Abstract
The primitive cardiac tube starts beating 6–8 weeks post fertilization in the developing embryo. In order to describe normal cardiac development during late first and early second trimester in human fetuses this study used microarray and pathways analysis and created a corresponding ‘normal’ database. Fourteen fetal hearts from human fetuses between 10 and 18 weeks of gestational age (GA) were prospectively collected at the time of elective termination of pregnancy. RNA from recovered tissues was used for transcriptome analysis with Affymetrix 1.0 ST microarray chip. From the amassed data we investigated differences in cardiac development within the 10–18 GA period dividing the sample by GA in three groups: 10–12 (H1), 13–15 (H2) and 16–18 (H3) weeks. A fold change of 2 or above adjusted for a false discovery rate of 5% was used as initial cutoff to determine differential gene expression for individual genes. Test for enrichment to identify functional groups was carried out using the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG). Array analysis correctly identified the cardiac specific genes, and transcripts reported to be differentially expressed were confirmed by qRT–PCR. Single transcript and Ontology analysis showed first trimester heart expression of myosin-related genes to be up-regulated >5-fold compared with second trimester heart. In contrast the second trimester hearts showed further gestation-related increases in many genes involved in energy production and cardiac remodeling. In conclusion, fetal heart development during the first trimester was dominated by heart-specific genes coding for myocardial development and differentiation. During the second trimester, transcripts related to energy generation and cardiomyocyte communication for contractile coordination/proliferation were more dominant. Transcripts related to fatty acid metabolism can be seen as early as 10 weeks and clearly increase as the heart matures. Retinol receptor and gamma-aminobutyric acid (GABA) receptor transcripts were detected, and have not been described previously in human fetal heart during this period. For the first time global gene expression of heart has been described in human samples to create a database of normal development to understand and compare with known abnormal fetal heart development.
Keywords: fetal, human, microarray, renin-angiotensin, cardiac development
Introduction
Six to eight weeks after fertilization and toward the end of the embryonic period, the primitive cardiac tube, with four chambers, starts beating. At this time, the remaining cardiac stem cells became differentiated cardiomyocytes. Through the remaining pregnancy period, the cardiomyocytes then undergo differentiation and specialization into specific cardiac tissues (Wheater et al., 1987; Moore, 1989; Gratacos et al., 2007). Due to the obvious difficulty in obtaining human cardiac tissue, most of the literature in early cardiac profiling is based on animal models, such as mice and sheep. Studies looking for common pathways that may explain cardiac failure have been performed in human fetal hearts compared with failing adult heart profiles (Razeghi et al., 2001), but such studies were limited to the 18- to 28-week period of gestation.
Previous cardiac development studies have elucidated, in part, the metabolic profile of the early human heart. Fetal heart energy production has been described as dependent on glucose metabolism during the whole fetal life, which changes to fatty acid oxidation during the first weeks of the neonatal period (Lopaschuk and Jaswal, 2010). The adult heart in turn depends on fatty acid oxidation for energy generation. Previous experiments using mouse models have also assessed the transition in energy profile from embryonic stem cells to fetal cardiomyocyte during the first stages of pregnancy. Cardiac stem cells appear to obtain energy from anaerobic metabolism that transforms into a mitochondria-mediated oxidative metabolism to meet increases in energy demand during cardiomyocyte differentiation and development during fetal life (Chung et al., 2007; Lopaschuk and Jaswal, 2010). No data are available to verify if this is also true in humans.
The regulation of fetal cardiomyocyte growth is essential since the number of cardiac cells at birth predetermines the number of cardiomyocytes for life, which is a predictor for cardiac disease (Thornburg et al., 2010). Studies in sheep have shown that proliferation of cardiomyocytes is stimulated by angiotensin II, cortisol and insulin-like growth factor-1 in the womb (Thornburg et al., 2010). Thus, in order to understand cardiac disease in humans, it is imperative to study if these same endocrine systems are present in early fetal life.
In the current study, the gene expression profile of human fetal heart between 10 and 18 weeks' gestation is presented. The working hypothesis was that there is a developmental change in gene profile between fetal hearts in the late first trimester (10–12 weeks' gestation) compared with the early second trimester (16–18 weeks' gestation), which would be detectable by transcript analysis and/or pathway analysis. To this end we have analyzed fetal heart mRNA using Affymetrix arrays in an attempt to both investigate changes in gene expression in human fetal heart and provide a much needed reference database to guide future studies in normal and abnormal human fetal development.
Methods
Tissue collection and RNA isolation
Human fetal cardiac tissue was collected after informed consent following institutional guidelines, from patients older than 18 years of age undergoing an elective dilation and curettage for termination of pregnancy due to unwanted pregnancy between 10 and 18 weeks of gestational age. Patients had already undergone the surgical consent process with their health team before they were approached to donate fetal tissue samples under an IRB approved protocol. Those who expressed willingness for such research tissue donation then underwent a research informed consent process. All pregnancies had a dating ultrasound done within a week of the procedure. The suction was generated by a vacuum with a standard suction unit providing variable controlled suction up to 550 mmHg, and a suction flow rate of 30 lpm.
Before the procedure, collection bottles were placed at 4°C for 1 h. At the time of the procedure, 100 ml (at 4°C), RNAse-free water solution (BP561-1 Water, Sterile for RNA work, Fisher Scientific, USA) mixed with 10× PBS buffer (Ambion, USA) at a final concentration of 10%, was added to the collecting bottle.
The procedure was performed within 5 min of adding the cold water. After the procedure, the heart was grasped with sterilized surgical instruments and carefully isolated from the rest of the thoracic content to be placed in RNAse-free plastic tubes. Fetal brain was also collected in the same fashion. No karyotype was done to confirm normal chromosomes. The brain tissue was used for comparison looking for major differences among the study group and the brain group as an internal validation.
These tubes were immediately placed in liquid nitrogen for transport. The tissue was then stored in a −80°C freezer.
Tissue RNA was extracted using RNA STAT-60 reagents, as recommended by the manufacturer (Tel-Test, Inc., Friendswood, TX, USA). All procedures were run on ice. A starting ratio of 1 ml reagent/100 mg tissue was used to ensure efficient, intact RNA recovery. RNA concentration and purity were initially determined using spectrophotometric A260 and A260/280 ratios (NanoDrop Products, Thermo Fisher Scientific, Inc., Wilmington, DE, USA). RNA quantification and quality control were performed using the Agilent Bioanalyzer (Agilent Technology, Santa Clara, CA, USA) 260/280 ratios and standard RIN-values (RNA integrity number). A value of 1.7–2.1 was considered workable for further testing.
Array preparation
Fourteen hearts RNA samples were divided into three Affymetrix ST 1.0 arrays according to gestational age. Array #1 analyzed six hearts RNA samples from 10 to 12 weeks' gestation. Array #2 analyzed six hearts RNA samples from 13 to 15 weeks' gestation, and Array #3 analyzed two hearts RNA samples from 16 to 18 weeks' gestation. Ten brain samples from the same fetuses were used as internal controls. cDNA was synthesized from extracted RNA (300 ng) using the Ambion WT Expression Kit (Expression Kit Manual P/N 4425209 Rev. C). cDNA yield quantity and quality were assessed using NanoDrop Spectrophotometer (NanoDrop Products, Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and Agilent Bioanalyzer (Agilent Technology, Santa Clara, CA, USA). Extraction RNA samples with an A260/280 ratio of 1.7–2.1 were further analyzed by gel electrophoresis at the Biotech Center. Samples were considered acceptable for testing when 28S and 18S rRNA bands resolved into two discrete bands that had no significant smearing below each band. The 28S rRNA band had intensity approximately twice that of the 18S rRNA band. These samples were then further tested by cDNA generation for hybridization. cDNA was checked for yield and size distribution using NanoDrop Spectrophotometer (NanoDrop Products, Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and Agilent profile (Agilent Bioanalyzer, Agilent Technology, Santa Clara, CA, USA). Fragmentation check revealed a cDNA <500 bp reliable for hybridization to Human Gene 1.0 ST arrays. All subsequent experiments were then undertaken using RNA samples matching these requirements.
Fragmentation and end-terminus labeling of cDNAs were done at the Biotechnology Center of the University of Wisconsin, Madison, using the Affymetrix WT Terminal Labeling Kit [see GeneChip® WT Terminal Labeling and Hybridization User Manual (P/N 702808 Rev. 4)]. The samples were then hybridized to Human Gene 1.0 ST arrays at 45°C for 16 h O/N, following all procedures outlined in the GeneChip® WT Terminal Labeling and Hybridization User Manual (P/N 702808 Rev. 4) for Human Gene 1.0 ST Arrays. GeneChips post-processed were done on the AFX Fluidics 450 Station, according to all AFX protocols and procedures defined for the Human Gene 1.0 ST Array (FS450_0007), as outlined in the GeneChip Expression Wash, Stain and Scan User Manual (P/N 702731 Rev. 3). GeneChips were scanned on the GC3000 G7 Scanner. Data were extracted and processed using Affymetrix Command Console version 3.1.1.1229.
Heart tissue was compared with brain tissue looking for organ-specific genes. The heart's top signals detected were Myosin light chain 7 (MYL7), Myosin heavy chain 7 (MYH7), T-box 20 and Popeye domain which are clearly appropriate to the heart. The brain's top signals detected were Internexin, Septin, Doublecortin, Stathmin and NEL2 which are appropriate to the heart.
Brain tissue was compared with heart tissue looking for organ-specific genes. In the brain, the top signals detected are summarized in Fig. 1B. Note the abundant expression of Internexin, Septin, Doublecortin, Stathmin and NEL2, which are all clearly appropriate.
Figure 1.
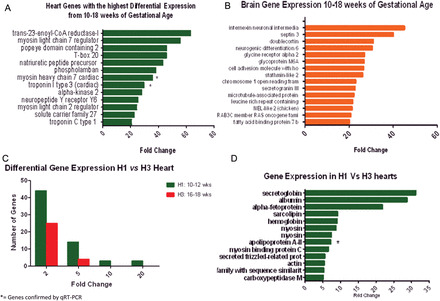
(A) Genes with the highest differential expression in the heart. This figure is based on pooled tissue samples from 14 fetal hearts from 10 to 18 weeks of gestational age for genes with 20-fold change or greater expression level. Human Gene 1.0 ST array (Affymetrix) was used. Genes were chosen based on fold change compared with mRNA, after confirming a differential expression >95% with an false discover rate (FDR) of 5%. * = genes verified by qRT–PCR. (B) Genes with the highest differential expression in the brain. This figure is based on pooled tissue samples from 10 fetal brains from 10 to 18 weeks of gestational age for genes with 20-fold change or greater expression level. Human Gene 1.0 ST array (Affymetrix) was used. Genes were chosen based on fold change compared with mRNA, after confirming a differential expression >95% with an FDR of 5%. * = genes verified by qRT–PCR. (C) Differentially expressed genes measured by fold change in mRNA levels between the hearts at different gestational ages. The late first trimester hearts (H1) showed an increased number of differentially expressed genes (green bars) when compared with the early second trimester hearts (H3). Tissue samples correspond to hearts from 10–12 weeks of gestational age (H1) and 16–18 weeks of gestational age (H3). Differences are measured by fold change (>2), after adjusting for an FDR of 5% with a differential expression >95% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), to identify differentially expressed genes in any two conditions. (D) Genes with the highest differential expression in the late first trimester hearts (H1). Tissue samples correspond to hearts from 10–12 weeks of gestational age (H1) and 16–18 weeks of gestational age (H3) pooled on two separate Human Gene 1.0 ST array, according to gestational age. Differences are measured by fold change (>2), after adjusting for an FDR of 5% with a differential expression >95% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), to identify differentially expressed genes in any two conditions. * = gene verified by qRT–PCR.
The array data can be found at GEO; the accession number is GSE56048.
Statistical methods
All analyses of GeneChip data were carried out in R (R Development Core Team, 2007), a publicly available statistical analysis environment (available at www.r-project.org). Specific software packages (affy, EBarrays, allez) are available at Bioconductor (http://bioconductor.org/), an online suite of tools for the analysis of genomic data (Huber and Gentleman, 2004). The Affymetrix probe level data were processed using Robust Multi-Array Analysis (RMA) as implemented in the affy package, in order to obtain normalized summary expression scores for each probe set on each array (Irizarry et al., 2003). Cutoffs for sensitivity, ensuring a reliable positive detection result, were 0.95. Cutoffs for a significant change in expression were initially set at 2-fold.
Initial pools of the brain and heart derived from RNA were prepared for validation using methods allowing for verification of the characteristics of both the brain and heart. Results were those expected in the brain and heart RNA.
In order to draw any conclusion, parameters had to first be established as to how the output chips would be analyzed. In this presentation, the probe sets are referred to as genes. RMA fits a linear model to the log probe intensities for each probe set. The linear model includes a sample effect (the parameter of interest), a probe effect and an error term. The standard errors are normalized so that each probe set has the same median standard error across arrays.
EBarrays was used to identify differentially expressed (DE) genes (Newton et al., 2001; Kendziorski et al., 2003). EBarrays is an empirical Bayes approach which models the probability distribution of a set of expression measurements. It accounts generally for differences in specific genes with regard to their true underlying expression levels, measurement fluctuations and distinct expression patterns for a given gene among conditions (Kendziorski et al., 2003). An expression pattern is an arrangement of the true underlying intensities (μ) in each condition. The number of patterns possible depends on the number of conditions from which the expression measurements were obtained. For example, when measurements are taken from two conditions, two patterns of expression are possible; equivalent expression (!μ1 = μ2) and differential expression (!μ1 ≠ μ2). Since there is no priori stating which genes are in which patterns, the marginal distribution of the data is a mixture of all possible patterns with model parameters being determined by the full set of array data. This approach utilizes information across a set of arrays optimizing model fit and is, therefore, more efficient than a number of methods that make gene inferences one gene at a time (Kendziorski et al., 2003). Fitted model parameters provide information on the number of genes expected in each expression pattern. Furthermore, the fitted model is used to assign probability distributions to every gene. Each gene-specific distribution gives the posterior probability of that gene's individual expression pattern.
Thresholds were chosen to target an FDR of 5% when more than one sample was available within the condition (e.g. heart versus brain), the lognormal normal with moderated variance (LNNMV) model was used in EBarrays; otherwise, a lognormal normal model was used.
EBarrays then provides the posterior probability of differential expression (DE) as well as equivalent expression (EE) for every gene (Table I). A gene was defined as DE if its posterior probability of DE exceeded 0.95. This is analogous to its posterior probability of EE being ≤0.05. This threshold targets an overall false discovery rate (FDR) of 5%. In some cases (specified in the text), a second fold-change based filter was applied to the list of DE genes obtained from EBarrays.
Table I.
Transcripts detected by array analysis with a fold change >5.
| Entrez ID | Gene | Gene.Name | PP.LNNMV. EE |
PP.LNNMV. DE |
FC |
|---|---|---|---|---|---|
| 253017 | TECRL | trans-2,3-enoyl-CoA reductase-like | 0 | 1 | 63 |
| 58498 | MYL7 | Myosin, light chain 7, regulatory | 0 | 1 | 56 |
| 64091 | POPDC2 | Popeye domain containing 2 | 0 | 1 | 46 |
| 57057 | TBX20 | T-box 20 | 0 | 1 | 46 |
| 4878 | NPPA | Natriuretic peptide precursor A | 0 | 1 | 43 |
| 5350 | PLN | phospholamban | 0 | 1 | 38 |
| 4625 | MYH7 | Myosin, heavy chain 7, cardiac muscle, beta | 0 | 1 | 36 |
| 7137 | TNNI3 | Troponin I type 3 (cardiac) | 0 | 1 | 30 |
| 115701 | ALPK2 | alpha-kinase 2 | 0 | 1 | 28 |
| 4888 | NPY6R | Neuropeptide Y receptor Y6 (pseudogene) | 0 | 1 | 25 |
| 4633 | MYL2 | Myosin, light chain 2, regulatory, cardiac, slow | 0 | 1 | 24 |
| 28965 | SLC27A6 | Solute carrier family 27 (fatty acid transporter), member 6 | 0 | 1 | 23 |
| 7134 | TNNC1 | Troponin C type 1 (slow) | 0 | 1 | 21 |
| 4634 | MYL3 | Myosin, light chain 3, alkali; ventricular, skeletal, slow | 0 | 1 | 21 |
| 137835 | TMEM71 | Transmembrane protein 71 | 0 | 1 | 20 |
| 51086 | TNNI3K | TNNI3 interacting kinase | 0 | 1 | 19 |
| 10699 | CORIN | Corin, serine peptidase | 0 | 1 | 19 |
| 4624 | MYH6 | Myosin, heavy chain 6, cardiac muscle, alpha | 0 | 1 | 18 |
| 93649 | MYOCD | Myocardin | 0 | 1 | 17 |
| 4607 | MYBPC3 | Myosin binding protein C, cardiac | 0 | 1 | 17 |
| 27063 | ANKRD1 | Ankyrin repeat domain 1 (cardiac muscle) | 0 | 1 | 16 |
| 27129 | HSPB7 | Heat shock 27 kDa protein family, member 7 (cardiovascular) | 0 | 1 | 16 |
| 7139 | TNNT2 | Troponin T type 2 (cardiac) | 0 | 1 | 16 |
| 150572 | SMYD1 | SET and MYND domain containing 1 | 0 | 1 | 15 |
| 4151 | MB | Myoglobin | 0 | 1 | 14 |
| 51725 | FBXO40 | F-box protein 40 | 0 | 1 | 14 |
| 2170 | FABP3 | Fatty acid binding protein 3, muscle and heart (mammary-derived growth inhibitor) | 0 | 1 | 13 |
| 376132 | LRRC10 | Leucine rich repeat containing 10 | 0 | 1 | 13 |
| 145781 | GCOM1 | GRINL1A complex locus | 0 | 1 | 13 |
| 6523 | SLC5A1 | Solute carrier family 5 (sodium/glucose cotransporter), member 1 | 0 | 1 | 12 |
| 23676 | SMPX | Small muscle protein, X-linked | 0 | 1 | 12 |
| 29119 | CTNNA3 | Catenin (cadherin-associated protein), alpha 3 | 0 | 1 | 12 |
| 8048 | CSRP3 | Cysteine and glycine-rich protein 3 (cardiac LIM protein) | 0 | 1 | 12 |
| 8736 | MYOM1 | Myomesin 1, 185 kDa | 0 | 1 | 11 |
| 5318 | PKP2 | Plakophilin 2 | 0 | 1 | 11 |
| 1160 | CKMT2 | Creatine kinase, mitochondrial 2 (sarcomeric) | 0 | 1 | 11 |
| 11149 | BVES | Blood vessel epicardial substance | 0 | 1 | 11 |
| 10529 | NEBL | nebulette | 0 | 1 | 11 |
| 2274 | FHL2 | Four and a half LIM domains 2 | 0 | 1 | 10 |
| 23493 | HEY2 | Hairy/enhancer-of-split related with YRPW motif 2 | 0 | 1 | 10 |
| 285025 | CCDC141 | Coiled-coil domain containing 141 | 0 | 1 | 10 |
| 27302 | BMP10 | Bone morphogenetic protein 10 | 0 | 1 | 10 |
| 64208 | POPDC3 | Popeye domain containing 3 | 0 | 1 | 9 |
| 221662 | RBM24 | RNA binding motif protein 24 | 0 | 1 | 8 |
| 10611 | PDLIM5 | PDZ and LIM domain 5 | 0 | 1 | 8 |
| 146862 | UNC45B | Unc-45 homolog B (C. elegans) | 0 | 1 | 8 |
| 1410 | CRYAB | Crystallin, alpha B | 0 | 1 | 8 |
| 6262 | RYR2 | Ryanodine receptor 2 (cardiac) | 0 | 1 | 8 |
| 123722 | FSD2 | Fibronectin type III and SPRY domain containing 2 | 0 | 1 | 8 |
| 8988 | HSPB3 | Heat shock 27 kDa protein 3 | 0 | 1 | 8 |
| 7135 | TNNI1 | Troponin I type 1 (skeletal, slow) | 0 | 1 | 7 |
| 23566 | LPAR3 | Lysophosphatidic acid receptor 3 | 0 | 1 | 7 |
| 6331 | SCN5A | Sodium channel, voltage-gated, type V, alpha subunit | 0 | 1 | 7 |
| 27145 | FILIP1 | Filamin A interacting protein 1 | 0 | 1 | 7 |
| 167681 | PRSS35 | Protease, serine, 35 | 0 | 1 | 7 |
| 91624 | NEXN | Nexilin (F actin binding protein) | 0 | 1 | 7 |
| 84803 | AGPAT9 | 1-acylglycerol-3-phosphate O-acyltransferase 9 | 0 | 1 | 7 |
| 11155 | LDB3 | LIM domain binding 3 | 0 | 1 | 7 |
| 116496 | FAM129A | Family with sequence similarity 129, member A | 0 | 1 | 7 |
| 1E+08 | OCR1 | Ovarian cancer-related protein 1 | 0 | 1 | 7 |
| 6450 | SH3BGR | SH3 domain binding glutamic acid-rich protein | 0 | 1 | 7 |
| 59272 | ACE2 | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 2 | 0 | 1 | 7 |
| 91807 | MYLK3 | Myosin light chain kinase 3 | 0 | 1 | 7 |
| 2201 | FBN2 | Fibrillin 2 | 0 | 1 | 6 |
| 140456 | ASB11 | Ankyrin repeat and SOCS box-containing 11 | 0 | 1 | 6 |
| 6546 | SLC8A1 | Solute carrier family 8 (sodium/calcium exchanger), member 1 | 0 | 1 | 6 |
| NA | NA | NA | 0 | 1 | 6 |
| NA | NA | NA | 0 | 1 | 6 |
| 8470 | SORBS2 | Sorbin and SH3 domain containing 2 | 0 | 1 | 6 |
| 6910 | TBX5 | T-box 5 | 0 | 1 | 6 |
| 84675 | TRIM55 | Tripartite motif-containing 55 | 0 | 1 | 6 |
| 116362 | RBP7 | Retinol binding protein 7, cellular | 0 | 1 | 6 |
| 84940 | CORO6 | Coronin 6 | 0 | 1 | 6 |
| 1573 | CYP2J2 | Cytochrome P450, family 2, subfamily J, polypeptide 2 | 0 | 1 | 6 |
| 144100 | PLEKHA7 | Pleckstrin homology domain containing, family A member 7 | 0 | 1 | 6 |
| 84676 | TRIM63 | Tripartite motif-containing 63 | 0 | 1 | 6 |
| 4286 | MITF | Microphthalmia-associated transcription factor | 0 | 1 | 6 |
| 345557 | PLCXD3 | Phosphatidylinositol-specific phospholipase C, X domain containing 3 | 0 | 1 | 6 |
| 9200 | PTPLA | Protein tyrosine phosphatase-like (proline instead of catalytic arginine), member A | 0 | 1 | 6 |
| 26548 | ITGB1BP2 | Integrin beta 1 binding protein (melusin) 2 | 0 | 1 | 6 |
| 88 | ACTN2 | Actinin, alpha 2 | 0 | 1 | 6 |
| 1832 | DSP | Desmoplakin | 0 | 1 | 6 |
| 8490 | RGS5 | Regulator of G-protein signaling 5 | 0 | 1 | 6 |
| NA | NA | NA | 0 | 1 | 5 |
| 10398 | MYL9 | Myosin, light chain 9, regulatory | 0 | 1 | 5 |
| 283358 | B4GALNT3 | beta-1,4-N-acetyl-galactosaminyl transferase 3 | 0 | 1 | 5 |
| 161247 | FITM1 | Fat storage-inducing transmembrane protein 1 | 0 | 1 | 5 |
| 387700 | SLC16A12 | Solute carrier family 16, member 12 (monocarboxylic acid transporter 12) | 0 | 1 | 5 |
| 11030 | RBPMS | RNA binding protein with multiple splicing | 0 | 1 | 5 |
| 90523 | C6orf142 | Chromosome 6 open reading frame 142 | 0 | 1 | 5 |
| 5322 | PLA2G5 | Phospholipase A2, group V | 0 | 1 | 5 |
| 2702 | GJA5 | Gap junction protein, alpha 5, 40 kDa | 0 | 1 | 5 |
| 51705 | EMCN | endomucin | 0 | 1 | 5 |
| 8395 | PIP5K1B | Phosphatidylinositol-4-phosphate 5-kinase, type I, beta | 0 | 1 | 5 |
| 606553 | C8orf49 | Chromosome 8 open reading frame 49 | 0 | 1 | 5 |
| 442721 | LMOD2 | Leiomodin 2 (cardiac) | 0 | 1 | 5 |
| 358 | AQP1 | Aquaporin 1 (Colton blood group) | 0 | 1 | 5 |
Tests for enrichment of common function among sets of differentially expressed genes were carried out using data from the Gene Ontology (GO) annotations and the Kyoto Encyclopedia of Genes and Genomes (KEGG). In GO, transcripts genes are categorized at varying levels of biological detail. The three broadest levels are molecular function, cellular component and biological process. There are many subcategories within each detail. The R package allez was used to perform tests of enrichment for each GO category and KEGG pathway (Newton et al., 2001). In general, the interpretation of P-values resulting from enrichment tests is not straightforward due to the many dependent hypotheses tested. Furthermore, the enrichment test tends to result in small P-values when groups with few transcripts are considered. The statistical methods underlying allez adjust for these factors, allowing increased power and sensitivity for identifying sets that are biologically meaningful (Tables II and III).
Table II.
Genontology classification.
| GO | GO.term | n.genes | z.score | |
|---|---|---|---|---|
| GO:0042627 | CC | Chylomicron | 12 | 12.01 |
| GO:0010886 | BP | Positive regulation of cholesterol storage | 6 | 11.26 |
| GO:0008307 | MF | Structural constituent of muscle | 43 | 10.29 |
| GO:0032982 | CC | Myosin filament | 18 | 10.08 |
| GO:0042159 | BP | Lipoprotein catabolic process | 6 | 9.96 |
| GO:0033033 | BP | Negative regulation of myeloid cell apoptosis | 6 | 9.92 |
| GO:0008035 | MF | High-density lipoprotein binding | 7 | 9.81 |
| GO:0034361 | CC | Very-low-density lipoprotein particle | 20 | 9.09 |
| GO:0034385 | CC | Triglyceride-rich lipoprotein particle | 20 | 9.09 |
| GO:0031983 | CC | Vesicle lumen | 38 | 8.64 |
Table III.
Geontology classification by heart group.
| H1 |
H3 |
||||
|---|---|---|---|---|---|
| GO:0008307 | Structural constituent of muscle | 43 | GO:0042627 | Chylomicron | 12 |
| GO:0032982 | Myosin filament | 18 | GO:0010886 | Positive regulation of cholesterol storage | 6 |
| GO:0033033 | Negative regulation of myeloid cell apoptosis | 6 | GO:0042159 | Lipoprotein catabolic process | 6 |
| GO:0008035 | High-density lipoprotein binding | 7 | |||
| GO:0034361 | Very-low-density lipoprotein particle | 20 | |||
| GO:0034385 | Triglyceride-rich lipoprotein particle | 20 | |||
| GO:0031983 | Vesicle lumen | 38 | |||
| Total genes | 67 | Total genes | 109 | ||
H1: 10-12wks; H3: 16-18wks.
qRT–PCR
Seven selected genes (FABP4, MYH7B, APOA2, TNNI3, TMOD2, NRXN1, RPLO) were further validated by quantitative real-time PCR (qRT–PCR) in order to validate the array data. Human reference cDNA, oligo-dT primed (Clontech) was used to create a standard dilution series for analysis in parallel with samples on a Roche LightCycler480 using sequence-specific primers. Each assay per sample was performed in triplicate on 96-well plates with 480 Sybr Green I Master (Roche), and 500 nM forward and reverse primers. Cycling conditions were as follows: 10 min at 95°C; 40 cycles at 95°C for 10 s, 57°C for 10 s and 72°C for 5 s; followed by melt curve analysis.
Ethical approval
Sample collection was started after receiving Institutional Review Board approval (H-2009-0071) over a 2-year period from 2009 to 2011.
Results
Confirmation of cardiac tissue
Pooled RNA from all cardiac tissue (10–18 weeks) was compared with a control tissue (fetal brain) to confirm specificity of the tissue. Genes like natriuretic peptide (NPPA), troponin (TNNI3) and myosin heavy chain 7 (MYH7) cardiac muscle were readily detected (differential expression >95%, after adjusting for an FDR of 5%) and correctly reported as ‘up-regulated’ relative to brain tissue (Fig. 1A). Subsequent qRT–PCR confirmed expression of TNNI3 and MYH7. Brain tissue genes are shown in Fig. 1B (Iruretagoyena et al., 2014).
In order to simplify the analysis of the data, samples were then subgrouped by gestational age within 2-week periods. H1 (10–12 weeks), H2 (13–15 weeks) and H3 (16–18 weeks). Given the magnitude of the data set, statistical analysis was done comparing groups H1 versus H3 (H2 group is shown in the figures to further illustrate the normal progression from first trimester to second trimester).
Changes according to gestational age on global expression of genes
When fold changes of 2, 5 and above 20 were used to compared H1 to H3 gene expression, there were 43, 13 and 5 individual gene transcripts specifically up-regulated, respectively. Conversely, when H3 was compared with H1 24, 4, 0 genes were up-regulated, respectively (Fig. 1C).
Heart contractile proteins
MYL1, MYH3, MYBPC1, SLN and ACTG2 genes coding for sarcoplasmic reticulum, actin and essential myosin contractile protein constituents of cardiac muscle were more highly expressed (differential expression >95%, after adjusting for an FDR of 5%) in hearts of a younger gestational age (Figs 1D and 2A). When gene ontology analysis was applied, terms for structural constituents of muscle and myosin filament were revealed, further supporting single transcript analysis (Fig. 2B). qRT–PCR analysis was also confirmatory when MYH7 was used as a representative target.
Figure 2.
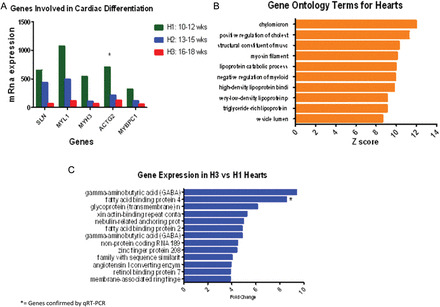
(A) mRNA gene expression of regulators of cardiomyocyte differentiation. All transcripts are increased in the late first trimester (H1) versus the early second trimester (H3). Differences are measured by mRNA level on genes with a differential expression >95%, after adjusting for an FDR of 5% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), to identify differentially expressed genes in any two conditions. SLN, Sarcolipin; MYL1, Myosin light chain 1; MYH3, Myosin heavy chain 3; ACTG2, Actin gamma. * = gene verified by qRT–PCR. (B) Bar chart showing terms from Gene Ontology in the heart, classified according to biological process, cellular component and molecular function using test for enrichment of common functions among sets of differentially expressed genes (Gene Ontology Annotations and the KEGG). Gene families involved in common functions for muscular development like myosin filament and fatty acid metabolism including chylomicron, cholesterol, lipoprotein binding proteins and triglyceride lipoprotein are the highest ranked in the cardiomyocytes from 10 to 18 weeks of gestational age (pooled data). (C) Genes with the highest differential expression in the early second trimester hearts (H3). Tissue samples correspond to hearts from 10–12 weeks of gestational age (H1) and 16–18 weeks of gestational age (H3) pooled on two separate Human Gene 1.0 ST array, according to gestational age. Differences are measured by fold change (>2), after adjusting for an FDR of 5% with a differential expression >95% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), to identify differentially expressed genes in any two conditions. *= gene verified by qRT–PCR.
Energy generation metabolism in the heart
Individual transcript analysis showed that genes coding for FABP4, FABP2, NRAP and ACE2, all part of either cardiac fatty acid metabolism or structural remodeling, were among the highest expressed in H3 hearts. (Figs 2C and 3A). Gene ontology analysis showed ontology terms in agreement with single transcript finding involving fatty acid metabolism including chylomicron, positive regulation of cholesterol, lipoprotein catabolic process, high and very low lipoprotein and triglyceride-rich lipoprotein (Fig. 2B). qRT–PCR confirmed expression of FABP4 and APOA2.
Figure 3.
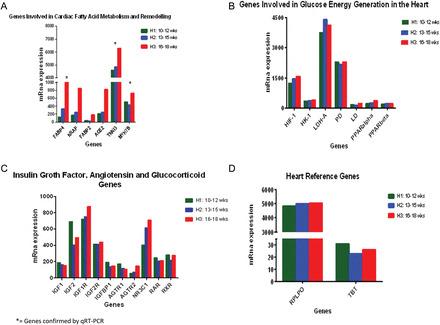
(A) mRNA gene expression of regulators of the cardiac fatty acid metabolism and remodeling. All transcripts are increased in the early second trimester as the heart matures, compared with the late first trimester. Differences are measured by mRNA level on genes with a differential expression >95%, after adjusting for an FDR of 5% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), to identify differentially expressed genes in any two conditions. *= genes verified by qRT–PCR. (B) mRNA expression of regulators of glucose energy generation by the cardiomyocytes. All transcripts are high in both groups showing no significant differential expression (<0.005), after adjusting for an FDR of 5%. (C) mRNA gene expression of insulin growth factor and angiotensin. Specific transcripts were examined due to the their suggested importance in previous studies (see introduction). mRNA levels detected in each group are shown. Differential expression >95%, after adjusting for an FDR of 5% using EBarrays (Newton et al., 2001; Kendziorski et al., 2003), was not achieved suggesting that these genes are not differentially controlled across early gestation. (D) Reference genes in the heart. The graph shows mRNA expression of two candidate genes without significant change in expression, regardless of the experimental group (H1, H2 or H3). Reference genes were validated by the method of Stern-Straeter et al. (2009). All H1, H2 and H3 groups of hearts were analyzed under exact conditions. Differential expression was <0.005 for both genes under the two conditions. RPLP0: Ribosomal protein, large; TBS: TATA box binding protein.
Complementing our findings on transcripts for proteins involved in fatty acid metabolism as an energy source, single gene transcript analysis for genes involved in glucose energy production including PPARα, PPARβ, PD, LD, LDH-A, HK-1 and HIF-1 also was present but not differentially expressed between H1-H3 groups, after adjusting for an FDR of 5% (Fig. 3B). Thus expression of the mRNA encoding these pathways appears more constant with gestation.
Miscellaneous transcripts
GABRA4, GABRB1 and RBP7 genes were highly expressed in the H3 group, with a differential expression >95%, after adjusting for an FDR of 5% (Fig. 2C). GABRA4 or GABRA1 were not found in the gene ontology terms. Although RBP7 is not a direct participant in the ontology terms described, it is indirectly involved in the Chylomicron and lipoproteins terms as part of fatty acid metabolism.
Retinol receptors (RAR, RXR), angiotensin receptors 1-2, IGF1-2, IGFR1-2, IGFBP1 and glucocorticoid receptor NR3C1 were found to be present but not differentially expressed (differential expression <95%, after adjusting for an FDR of 5%) (Fig. 3C).
Housekeeping genes
For validation purposes, mRNA expression of reference genes RPLO and TBT (Stern-Straeter et al., 2009) did not show changes among the different groups as expected (Fig. 3D).
Discussion
Our findings have shown that there are characteristic dynamic changes in gene expression during early development in the human heart. While H1 heart gene expression profiles showed dominance by genes involved in myocardium development and differentiation, H3 hearts showed relatively increased expression of genes not only actively involved in energy generation, but also advanced cellular functions like cardiomyocyte communication and proliferation.
Heart contractile and remodeling proteins
A heart's ultimate destiny is myocardial contraction and so it is clearly of interest that various subtypes of myosin, the principal component of muscle, are highly and differentially expressed in H1 hearts compared with H3 hearts. Light and heavy chains of myosin and actin genes involved in the cytoskeleton formation dominate this period (Tsuchimochi et al., 1986; Yazaki et al., 1987). Genes like the Secreted Frizzled-Related Protein 2 (SFRP2), which function to inhibit fibroblast growth (He et al., 2010) and sarcolipin, which function to reduce the accumulation of calcium in the sarcoplasmic reticulum (Babu et al., 2007) are differentially expressed in the young hearts, potentially contributing to the right proportion of muscle and collagen, as well as calcium dynamics based in the findings of our study.
The renin-angiotensin system, specifically through ACE2 (angiotensin converting enzyme), is one of the pathways hypothesized to play an increased role in H3 hearts when compared with the H1 heart, based on differential gene expression revealed in our studies. The H3 heart expresses genes involved in advanced functions such as contractile coordination, angiogenesis and utilization of energy. Studies in ovine animal models have already shown angiotensin II acts in conjunction with insulin-like growth factor (IGF) to control proliferation of cardiomyocytes during the prenatal period through late gestation, when cardiomyocytes undergo terminal differentiation and proliferation diminishes (Reini et al., 2009). Data from our study further suggest the initiation of cardiomyocyte proliferation and remodeling in humans occurs around weeks 16–18 of gestation. Prior to this period, in the second trimester, more specifically at the end of the first trimester (10–12 weeks' gestation), the major events occurring in the cardiomyocyte relate more to the early differentiation of stem cells into active contractile cardiomyocytes.
Energy generation metabolism in the heart
Fetal cardiac metabolism has been characterized in animal models as dependent on glucose metabolism for energy from the very early stages of cardiac differentiation. This profile changes shortly after birth when the heart energy metabolism depends on fatty acid oxidation in the mitochondria, and persists into adulthood (Kong et al., 2008). Our findings using transcriptome analysis approaches in humans are entirely consistent with Kong et al. findings.
Single gene expression analysis showed H3 heart genes were differentially expressed, favoring fatty acid oxidation metabolism (FABP 4, FABP 2 and RBP) (Figs 2C and 3A) during the H3 period otherwise not apparent in the H1 hearts. Nonetheless this pathway was revealed when grouped analysis was performed. This may be interpreted as further evidence that an early sign of emerging fatty acid oxidation metabolism in the H1 hearts that is not significant when transcripts are evaluated individually. At a later gestational age, when single gene expression is robust enough to create a difference, such changes can be observed individually.
When gene ontology was further applied to a pooled sample of hearts from 10 to 18 weeks of gestational age (Tables II and III), genes involved in fatty acid metabolism either in cell components, molecular function or biological processes were again identified as the major active pathways. One remarkable finding was that genes driving metabolism of fatty acid were highly differentially expressed in the H3 hearts, compared with the H1 hearts. KEGG pathway analysis also showed pathways involved in fatty acid metabolism and oxidative stress controls (ascorbate and aldarate metabolism, retinol metabolism and PPAR signaling) are more dominant in the H3 period.
In contrast to our findings on fatty acid metabolism, key genes coding for HIF, HK1, LDH-A, and lactate dehydrogenase, as well as PPAR-alpha and beta were more uniformly present and highly expressed in both H1 and H3 hearts. The apparent lack of differential gene expression for genes involved in glucose metabolism is again consistent with previous findings in animal studies of the heart as being dependent on glucose utilization for basal energy generation during the fetal period (Lopaschuk and Jaswal, 2010). Combined, these observations may reflect the fact that the fetal period is dominated by glucose energy production until after birth, and no differential changes in the genes regulating this process should be expected from 10 to 18 weeks of gestation. Interestingly, however, three genes involved in fatty acid oxidation ranked among those with more than a 5-fold change in the H3 period compared with H1. These genes are the FABP 4, FABP 2 and RBP.
Other specific transcripts
Beyond energy metabolism, transcript analysis also revealed many other developmental changes of relevance. Retinol receptors are grouped in two families; the RXRs and the RARs (Mangelsdorf et al., 1992). RARs may function as receptors for thyroid hormones, vitamin D and peroxisome proliferators (PPAR) and co-operate with RXRs. Retinol and its receptors may play an important role in cardiac development through hormonal signaling and as cofactors in fatty acid oxidation for energy generation (Sucov et al., 1994). Although RXR presence has been documented in adult hearts, it has not been shown in the human fetal heart. In this study, experiments found the presence mRNA coding for both RXR and RAR in the human embryonic heart. There does not appear to be a difference in mRNA expression when compared by gestational age. However, when KEGG pathways are implemented, the retinol metabolism and PPAR signaling pathway appear to be among the main pathways activated in fetal hearts from 10 to 18 weeks. This finding may be linked, since retinol is a cofactor in many peroxidation reactions where PPAR is involved (Lopaschuk and Jaswal, 2010). Fatty acid metabolism and gluconeogenesis are major processes regulated by these pathways.
A further intriguing finding in the H3 heart was the higher expression of GABA receptors. These receptors have been extensively described in the brain and pre/post synaptic environment as neurotransmitters, but have not been described in the heart. Kang et al. (2011) recently showed an association between neurotransmitters as GABA and sympathoexcitation of the heart in rats with heart failure. It would be reasonable to associate the high expression of GABA receptors in the H3 heart with sympathetic innervation control of the heart and advanced functions such as heartbeat variability and blood pressure control.
Insulin growth factor-1 (IGF-1), cortisol receptor (NR3C1) and angiotensin receptors 1 and 2 (AGT1R, AGT2R), previously described by Thornburg et al. (2010) in sheep as important factors stimulating cardiomyocyte proliferation, were found to be present in human fetal heart as early as 10 weeks of gestation. These genes were not differentially expressed when comparison was made among groups based on gestational age. The mRNA expression though, showed that IGF-1 and IGFBP1 were present in early gestational age (10–12 weeks) as opposed to IGF-1 receptor that appeared to be mostly present at a later stage (16–18 weeks).
The angiotensin receptor 1 (AGT1R) mRNA expression appears to decrease with gestational age, the opposite of AGT2R which increases with gestational age. These changes may be related to the function of angiotensin II in cardiac remodeling during fetal life. AGT1R has been associated with vasoconstriction and hypertrophy while AGT2R has been associated with vasodilation counteracting AGT1R (Savoia and Volpe, 2011). IGF and AGTR may be related. IGF has been previously described (Thornburg, 2011) to inhibit the expression of AGT2R during hypoxic conditions in fetal rats. To our knowledge, none of these genes have been studied during early human fetal life.
Reference genes
In all array studies it is important to run negative controls. Interrogated housekeeping genes, or reference genes, were indeed found to be unchanged in both groups. While it was not clear in advance that this would be the case for all housekeeping genes, it is reassuring that this is the outcome, since it provides an internal quality control for this and indeed future analysis. Previous studies validating reference genes in myoblast development, utilizing different software, indicated that TBP (TATA box binding protein) and RPLP0 (ribosomal protein large P0) were the only two consistent genes that could be used to validate changes in other genes (Stern-Straeter et al., 2009).
In conclusion, these experiments using fetal human hearts spanning between 10 and 18 weeks of gestation confirmed previous findings in metabolic profile showing, during this period, that energy derived from glucose was the main source of fuel for the heart. Although the major source of energy is glucose, it is not the only source. Fatty acid metabolism appears to also be a source of energy and transcripts related to that metabolic pathway can be found to be present as early as 10 weeks of gestation. These genes involved in fatty acid metabolism thereafter show a stronger expression as the fetus develops, and this finding is consistent with our current knowledge showing a transition to a full utilization of fatty acid oxidation as the energy source in the neonatal period and adulthood. Many other individual transcript changes have also been identified that relate to the altered endocrine control of the heart and contractile proteins underlying its function. These findings not only serve as a database for comparison with older gestational ages and adults, but give a picture of the heart at a developmental stage not previously well characterized and yet critical to developmental success. It is tempting to hypothesize that, between the gestational ages of 10–18 weeks, the heart fully differentiates and starts specializing, remodeling and adjusting to finally become the hemodynamic controller. Further studies will be necessary to both validate these findings at a functional level and establish physiologic correlates suggested by this data set.
Supplementary Material
Acknowledgements
We thank Mark Garthwaite for assistance with mRNA preparation.
Contributor Information
J.I. Iruretagoyena, MFM Division, OBGYN Department, University of Wisconsin, Madison, WI, USA
W. Davis, Gene Expression Center, University of Wisconsin, Madison, WI, USA
C. Bird, MFM Division, OBGYN Department, University of Wisconsin, Madison, WI, USA
J. Olsen, Gene Expression Center, University of Wisconsin, Madison, WI, USA
R. Radue, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
A. Teo Broman, Department of Biostatistics and Medical Informatics, University of Wisconsin, Madison, WI, USA
C. Kendziorski, Department of Biostatistics and Medical Informatics, University of Wisconsin, Madison, WI, USA
S. Splinter BonDurant, Gene Expression Center, University of Wisconsin, Madison, WI, USA
T. Golos, National Primate Research Center, and Department of Comparative Biosciences, University of Wisconsin, Madison, WI, USA
I. Bird, Reproductive Sciences Division, OBGYN Department, University of Wisconsin, Madison, WI, USA
D. Shah, MFM Division, OBGYN Department, University of Wisconsin, Madison, WI, USA
Authors' roles
J.I.I.: substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. W.D.: substantial contributions to analysis and interpretation of data, drafting the article and final approval of the version to be published. C.B.: substantial contributions to acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. J.O.: substantial contributions to analysis and interpretation of data, drafting the article and final approval of the version to be published. R.R.: substantial contributions to acquisition of data, drafting the article and final approval of the version to be published. A.T.B.: substantial contributions to analysis and interpretation of data, drafting the article and final approval of the version to be published. C.K.: substantial contributions to design, analysis and interpretation of data, drafting the article and final approval of the version to be published. S.S.B.D.: substantial contributions to analysis and interpretation of data, drafting the article and final approval of the version to be published. T.G.: substantial contributions to conception and design and interpretation of data, drafting the article and final approval of the version to be published. I.B.: substantial contributions to conception and design, analysis and interpretation of data, drafting the article and final approval of the version to be published. D.S.: substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published.
Funding
Maternal Fetal Medicine Research and Development Grant. Dept. OBGYN, University of Wisconsin.
Conflict of interest
None declared.
References
- Babu GJ Bhupathy P Carnes CA Billman GE Periasamy M Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S Dzeja PP Faustino RS Perez-Terzic C Behfar A Terzic A Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacos E Gomez R Nicolaides K Romero R Cabero L. Medicina Fetal. Spain: Editorial Medica Panamericana; 2007. 822. [Google Scholar]
- He W Zhang L Ni A Zhang Z Mirotsou M Mao L Pratt RE Dzau VJ Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci USA. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W Gentleman R matchprobes: a Bioconductor package for the sequence-matching of microarray probe elements. Bioinformatics. 2004;20:1651–1652. doi: 10.1093/bioinformatics/bth133. [DOI] [PubMed] [Google Scholar]
- Irizarry RA Hobbs B Collin F Beazer-Barclay Y Antonellis K Scherf U Speed T Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Iruretagoyena J.I Davis W Bird C Olsen J Radue R Teo Broman A Kendziorski C Splinter BonDurant S Golos T Bird I et al. Differential changes in gene expression in human brain during late first trimester and early second trimester of pregnancy. Prenat Diagn. 2014 doi: 10.1002/pd.4322. Jan 16 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kang YM Zhang AQ Zhao XF Cardinale JP Elks C Cao XM Zhang ZW Francis J Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol. 2011;106:473–483. doi: 10.1007/s00395-011-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendziorski CM Newton MA Lan H Gould MN On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat Med. 2003;22:3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]
- Kong B Liu YL Lu XD Microarray-bioinformatics analysis of altered genomic expression profiles between human fetal and infant myocardium. Chin Med J (Engl) 2008;121:1257–1264. [PubMed] [Google Scholar]
- Lopaschuk GD Jaswal JS Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ Borgmeyer U Heyman RA Zhou JY Ong ES Oro AE Kakizuka A Evans RM Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Moore K. Embriologia Clinica. 4th edn. Mexico: Interamericana; 1989. [Google Scholar]
- Newton MA Kendziorski CM Richmond CS Blattner FR Tsui KW On differential variability of expression ratios: improving statistical inference about gene expression changes from microarray data. J Comput Biol. 2001;8:37–52. doi: 10.1089/106652701300099074. [DOI] [PubMed] [Google Scholar]
- Razeghi P Young ME Alcorn JL Moravec CS Frazier OH Taegtmeyer H Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- Reini SA Wood CE Keller-Wood M The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patterns. 2009;9:122–128. doi: 10.1016/j.gep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C Volpe M Angiotensin receptor modulation and cardiovascular remodeling. J Renin Angiotensin Aldosterone Syst. 2011;12:381–384. doi: 10.1177/1470320311417750. [DOI] [PubMed] [Google Scholar]
- Stern-Straeter J Bonaterra GA Hormann K Kinscherf R Goessler UR Identification of valid reference genes during the differentiation of human myoblasts. BMC Mol Biol. 2009;10:66. doi: 10.1186/1471-2199-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM Dyson E Gumeringer CL Price J Chien KR Evans RM RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Thornburg KL Foetal programming reveals the dark side of AT(2)R. Cardiovasc Res. 2011;89:260–261. doi: 10.1093/cvr/cvq387. [DOI] [PubMed] [Google Scholar]
- Thornburg K Jonker S O'Tierney P Chattergoon N Louey S Faber J Giraud G Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol. 2010;106:289–299. doi: 10.1016/j.pbiomolbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H Kuro-o M Takaku F Yoshida K Kawana M Kimata S Yazaki Y Expression of myosin isozymes during the developmental stage and their redistribution induced by pressure overload. Jpn Circ J. 1986;50:1044–1052. doi: 10.1253/jcj.50.1044. [DOI] [PubMed] [Google Scholar]
- Wheater PR Burkitt HG Daniels VG. Histologia Funcional. 2nd edn. Spain: JIMS; 1987. [Google Scholar]
- Yazaki Y Tsuchimochi H Kurabayashi M Kawana M Kimata S Distribution of cardiac myosin isozymes in cardiomyopathy: immunohistochemical and gene analysis. Jpn Circ J. 1987;51:676–681. doi: 10.1253/jcj.51.676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


