Abstract
Chloroquine is still used as a first-line treatment for uncomplicated Plasmodium vivax malaria in India and resistance to this therapy can act as a major hurdle for malaria elimination. It is difficult to monitor drug-efficacy and drug resistance through in vivo and in vitro studies in case of Plasmodium vivax so analysis of molecular markers serves as an important tool to track resistance. Molecular methods that are currently in use for detecting single nucleotide polymorphisms in resistant genes including Polymerase chain reaction (PCR), Realtime-Polymerase chain reaction require highly sophisticated labs and are time consuming. So, with this background the study has been designed to optimize Loop Mediated Isothermal Amplification Assay to detect single nucleotide polymorphisms in chloroquine resistance gene of Plasmodium vivax in field settings. Eighty-eight Plasmodium vivax positive samples were collected. Pvmdr1 gene was amplified for all the samples and sequenced. Obtained sequences were analyzed for the presence of single nucleotide polymorphisms in the target gene. Further Loop Mediated Isothermal Amplification Assay primer sets were designed for the target mutants and the assay was optimized. Clinical as well as analytical sensitivity and specificity for the assay was calculated. Double mutants with variations at T958M and F1076L were detected in 100% of the Plasmodium vivax clinical isolates with haplotype M958 Y976 Y1028 L1076. Designed primers for Loop Mediated Isothermal Amplification Assay successfully detected both the mutants (T958M and F1076L) in 100% of the isolates and do not show cross-reactivity with other strains. So, the assay was 100% sensitive and specific for detecting single nucleotide polymorphisms in the target Pvmdr1 gene. Limit of detection was found to be 0.9 copies/µl and lowest DNA template concentration detected by designed assay was 1.5 ng/µL. Observed prevalence of single nucleotide polymorphisms in Pvmdr 1 gene is indicating a beginning of trend towards chloroquine resistance in Plasmodium vivax. The present study optimized LAMP for detecting single nucleotide polymorphisms in Plasmodium vivax cases in field settings, thus would help in finding significant hubs of emerging chloroquine drug resistance and ultimately helping in the management of suitable antimalarial drug policy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76479-7.
Subject terms: Microbiology, Molecular biology
Introduction
Malaria is one of the fast-emerging deadliest disease and resistance to antimalarials is the major hurdle for eliminating malaria. World Health Organization reports, 247 million malaria cases from 84 endemic countries all over the world in 2021, and in the year 2020 the cases were 245 million in number1. During 2019–2021 period additional 13.4 million malaria cases were reported and the disruption was due to COVID-19. Among globally reported malaria cases 95% of the cases were from WHO African region alone. Number of deaths occurring due to malaria were declined during the period 2000–2019 from 897,000 to 568,000. But disruptions due to COVID-19 pandemic in the malaria related services led to rose up number of deaths again to 63,000 during the period 2019–20211.
According to earlier studies only P. falciparum was known for causing fatal severities and P. vivax was known for causing mild form of malaria without any fatality. Recent studies have reported severe complications occurring in case of P. vivax malaria including anaemia, respiratory distress, coma ultimately causing fatality2. Host, parasite and environmental factors together contribute to clinical outcomes of malaria3. Major hurdle to the aim of eliminating malaria is increasing resistance to antimalarials and insecticides. First-line treatment in India for uncomplicated P. vivax malaria include chloroquine (for eliminating blood stages of parasite) along with Primaquine (for treating hypnozoite stage). Complicated P. vivax malaria is treated with artemisinin combination therapy4. Although these drugs are effective in treating P. vivax malaria but reports of resistance from different areas is causing difficulty in malaria elimination5.
Drug resistance in malaria is very complex phenomenon that require different parameters and approaches for detection. Taking general example for P. falciparum, there are three major approaches that are used for identifying and checking the drug resistance level which includes in vivo, in vitro tests and molecular marker analysis6. Parasite biology limits in vivo tests for P. vivax due to the presence of hypnozoite stage that most commonly escape the drugs and cause relapse in later time. So, it becomes difficult to differentiate between relapse, re-infection and infection by a resistant parasite7. For P. falciparum standard methods of maintaining continuous in vitro culture are available making evaluation of resistance to different drugs easy while in case of P. vivax in vitro tests for monitoring drug resistance encounters difficulty due to challenges in maintaining in vitro culture8. Detection of variations in molecular markers of resistance serves as an important tool for the surveillance of drug resistance and in case of P. falciparum and acts as support for in vitro and in vivo tests to identify resistance to particular drug treatment9. In P. falciparum gene showing association with chloroquine resistance (CQR) is localized on food vacuole transmembrane and is known as P. falciparum chloroquine transporter (Pfcrt) gene and another gene P. falciparum multidrug resistance gene which plays role in modulating CQR10,11. Although mechanism of action of chloroquine is same in both the Plasmodium species but the mechanism of resistance is quite different6. Orthologues to P. falciparum chloroquine resistance genes in P. vivax are reported as Pvcrt-o and Pvmdr 1 genes. One recent study had shown that upregulation of Pvcrt-o gene expression is associated with CQR and further evidences are given by study from Brazilian Amazon where multicopy Pvcrt-o was reported in high frequency from CQR P. vivax12,13. P. vivax orthologue for Pfmdr-1 another factor associated with CQR in P. falciparum was detected for the first time in the 2005 and was known as Pvmdr-114. Recent studies have shown association of SNPs (at codons, 976 and 1076) in Pvmdr1 gene and in vitro as well in vivo CQR in countries of Southeast Asia15,16. A study conducted in four different regions of India including, Mangaluru (MAQ), Puducherry (PDY), Jodhpur (JDH), Cuttack (CTC), showed the prevalence of F1076L mutation in Pvmdr-1 gene was 100% in both JDH and CTC, 93.3% in MAQ and 73.3% in PDY17.
Molecular methods that are currently used for tracking anti-malarial drug resistance are based on gene markers of the parasites. These methods are very useful for detecting markers of resistance but on the other hand faces certain limitations due to high cost, consume more time, requirement of well-equipped laboratories and trained personnel. So, these methods are limited to cope with the current demand of field-based assay that is required for surveillance of markers of resistance in endemic areas. Thus, there is an urgent need to design a field deployable assay to perform surveillance studies in the endemic areas. According to recent studies Isothermal methods including Loop Mediated Isothermal Amplification Assay (LAMP) is emerging as a field friendly tool for diagnosis of various parasites including Plasmodium species18,19. LAMP has been proven to be a cost effective, rapid, simple and user friendly assay for resource limited setups as it eliminates the need of highly sophisticated thermal cyclers as prior denaturation is not required and complete the whole process in single step20. In comparison to other available molecular methods, LAMP is found to be more sensitive and specific. The basic principle of LAMP depends upon the strand displacement-based synthesis by Bst DNA polymerase which are primed by different sets of outer (F3, B3) and inner (B3, BIP) primers that are specifically designed for the target regions20.
LAMP has been previously used for detecting SNPs in P. falciparum isolates for chloroquine resistant, sulphadoxine-pyrimethamine and artemisinin resistant genes in field settings with very high sensitivity as well as specificity21–23. This study has investigated the application of LAMP to detect SNPs in chloroquine resistant gene of P. vivax isolates. Data from our study have provided evidence that LAMP can be used for surveillance of SNPs in Pvmdr1 chloroquine resistant gene in field settings. It can serve as early warning sign for the emerging chloroquine resistance in areas like India where chloroquine is still used as a first-line treatment for the P. vivax malaria cases.
Methodology
Ethics
The proposal was reviewed and approved by Institutional ethics committee of Postgraduate Institute of Medical Education and Research. (INT/IEC/2020/SPL-1480). All methods were performed in accordance with the relevant guidelines/regulation as approved by ethics committee and written informed consent was obtained from the participants and/or guardian.
Sample collection and study site
Eighty-eight P. vivax positive samples were collected from patients visiting Out Patient Department (OPD) or admitted at Nehru Hospital, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, All India Institute of Medical Sciences (AIIMS), Jodhpur and from different districts of Punjab, during the period 2019 to 2022. Candidates eligible for the study were enrolled and 2–3 ml of blood was collected in EDTA vials from each patient along with written consents.
Patient screening
All the collected samples suspected for malaria were further screened for species identification by Rapid diagnostic test (RDT), microscopy and nested Polymerase chain reaction (nested PCR).
Antigen detection and microscopy
RDTs was performed by detecting Plasmodium lactate dehydrogenase (pLDH) in collected samples as per manufacturer’s instructions (Alere Medical Pvt. Ltd., India). For microscopic examination, parasite smears were prepared by using Giemsa stain and the prepared slides were observed.
DNA extraction, quantification and nested PCR for species identification
Qiagen (QIAamp, DNA Blood) DNA isolation kit was used to extract DNA from the whole blood by following manufacturer’s instructions. Concentration of extracted DNA was measured by checking optical density of extracted DNA using spectrophotometer (Nanodrop technologies).
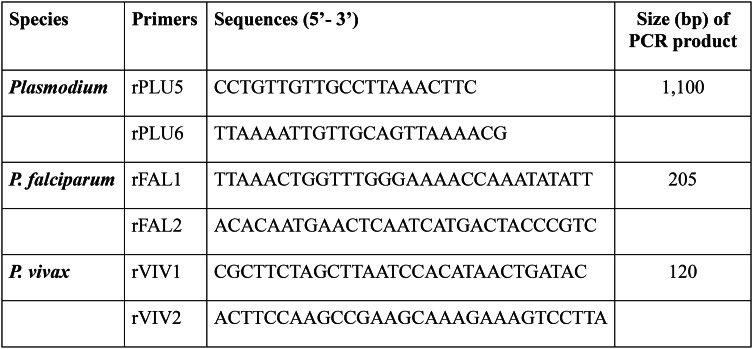
Nested PCR targeting 18srRNA was performed for detecting Plasmodium species using previously published primers given in Table 124. Amplified products were resolved on 1.5–2% of agarose gel and were observed under UV transilluminator. P. vivax positive isolates were included in the study and P. falciparum positive isolates were excluded.
Table 1.
Primers used for Plasmodium species identification.
Amplification of P. vivax drug resistant gene
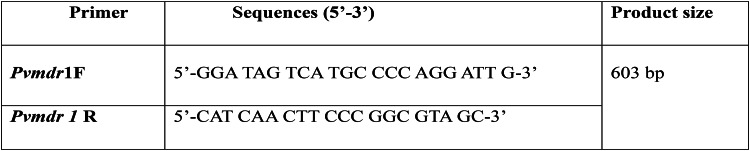
Pvmdr1 gene was amplified in all P. vivax positive samples using already published primers given in the Table 225. PCR reaction mixture was prepared by using Go Taq Green Mastermix (Promega, Madison, WI, USA), as summarized in Additional file No. 1 and optimized thermocycling conditions that were used are detailed in Additional file No. 2. Amplified products were resolved on 2–2.5% of agarose gel and were seen under UV transillumination.
Table 2.
Primer sequences for Pvmdr1 gene.
Qiagen PCR purification kit (QIAGEN, CA, USA) was used to purify amplified PCR products which were sequenced through Sanger sequencing (Genewiz INC, NJ, USA). Sequenced products were then analyzed through Finch TV 1.4.0 (Geospiza Inc.) (https://digitalworldbiology.com/FinchTV) and Clustal X 2.1 (http://www.clustal.org/clustal2/) was used for Multiple sequence alignment of the sequences for detecting variations (SNPs) in comparison with the reference Sal-I (GeneBank: AY571984.1). Accession numbers were obtained from GeneBank for all the sequences.
LAMP for detecting SNPs in Pvmdr1 gene
Primer designing
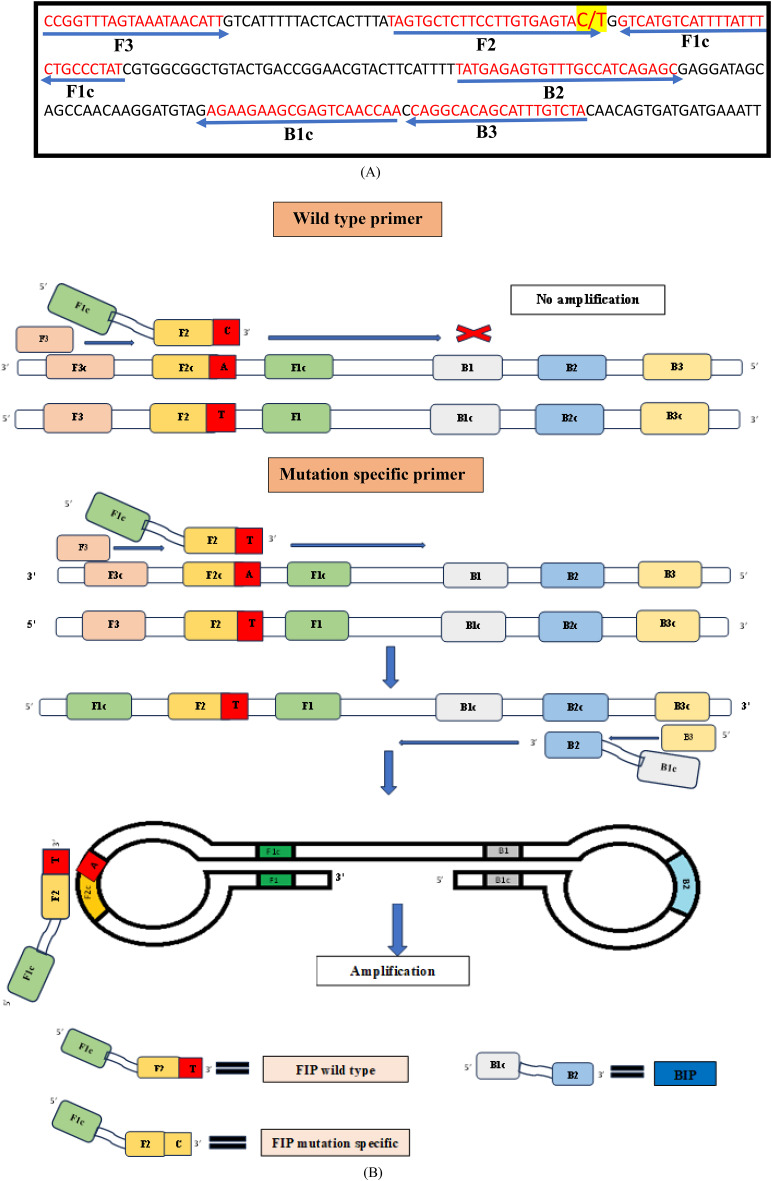
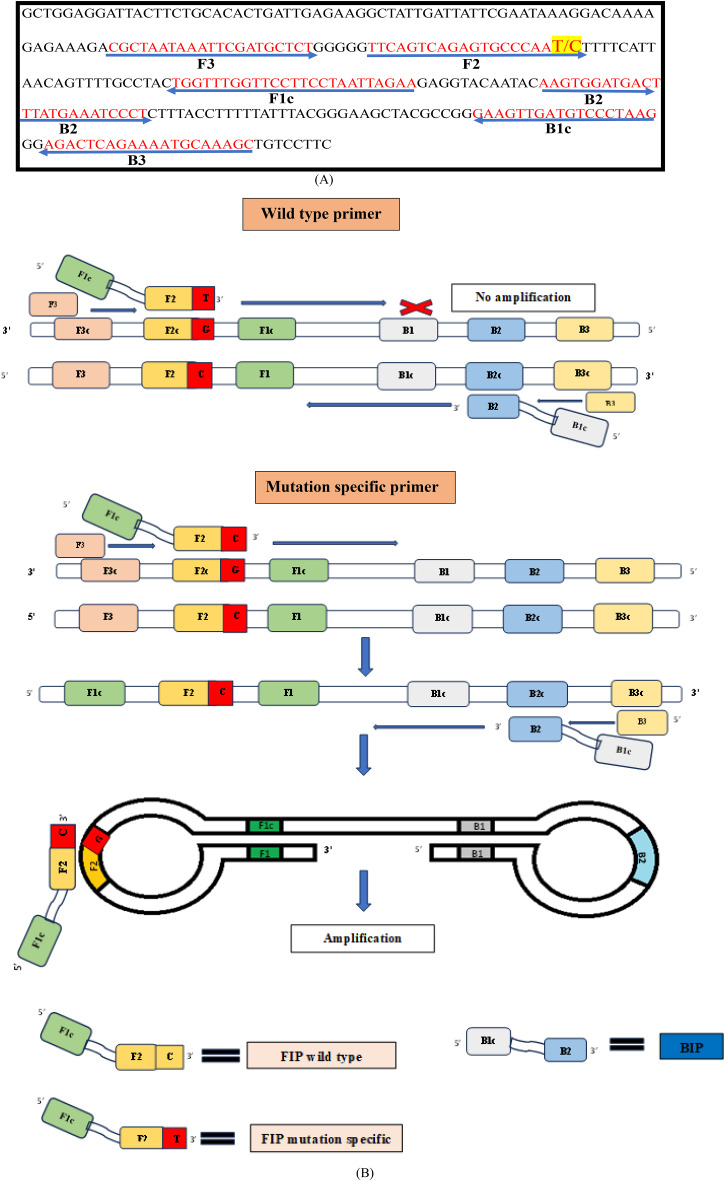
For SNP-LAMP assay initially primers were designed by using NEB LAMP primer designing tool (New England Biolabs.) for the two most prevalent SNPs in Pvmdr1 gene. Each designed primer set includes four primers: F3, B3, FIP and BIP for T98M mutations (Fig. 1) and F1076L mutations (Fig. 2). Bst polymerase which helps in strand displacement activity during the amplification lacks 5’ to 3’ DNase activity so the amplification with LAMP primers proceeds in 3’ to 5’ ends and primers designed were complementary to 3’-5’ end26. Mismatches were introduced at 3’ end of FIP for the amplification of mutants over the wild types. Separate sets of primers were designed for the T958M and F1076L mutants of Pvmdr1gene (Additional File No. 3).
Fig. 1.
(A) Alignment of nucleotide sequence for target Pvmdr 1 gene (Accession number AY571984.1). Arrows indicate sequences of designed F3, B3, FIP and BIP primers used in the study for LAMP assay. Point mutation highlighted by yellow (T958M → ACG-ATG) was inserted at 3’ end of FIP in mutation specific primers. (B) Schematic representation of designed LAMP primers F3, B3, FIP (F1c + F2) and BIP (B1c + B2) specific for amplifying wild types (T958) and for mutation (958 M). F1c and B1c sequences are complementary to F1 and B1.
Fig. 2.
(A) Alignment of nucleotide sequence for target F1076L gene (Accession number AY571984.1). Arrows indicate sequences of designed F3, B3, FIP and BIP primers used in the study for LAMP assay. Point mutation highlighted by yellow (F1076L → TTT-CTT) was inserted at 3’ end of FIP in mutation specific primers. (B) Schematic representation of designed LAMP primers F3, B3, FIP (F1c + F2) and BIP (B1c + B2) specific for amplifying wild types (F1076) and for mutation (1076L). F1c and B1c sequences are complementary to F1 and B1.
Optimization of LAMP conditions
Conditions for SNP-LAMP assay were optimized in reaction volume of 20 µl. Isothermal reaction mix (Ampligene India Biotech Pvt.Ltd., Ahmedabad India), was used in the reaction and 1 µl template DNA was used. For optimization different ranges of concentration were tested for designed primers including 0.2–0.8 µM for F3, B3 and 0.6 µM-2.0 µM for FIP, BIP, temperature range of 58℃-64℃ for 60–120 min were tested to find suitable conditions for Bst 2.0 Warmstart DNA polymerase to start the reaction. Nuclease free water was added for making the total reaction volume to 20 µl. Results were obtained as fluorescent peaks on Genei III fluorimeter (Optigene, UK, https://www.optigene.co.uk/instruments/genie-iii/) due to presence of dsDNA binding intercalating dye in the Ampligene Isothermal mix (ISO-001).
Sensitivity and specificity of SNP-LAMP based assay
SNP-LAMP assay was performed on 88 P. vivax positive samples with both mutation specific set of primers as well as wild type set of primers and to calculate the clinical sensitivity sequencing was considered as gold standard.
For analytical sensitivity amplified PCR product of Pvmdr1 gene was cloned and serial dilutions of the cloned gene were detected by the LAMP assay to check its analytical sensitivity. Firstly pGEM-T Easy vector (Promega corporation Madison, Wisconsin, United States) was added to tubes containing amplified PCR product and incubated for ligation. Ligated reaction was mixed with competent cells and heat shock was given followed by addition 1 ml LB medium (Lysogeny broth) and incubation at 37 ℃ for 1 h on shaker. Then the tube was centrifuged and the culture was plated on LB agar plates containing Xgal + Isopropyl β-D-1-thiogalactopyranoside (IPTG). Plate was kept at 37℃ for overnight incubation and white colonies were selected from the grown colonies. Picked colonies were resuspended in 10 ml of LB with 100 µg ampicillin and again kept at 37℃ for overnight incubation in shaker incubator (250 rpm). Plasmid was extracted by using Qiagen plasmid extraction kit by following manufacturer’s instructions and copy number was calculated for the cloned DNA.
Ten-fold serial dilutions were prepared and tested by designed sets of mutation specific primers to determine the limit of detection of the assay and by wild type set of primers to check the cross-amplification by the assay. Assay was also performed on P. falciparum (3D7 strain), Leishmania major and Toxoplasma gondii isolates with the designed primers to check the accuracy of these primers.
Results
Nested PCR for species identification
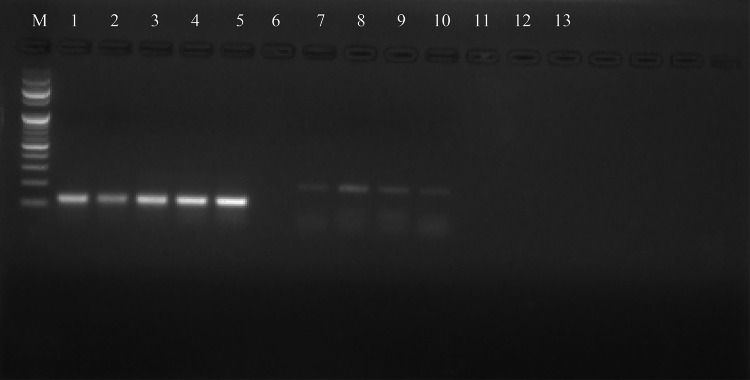
Samples enrolled in the study were confirmed by nested PCR and product of 120 bp was obtained for all the P. vivax positive samples (Fig. 3) (original gel picture Additional file No. 4).
Fig. 3.
Gel electrophoresis picture for amplified products of P. vivax positive samples. M-Marker 100 bp, Lane 1–4 P. vivax clinical isolates, Lane 5: Positive control, Lane 6: Negative control, Lane 7–10 P.falciparum positive samples (excluded from study).
Detection of SNPs in Pvmdr1 gene using PCR and sequencing
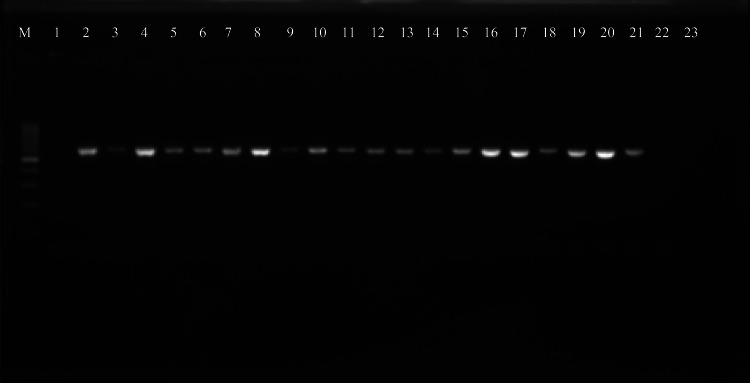
Pvmdr-1 gene was successfully amplified into a 603 bp product using PCR (Fig. 4) (original gel picture Additional File No. 5). Sequencing data was successfully obtained for all the amplified products and were aligned using Multiple sequences alignment tool (Clutal X2.1) to compare with reference wild type (AY571984.1) as well as mutant strains (MZ746915.1) for analyzing SNPs. Double mutants with variations at T958M and F1076L in Pvmdr1 gene were observed from all the sequenced clinical isolates. (Accession numbers in Additional file No. 6).
Fig. 4.
Gel electrophoresis picture for amplified Pvmdr1 gene of P. vivax clinical isolates. M = 100 bp marker, Lane 1-Negative control, Lane 2–22 = amplified Pvmdr1 gene products (603 bp).
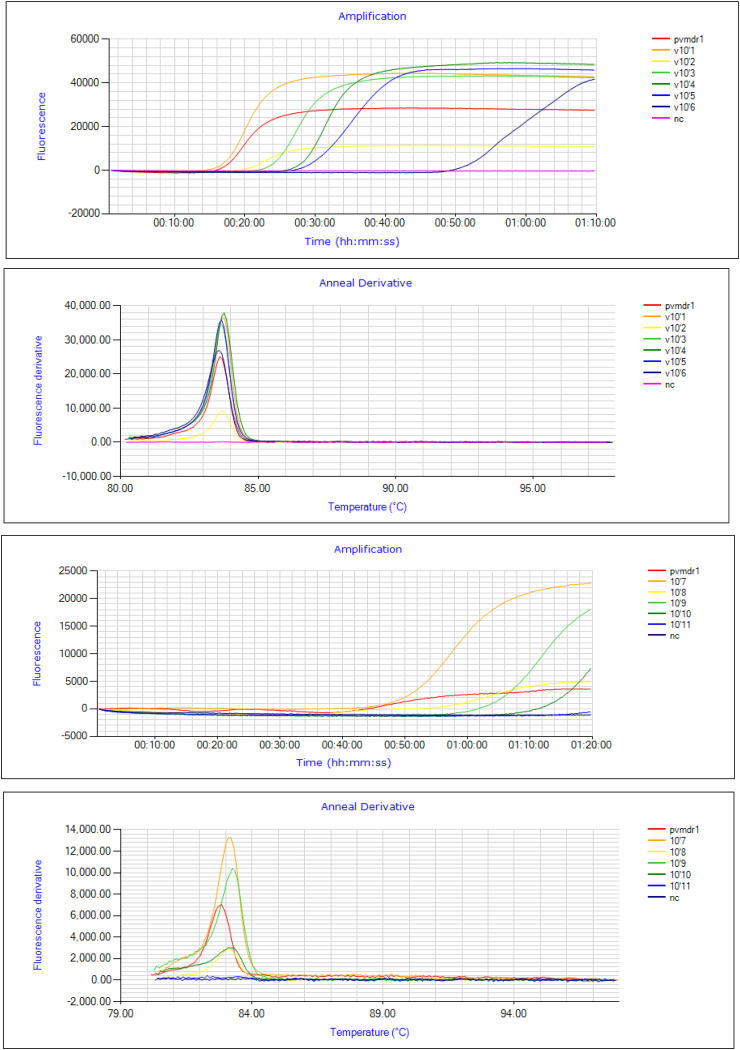
Optimization of designed primers for real-time LAMP assay
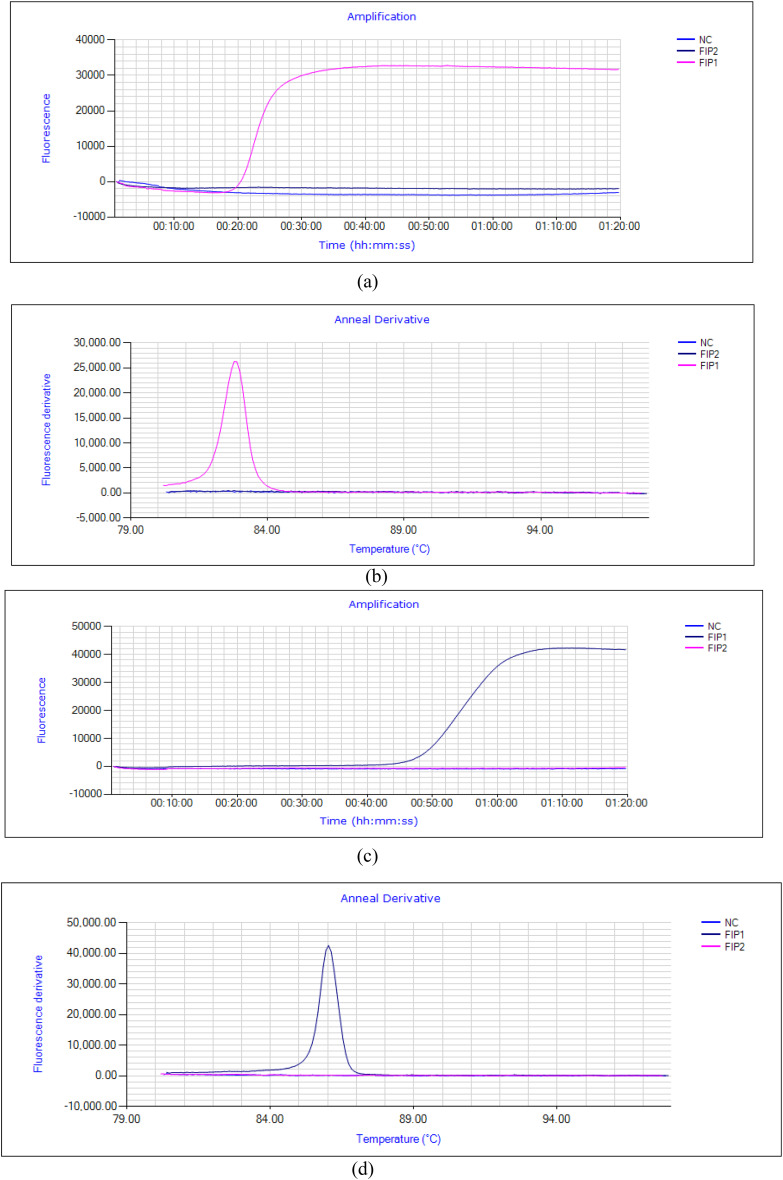
LAMP primers were designed by using NEB LAMP primer designing tool (New England Biolabs.). Two sets of FIP were designed to amplify mutations over wild types specifically in Pvmdr 1 gene by introducing mismatches specific for the target mutation at 3’end of FIP (FIP1 and FIP2). FIP1 successfully amplified the mutation at T958M over the wild as shown in (Fig. 5a,b) and no such amplification was observed for FIP2. FIP (W) set was designed for wild type amplification and all the samples were tested with this set also to check for cross-amplification and no such cross-amplification was observed for any of the mutant samples (Additional file No. 7). For mutation at F1076L (TTT CTT) also two sets of FIP were designed with mismatch at 3’end (FIP1 and FIP2). Mutation was successfully amplified by FIP1 and no such amplification was observed with other set (FIP2) (Fig. 5c,d). All the samples were also tested for cross amplification with designed set of wild type FIP(W) and no cross amplification was observed among the tested samples, (Additional file No. 7).
Fig. 5.
(a,b) Amplification fluorescence peaks for T958M mutation with designed primer sets, FIP1 (Pink), FIP2 (Violet), NC-Negative control (Blue) (c,d) Amplification fluorescence peaks for F1076L mutation with designed primer sets, NC-Negative control (Blue), FIP1 (Violet), FIP2 (Pink).
Optimization of LAMP conditions and reaction
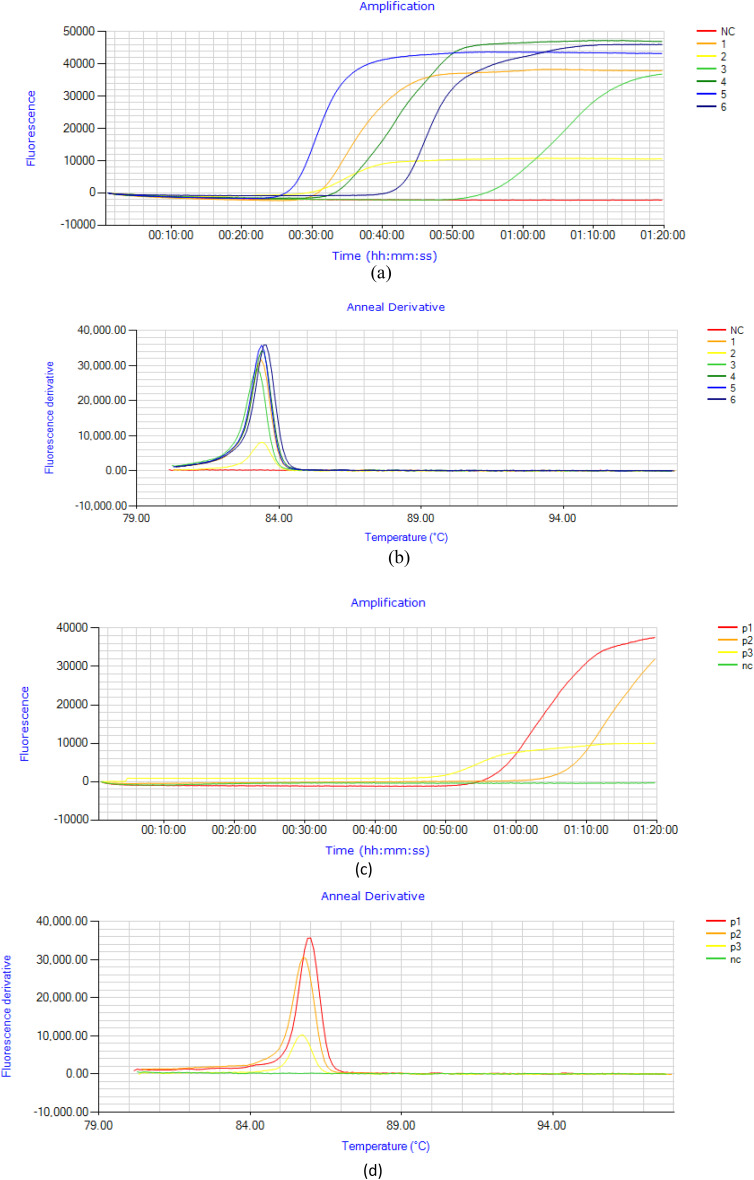
LAMP assay was standardized on purified Pvmdr1 gene and tested on “eighty-eight” P. vivax positive samples. For the selected set of primers SNP-LAMP was optimized and optimum incubation temperature was at 63 ℃ for T958M (Fig. 6a,b) mutations and 62 ℃ for F1076L mutations (Fig. 6c,d). Optimum incubation time for SNP-LAMP based assay was found to be 60 min. The final concentrations of the primers were 0.5 µM for F3 and B3, 2.0 µM for FIP and BIP in 20 µl of total reaction mixture.
Fig. 6.
Representation of SNP-LAMP reaction optimization for reaction temperature. (a,b) Optimum temperature for T958M mutation amplification was observed to be 63 ℃, NC-Negative control, 1–6 fluorescence peaks observed for samples with mutation at T958M (c,d) Optimum temperature for F1076L mutant amplification was observed to be 62 ℃, p1-p3 Fluorescence peaks at 62℃ of incubation temperature for samples with mutation at F1076L.
Clinical sensitivity and specificity
For clinical sensitivity and specificity, the SNP-LAMP assay was tested on 88 P. vivax positive clinical isolates to amplify T958M and F1076L mutations of Pvmdr-1 gene. Sanger Sequencing was considered as reference technique for calculating clinical sensitivity of the SNP-LAMP assay. Both the SNPs were successfully amplified in all P. vivax (88/88) clinical isolates. Sensitivity and specificity of the SNP-LAMP assay were found to be 100% as there were no false positives or false negatives by the SNP-LAMP assay.
Limit of detection and confirmation of specificity
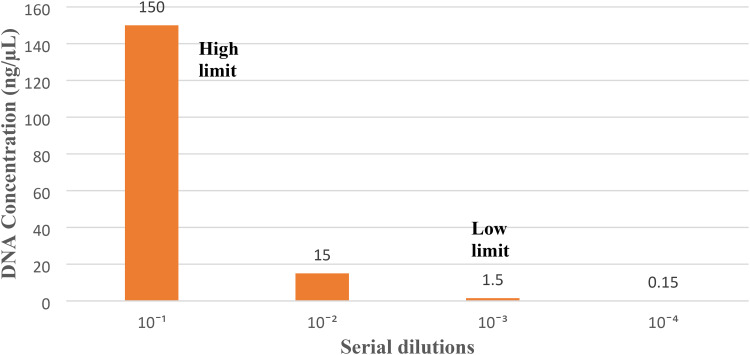
DNA concentration for all samples was measured by spectrophotometer (Nanodrop technologies). SNP-LAMP assay successfully detected the highest concentration of 150 ng/µl of DNA template and to check the lowest limit serial dilutions were prepared and the lowest limit was found to be 1.5 ng/µl (Fig. 7). LAMP amplification for serial dilutions were monitored as fluorescent peaks on Genei III fluorimeter (Optigene, UK) (Fig. 8). Wild type set of primers was also tested with highest and lowest concentration of DNA template and no cross-amplification was observed with the mutations even at highest concentration (Additional file No. 8).
Fig. 7.
Graphical representation of highest and lowest concentration of DNA template detected by LAMP (150 ng/µl-Highest limit, 1.5 ng/µl-Lower limit).
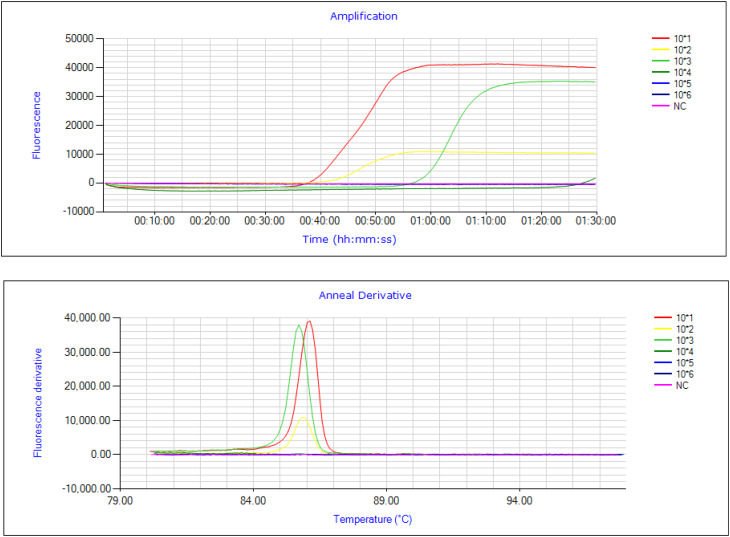
Fig. 8.
Sensitivity detection for the highest and lowest DNA template concentrations. Fluorescence peaks were observed for target mutations at DNA concentration of 150 ng/µL (Red peak), 15 ng/µL (Yellow peak) and the lowest concentration detected by SNP-LAMP assay was 1.5 ng/µL (Green peak).
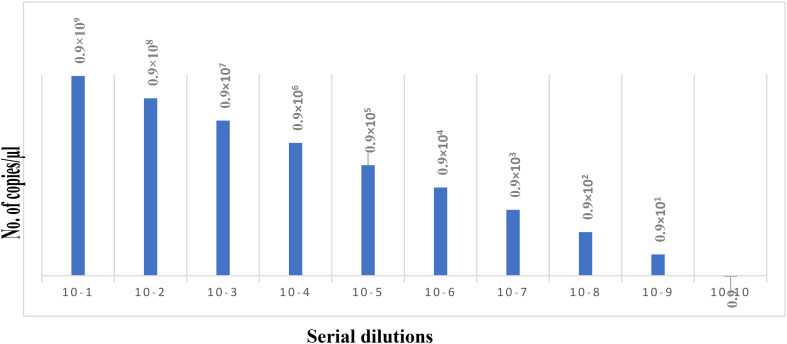
For analytical sensitivity Pvmdr 1 gene was cloned and copy number for the cloned plasmid DNA was found to be 0.9˟1010 copies/µL. Tenfold serial dilutions were prepared from cloned Pvmdr1 gene and tested by the SNP-LAMP assay to check limit of detection. Fluorescence graphs were obtained on the Genei III fluorimeter (Optigene, UK) for the amplification of mutations in serially diluted cloned DNA (Fig. 9). SNP-LAMP assay successfully detected up to 0.9 copies/µl of target gene (Fig. 10).
Fig. 9.
Analytical sensitivity detection for designed SNP-LAMP assay. Analytical sensitivity of SNP-LAMP assay detected for various dilutions of cloned Pvmdr-1 gene (10–1 to 10–11). Lowest limit of detection for the assay was up to 10–tenfold serial dilution (0.9 copies/µL).
Fig. 10.
Graphical representation of limit of detection of SNP-LAMP assay. Lowest limit of assay was 0.9 copies/µl.
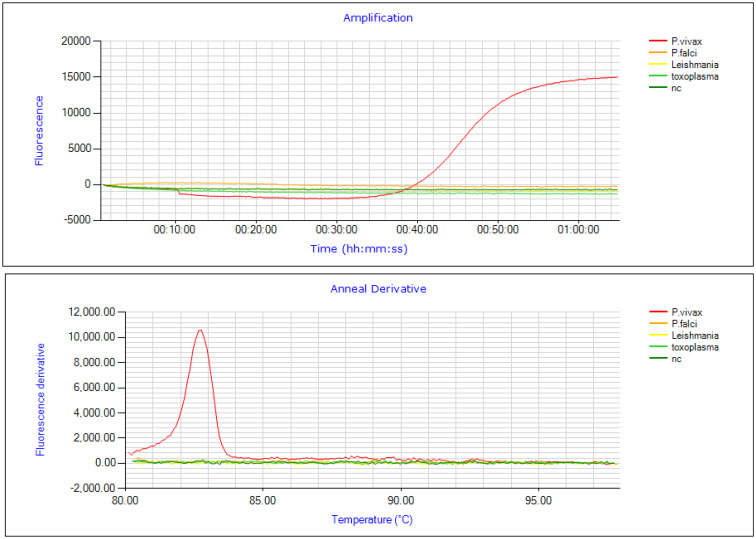
To check the accuracy of designed SNP-LAMP assay cross-reactivity was tested with P. falciparum (3D7 strain), Leishmania major and Toxoplasma gondii. No cross-reactivity was observed with the designed primers (Fig. 11).
Fig. 11.
Assessment of cross-reactivity of the SNP-LAMP assay in detecting SNPs. Fluorescence peak was seen for P.vivax (Red) only and no cross-reactivity was detected with P.falciparum (Orange), L. major (Yellow) and T.gondii (Light green).
Discussion
Emerging anti-malarial drug resistance poses a major hurdle for eliminating malaria. LAMP based assay is emerging as a useful alternative tool to other molecular methods in field settings due to its simplicity, cost-effectiveness, rapidity and near to patient molecular test27,28. There are earlier reports on the use of LAMP for detecting fungal, bacterial, and parasitic strains18,19,29–31. Studies on detection of SNPs in P. falciparum chloroquine transporter resistance gene (Pfcrt), P. falciparum dihydrofolate reductase gene (Pfdhfr) and artemisinin resistance gene (Kelch13) using visual SNP-LAMP assay have been reported21–23. No such reports are available for detecting drug resistance markers using Real-Time LAMP in P.vivax. In India, chloroquine is still a first-line treatment, but there are reports for emerging SNPs in marker genes of chloroquine resistance which need to be monitored at large scale17. So, this study was designed to develop a Real-Time SNP-LAMP assay to detect SNPs in chloroquine multi-drug resistance gene in field settings.
“Eighty-eight” P. vivax positive clinical isolates were enrolled in the study confirmed by nested PCR. For detection of SNPs Pvmdr 1 gene was amplified in P.vivax positive clinical isolates through PCR and was sequenced. All the isolates were found to be double mutant for Pvmdr1 gene with variations at T958M and F1076L (M958 Y976 Y1028 L1076). Earlier studies reported an association between CQR and SNPs in Pvmdr 1 gene from the year 2007 where in vitro susceptibility for chloroquine tested in Indonesia and Thailand had suggested an association between Pvmdr 1 polymorphism and CQR16. Another study supports the results of present study where double mutants M958 Y976 Y1028 L1076 for Pvmdr1 gene were present in 100% of the complicated cases and 98.7% in uncomplicated cases, and a triple mutant (T958M/F1076L/Y1028C) was observed only in single uncomplicated isolate30. So, the current study is supporting the increasing prevalence of SNPs in Pvmdr 1 gene thus following the track towards hypothesis from the previous studies stating that resistance to chloroquine follows a two-step trajectory of SNPs F1076L followed by Y976F in Pvmdr1 gene14. The data from this study for F1076L mutants is an alarming stage for emerging resistance in P. vivax for chloroquine suggesting a need for regular surveillance.
For Real-Time SNP-LAMP assay primers were designed using NEB LAMP primer designing tool (New England Biolabs) targeting both SNPs. In the primers designed for amplifying target mutations FIP was modified by adding mismatch at its 3’end. Suitable conditions for reaction and concentration of reagent and primers were optimized to obtain fluorescence peaks for the SNPs. Optimum incubation temperature was 63℃ for T958M and 62℃ for F1076L mutants with incubation time of 60 min, so the designed SNP-LAMP works on isothermal temperature and do not require sophisticated labs as it can be performed on simple water bath also. SNP-LAMP assay is also found to be faster as compared to traditional PCR methods. There were no false positives or false negatives by designed set of primers, so the sensitivity and specificity of the assay was found to be 100%. Analytical sensitivity of SNP-LAMP based detected from serial dilutions of cloned Pvmdr1 gene was 0.9 copies/µl which was ten times better than that of PCR. Lowest concentration of template DNA detected by designed set of primers was 1.5 ng/µL. To check accuracy the primers were tested on P. falciparum, Leishmania major and Toxoplasma gondii isolates, and no cross-reactivity was observed with any of these parasitic strains. So, in comparison to older molecular methods including PCR the results from this study prove the robustness of the assay with high sensitivity with low limit of detection and specificity.
In conclusion, LAMP assay can be used for large field-based surveillance studies to check the prevalence of SNPs in Pvmdr1 of chloroquine resistance in case of P. vivax positive clinical isolates. The study proves LAMP to be a reliable, field friendly, economic and more sensitive as well as specific tool to detect SNPs in chloroquine resistant Pvmdr1 gene. This method can be used as a powerful tool for finding the hubs of CQ resistance in endemic as well as resource limited countries and facilitate the management of correct treatment. The assay further needs to be tested on larger sample size to check the suitability of the assay.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Department of Medical Parasitology at the Post Graduate Institute of Medical Education and Research for providing the platform necessary for conducting the project.
Abbreviations
- Pvmdr1
Plasmodium vivax multi-drug resistance
- LAMP
Loop mediated isothermal amplification assay
- WHO
World Health Organization
- P. falciparum
Plasmodium falciparum
- P. vivax
Plasmodium vivax
- Pfcrt
Plasmodium falciparum chloroquine resistance transporter
- Pfmdr
Plasmodium falciparum multi-drug resistance
- Pvcrt
Plasmodium falciparum chloroquine resistance transporter
- PCR
Polymerase chain reaction
- F3
Forward
- B3
Backward
- FIP
Forward inner primer
- BIP
Backward inner primer
- PPV
Positive predictive value
- NPV
Negative predictive value
Author contributions
Davinder Kaur: Conception of study, Sample collection & processing, Investigation, Experimentation, acquisition of data, analysis and interpretation of data, Draft writing. Upninder Kaur: Supervision and review of the study, Revised the manuscript, Final Editing. Chandra Kanta Bhusal: Writing manuscript, analysis, Review. Vibhor Tak: Sample collection, Patient data collection. Rakesh Sehgal: Supervision and review of the study, Contributed to conception of study, Validation of results, Revised manuscript.
Funding
Davinder Kaur is thankful to Council of Scientific & Industrial Research (CSIR), New Delhi, for providing Fellowship: 09/141(0227)/2019-EMR-1.
Data availability
The datasets analyzed during the current study are available in the present study results and the supporting data which is related to the present study is available with the corresponding author. The sequences obtained during the study were submitted to the GenBank (Accession number given in additional file No. 6).
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The ethical clearance (INT/IEC/2020/SPL-1480) was given by Postgraduate Institute of Medical Education and Research (PGIMER) ethical committee. The committee members have reviewed the study protocol and research proposal thoroughly. Prior to sample collection informed consents were obtained from all the enrolled patients.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. World Malaria Report 2022 (World Health Organization, 2022). [Google Scholar]
- 2.Price, R. N. et al. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg.77, 79 (2007). [PMC free article] [PubMed] [Google Scholar]
- 3.Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature415, 673–679 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Kaur, H. et al. Distribution pattern of amino acid mutations in chloroquine and antifolate drug resistance associated genes in complicated and uncomplicated Plasmodiumvivax isolates from Chandigarh, North India. BMC Infect. Dis.20, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnadas, C. et al. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. J. Antimicrob. Agents52, 4233–4240 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur, D., Sinha, S. & Sehgal, R. Global scenario of Plasmodiumvivax occurrence and resistance pattern. J. Basic Micribiol.62, 1417–1428 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Simpson, J. A. et al. Mefloquine pharmacokinetic-pharmacodynamic models: implications for dosing and resistance. J. Antimicrob. Agents44, 3414–3424 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noulin, F., Borlon, C., Van Den Abbeele, J., D’Alessandro, U. & Erhart, A. 1912–2012: a century of research on Plasmodiumvivax in vitro culture. Trends Parasitol.29, 286–294 (2013). [DOI] [PubMed] [Google Scholar]
- 9.L’Episcopia, M., Perrotti, E., Severini, F., Picot, S. & Severini, C. An insight on drug resistance in Plasmodiumvivax, a still neglected human malaria parasite. Ann. Ist. Super Sanità56, 403–408 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Fidock, D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell6, 861–71 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babiker, H. A. et al. High-level chloroquine resistance in Sudanese isolates of Plasmodiumfalciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J. Infect. Dis.183, 1535–1538 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Sá, J. M. et al. Plasmodium vivax chloroquine resistance links to pvcrt transcription in a genetic cross. Nat. Commun.10, 4300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva, S. R. et al. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Mal. J.17, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brega, S. et al. Identification of the Plasmodiumvivax mdr-like gene (pvmdr1) and analysis of single-nucleotide polymorphisms among isolates from different areas of endemicity. J. Infect. Dis.191, 272–277 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Russell, B. et al. Determinants of in vitro drug susceptibility testing of Plasmodiumvivax. J. Antimicrob. Agents52, 1040–1045 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suwanarusk, R. et al. Chloroquine resistant Plasmodiumvivax: in vitro characterisation and association with molecular polymorphisms. PLoS One2, e1089 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anantabotla, V. M. et al. Polymorphisms in genes associated with drug resistance of Plasmodiumvivax in India. J. Parasitol. Int.70, 92–97 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Kaur, H. et al. Development of visually improved loop mediated isothermal amplification for the diagnosis of Plasmodiumvivax malaria in a tertiary hospital in Chandigarh, North India. Am. J. Trop. Med. Hyg.98, 1374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versalovic, J. & Lupski, J. R. Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol.10, s15–s21 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res.28, e63 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chahar, M., Mishra, N., Anvikar, A., Dixit, R. & Valecha, N. J. Establishment and application of a novel isothermal amplification assay for rapid detection of chloroquine resistance (K76T) in Plasmodiumfalciparum. Sci. Rep.7, 41119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohon, A. N. et al. A novel single-nucleotide polymorphism loop mediated isothermal amplification assay for detection of artemisinin-resistant Plasmodium falciparum malaria. Open forum Infect. Dis.5, ofy011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yongkiettrakul, S. et al. Simple detection of single nucleotide polymorphism in Plasmodium falciparum by SNP-LAMP assay combined with lateral flow dipstick. J. Parasitol. Int.66, 964–971 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Shahzadi, S. et al. Molecular detection of malaria in South punjab with higher proportion of mixed infections. Iran. J. Parasitol.9, 37 (2014). [PMC free article] [PubMed] [Google Scholar]
- 25.Garg, S. et al. Novel mutations in the antifolate drug resistance marker genes among Plasmodiumvivax isolates exhibiting severe manifestations. Exp. Parasitol.132, 410–416 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Park, J.-W. Principles and applications of loop-mediated isothermal amplification to point-of-care tests. Biosensors12, 857 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohon, A. N. et al. NINA-LAMP compared to microscopy, RDT, and nested PCR for the detection of imported malaria. Diagn. Microbiol.85, 149–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tegegne, B., Getie, S., Lemma, W., Mohon, A. N. & Pillai, D. R. Performance of loop-mediated isothermal amplification (LAMP) for the diagnosis of malaria among malaria suspected pregnant women in Northwest Ethiopia. Mal. J.16, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori, Y. & Notomi, T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Inf. Chemo15, 62–69 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niessen, L. & Vogel, R. F. Detection of Fusariumgraminearum DNA using a loop-mediated isothermal amplification (LAMP) assay. Int. J. Food Microbiol.140, 183–91 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Saito, R. et al. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of Mycoplasma pneumoniae. J. Med. Microbiol.54, 1037–41 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the present study results and the supporting data which is related to the present study is available with the corresponding author. The sequences obtained during the study were submitted to the GenBank (Accession number given in additional file No. 6).