Abstract
Dogs are the primary reservoirs of Leishmania infantum (L. infantum), but Leishmania tropica (L. tropica) infection is also possible in dogs and can transmitted to humans. The southeast of Iran experiences a high prevalence of canine leishmaniasis (CanL), and veterinarians frequently encounter symptomatic cases. Therefore, from December 2017 to November 2022, the present case series was designed to assess the prevalence of CanL in owned dogs with cutaneous manifestations resembling CanL. A total of 500 owned dogs with dermal lesions from two endemic cities, Zabol and Kerman, were enrolled. Impression smears from skin lesions and popliteal lymph nodes were prepared from all cases, whereas blood samples were gathered from amastigote-positive dogs for serological and molecular surveys. Commercial ELISA was done on sera samples, and two-step nested PCR was used on extracted DNA to amplify variable fragments of the Leishmania species’ kinetoplast DNA (kDNA). Leishman bodies were microscopically detected in 7.2% (36/500) of dermal smears. Of the 360 owned dogs from Zabol, 2 have been diagnosed with L. tropica, and 10 have been diagnosed with L. infantum. Out of 140 owned dogs from Kerman, 8 were identified with L. tropica infection, and 16 were infected with L. infantum. Molecular results revealed the presence of 750 full dual-band bases related to the L. tropica species in 10/36 (27.7%) cases, which showed a considerable increase in canine cutaneous leishmaniosis compared with previous studies in southeastern Iran. The noticeable prevalence of L. tropica in owned dogs indicated that the dog’s role in cutaneous leishmaniosis should be re-evaluated as a possible animal reservoir in endemic areas.
Keywords: Cutaneous leishmaniasis, Dog, Leishmania Tropica, Iran
Subject terms: Microbiology, Diseases, Medical research, Molecular medicine, Pathogenesis
Introduction
Six critical tropical diseases have the potential to cause human death, including Visceral Leishmaniasis (VL)1,2. More than 72 developing countries are affected by leishmaniasis3. It is estimated that Cutaneous Leishmaniasis (CL) is affected yearly by 1-1.5 million people worldwide4,5. In addition, it is estimated that around 1 billion people are at risk of infection by the disease6,7. It has been reported that 90% of the global cases of VL are found in Iran, Saudi Arabia, Afghanistan, Algeria, Brazil, Peru, and Syria, among which the incidence of the disease in Iran is among the highest8–10. Cutaneous leishmaniasis and VL are both prevalent in Iran11,12. In Iran, CL and VL were reported from many provinces, with emphasis on Kerman13,14, Razavi Khorasan15, North Khorasan16–18, Sistan-va-Baluchestan19,20, Golestan21, Isfahan22–24, Semnan25, Yazd26–28, Fars29, Bushehr30–33, Hormozgan34–36, Khuzestan37,38, Ardebil39,40, Ilam41–46 and other parts47–56. Canine leishmaniasis is an important disease reported many times in Zabol and Kerman cities13,57–70 (Table 1).
Table 1.
Prevalence of CL and VL in animal and human population in Kerman and Sistan & Baluchestan Provinces, southeastern Iran.
| Province | Sample | CL/VL | Agent | Infection rate | Methodology | Author & year |
|---|---|---|---|---|---|---|
| Kerman | Human | CL | L. major | 4.7% | Direct smear microscopy / nested-PCR | (Khosravi et al., 2013) |
| Kerman | Human | CL | L. tropica L. major | (95.6%), (4.4%). | Skin scraping smears / nested-PCR | (Sharifi et al., 2012) |
| Kerman | Rodents / Stray dogs | CL | L. major |
33% rodents 0% dogs |
Smear microscopy / histopathological / PCR | (Ghaffari et al., 2014) |
| Kerman | Humans/ owned dogs | VL | L. infantum |
3.22% Children 23% dogs |
Direct agglutination test | (Mahmoudvand et al., 2011) |
| Kerman | Stray cat | VL | L. infantum | %6.7, 16.7% | ELISA, PCR | (Akhtardanesh et al., 2017) |
| Kerman | Stray dog | VL | L. infantum | 11.25% | Direct agglutination test / Pathology | (Bamorovat et al., 2014) |
| Kerman | Humans | CL | L. tropica | 5.3% | Smears / cultures / PCR | (Sharifi et al., 2011) |
| Kerman | Sand flies, Phlebtomus sergenti | CL | L. tropica | (3.6%) | Nested-PCR | (Mozafary et al., 2016) |
| Kerman | Canine | VL | L. infantum |
11% stray dogs 3% sheepdogs |
Direct agglutination test | (Afshar et al., 2018) |
| Kerman | Human | VL | L. infantum |
2.6% titers ≥ 1:800 0.6% titers ≥ 1:3200 |
(Abbaszadeh-Afshar et al., 2015) | |
| Kerman | Human | CL | L. tropica | 4.9% | Smear scrapings | (Aflatoonian & Sharifi, 2007) |
| Kerman | Human | CL | L. major | 0.34% | Smear scrapings | (Askari et al., 2018) |
| Kerman | Human | CL |
L. tropica L. major |
(88.5%) (11.5%) |
Smear scrapings / PCR | (Ramezany et al., 2018) |
| Kerman | Dogs | CL | L. infantum | 15.4% | ELISA | (Mahshid et al., 2014) |
| Sistan & Baluchestan | Dogs | VL | L. infantum | 5.33% | IFA | (Akhtardanesh et al., 2021) |
Canine leishmaniasis is a chronic pansystemic disease manifested by many clinical signs and clinical findings71,72. One of dogs’ most prevalent clinical findings are skin lesions and local or generalized lymphadenomegaly73. Several species of Leishmania can cause various forms of the disease, and leishmaniasis will probably become an emerging zoonosis and a public health problem in the coming years74,75. It is important to note that while dogs are the primary sources for transmitting L. infantum, clinical diseases associated with L. tropica are also possible in dogs and can be transmitted to humans76–78. Diagnosing CL in dogs is usually possible by observing the impression smears of the skin lesions, which are stained with Giemsa stain. Molecular methods could confirm the infection and specify the species79,80. Although PCR is the most effective method of detecting CVL, it is still applied only for research purposes and individual diagnosis because it lacks standardization81. Several target genes have been identified for detecting Leishmania using the PCR procedure82. The most specific tests are used for species identification, while the most sensitive tests are used for generic diagnosis.
To the best of our knowledge, a few cases of L. tropica-infected dogs were reported from Morocco and Iran83–86. In the current case series, several dogs with skin lesions resembling VL were recently noted in teaching veterinary hospitals in southeastern Iran, and they are being investigated for possible links to leishmaniasis.
Methods and materials
Study area
The study was conducted between December 2017 and November 2022 in the Zabol and Kerman provinces of Iran, situated in the country’s southeast. Sistan and Baluchestan are situated between pivot points of 30 and 18 min in the northern region and 31 and 20 min in the eastern region. Sistan and Baluchestan, from the north and east, are neighbors of Afghanistan. Deserts bound its west and northwest. Sistan and Baluchestan are between 475 and 500 m above sea level. Zabol city is the capital of Sistan and Baluchestan. It has been reported for 20 years that maximum temperatures are between 22 and 49 °C and minimum temperatures are between − 8 °C and 15 °C. Approximately 59 milliliters of rain are reported to fall in Sistan and Baluchestan annually, and the humidity is also 40%.
The latitude of Kerman is 30.283938, and the longitude is 57.083363, with elevations ranging from 1700 to 2700 m above mean sea level and an average annual rainfall of about 120 mm. As a province of 180,726 km2 with a population of nearly 3 million, Kerman is Iran’s largest province. The city of Kerman embraces about 80% of the provincial urban population, making it the most developed and the largest city in the province, with a current population of about 550,000 (Fig. 1).
Fig. 1.
Map of Iran. This figure illustrates the sampling area in the present study. The map was drawn by using ArcGIS software version 10.3 (https://enterprise.arcgis.com/en/portal/).
Sampling
A total of 360 owned dogs by Zabol and 140 by Kerman were enrolled in the study. They had skin manifestations resembling leishmaniasis, including focal alopecia, nose and pad hyperkeratosis, dermal lesions on the muzzle, eyelids, pinnae, and onychogryphosis. They were all referred for dermatologic problems to the teaching veterinary hospitals. Demographic data, including age, breed, sex, and location of habitation, were documented. Physical examination data were recorded in a questionnaire.
Microscopic examination
Impression smears from all 500 cases with dermal lesions were prepared. Afterward, 70% ethanol was used to fix the sample for 30 to 60 s. The smear dried, and Giemsa solution was poured onto it for 30–50 min. Finally, the slides were immersed in water for a short time, then quickly removed from the water and air-dried. Microscopic examination was done with eyepieces 10, objective lenses 10, 40, and 100, and immersion oil without coverslips.
Fine needle aspiration of the popliteal lymph node was also prepared for microscopic examination in all 500 cases under short chemical restraint by IV injection of 5 mg/kg ketamine (Alfasan, Nederland) and 0.5 mg/kg midazolam (Caspian Pharmaceutical Company, Iran).
Skin biopsy
In the next step, a full-thickness punch biopsy of the skin lesion was taken under short general anesthesia induced by IV injection of 5 mg/kg ketamine and 0.5 mg/kg midazolam and submitted for PCR (ribosomal DNA testing).
Serological examination
From suspected dogs (36/500; 7.2%), in which amastigotes were seen in their impression smear, 3 ml blood samples were taken from the cephalic veins. Serum samples were separated by centrifugation at 3000 rpm for 3–5 min and stored at -20 °C for serological examination. The ELISA was performed using an indirect kit (ID Screen Canine Leishmaniasis, ID-Vet Company, France) as instructed in the manufacturer’s manual, and the samples were read at 450 nm using an ELISA reader (ELX800 BioTek, USA). Validation of the ELISA test was determined by the difference between the mean optical density of the positive control (ODPC) and the mean optical density of the negative control (ODNC) being > 3 (ODPC/ODNC > 3).
Molecular techniques
DNA extraction
DNA extraction was performed using the Gene All Biotechnology commercial kit (South Korea) on cutaneous ulcer fragments. Following the manufacturer’s instructions, 15µL of proteinase K was initially added to 1.5 ml microtubes. Then, full-thickness skin samples with diameters of 3 mm to 4 mm and 200µL of BL buffer were added to each microtube. The microtubes were vortexed and kept in a Bain-marie at 56 °C for half an hour. Subsequently, the centrifugation steps were carried out according to the kit’s instructions, and the DNA was transferred to a freezer at -18 °C.
Nested PCR assay
Using extracted DNA, Leishmania species’ kinetoplast DNA (kDNA) was amplified by two-step nested PCR. As described in a recent study, the primers were designed specifically for this analysis87. In the first step, the external primers CSB2XF (5′-CGAGTAGCAGAAACTCCCGTTCA-3′) and CSB1XR (5′-ATTTTTCGCGATTTT CGCAGAACG-3′) and the second-step, the internal primers 13Z (5′-ACTGGGGGTTGGTGTAAAATAG-3′) and LiR (5′-TCGCAGAACGCCCCT-3′) were utilized. In the first round of each, 25 µl reaction mixture, 5 µl template DNA, 12.5 µl Taq DNA Polymerase Master Mix Red (Ampliqon, Denmark), and 30 picoM of each primer of CSB2XF and CSB1XR were applied together. After 5 min at 95 °C, 35 cycles of 30 s at 94 °C, 1 min at 55 °C, and 1 min at 72 °C were carried out. This was followed by a 5-minute extension at 72 °C. 1 µl of 1:9 diluted PCR product of the first round was used as a template for the second PCR round. LiR and 13Z primers were used in this step, with the same conditions and reaction mixture as in the first. Amplified DNA was electrophoretically separated using an agarose gel electrophoresis, ethidium-bromide pre-staining, and ultra-violet analysis. The presence of 750 bp bands for L. tropica and 560 bp for L. infantum was recorded in positive samples.
Results
Amastigotes were seen in the impression smear of 36 dogs, and FNA of the popliteal lymph node was positive in 28 cases. Molecular investigation of skin biopsy samples showed that among the target population in Zabol, 0.55% (2/360) have been diagnosed with L. tropica, and 2.77% (10/360) have been diagnosed with L. infantum. In Kerman, 5.71% (8/140) were identified with L. tropica, and 11.42% (16/140) were infected with L. infantum (Table 2).
Table 2.
Signalment of 36 leishmania-infected dogs with skin lesions in this study.
| Parameter | L. infantum number (%) | L. tropical number (%) | |
|---|---|---|---|
| Age | > 3 years | 10 (27.7%) | 4 (19.9%) |
| 3–6 years | 10 (27.7%) | 4 (56.2%) | |
| 6 years < | 6 (16.66%) | 2 (8.4%) | |
| Sex | Male | 16 (10.4%) | 6 (49.8%) |
| Female | 10 (5%) | 4 (34.8%) | |
| Location | Kerman | 16 (44.44%) | 8 (22.22%) |
| Zabol | 10 (27.77%) | 2 (5.55%) | |
| Clinical findings | Focal alopecia | 5 (19.23%) | 3(30%) |
| Hyperkeratosis/foot pad | 5(19.23%) | - | |
| Hyperkeratosis/plenum nasal | 10 (38.46%) | 1(10%) | |
| Erosive lesions/ muzzle | 6 (23.07%) | 5(50%) | |
| Erosive lesions/ planum nasal | 10 (38.46%) | 3(30%) | |
| Erosive lesions/ eyelids | 3 (11.53) | 1(10%) | |
| Erosive lesions/ pinnae, | 2 (7.69%) | 1(10%) | |
| Onychogryphosis | 3 (11.53%) | - | |
| Pododermatitis | - | 1(10%) | |
| Lymphadenomegaly | 22(84.61%) | 1(10%) | |
| Cachexia | 5(19.23%) | 1(10%) | |
| Nasal bleeding | 3(11.53) | 1(10%) | |
| Hepatosplenomegaly | 5(19.23%) | 1(10%) | |
Zinc-responsive alopecia, discoid lupus erythematosus, localized demodicosis, dermatophytosis, and bullous pemphigoid, were the most common other skin disorders that were differentiated in suspected cases in the present study.
One case of L. tropica-infected dogs from Zabol had hyperkeratosis lesions, pustular dermatitis on the muzzles, and pododermatitis, and the second case had mucocutaneous ulceration on the muzzle. Leishmania tropica-infected cases from Kerman had different clinical signs, such as erosive to ulcerative lesions on the planum nasal, lower lip, and supra-ocular regions. Clinical manifestations of all infected dogs with L. tropica were presented in serial images (Fig. 2) (Table 3). Among L. infantum-infected dogs of Zabol, lymphadenomegaly and erosive lesions on the planum nasal and muzzle were the common clinical findings.
Fig. 2.
Case series of the current study. The present study cases that were infected with L. tropica. Location: K = Kerman Z = zabol.
Table 3.
Clinical and cytological findings in 10 Leishmania Tropica-infected dogs.
| Dog ID | Signalment (Breed, gendera, ageb) | Clinical findings | ELISA | Popliteal lymph node impressions |
|---|---|---|---|---|
| 1 K | Mix, F, 9yr | Focal erosive lesions on planum nasal and lower lip, focal alopecia | N | P |
| 2k | Mix, M, 2yr | Erosive lesion on supra-ocular regions | N | P |
| 3 K | Pomeranian, F, 3yr | Severe ulcers on nasal planum, generalized lympadenomegaly, cachexia | P | P |
| 4 K | Husky, M, 9yr | Erosive lesions on the planum nasal | N | N |
| 5 K | German shepherd, M, 6yr | Hyperkeratosis in muzzle | N | N |
| 6 K | Alaskan malamute, M, 3yr | severe crusting and ulceration of the face, focal alopecia | N | N |
| 7 K | Labrador, F, 3yr | Mucocutaneous ulceration on the muzzle | N | N |
| 8 K | Mix, M, 2yr | Severe mucocutaneous ulceration on the muzzle, epistaxis, hepatosplenomegaly | P | P |
| 9z | Mix, F, 2yr | Mucocutaneous ulceration on the muzzle, pododermatitis | N | N |
| 10z | Mix, F, 1yr | Mucocutaneous ulceration on the muzzle, focal alopecia | N | N |
aM=Male F = Female.
byr=Year.
location: K = Kerman Z = zabol.
N = Negative.
P = positive.
Among 16 L. infantum-infected dogs of Kerman, 11 cases showed no other clinical signs except mild skin lesions and generalized lymphadenomegaly. However, 5 cases were in poor health condition and were euthanatized after obtaining owner consent.
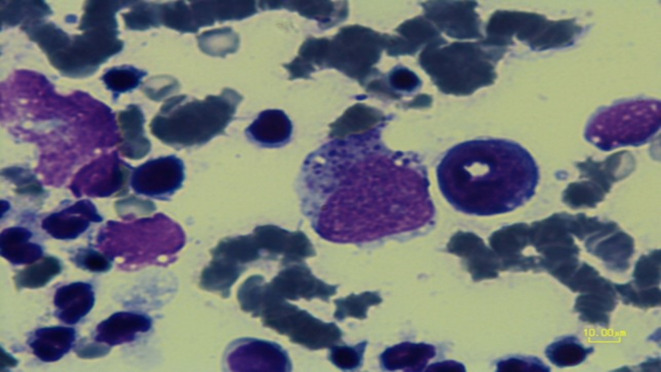
All L. infantum-infected cases and two L. tropica-infected cases from Kerman were seropositive in the ELISA test. In these cases, amastigote was seen in a popliteal lymph node in cytology. Based on other systemic signs like cachexia and generalized lymphadenopathy, visceral involvement with L. tropica was suspected, which may cause a false positive reaction in the ELISA test. Bone marrow aspiration was done, which was positive in two cases. However, euthanasia was not accepted by owners (Fig. 3).
Fig. 3.
Bone marrow aspiration. Bone marrow aspiration was positive in case 3.
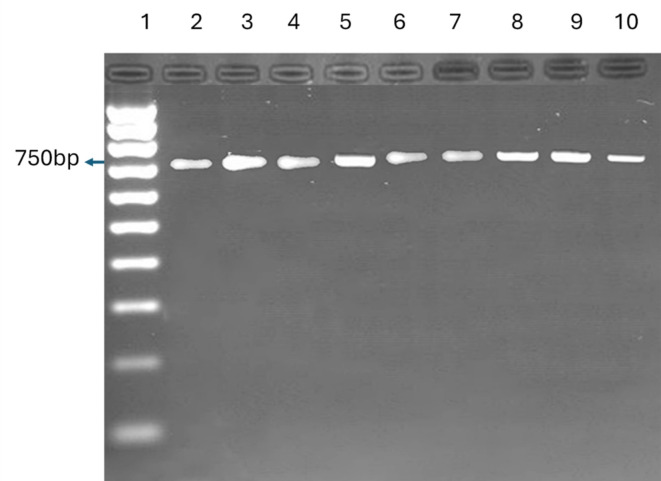
Molecular results showed the presence of 750 full dual-band bases related to the L. tropica species in 10 infected cases and a band of 560 bp, corresponding to L. infantum in 26 dogs (Figs. 4, 5 and 6).
Fig. 4.
Leishmania spp positive samples of Zabol. Sample 1 is a reference strain, samples 3 and 4 are two Leishmania tropica positive samples of Zabol, and samples 6 to 16 are ten Leishmania infantum positive ones in Zabol.
Fig. 5.
Leishmania tropica-positive samples of Kerman. Sample 2 is a reference strain, and samples 3 to 10 are Eight Leishmania tropica-positive samples of Kerman.
Fig. 6.
Leishmania infantum-positive samples of Kerman. Sixteen Leishmania infantum-positive samples of Kerman.
Excellent clinical improvement and initial response to allopurinol were seen in three cases of L. tropica-infected dogs (cases 1, 4, 7) that participated in a treatment protocol with 10 mg/kg allopurinol, given twice daily after two months. Owners did not report recurrence.
Discussion
In tropical and subtropical regions, leishmaniasis is one of the most important diseases88. It has diverse prevalence worldwide, and L. tropica is one of the leading causes of the disease89. The CL in dogs is emerging in some parts of the world90,91. In certain parts of the world, dogs infected with CL are important reservoirs for transmitting the disease from dogs to humans92,93. Clinical Symptoms are local lesions on the skin that can heal spontaneously or grow into chronic mucocutaneous lesions that eventually cause deformities in affected areas. The epidemiological patterns of CL in Iran have changed over the past two decades due to population migration between urban and rural areas and refugee flow from Afghanistan and Iraq to Iran94,95.
The results of our study show that among 360 owned dogs from Zabol, two were infected with L. tropica. The mucocutaneous lesion was seen around the lips, nasal planum, and toenails. Infected cases from Kerman had clinical signs such as alopecia, upper and lower lip, erythema, and erosive dermatitis on supra-ocular regions. Nasal planum and hard pads, which are not hair-covered, are an extremely higher risk of bites and injuries than other body parts. Responsible pet ownership has a crucial impact on VL control. Still, unfortunately, the absence of mandatory legal considerations for isolation, treatment, or euthanasia for infected dogs in Iran presented a significant obstacle to the follow-up of many cases. Some owners were not responsive to follow-up efforts, abandoned treatment midway, or faced other challenges that hindered effective monitoring.
According to a study conducted in Morocco, seven dogs were diagnosed with cutaneous leishmaniasis caused by L. tropica86. Four dogs in Essaouira and four in Azilal had cutaneous lesions out of 313 dogs examined. Solitary or clustered small ulcers were present around the muzzle. Samples were collected under general anesthesia for examination and culture using an Arquette scalpel. Lymphadenopathy was diagnosed by aspirating lymph nodes. Isoenzyme electrophoresis identified 3 strains from Azilal as L. tropica MON-102 and 4 from Essaouira as L. tropica MON-113. These seven dogs showed no signs of lymphadenopathy. In one dog from Azilal, an ulcer on the muzzle was caused by L. infantum MON-1. The animal also had lymphadenopathy, and L. infantum was isolated.
In the current study, 2 L. tropica-infected cases were positive in the ELISA test, which shows the cross-reaction between the two species; however, molecular tests are needed to differentiate between viscerotropic L. tropica and L. infantum. Furthermore, in another study of 471 dogs in the Kerman province, two were infected with a strain of L. tropic85. Clinical examination revealed myiasis, alopecia, and hyperkeratosis of the footpads in one of the dogs. In addition to weight loss, cachexia, splenomegaly, and lymphadenomegaly, these manifestations were particularly prominent in the prescapular and popliteal lymph nodes. The phylogenetic analysis revealed that the positive sample in this research was closely related to the Iranian L. tropica kDNA with accession number AB678350. The present study confirms the emergence of canine CL caused by L. tropica, previously reported in western India96.
The study presented herein delves into the prevalence of L. infantum and L. tropica infections in owned dogs exhibiting cutaneous manifestations in southeast Iran. Dogs, particularly in endemic regions, serve as the primary reservoirs for L. infantum, and this study reveals the possibility of L. tropica infections in owned dogs, emphasizing the potential transmission risk to humans. Skin impressions and blood samples were obtained from dogs with dermal lesions for serological and molecular examinations, respectively. The study’s molecular analysis, specifically Two-step nested PCR targeting variable fragments of Leishmania species’ kinetoplast DNA, revealed the presence of L. tropica and L. infantum in the sampled dogs. The results elucidate a significant prevalence of L. tropica in owned dogs, particularly in Kerman province. Moreover, 10 dogs in Zabol and 16 dogs in Kerman were infected with L. infantum, and these cities were considered endemic for canine leishmaniosis in previous studies13,58.
These findings underscore the importance of re-evaluating dogs as potential animal reservoirs in endemic areas. Given the notable prevalence of L. tropica in owned dogs, the implications extend to public health, highlighting the need for comprehensive surveillance and control measures to mitigate the transmission risk to animals and humans in these regions97,98. The potential transmission of these infections from dogs to humans underscores the interconnectedness of animal and human health, necessitating comprehensive surveillance and control measures99. Canine and human leishmaniasis can be controlled by controlling sand flies and avoiding exposure using repellent antiparasitic collars in endemic areas and vaccinations100–103. The study’s findings contribute to the scientific understanding of zoonotic diseases and advocate for a holistic approach that recognizes the pivotal role of animals in mitigating the impact of infectious diseases on both veterinary and human populations worldwide.
Conclusion
This case series showed that CL must be noticed as an emerging disease in owned dogs in Iran. Detection of new reservoirs of L. tropica in endemic CL regions is necessary to control and prevent the disease. As dogs may carry L. tropica asymptomatically, further investigation must be performed by aphidological surveys to assess and understand the real prevalence of this species in endemic areas, especially in free-ranging dogs. Complete surveillance of L. tropica-infected dogs for the progression of skin lesions or visceral invasion of this species must be evaluated in experimental studies.
Acknowledgements
Not applicable.
Author contributions
Conceptualization by Baharak AkhtardaneshMethodology by Baharak Akhtardanesh, Soheil Sadr, Javad Khedri, Mehdi Bamorovat, Ehsan Salarkia, Iraj SharifiFormal analysis and investigation by All AuthorsWriting - original draft preparation by Soheil SadrWriting - review and editing by Baharak Akhtardanesh, Soheil Sadr, Javad Khedri, Mehdi Bamorovat, Ehsan Salarkia, Iraj Sharifi.
Data availability
The data presented in this study are contained within the article. Additional data can be provided on request from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and we confirm the study was carried out in compliance with the ARRIVE guidelines. Moreover, all experimental protocols were approved by the ethical committee of the Kerman University of Medical Sciences (ID: 910056).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dehghan, A., Ghahramani, F. & Hashemi, B. The epidemiology of anthroponotic cutaneous leishmaniasis (ACL) in Larestan, 2006–2008. Pars J. Med. Sci.8, 8–11. 10.29252/jmj.8.3.8 (2022). [Google Scholar]
- 2.Tabbabi, A. Review of leishmaniasis in the Middle East and North Africa. Afr. Health Sci.19, 1329–1337. 10.4314/ahs.v19i1.4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi, I. et al. Cutaneous leishmaniasis situation analysis in the Islamic Republic of Iran in preparation for an elimination plan. Front. Public Health11, 1091709. 10.3389/fpubh.2023.1091709 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Beshbishy, H. A., Al-Ali, K. H. & El-Badry, A. A. Molecular characterization of cutaneous leishmaniasis in Al-Madinah Al-Munawarah province, western Saudi Arabia. Int. J. Infect Dis.17, e334–e338. 10.1016/j.ijid.2012.11.015 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Mollalo, A., Alimohammadi, A., Shirzadi, M. R. & Malek, M. R. Geographic information system-based analysis of the spatial and spatio‐temporal distribution of zoonotic cutaneous leishmaniasis in Golestan Province, north‐east of Iran. Zoonoses Public. Health62, 18–28. 10.1111/zph.12109 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Choi, H. L., Jain, S., Ruiz Postigo, J. A., Borisch, B. & Dagne, D. A. The global procurement landscape of leishmaniasis medicines. PLoS Negl. Trop. Dis.15, e0009181. 10.1371/journal.pntd.0009181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wamai, R. G., Kahn, J., McGloin, J. & Ziaggi, G. Visceral leishmaniasis: A global overview. J. Glob Health Sci.10.35500/jghs.2020.2.e3 (2020).
- 8.Alvar, J. et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE7, e35671. 10.1371/journal.pone.0035671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Mondiale, O. & Organization, W. H. Leishmaniasis in high-burden countries: An epidemiological update based on data reported in 2014. Wkly. Epidemiol. Rec Relevé épidémiol Hebdomadaire. 91, 286–296 (2016). [PubMed] [Google Scholar]
- 10.Torres-Guerrero, E., Quintanilla-Cedillo, M. R., Ruiz-Esmenjaud, J. & Arenas, R. Leishmaniasis: A review. F1000Res. 10.12688/f1000research.11120.1 (2017). [DOI] [PMC free article] [PubMed]
- 11.Hajjaran, H. et al. The geographical distribution of human cutaneous and visceral leishmania species identified by molecular methods in Iran: A systematic review with meta-analysis. Front. Public. Health9, 661674. 10.3389/fpubh.2021.661674 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alborzi, A., Pouladfar, G. R. & Aalami, M. H. Visceral leishmaniasis; literature review and Iranian experience. Iran. J. Clin. Infect. Dis.2(2), 99–108 (2007). [Google Scholar]
- 13.Bamorovat, M. et al. Canine visceral leishmaniasis in Kerman, southeast of Iran: A seroepidemiological, histopathological and molecular study. Iran. J. Parasitol.9(3), 3242–3349 (2014). [PMC free article] [PubMed] [Google Scholar]
- 14.Sharifi, I. et al. A comprehensive review of cutaneous leishmaniasis in Kerman province, southeastern iran-narrative review article. Iran. J. Public Health44(3), 299–307 (2015). [PMC free article] [PubMed] [Google Scholar]
- 15.Farash, B. R. H. et al. Changes in the epidemiology of cutaneous leishmaniasis in northeastern Iran. Turk. Parazitol. Dergisi44(1), 52–57. 10.4274/tpd.galenos.2019.6137 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Sabzevari, S., Mohebali, M. & Hashemi, A. Cutaneous and visceral leishmaniasis: Parasites, vectors and reservoir hosts in endemic foci of North Khorasan, northeastern Iran-a narrative review. J. Med. Microbiol. Infect. Dis.8, 40–44. 10.29252/JoMMID.8.2.40 (2020). [Google Scholar]
- 17.Arzamani, K. Visceral leishmaniasis in North Khorasan Province, North east of Iran. Int. J. Infect. Dis.16, e340–e341. 10.1016/j.ijid.2012.05.407 (2012). [Google Scholar]
- 18.Salehi, M., Saadati, S. M., Arzamani, K., Shafiei, R. & Shoraka, H. R. Some epidemiological feature of human visceral leishmaniasis in North Khorasan Province during 2010–2018. Health Sci. Monit.3, 162–169. 10.61186/hsm.3.2.162 (2024). [Google Scholar]
- 19.Motalleb, G., Mirahmadi, H., Ahmad, Z. Z. & Mehravaran, A. Cytochrome b and molecular typing of Leishmania spp. in a passive sampling of suspected patients with cutaneous leishmaniasis in Sistan and Baluchestan Province, Eastern Iran. Iran. J. Parasitol.12(4), 534–543 (2017). [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtardanesh, B. et al. Seroepidemiology of visceral leishmaniasis among free-roaming dogs and children in Zahedan city, southeast of Iran, 2018–2020. Micob. Pathog.161, 105234. 10.1016/j.micpath.2021.105234 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Fakhar, M. et al. Emergence of a new focus of visceral leishmaniasis due to Leishmania infantum in Golestan Province, north-eastern of Iran. J. Parasitic Dis.38, 255–259. 10.1007/s12639-013-0307-4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karami, M., Doudi, M. & Setorki, M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J. Vector Borne Dis.50, 30–37. 10.4103/0972-9062.112532 (2013). [PubMed] [Google Scholar]
- 23.Nilforoushzadeh, M. A., Shirani-Bidabadi, L., Hosseini, S. M., Fadaei-Nobari, R. & Jaffary, F. The epidemiology of cutaneous leishmaniasis in Isfahan province, Iran, during 2001–2011. J. Isfahan Med. School. 32, 2241–2251. 10.17795/jssc23303 (2015). [Google Scholar]
- 24.Momeni, A. & Aminjavaheri, M. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int. J. Dermatol.33, 260–265. 10.1111/j.1365-4362.1994.tb01039.x (1994). [DOI] [PubMed] [Google Scholar]
- 25.Rasi, Y. et al. Study on sand flies as a vector (s) of cutaneous leishmaniasis by nested PCR in rural areas of Damghan District, Semnan Province. Avic. J. Clin. Med.18, 47–52 (2012). [Google Scholar]
- 26.Jafari, R., Najafzadeh, N., Sedaghat, M. & Parvizi, P. Molecular characterization of sandflies and Leishmania detection in main vector of zoonotic cutaneous leishmaniasis in Abarkouh district of Yazd province, Iran. Asian Pac. J. Trop. Med.6, 792–797. 10.1016/S1995-7645(13)60140-6 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Mirzaie, F., Eslami, G., Yosefi, M. H. & Pestehchian, N. Molecular identification of Leishmania isolates obtained from patients suspected as having cutaneous leishmaniasis referred to reference laboratories from Yazd province in central Iran. Adv. Biomed. Res.2(4), 92–96. 10.4103/2277-9175.122525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barati, H. et al. Epidemiological aspects of cutaneous leishmaniasis in Yazd province within 2004–2013. J. Community Health Res.5(2), 131–139 (2016). [Google Scholar]
- 29.Sarkari, B., Hatam, G. & Ghatee, M. Epidemiological features of visceral leishmaniasis in Fars province, southern Iran. Iran. J. Public. Health. 41(4), 94–99 (2012). [PMC free article] [PubMed] [Google Scholar]
- 30.Mohebali, M., Hamzavi, Y., Edrissian, G. H. & Forouzani, A. Seroepidemiological study of visceral leishmaniasis among humans and animal reservoirs in Bushehr province, Islamic Republic of Iran. EMHJ-Eastern Mediterr. Health J.7(6), 912–917. 10.26719/2001.7.6.912 (2001). [PubMed] [Google Scholar]
- 31.Darvishi, M. et al. Epidemiological study on sand flies in an endemic focus of cutaneous leishmaniasis, Bushehr city, southwestern Iran. Front. Public Health3, 14. 10.3389/fpubh.2015.00014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgipoor, M. et al. Human visceral leishmaniasis: A serological survey in rural areas of dashti district of Bushehr province, southern Iran. Novel Biomed.5, 54–58 (2017). [Google Scholar]
- 33.Yaghoobi-Ershadi, M. R. et al. Molecular epidemiological study of cutaneous leishmaniasis in the focus of Bushehr city, southwestern Iran. J. Arthropod-borne Dis.s7(2), 113–121 (2013). [PMC free article] [PubMed]
- 34.Azizi, K., Soltani, A. & Alipour, H. Molecular detection of Leishmania isolated from cutaneous leishmaniasis patients in Jask County, Hormozgan Province, Southern Iran, 2008. Asian Pac. J. Trop. Med.5, 514–517. 10.1016/S1995-7645(12)60090-X (2012). [DOI] [PubMed] [Google Scholar]
- 35.Hanafi-Bojd, A. A. et al. Species composition of sand flies (Diptera: Psychodidae) and modeling the spatial distribution of main vectors of cutaneous leishmaniasis in Hormozgan Province, Southern Iran. J. MedM Entomol.55, 292–299. 10.1093/jme/tjx205 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Ahmadizadeh, A. et al. Epidemiology of cutaneous leishmaniasis in Hormozgan province (2007–2011). Life Sci. J.10, 1473–1475 (2013). [Google Scholar]
- 37.Vazirianzadeh, B. et al. Epidemiology of cutaneous leishmaniasis in west of Ahvaz district, Khuzestan Province, southwestern of Iran. J. Exp. Zool. India17, 219–222 (2014). [Google Scholar]
- 38.Kassiri, H., Mortazavi, H. S. & Kazemi, S. The epidemiological study of cutaneous leishmaniasis in Khorram-Shahr City, Khuzestan Province, south-west of Iran. Jundishapur J. Health Sci.3, 11–20 (2011). [Google Scholar]
- 39.Adham, D., Moradi-Asl, E., Dorosti, A. & Khaiatzadeh, S. Spatial autocorrelation and epidemiological survey of visceral leishmaniasis in an endemic area of Azerbaijan region, the northwest of Iran. PLoS ONE15, e0236414. 10.1371/journal.pone.0236414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molaei, S. et al. Visceral leishmaniasis in Ardabil Province, Northwest of Iran: A retrospective epidemiological, clinical and paraclinical study (1985–2018). Iran. J. Public Health51(8), 1875–1885. 10.18502/ijph.v51i8.10274 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahangir, A. et al. Seroepidemiological survey of human visceral leishmaniasis in Ilam Province, west of Iran in 2013. Iran. J. Parasitol.10(1), 56–61 (2015). [PMC free article] [PubMed] [Google Scholar]
- 42.Mokhtari, M. et al. Cutaneous leishmaniasis prevalence and morbidity based on environmental factors in Ilam, Iran: Spatial analysis and land use regression models. Acta Trop.163, 90–97. 10.1016/j.actatropica.2016.08.002 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Kassiri, H., Sharifinia, N., Jalilian, M. & Shemshad, K. Epidemiological aspects of cutaneous leishmaniasis in Ilam Province, west of Iran (2000–2007). Asian Pac. J. Trop. Dis.2, 382–S386. 10.1016/S2222-1808(12)60186-8 (2012). [Google Scholar]
- 44.Shahidi-Hakak, F. et al. Typical features of cutaneous leishmaniasis in the Ilam Province, Iran. J. Parasit. Dis.44, 748–753. 10.1007/s12639-020-01258-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeinali, M., Mohebali, M., Mahmoudi, M., Hassanpour, G. R. & Shirzadi, M. R. Study on knowledge, attitude and practice of health workers of East Azerbaijan, Ilam and Khorasan Razavi provinces about leishmaniasis during 2015–2016: A comparative study before and after intervention. Arch. Clin. Infect. Dis.14(1), e64282. 10.5812/archcid.64282 (2019). [Google Scholar]
- 46.Kermanjani, A., Akhlaghi, L., Oormazdi, H. & Hadighi, R. Isolation and identification of cutaneous leishmaniasis species by PCR–RFLP in Ilam Province, the west of Iran. J. Parasit. Dis.41, 175–179. 10.1007/s12639-016-0772-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghatee, M. A., Taylor, W. R. & Karamian, M. The geographical distribution of cutaneous leishmaniasis causative agents in Iran and its neighboring countries, a review. Front. Public Health8, 11. 10.3389/fpubh.2020.00011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parhizgari, N., Piazak, N. & Mostafavi, E. Vector-borne diseases in Iran: Eidemiology and key challenges. Future Microbiol.16, 51–69. 10.2217/fmb-2019-0306 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Golpayegani, A. A. et al. Modeling of environmental factors affecting the prevalence of zoonotic and anthroponotic cutaneous, and zoonotic visceral leishmaniasis in foci of Iran: A remote sensing and GIS based study. J. Arthropod-borne Dis.12(1), 41–66 (2018). [PMC free article] [PubMed] [Google Scholar]
- 50.Mohebali, M. et al. Visceral leishmaniasis in Iran: An update on Epidemiological Features from 2013 to 2022. Iran. J. Parasitol.18(3), 279–293. 10.18502/ijpa.v18i3.13751 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabzevari, S., Teshnizi, S. H., Shokri, A., Bahrami, F. & Kouhestani, F. Cutaneous leishmaniasis in Iran: A systematic review and meta-analysis. Microb. Pathog.152, 104721. 10.1016/j.micpath.2020.104721 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Nasiri, Z. et al. Cutaneous leishmaniasis in Iran: A review of epidemiological aspects, with emphasis on molecular findings. Parasit. 29, 47. 10.1051/parasite/2022047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saberi, R. et al. Identification of Leishmania species using N-acetylglucosamine-1-phosphate transferase gene in a zoonotic cutaneous leishmaniasis focus of Iran. J. Vector Borne Dis.55, 14–19. 10.4103/0972-9062.234621 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Teimouri, A., Mohebali, M., Kazemirad, E. & Hajjaran, H. Molecular identification of agents of human cutaneous leishmaniasis and canine visceral leishmaniasis in different areas of Iran using internal transcribed spacer 1 PCR-RFLP. J. Arthropod-borne Dis.12(2), 162–171. 10.18502/jad.v12i2.42 (2018). [PMC free article] [PubMed] [Google Scholar]
- 55.Hosseini-Safa, A. et al. High resolution melting analysis as an accurate method for identifying Leishmania infantum in canine serum samples. J. Vector Borne Dis.55, 315–320. 10.4103/0972-9062.256568 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Najafzadeh, N., Taslimian, R., Fotouhi-Ardakani, R., Spotin, A. & Parvizi, P. Detection and differentiation of Leishmania parasites in asymptomatic canine by high-resolution melting analysis of microsatellite fragment in ITS gene. Microb. Pathog. 162, 105300. 10.1016/j.micpath.2021.105300 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Mahshid, M. et al. Seroprevalence of canine visceral leishmaniasis in southeast of Iran. J. Parasit. Dis.38, 218–222. 10.1007/s12639-012-0226-9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khedri, J., Radfar, M. & Sharifi, I. Potential demographic, clinical, and environment risk factors for canine visceral leishmaniasis using IFAT and nested PCR, southeastern Iran. Comp. Clin. Path.27, 275–281. 10.1007/s00580-017-2569-1 (2018). [Google Scholar]
- 59.Khosravi, A., Sharifi, I., Dortaj, E., Afshar, A. A. & Mostafavi, M. The present status of cutaneous leishmaniasis in a recently emerged focus in south-west of Kerman Province, Iran. Iran. J. Public Health42(2), 182–187 (2013). [PMC free article] [PubMed] [Google Scholar]
- 60.Sharifi, F. et al. Spatial distribution and molecular identification of Leishmania species from endemic foci of south-eastern Iran. Iran. J. Parasitol.7(1), 45–52 (2012). [PMC free article] [PubMed] [Google Scholar]
- 61.Ghaffari, D., Hakimi Parizi, M., Yaghoobi Ershadi, M. R., Sharifi, I. & Akhavan, A. A. A survey of reservoir hosts in two foci of cutaneous leishmaniasis in Kerman province, southeast of Iran. J. Parasit. Dis.38, 245–249. 10.1007/s12639-013-0275-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahmoudvand, H. et al. Epidemiological aspects of visceral leishmaniasis in Baft District, Kerman Province, Southeast of Iran. Iran. J. Parasitol.6 (1), 1–11 (2011). [PMC free article] [PubMed] [Google Scholar]
- 63.Akhtardanesh, B. et al. Feline visceral leishmaniasis in Kerman, southeast of Iran: Serological and molecular study. J. Vector Borne Dis.54, 96–102. 10.4103/0972-9062.203190 (2017). [PubMed] [Google Scholar]
- 64.Sharifi, I. et al. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania Tropica in rural communities of Bam district after the earthquake, Iran. Trop. Med. Int. Health. 16(4), 510–513. 10.1111/j.1365-3156.2011.02729.x (2011). [DOI] [PubMed] [Google Scholar]
- 65.Mozafary, M., Dayer, M. S., Afshar, A. A. & Mollaie, H. R. Molecular characterization of Leishmania parasites in naturally infected sand flies from the endemic focus of Kerman City, Southeastern Iran. Asian Pac. J. Trop. Dis.6, 188–192. 10.1016/S2222-1808(15)61011-8 (2016). [Google Scholar]
- 66.Afshar, M. J. A. et al. Canine visceral leishmaniasis; a seroepidemiological survey in Jiroft district, southern Kerman province, southeastern Iran in 2015. Iran. J. Parasitol.13 (1), 67–71 (2018). [PMC free article] [PubMed] [Google Scholar]
- 67.Abbaszadeh-Afshar, M. J. et al. Seroepidemiological survey of visceral leishmaniasis among nomadic tribes of Kerman Province, Southeastern Iran: An observational study for implication to health policy. J. Biostat Epidemiol.1 (3/4), 105–111 (2015). [Google Scholar]
- 68.Aflatoonian, M. & Sharifi, I. Prevalence of cutaneous leishmaniasis in school children in Bam and Barawat/Iran in 2006. J. Kerman Uni Med. Sci.14(4), 82–89 (2007). [Google Scholar]
- 69.Askari, A. et al. A newly emerged focus of zoonotic cutaneous leishmaniasis in South-Western Iran. Microb. Pathog.121, 363–368. 10.1016/j.micpath.2018.04.053 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Ramezany, M. et al. Geographical distribution and molecular characterization for cutaneous leishmaniasis species by sequencing and phylogenetic analyses of kDNA and ITS1 loci markers in south-eastern Iran. Pathog. Glob. Health112, 132–141. 10.1080/20477724.2018.1447836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mann, S. et al. A review of leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep.8, 121–132. 10.1007/s40475-021-00232-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baneth, G., Petersen, C., Solano-Gallego, L. & Sykes, J. E. Leishmaniosis Greene’s Infect. Dis. Dog Cat 1179–1202 10.1016/B978-0-323-50934-3.00096-3 (2021).
- 73.Cardoso, L., Schallig, H., Persichetti, M. F. & Pennisi, M. G. New epidemiological aspects of animal leishmaniosis in Europe: The role of vertebrate hosts other than dogs. Pathog. 10, 307. 10.3390/pathogens10030307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montaner-Angoiti, E. & Llobat, L. Is leishmaniasis the new emerging zoonosis in the world? Vet. Res. Commun.47, 1–23. 10.1007/s11259-023-10171-5 (2023). [DOI] [PubMed] [Google Scholar]
- 75.Sasidharan, S. & Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res.120, 1541–1554. 10.1007/s00436-021-07139-2 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol.149, 139–146. 10.1016/j.vetpar.2007.07.007 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Morales-Yuste, M., Martín-Sánchez, J. & Corpas-Lopez, V. Canine leishmaniasis: Update on epidemiology, diagnosis, treatment, and prevention. Vet. Sci.9, 387. 10.3390/vetsci9080387 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morelli, S., Diakou, A., Di Cesare, A., Colombo, M. & Traversa, D. Canine and feline parasitology: Analogies, differences, and relevance for human health. Clin. Microbiol. Rev.34, e00266–e00220. 10.1128/CMR.00266-20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Vries, H. J. & Schallig, H. D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol.23, 823–840. 10.1007/s40257-022-00726-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reimão, J. Q., Coser, E. M., Lee, M. R. & Coelho, A. C. Laboratory diagnosis of cutaneous and visceral leishmaniasis: Current and future methods. Microorganism. 8, 1632. 10.3390/microorganisms8111632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcelino, A. P. et al. Comparative PCR-based diagnosis for the detection of Leishmania infantum in naturally infected dogs. Acta Trop.207, 105495. 10.1016/j.actatropica.2020.105495 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Maurelli, M. P. et al. Clinical, molecular and serological diagnosis of canine leishmaniosis: An integrated approach. Vet. Sci.7, 43. 10.3390/vetsci7020043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajaran, H., Mohebali, M., Zareei, Z. & Edrisian, G. H. LeishTropicaropica: Another etiological agent of canine visceral leishmaniasis in Iran. Iran. J. Public. Health. 36 (1), 85–88 (2007). [Google Scholar]
- 84.Mohebali, M. et al. Disseminated leishmaniasis caused by Leishmania Tropica in a puppy from Karaj, Central Iran. Iran. J. Parasitol.6(2), 69–73 (2011). [PMC free article] [PubMed] [Google Scholar]
- 85.Bamorovat, M. et al. Leishmania Tropica in stray dogs in southeast Iran. Iran. J. Public Health44(10), 1359–1366 (2015). [PMC free article] [PubMed] [Google Scholar]
- 86.Dereure, J. et al. LeishTropicaropica in Morocco: Infection in dogs. Trans. R Soc. Trop. Med. Hyg.85 (5), 595–595. 10.1016/0035-9203(91)90356-4 (1991). [DOI] [PubMed] [Google Scholar]
- 87.Noyes, H. A., Reyburn, H., Bailey, J. W. & Smith, D. A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania Tropica in Pakistan. J. Clin. Microbiol.36, 2877–2881. 10.1128/JCM.36.10.2877-2881.1998 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valero, N. N. H. & Uriarte, M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: A systematic review. Parasitol. Res.119, 365–384. 10.1007/s00436-019-06575-5 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Özbilgin, A. et al. Visceral leishmaniasis caused by Leishmania Tropica. Acta Parasitol.68, 699–704 (2023). [DOI] [PubMed] [Google Scholar]
- 90.Pal, M., Gutama, K. P., Steinmetz, C. & Dave, P. Leishmaniasis: An emerging and re-emerging disease of global public health concern. Am. J. Infect. Dis.10 (1), 22–25. 10.12691/ajidm-10-1-4 (2022). [Google Scholar]
- 91.Abadías-Granado, I., Diago, A., Cerro, P., Palma-Ruiz, A. & Gilaberte, Y. Cutaneous and mucocutaneous leishmaniasis. Actas Dermo-Sifiliográficas (English Edition)112, 601–618. 10.1016/j.ad.2021.02.008 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Kayani, B. et al. Cutaneous leishmaniasis in Pakistan: A neglected disease needing one health strategy. BMC Infect. Dis.21, 622. 10.1186/s12879-021-06327-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohammadi, J. et al. Review and a brief report on the health perspective, causative agents, vectors, and reservoirs of visceral leishmaniasis in Iran. J. Health Sci. Surveillance Sys. 10 (3), 257–265 (2022). [Google Scholar]
- 94.Sanei-Dehkordi, A., Soleimani-Ahmadi, M., Zare, M. & Mirzaei, H. Epidemiological features of cutaneous leishmaniasis and distribution of sand flies in an endemic area in southeast of Iran. Parasit. Epidemiol. Control. 14, e00220. 10.1016/j.parepi.2021.e00220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knight, C. A. et al. Recent epidemiological studies in the Middle East. Front. Microbiol.13, 1052478. 10.3389/fmicb.2022.1052478 (2023). Leishmaniasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters, W. et al. The identity of some stocks of Leishmania isolated in India. Ann. Trop. Med. Parasitol.75, 247–249. 10.1080/00034983.1981.11687435 (1981). [Google Scholar]
- 97.Miró, G. et al. Novel areas for prevention and control of canine leishmaniosis. Trend Parasitol.33, 718–730. 10.1016/j.pt.2017.05.005 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Otranto, D. & Dantas-Torres, F. The prevention of canine leishmaniasis and its impact on public health. Trend Parasitol.29, 339–345. 10.1016/j.pt.2013.05.003 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Maroli, M. et al. Guidelines for prevention of leishmaniasis in dogs. J. Am. Vet. Med. Assoc.236, 1200–1206. 10.2460/javma.236.11.1200 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Brianti, E. et al. Field evaluation of two different treatment approaches and their ability to control fleas and prevent canine leishmaniosis in a highly endemic area. PLoS Negl. Trop. Dis.10, e0004987. 10.1371/journal.pntd.0004987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marcondes, M. & Day, M. J. Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci.123, 261–272. 10.1016/j.rvsc.2019.01.022 (2019). [DOI] [PubMed] [Google Scholar]
- 102.da Fonseca-Martins, A. M. et al. Immunotherapy using anti-PD-1 and anti-PD-L1 in Leishmania amazonensis-infected BALB/c mice reduce parasite load. Sci. Rep.9, 20275. 10.1038/s41598-019-56336-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinheiro, R. O. et al. Protection against cutaneous leishmaniasis by intranasal vaccination with lipophosphoglycan. Vaccine. 25, 2716–2722. 10.1016/j.vaccine.2006.05.093 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are contained within the article. Additional data can be provided on request from the corresponding author upon reasonable request.