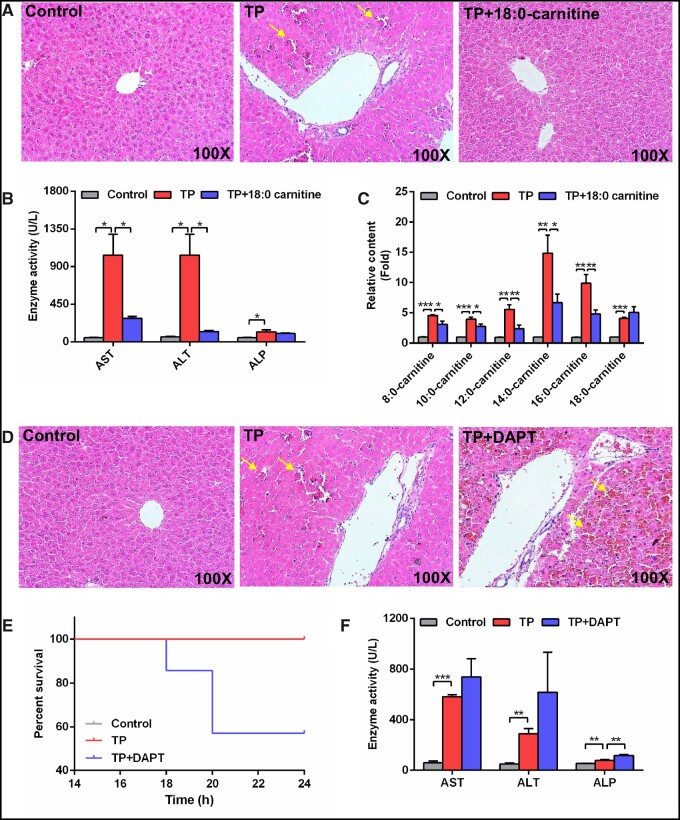
Experiment 7: To evaluate the role of 18:0-carnitine in TP-induced liver injury, the C57BL/6J mice were randomly assigned into 3 groups (n = 7): (1) control; (2) TP; (3) TP + 18:0-carnitine. Triptolide + 18:0-carnitine group was treated with a single dose of 18: 0-carnitine (20 mg/kg dissolved in ethanol/Tween 80/water [1:1:10], IP, 30 min prior to TP treatment) (Gongora et al., 2000). Triptolide and TP + 18:0-carnitine groups were given a single dose of TP. All mice were killed after TP administration for 18 h. Serum samples were collected and stored at −80°C until analysis.
Experiment 8: DAPT is a γ-secretase inhibitor, which can block NOTCH signaling by preventing the final cleavage step of the precursor form of NOTCH to the active NOTCH intracellular domain (NICD) (Jiang et al., 2017). To evaluate the role of NOTCH pathway in TP-induced liver injury, the C57BL/6J mice were randomly assigned to 3 groups (n = 7): (1) control; (2) TP; (3) TP+DAPT. The TP+DAPT group was treated with a single dose of DAPT (10 mg/kg dissolved in DMSO, IP, 30 min prior to TP treatment) (Jiang et al., 2017). Triptolide and TP+DAPT groups were given a single dose of TP. All mice were killed after TP administration for 24 h to evaluate survival rates. Serum samples (18 h) were collected and stored at −80°C until analysis.
Histological examination and biochemical assay
To assess liver damage, fresh liver samples were fixed in 10% neutral buffered formalin. Sections were stained with hematoxylin and eosin (H&E). Aspartate aminotransferase, ALT, and ALP activities were measured following manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Metabolomics analysis and data processing
Serum samples used for metabolomics were prepared using the method previously described in Zhao et al. (2017). The UPLC system was Agilent equipment consisting of a reverse-phase XDB-C18 column (2.1 × 100 mm, 1.8 µm, Agilent, Santa Clara, California). The liquid flow rate was 0.3 ml/min. (A) mobile phase was 0.01% formic acid in water, and (B) mobile phase was 0.01% formic acid in acetonitrile. The elution gradient was as follows: 0.0–12.0 min, 2%–98% B; 12.0–14.0 min, 98% B; 14.0–16.0 min, 98%–2% B. The column temperature was kept at 45°C, and the data were collected in the positive ion mode with electrospray ionization. Nitrogen was applied as both collision gas and drying gas. Capillary voltage was 3.5 kV. Drying gas temperature was 350°C, and nebulizer pressure was 35 psi. Five micromolar chlorpropamide was used as the internal standard.
MassHunter Workstation Software (Agilent) was used to the chromatographic and spectral data analysis. The data matrix was generated using Mass Profinder software (Agilent), and all data were normalized to peak areas of the internal standards. Then the data matrix was analyzed by SIMCA-P + 13.0 software (Umetrics, Kinnelon, New Jersey) for principal component analysis (PCA).
Xenobiotic metabolite analysis
Fifteen minutes serum samples or 18 h urine samples were prepared through mixing the 40 µl serum or 80 µl urine with 360 µl ethyl acetate containing 5 µM chlorpropamide. After vortexed for 1 min, the samples were centrifuged at 15 000 rpm for 5 min. Then, 300 µl supernatants were dried by N2 at room temperature. Subsequently, the residue was dissolved in 100 µl methanol. After centrifugation at 15 000 rpm for 20 min, 5 µl supernatants were injected into LC/MS.
Feces samples were prepared through mixing the 50 mg feces with 400 µl ethyl acetate containing 5 µM chlorpropamide. After shaking at room temperature for 20 min, the samples were centrifuged at 15 000 rpm for 5 min. Then, 150 µl supernatants were dried by N2 at room temperature. Subsequently, the residue was dissolved in 100 µl methanol. After centrifugation at 15 000 rpm for 20 min, 5 µl supernatants were injected into LC/MS.
RT-qPCR analysis and Western blot analysis
Total liver RNA was extracted from frozen tissues using Trizol reagent (Lifetechnologies, Carlsbad, California). qPCR was carried out in a CFX Connect Real-Time System (Bio-Rad Laboratories, Hercules, California) with SYBR green PCR master mix (TaKaRa, Dalian, China). The results were normalized to Gapdh mRNAs. qPCR primer sequences are listed in Supplementary Table 2. Western blot analyses were carried as detailed in a previous report (Crespillo et al., 2011). The following antibodies were used: NOTCH3 (abs136713, ABSIN, Shanghai, China), NRF2 (D1Z9C, Cell Signaling Technology, Massachusetts), GAPDH (14C10, Cell Signaling Technology), and anti-rabbit peroxidase-conjugated second antibody (SA00001-2, Proteintech, Illinois).
Primary mouse hepatocytes isolation and culture
Primary mouse hepatocytes were isolated and cultured from male C57BL/6J mice using 2-step perfusion method (Li et al., 2015). Cells were harvested after incubation with 18:0-carnitine (75 and 150 µM) for 18 h (Primassin et al., 2008). The incubations were conducted in triplicates.
HepG2 cell culture
HepG2 was maintained in 1640 containing 10% fetal bovine serum. Cell were incubation with 12:0-carnitine, 14:0-carnitine, 16:0-carnitine, and 18:0-carnitine (20 and 100 µM) for 18 h.
Statistical analysis
All values were expressed as mean ± SEM. GraphPad Prism v.6 (GraphPad Software, Inc, San Diego, California) was applied for statistical analysis. p Value less than 0.05 was considered to be statistically significant.
RESULTS
Increased Serum Long-chain Acylcarnitines and Inhibition of PPARα in TP-induced Liver Injury
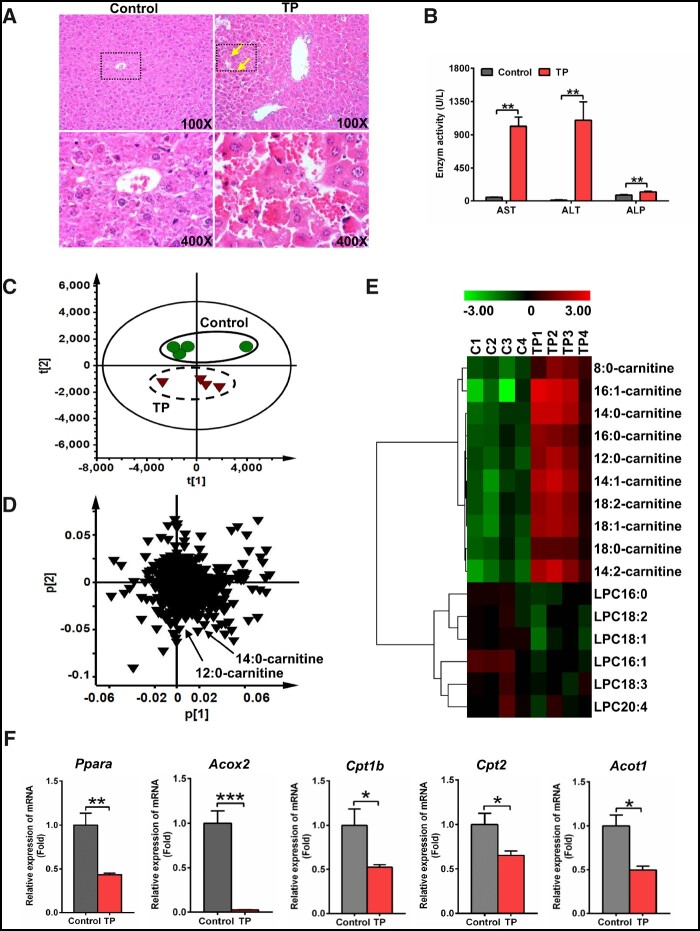
Hepatic histological and biochemical analysis showed that TP exposure dramatically increased hepatic congestion and AST, ALT, and ALP levels (Figs. 1A and 1B), indicating severe liver injury induced by TP. To further explore biomarkers of TP-induced liver injury, serum samples were examined by LC/MS-based metabolomics. The TP group deviated from the control group in PCA, indicating that TP dramatically altered the chemical composition of serum (Figure 1C). Two ions m/z 344.2794+ and 372.3107+ were found to deviate from the ions cloud in the loading scatter plot (Figure 1D). The MS/MS fragmentation identified these ions, m/z 344.2794+ (Rt = 8.43 min) and 372.3107+ (Rt = 9.38 min), as 12:0-carnitine and 14:0-carnitine, respectively. Target analysis showed that 10 medium- and long-chain acylcarnitines were significantly increased by TP (p < .05) (Figure 1E). Furthermore, long-chain acylcarnitines levels were increased within 2 h after TP treatment (Supplementary Figure 1). Further analysis revealed that the levels of long-chain acylcarnitines were positively correlated with the levels of AST and ALT (p < .001), which were 2 widely used markers for liver injury (Supplementary Figure 2).
Figure 1.
Triptolide (TP)-induced severe liver injury and inhibited peroxisome proliferator-activated receptor α signaling. A, Hematoxylin and eosin staining of liver. Solid arrow: congestion. B, Serum AST, ALT, and ALP enzymes activity. Principal component analysis score plot (C) and loading plot (D) derived from UPLC-QTOFMS data of serum ions. Each point represented an individual mouse serum sample (-top) and an ion in the sample (-bottom). Metabolites were labeled in the loading plot (●, control group; ▼, TP group). E, Heat map analysis of the relative abundance of medium- and long-chain acylcarnitines and LPCs in serum of control and TP groups. F, qPCR analysis was performed to measure the expression of Ppara mRNA and its target gene mRNAs (Acox2, Cpt1b, Cpt2, and Acot1). All data were expressed as mean ± SEM (n = 4). *p < .05, **p < .01, and ***p < .001.
Peroxisome proliferator-activated receptor α is the key regulator of fatty acid catabolism and thus modulates acylcarnitines levels. Thus, Ppara mRNA and its target gene mRNAs (acyl-CoA oxidase 2 [Acox2], Cpt1b, Cpt2, and Acyl-CoA thioesterase 1 [Acot1]) were measured in liver. Ppara, Acox2, Cpt1b, Cpt2, and Acot1 mRNAs were significantly downregulated after TP treatment (p < .05) (Figure 1F), indicating inhibition of PPARα signaling by TP exposure. Taken together, these data indicated that TP exposure induced liver injury, accompanied with increased long-chain acylcarnitines that result from inhibition of PPARα signaling.
A dose-response study showed that TP-induced hepatotoxicity and acylcarnitine levels were gradually increased (Supplementary Figure 3). Triptolide-induced hepatotoxicity and acylcarnitine levels were gradually increased within 24 h, and then decreased from 24 to 72 h (Supplementary Figs. 4A–F). Messenger RNAs encoding proliferating cell nuclear antigen (Pcna) and Cyclin D1 (Ccnd1), 2 markers of cell proliferation and liver repair (Bhushan et al., 2014, 2017) were measured after TP administration. Pcna and Ccnd1 mRNAs were markedly induced 18–48 h after TP treatment, and their levels decreased after 48 h (Supplementary Figs. 4G and 4H). However, activation of PPARα by fenofibrate did not affect cell proliferation and liver repair during TP-induced toxicity (Supplementary Figs. 4G and 4H).
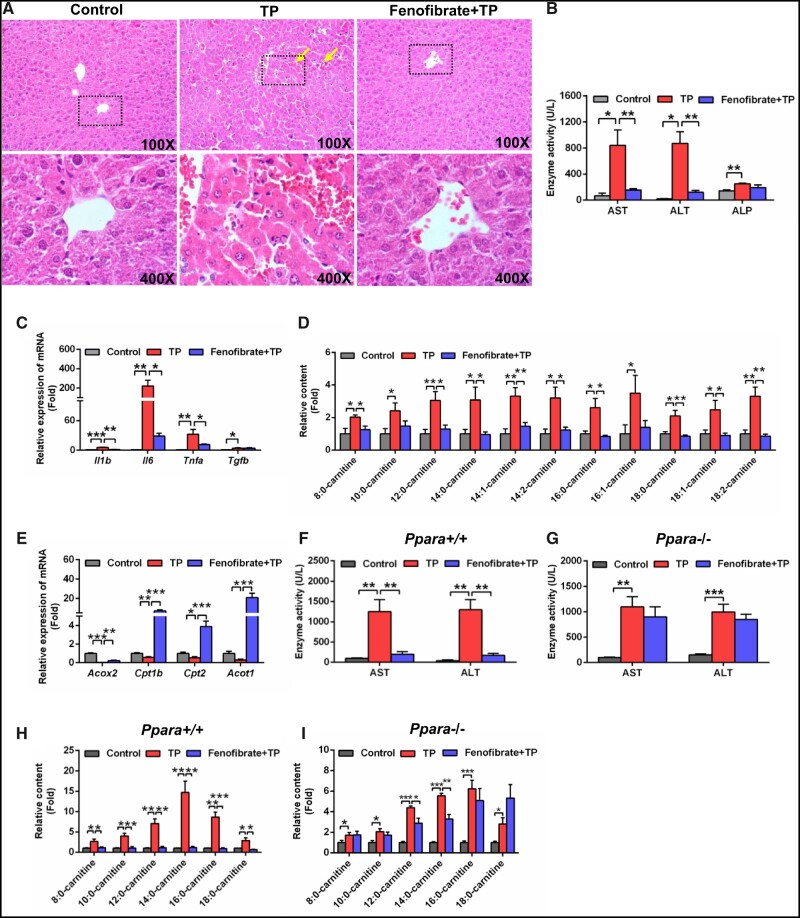
Activation of PPARα by Fenofibrate Ameliorated TP-induced Liver Injury
Because PPARα signaling was inhibited in TP-induced liver injury, activation of PPARα by fenofibrate might protect the mice from TP-induced liver damage. As expected, hepatic histological analysis showed that fenofibrate alleviated congestion induced by TP (Figure 2A). The elevations of AST and ALT in TP-induced liver injury were attenuated by fenofibrate (Figure 2B). In addition, fenofibrate treatment decreased the increased inflammatory cytokines interleukin-1β (Il1b), Il6, tumor necrosis factor α (p < .05) mRNAs after TP treatment (Figure 2C).
Figure 2.
Fenofibrate attenuated TP-induced liver injury. A, Hematoxylin and eosin staining of liver. Solid arrow: congestion. B, Serum AST, ALT, and ALP enzymes activity. C, qPCR analysis of inflammatory factor mRNAs in liver. D, Fenofibrate recovered the increased serum medium- and long-chain acylcarnitine levels. E, qPCR analysis was performed to measure the expression of peroxisome proliferator-activated receptor α target gene mRNAs. Serum AST and ALT activity in Ppara+/+ mice (F) and Ppara−/− mice (G). Serum medium- and long-chain acylcarnitine levels in Ppara+/+ mice (H) and Ppara−/− mice (I). All data were expressed as mean ± SEM (n = 5). *p < .05, **p < .01, and ***p < .001.
Serum metabolomics analysis indicated that the Fenofibrate+TP group was similar to the control group compared with the TP group (Supplementary Figure 5A). In the loading scatter plot of PCA, the 3 top increased ions 344.2794+ (Rt = 8.43), 372.3107+ (Rt = 9.38), and 428.3733+ (Rt = 11.28) in the serum of TP group were identified as 12:0-carnitine, 14:0-carnitine, and 18:0-carnitine, respectively (Supplementary Figure 5B). Further target analysis showed that fenofibrate recovered the levels of 9 medium- and long-chain acylcarnitines (Figure 2D). The lower expression of Acox2, Cpt1b, Cpt2, and Acot1 mRNA in TP-induced liver damage was recovered by fenofibrate (Figure 2E). These data suggested that PPARα activation ameliorated TP-induced liver injury.
To further determine the role of PPARα in TP-induced liver injury, Ppara−/− mice were treated with TP and Fenofibrate+TP. The decreased AST, ALT, and medium- and long-chain acylcarnitines levels in Ppara+/+ mice by fenofibrate were not observed in the Ppara−/− mice (Figs. 2F–I). These findings verified that PPARα played an important role in the therapeutic effect of fenofibrate.
To determine the role of PPARα on TP metabolism, Ppara−/− mice and fenofibrate treatment were used. The parent TP and its 3 hydroxylated metabolites were found in serum 15 min after administration (Supplementary Figure 6B). Three hydroxylated metabolites and a dihydroxylated metabolite were found in urine (Supplementary Figure 6C). No metabolites were detected in feces. These metabolites were identified in a previous report (Hu et al., 2018). No obvious metabolic difference was found between Ppara+/+ mice and Ppara−/− mice, and there was no obvious metabolic difference in Ppara+/+ mice treated with fenofibrate compared with no vehicle treatment (Supplementary Figure 6). These results suggested that the change of PPARα status did not affect TP metabolism.
Inhibition of PPARα by GW6471 Aggravated TP-induced Liver Injury
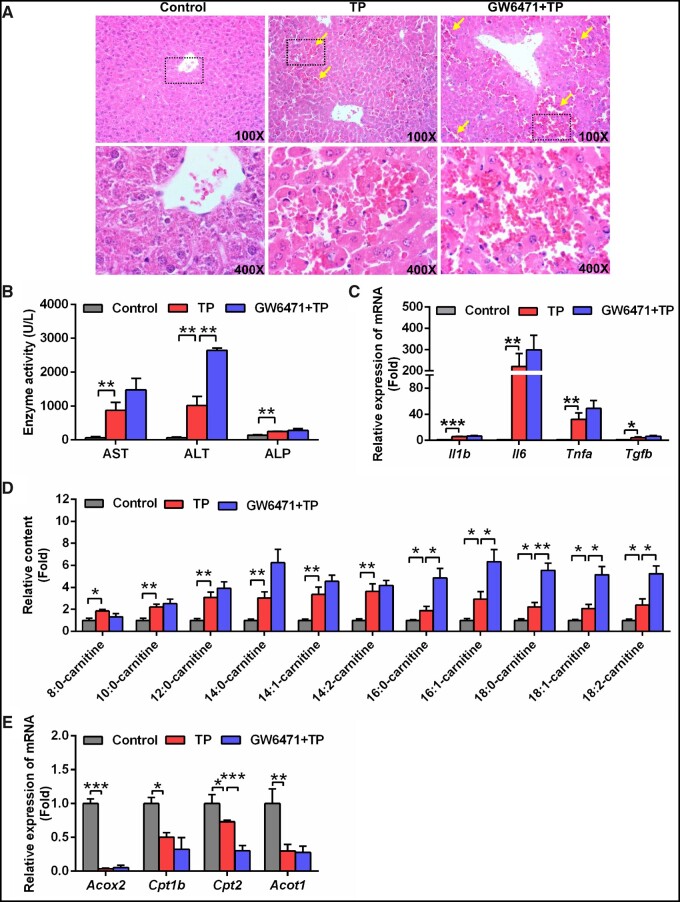
Conversely, TP-induced liver injury was significantly increased by using a selective PPARα inhibitor, GW6471. Hematoxylin and eosin staining showed more congestion accompanied by higher ALT levels in the GW6471+TP group compared with the TP group (p < .05) (Figs. 3A and 3B). The levels of AST, ALP, and inflammatory cytokines were also slightly increased, although no significance was noted (Figs. 3B and 3C).
Figure 3.
GW6471 aggravated TP-induced liver injury. A, Hematoxylin and eosin staining of liver. Solid arrow: congestion. B, Serum AST, ALT, and ALP enzymes activity. C, qPCR analysis of inflammatory factor mRNAs in liver. D, GW6471 aggravated the long-chain acylcarnitine levels in serum compared with TP group. E, QPCR analysis was performed to measure the expression of peroxisome proliferator-activated receptor α target gene mRNAs. All data were expressed as mean ± SEM (n = 5). *p < .05, **p < .01, and ***p < .001.
Serum metabolomics analysis showed that the GW6471+TP group was more deviated from control group compared with TP group (Supplementary Figure 7A), indicating that GW6471 treatment increased the toxicity of TP. In addition, long-chain acylcarnitines were significantly increased in the GW6471+TP group (Supplementary Figure 7B and Figure 3D). Finally, inhibition of Cpt2 mRNA, encoded by a PPARα target gene, after treatment with GW6471 was also found (Figure 3E). These data demonstrated that inhibition of PPARα by GW6471 increased TP-induced liver injury.
Long-Chain Acylcarnitines Activated NOTCH-NRF2 Signaling Pathway
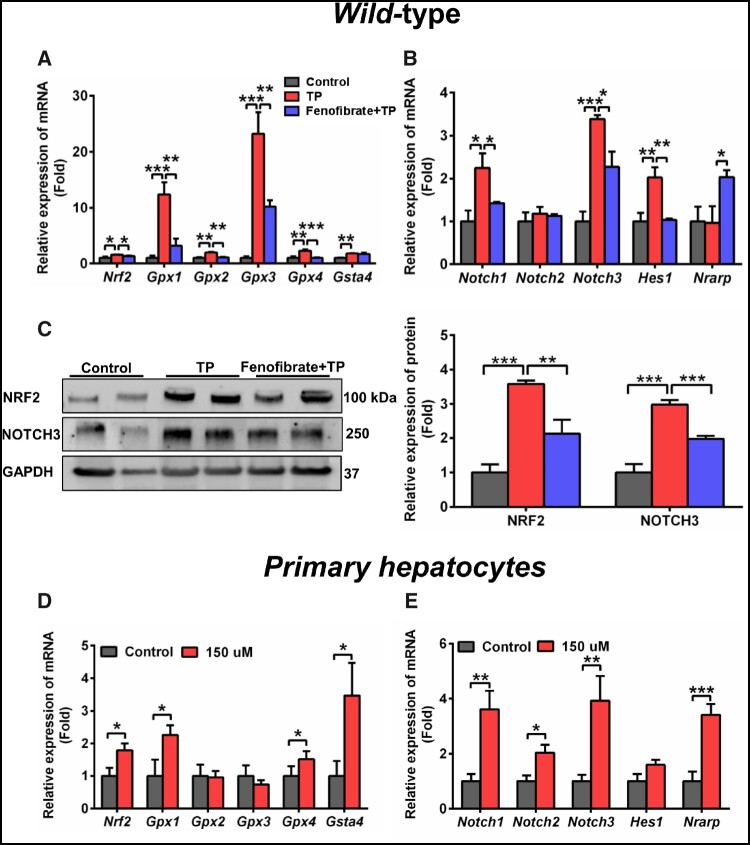
NOTCH signaling pathway could regulate development, differentiation, and homeostasis through cell-cell communication, which was associated with oxidative stress (Yaligar et al., 2016). It has been demonstrated that there was a cross-talk between NOTCH3 and NRF2 pathways (Wakabayashi et al., 2015). qPCR and WB analysis indicated that NOTCH-NRF2 pathway was activated by TP exposure (Figs. 4A–C), suggesting the increase in oxidative stress in TP-induced liver toxicity. Fenofibrate reduced the elevated expression of Nrf2, glutathione peroxidase 1 (Gpx1), Gpx2, Gpx3, Gpx4, Notch1, Notch3, and Hes1 mRNAs induced by TP (Figs. 4A and 4B). Furthermore, the elevated protein levels of NRF2 and NOTCH3 were also recovered following the activation of PPARα by fenofibrate (Figure 4C).
Figure 4.
Long-chain acylcarnitines developed defense response by activating NRF2 and NOTCH signaling. qPCR analysis of the gene expression of NRF2 (A) and NOTCH (B) pathway in WT mice. C, Western blot was used to measure NRF2 and NOTCH3 in WT mice. qPCR analysis of the Nrf2 (and NRF target genes) (D) and Notch (and NOTCH target genes) (E) mRNAs in primary hepatocytes after treatment with 150 µM 18:0-carnitine. All data were expressed as mean ± SEM. *p < .05, **p < .01, and ***p < .001.
The bioactivity of 12:0-carnitine, 14:0-carnitine, 16:0-carnitine, and 18:0-carnitine was evaluated in HepG2 cells. 18:0-Carnitine showed the strongest NRF2 activation compared with the other acylcarnitines (Supplementary Figure 8). Therefore, 18:0-carnitine was chosen for use in the following study. Expression of Nrf2, Notch1, Notch2, Notch3, and their target gene mRNAs Gpx1, Gpx4, Gsta4, and NOTCH-regulated ankyrin repeat protein (Nrarp), were dramatically increased following 18:0-carnitine exposure (Figs. 4D and 4E), suggesting that the increased long-chain acylcarnitines during liver injury would activate NRF2-NOTCH pathway.
Acylcarnitine-NOTCH Signaling Pathway Protected TP-Induced Liver Injury
To evaluate the important role of long-chain acylcarnitines, mice were treated with 20 mg/kg 18:0-carnitine. Hepatic histological analysis showed that 18:0-carnitine alleviated congestion induced by TP (Figure 5A). The elevations of AST, ALT, and medium- and long-chain acylcarnitines in TP-induced liver injury were attenuated by 18:0-carnitine treatment (Figs. 5B and 5C).
Figure 5.
18:0-Carnitine protected against TP-induced liver damage and blocked the NOTCH signaling pathway aggravated TP-induced liver damage. A, Hematoxylin and eosin staining of liver after 18:0-carnitine treatment. Solid arrow: congestion. B, Serum AST, ALT, and ALP enzymes activity after 18: 0-carnitine treatment. C, Medium- and long-chain acylcarnitine levels after 18: 0-carnitine treatment. D, Hematoxylin and eosin staining of liver after DAPT treatment. Solid arrow: congestion. E, Animal survival curves after DAPT treatment over 24 h. F, Serum AST, ALT, and ALP enzymes activity after DAPT treatment. All data were expressed as mean ± SEM. *p < .05, **p < .01, and ***p < .001.
Mice treated with DAPT, which can block NOTCH signaling, showed increased congestion, mortality, and ALP levels (Figs. 5D–F). These results indicate that the inhibition of NOTCH signaling increased TP-induced liver injury. In summary, acylcarnitine-NOTCH signaling protected from TP-induced liver injury through defense response.
PPARα and Long-Chain Acylcarnitines in Cholestatic Liver Injury Patients
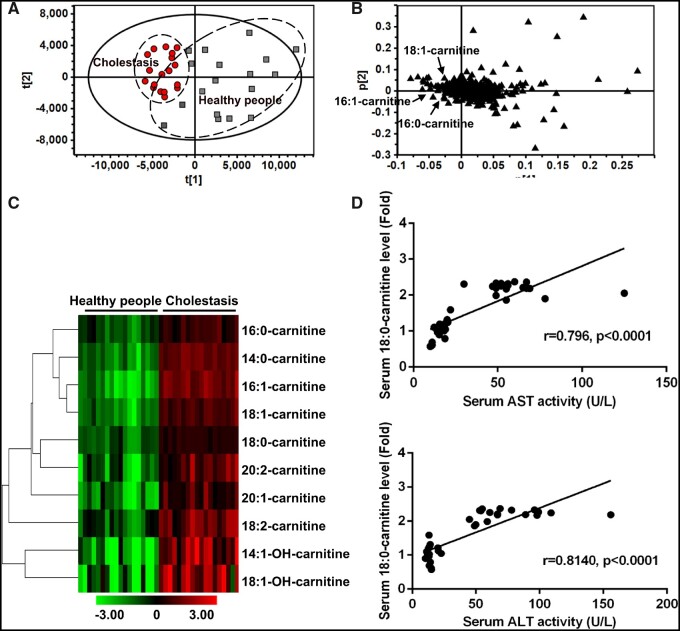
The cholestatic patients deviated from the normal subjects in PCA, indicating that cholestasis dramatically altered the chemical composition of serum (Figure 6A). Long-chain acylcarnitines were significantly increased in the cholestatic patients (Figs. 6B and 6C). Further analysis revealed that the levels of 18:0-carnitine were positively correlated with the levels of AST and ALT (p < .001) (Figure 6D). These findings demonstrated that PPARα and long-chain acylcarnitines played an important role in human liver injury.
Figure 6.
Long-chain acylcarnitines in cholestatic liver injury patients. Principal component analysis score plot (A) and loading plot (B) derived from UPLC-QTOFMS data of serum ions. Each point represented an individual serum sample (-left) and an ion in the sample (-right). Metabolites were labeled in the loading plot (■, Healthy people; ●, Cholestasis). C, Heat map analysis of long-chain acylcarnitines between cholestasis and healthy people. D, Correlation analysis between the levels of 18:0-carntine and serum AST and ALT. Correlation factor (r) and p value were estimated with Pearson’s correlation analysis. All data were expressed as mean ± SEM.
DISCUSSION
Metabolomics analysis revealed that serum long-chain acylcarnitines could be used as diagnostic biomarker for liver injury and could also induce a defense response through activating the NOTCH-NRF2 pathway. Furthermore, activation of PPARα by fenofibrate could improve TP-induced liver injury. A clinical pilot study found that liver injury patients also had higher serum long-chain acylcarnitines and lower hepatic PPARα protein levels compared with healthy volunteers. These findings provide the rationale for the use of long-chain acylcarnitines as diagnostic biomarkers for liver injury, and PPARα agonists as therapeutic alternatives for liver injury.
The levels of acylcarnitines in serum have been used as clinical biomarkers for screening inborn genetic defects in fatty acid β-oxidation pathways (Santra and Hendriksz, 2010). Except for their association with genetic defects, acylcarnitines usually result from liver injury. The concentration of acylcarnitines was significantly higher in human hepatocellular carcinoma patients and in mice with liver tumors compared with normal subjects and non-tumor bearing mice (Yaligar et al., 2016). α-Naphthylisothiocyanate-induced cholestasis also resulted in the accumulation of serum long-chain acylcarnitines (Zhao et al., 2017). However, accumulation of long-chain acylcarnitines had not been used as a common contributing factor in liver injury even through it was known that liver injury can cause severe disruption of lipid metabolism. In this study, long-chain acylcarnitines were significantly increased in liver injury patients and TP-induced mouse liver injury models. Therefore, long-chain acylcarnitines may be a common serum biomarker for liver injury.
A previous study found that long-chain acylcarnitines had the potential to mediate a cellular stress response (McCoin et al., 2015). They can activate some receptors, such as toll-like receptor 2 (TLR2), TLR4, NF-κB, cyclooxygenase-2, and JNK (McCoin et al., 2015). This study demonstrated that long-chain acylcarnitines could activate the NOTCH-NRF2 pathway and induce a defense response against liver injury. This suggests that patients with increased serum levels of long-chain acylcarnitines would have a better prognosis. An earlier study revealed that tissue injury and repair were involved in hepatotoxicity (Anand and Mehendale, 2004). Therefore, some increased metabolites such as acylcarnitines, glutamine, and ursodeoxycholic acid, that appear during the process of hepatotoxicity, may have a protective role. Notably, glutamine protected against CCl4-induced liver fibrosis, although glutamine was significantly increased in this model (Liang et al., 2016; Shrestha et al., 2016). Ursodeoxycholic acid, that is elevated in clinical cholestasis, is approved by the FDA to treat cholestatic liver injury (Zhao et al., 2019a). Acetylcarnitine may also protect against acetaminophen-induced hepatotoxicity, although acetylcarnitine was significantly increased in this hepatotoxicity model (Alotaibi et al., 2016; Bhattacharyya et al., 2014; Chen et al., 2009).
Peroxisome proliferator-activated receptor α is expressed in metabolically active tissues, especially liver, and regulates genes involved in lipid metabolism (Mandard et al., 2004). Peroxisome proliferator-activated receptor α plays a crucial role in liver injury. The deficiency and inhibition of PPARα would aggravate liver injury. Severe liver dysfunction was induced in Ppara−/− mice during cholic acid challenge comparing with Ppara+/+ mice, including disruption of bile acids, phospholipids, and cholesterol homeostasis (Li et al., 2012). Ppara−/− mice fed a high-fat diet accumulated more hepatic triglycerides compared with Ppara+/+ mice (Stienstra et al., 2007). Peroxisome proliferator-activated receptor α expression was lower in clinical patients with liver injury. Hepatic PPARα and CPT1α levels were profoundly lower in the patients with HCV infection compared with healthy people (Dharancy et al., 2005). The level of PPARα was downregulated in alcoholic liver disease (Wan et al., 1994). In humans with nonalcoholic fatty liver disease, hepatic expression of PPARα was also decreased (Francque et al., 2015). Using a PPARα agonist, inhibitor, and Ppara−/− mice, this study provided evidence that the PPARα signaling pathway played an important role in TP-induced liver injury.
Fenofibrate is a selective PPARα agonist widely used for the treatment of hypertriglyceridemia, hyperlipidemia, and cholestatic liver disease, such as primary biliary cirrhosis (Ghonem et al., 2015). Fenofibrate can improve various liver injury such as diet- or concanavalin A-induced hepatitis (Mohamed et al., 2013; Rajamoorthi et al., 2017), hepatic ischemia reperfusion (Boshra and Moustafa, 2011), cholestasis (Zhao et al., 2017), and sunitinib-induced hepatic injury (Zhao et al., 2019b). This study demonstrated that fenofibrate could protect TP-induced liver injury in animal models.
NOTCH signaling is an intracellular signaling pathway, related to liver injury that result from drug-induced hepatotoxicity, inflammation, hepatic ischemia reperfusion, and liver fibrosis (Bansal et al., 2015; Jiang et al., 2017; Wei et al., 2016; Yu et al., 2016). A previous study reported that TP treatment increased NOTCH signaling (Wang et al., 2016). Furthermore, NRF2-related genes could be upregulated by 16:0-carnitine (Zhao et al., 2017). NRF2 is a transcription factor that mediates a broad-based set of adaptive responses to cellular stresses (Wakabayashi et al., 2010), and is closely related to various liver diseases, including cholestasis, drug-induced hepatotoxicity, hepatic ischemia reperfusion, and hepatic iron overload (Kudoh et al., 2014; Silva-Gomes et al., 2014; Zollner et al., 2010). Therefore, NOTCH-NRF2 signaling is important in hepatoprotection. Both NOTCH and NRF2 are evolutionarily conserved among animals, and there exists transcriptional cross-talk between the NOTCH and NRF2 pathways. NOTCH signaling could directly activate NRF2 through combination of the NICD transcriptosome to the Nrf2 promoter (Wakabayashi et al., 2014). NRF2 can also directly regulate NOTCH signaling through an antioxidant response element sequences in the Notch promoter (Wakabayashi et al., 2010). In this study, NOTCH-NRF2 signaling was significantly activated by TP in murine models, which induced defense response.
PPARα signaling was significantly inhibited in TP-induced liver injury. Peroxisome proliferator-activated receptor α activation by fenofibrate markedly improved TP-induced liver injury, whereas PPARα inhibition by the antagonist GW6471 potentiated liver injury, indicating an important role for PPARα in the hepatoprotection. Increased long-chain acylcarnitines were further found to protect against TP-induced liver injury. These findings provide the evidence for the protective role of PPARα and long-chain acylcarnitines in TP-induced hepatotoxicity, and suggested that the modulation of PPARα may protect against clinical liver injury.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the National Key Research and Development Program of China (2017YFC1700906, 2017YFC0906903); CAS “Light of West China” Program (Y72E8211W1); Kunming Institute of Botany (Y76E1211K1, Y4662211K1); and State Key Laboratory of Phytochemistry and Plant Resources in West China (52Y67A9211Z1).
Supplementary Material
Contributor Information
Dan-Dan Hu, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; School of Pharmaceutical Science and Yunnan Key Laboratory of Pharmacology of Natural Products, Kunming Medical University, Kunming 650500, China.
Qi Zhao, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
Yan Cheng, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
Xue-Rong Xiao, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
Jian-Feng Huang, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
Yan Qu, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
Xian Li, School of Pharmaceutical Science and Yunnan Key Laboratory of Pharmacology of Natural Products, Kunming Medical University, Kunming 650500, China.
Ying-Mei Tang, Department of Gastroenterology, Yunnan Research Center for Liver Diseases, The 2nd Affiliated Hospital of Kunming Medical University, Kunming 650033, China.
Wei-Min Bao, Department of General Surgery, Yunnan Provincial 1st People’s Hospital, Kunming 650032, China.
Jin-Hui Yang, Department of Gastroenterology, Yunnan Research Center for Liver Diseases, The 2nd Affiliated Hospital of Kunming Medical University, Kunming 650033, China.
Tao Jiang, Department of Gastroenterology, Yunnan Research Center for Liver Diseases, The 2nd Affiliated Hospital of Kunming Medical University, Kunming 650033, China.
Jia-Peng Hu, Clinical Laboratory, The 2nd Affiliated Hospital of Kunming Medical University, Kunming 650033, China.
Frank J Gonzalez, Laboratory of Metabolism, National Cancer Institute, National Institutes of Health, Center for Cancer Research, Bethesda, Maryland 20892.
Fei Li, State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China.
The authors certify that all research involving human subjects was done under full compliance with all government policies and the Helsinki Declaration.
REFERENCES
- Alotaibi S. A., Alanazi A., Bakheet S. A., Alharbi N. O., Nagi M. N. (2016). Prophylactic and therapeutic potential of acetyl-l-carnitine against acetaminophen-induced hepatotoxicity in mice. J. Biochem. Mol. Toxicol. 30, 5–11. [DOI] [PubMed] [Google Scholar]
- Anand S. S., Mehendale H. M. (2004). Liver regeneration: A critical toxicodynamic response in predictive toxicology. Environ. Toxicol. Pharmacol. 18, 149–160. [DOI] [PubMed] [Google Scholar]
- Bansal R., van Baarlen J., Storm G., Prakash J. (2015). The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci. Rep. 5, 18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Yan K., Pence L., Simpson P. M., Gill P., Letzig L. G., Beger R. D., Sullivan J. E., Kearns G. L., Reed M. D. (2014). Targeted liquid chromatography-mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomarkers Med. 8, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B., Poudel S., Manley M. W. Jr, Roy N., Apte U. (2017). Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am. J. Pathol. 187, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B., Walesky C., Manley M., Gallagher T., Borude P., Edwards G., Monga S. P., Apte U. (2014). Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol. 184, 3013–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshra V., Moustafa A. M. (2011). Effect of preischemic treatment with fenofibrate, a peroxisome proliferator-activated receptor-α ligand, on hepatic ischemia-reperfusion injury in rats. J. Mol. Histol. 42, 113–122. [DOI] [PubMed] [Google Scholar]
- Chen C., Krausz K. W., Shah Y. M., Idle J. R., Gonzalez F. J. (2009). Serum metabolomics reveals irreversible inhibition of fatty acid β-oxidation through the suppression of PPARα activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem. Res. Toxicol. 22, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Q., Cao G., Chen H., Liu D., Su W., Yu X. Y., Vaziri N. D., Liu X. H., Bai X., Zhang L. (2017). Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/β-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol. 12, 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Cao G., Chen D. Q., Wang M., Vaziri N. D., Zhang Z. H., Mao J. R., Bai X., Zhao Y. Y. (2016). Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol. 10, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespillo A., Alonso M., Vida M., Pavon F. J., Serrano A., Rivera P., Romero-Zerbo Y., Fernandez-Llebrez P., Martinez A., Perez-Valero V. (2011). Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 164, 1899–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharancy S., Malapel M., Perlemuter G., Roskams T., Cheng Y., Dubuquoy L., Podevin P., Conti F., Canva V., Philippe D., et al. (2005). Impaired expression of the peroxisome proliferator-activated receptor α during hepatitis C virus infection. Gastroenterology 128, 334–342. [DOI] [PubMed] [Google Scholar]
- Francque S., Verrijken A., Caron S., Prawitt J., Paumelle R., Derudas B., Lefebvre P., Taskinen M. R., Van Hul W., Mertens I., et al. (2015). PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 63, 164–173. [DOI] [PubMed] [Google Scholar]
- Ghonem N. S., Assis D. N., Boyer J. L. (2015). Fibrates and cholestasis. Hepatology 62, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongora L., Manez S., Giner R. M., Recio M. C., Rios J. L. (2000). On the activity of trifluoperazine and palmitoylcarnitine in mice: Delayed hypersensitivity models. Life Sci. 66, Pl183–188. [DOI] [PubMed] [Google Scholar]
- Hu D. D., Chen X. L., Xiao X. R., Wang Y. K., Liu F., Zhao Q., Li X., Yang X. W., Li F. (2018). Comparative metabolism of tripolide and triptonide using metabolomics. Food Chem. Toxicol. 115, 98–108. [DOI] [PubMed] [Google Scholar]
- Jiang L., Ke M., Yue S., Xiao W., Yan Y., Deng X., Ying Q. L., Li J., Ke B. (2017). Blockade of Notch signaling promotes acetaminophen-induced liver injury. Immunol. Res. 65, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh K., Uchinami H., Yoshioka M., Seki E., Yamamoto Y. (2014). Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann. Surg. 260, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Patterson A. D., Krausz K. W., Tanaka N., Gonzalez F. J. (2012). Metabolomics reveals an essential role for peroxisome proliferator-activated receptor α in bile acid homeostasis. J. Lipid Res. 53, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Yang X. W., Krausz K. W., Nichols R. G., Xu W., Patterson A. D., Gonzalez F. J. (2015). Modulation of colon cancer by nutmeg. J. Proteome Res. 14, 1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. H., Tang C. L., Lu S. Y., Cheng B., Wu F., Chen Z. N., Song F., Ruan J. X., Zhang H. Y., Song H., et al. (2016). Serum metabonomics study of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced acute hepatotoxicity in rats by (1)H NMR analysis. J. Pharm. Biomed. Anal. 129, 70–79. [DOI] [PubMed] [Google Scholar]
- Liu J., Wu Q. L., Feng Y. H., Wang Y. F., Li X. Y., Zuo J. P. (2005). Triptolide suppresses CD80 and CD86 expressions and IL-12 production in THP-1 cells. Acta Pharmacol. Sin. 26, 223–227. [DOI] [PubMed] [Google Scholar]
- Mandard S., Muller M., Kersten S. (2004). Peroxisome proliferator-activated receptor α target genes. Cell. Mol. Life Sci. 61, 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin S. A., Belenguer A., Stacey V. E., Qlan S. Z., Xu Y., Zhang J. W., Sanders J. K. M., Amor S. R., Pearce C. M. (1993). Male antifertility compounds from Tripterygium wilfordii Hook F. Contraception 47, 387–400. [DOI] [PubMed] [Google Scholar]
- McCoin C. S., Knotts T. A., Adams S. H. (2015). Acylcarnitines—Old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 11, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M. R., Li F., Sharpe M. R., Williams C. D., Curry S. C., Ma X. C., Jaeschke H. (2014). Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 88, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed D. I., Elmelegy A. A., El-Aziz L. F., Abdel Kawy H. S., El-Samad A. A., El-Kharashi O. A. (2013). Fenofibrate A peroxisome proliferator activated receptor-α agonist treatment ameliorates concanavalin A-induced hepatitis in rats. Eur. J. Pharmacol. 721, 35–42. [DOI] [PubMed] [Google Scholar]
- Primassin S., Ter Veld F., Mayatepek E., Spiekerkoetter U. (2008). Carnitine supplementation induces acylcarnitine production in tissues of very long-chain acyl-CoA dehydrogenase-deficient mice, without replenishing low free carnitine. Pediatr. Res. 63, 632–637. [DOI] [PubMed] [Google Scholar]
- Rajamoorthi A., Arias N., Basta J., Lee R. G., Baldan A. (2017). Amelioration of diet-induced steatohepatitis in mice following combined therapy with ASO-Fsp27 and fenofibrate. J. Lipid Res. 58, 2127–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S., Hendriksz C. (2010). How to use acylcarnitine profiles to help diagnose inborn errors of metabolism. Arch. Dis. Child Educ. 95, 151–156. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Yao D., Gosnell B. A., Chen C. (2012). Lipidomic profiling reveals protective function of fatty acid oxidation in cocaine-induced hepatotoxicity. J. Lipid Res. 53, 2318–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha N., Chand L., Han M. K., Lee S. O., Kim C. Y., Jeong Y. J. (2016). Glutamine inhibits CCl4 induced liver fibrosis in mice and TGF-β1 mediated epithelial-mesenchymal transition in mouse hepatocytes. Food Chem. Toxicol. 93, 129–137. [DOI] [PubMed] [Google Scholar]
- Silva-Gomes S., Santos A. G., Caldas C., Silva C. M., Neves J. V., Lopes J., Carneiro F., Rodrigues P. N., Duarte T. L. (2014). Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J. Hepatol. 60, 354–361. [DOI] [PubMed] [Google Scholar]
- Soga T., Sugimoto M., Honma M., Mori M., Igarashi K., Kashikura K., Ikeda S., Hirayama A., Yamamoto T., Yoshida H., et al. (2011). Serum metabolomics reveals gamma-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J. Hepatol. 55, 896–905. [DOI] [PubMed] [Google Scholar]
- Stienstra R., Mandard S., Patsouris D., Maass C., Kersten S., Müller M. (2007). Peroxisome proliferator-activated receptor α protects against obesity-induced hepatic inflammation. Endocrinology 148, 2753–2763. [DOI] [PubMed] [Google Scholar]
- Tao X., Cush J. J., Garret M., Lipsky P. E. (2001). A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii Hook F in rheumatoid arthritis. J. Rheumatol. 28, 2160–2167. [PubMed] [Google Scholar]
- Wakabayashi N., Chartoumpekis D. V., Kensler T. W. (2015). Crosstalk between Nrf2 and Notch signaling. Free Radic. Biol. Med. 88, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Skoko J. J., Chartoumpekis D. V., Kimura S., Slocum S. L., Noda K., Palliyaguru D. L., Fujimuro M., Boley P. A., Tanaka Y., et al. (2014). Notch-Nrf2 axis: Regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 34, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Slocum S. L., Skoko J. J., Shin S., Kensler T. W. (2010). When NRF2 talks, who’s listening? Antioxid. Redox Signal. 13, 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., Morimoto M., Thurman R. G., Bojes H. K., French S. W. (1994). Expression of the peroxisome proliferator-activated receptor gene is decreased in experimental alcoholic liver disease. Life Sci. 56, 307–317. [DOI] [PubMed] [Google Scholar]
- Wang X., Sun L., Zhang L., Jiang Z. (2016). Effect of adoptive transfer or depletion of regulatory T cells on triptolide-induced liver injury. Front. Pharmacol. 7, 99.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Wang J. P., Hao C. Q., Yang X. F., Wang L. X., Huang C. X., Bai X. F., Lian J. Q., Zhang Y. (2016). Notch signaling contributes to liver inflammation by regulation of interleukin-22-producing cells in hepatitis B virus infection. Front. Cell. Infect. Microbiol. 6, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaligar J., Teoh W. W., Othman R., Verma S. K., Phang B. H., Lee S. S., Wang W. W., Toh H. C., Gopalan V., Sabapathy K. (2016). Longitudinal metabolic imaging of hepatocellular carcinoma in transgenic mouse models identifies acylcarnitine as a potential biomarker for early detection. Sci. Rep. 6, 20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Chen J., Guo Z., Xu X. M., Wang L., Pei X. F., Yang J., Underhill C. B., Zhang L. (2003). Triptolide inhibits the growth and metastasis of solid tumors. Mol. Cancer Ther. 2, 65–72. [PubMed] [Google Scholar]
- Yu H. C., Bai L., Yang Z. X., Qin H. Y., Tao K. S., Han H., Dou K. F. (2016). Blocking Notch signal in myeloid cells alleviates hepatic ischemia reperfusion injury by repressing the activation of NF-kappaB through CYLD. Sci. Rep. 6, 32226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li F., Patterson A. D., Wang Y., Krausz K. W., Neale G., Thomas S., Nachagari D., Vogel P., Vore M., et al. (2012). Abcb11 deficiency induces cholestasis coupled to impaired β-fatty acid oxidation in mice. J. Biol. Chem. 287, 24784–24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Liu F., Cheng Y., Xiao X. R., Hu D. D., Tang Y. M., Bao W. M., Yang J. H., Jiang T., Hu J. P., et al. (2019a). Celastrol protects from cholestatic liver injury through modulation of SIRT1-FXR signaling. Mol. Cell. Proteomics 18, 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yang R., Wang J., Hu D. D., Li F. (2017). PPARα activation protects against cholestatic liver injury. Sci. Rep. 7, 9967.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zhang J. L., Li F. (2018). Application of metabolomics in the study of natural products. Nat. Prod. Bioprospect. 8, 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zhang T., Xiao X. R., Huang J. F., Wang Y., Gonzalez F. J., Li F. (2019b). Impaired clearance of sunitinib leads to metabolic disorders and hepatotoxicity. Br. J. Pharmacol. 176, 2162–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G., Wagner M., Trauner M. (2010). Nuclear receptors as drug targets in cholestasis and drug-induced hepatotoxicity. Pharmacol. Ther. 126, 228–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.