Abstract
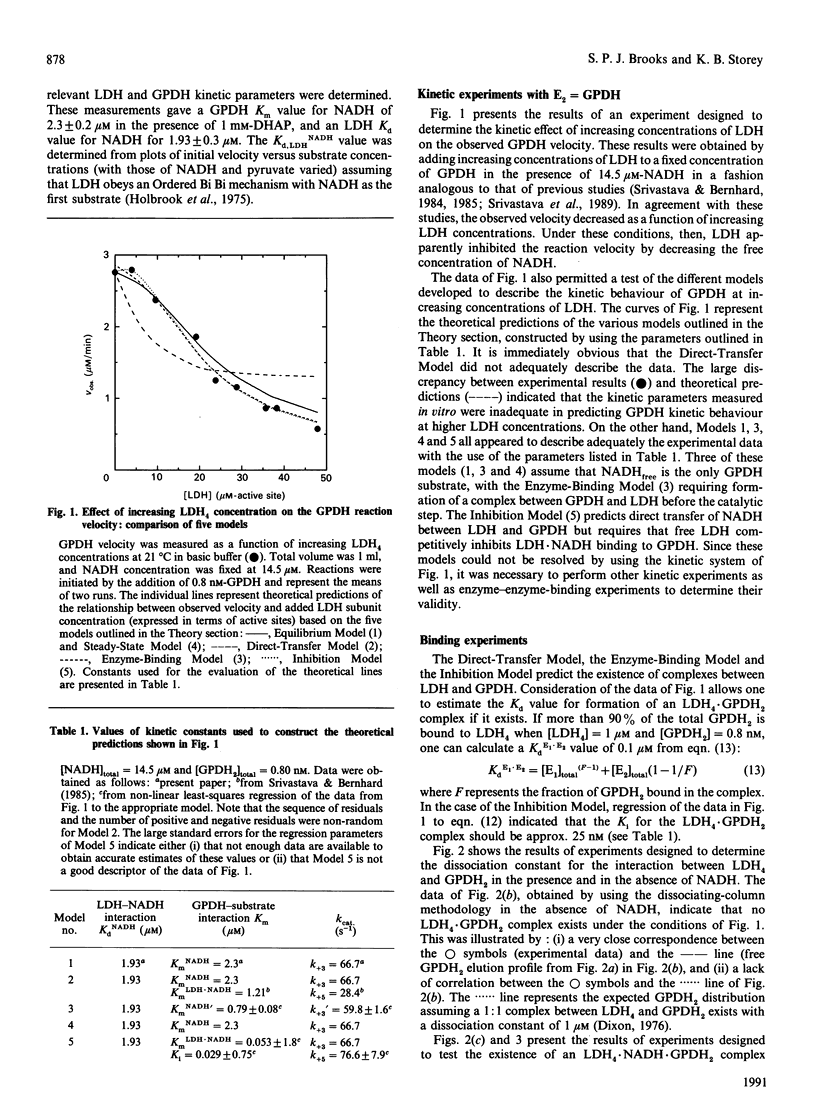
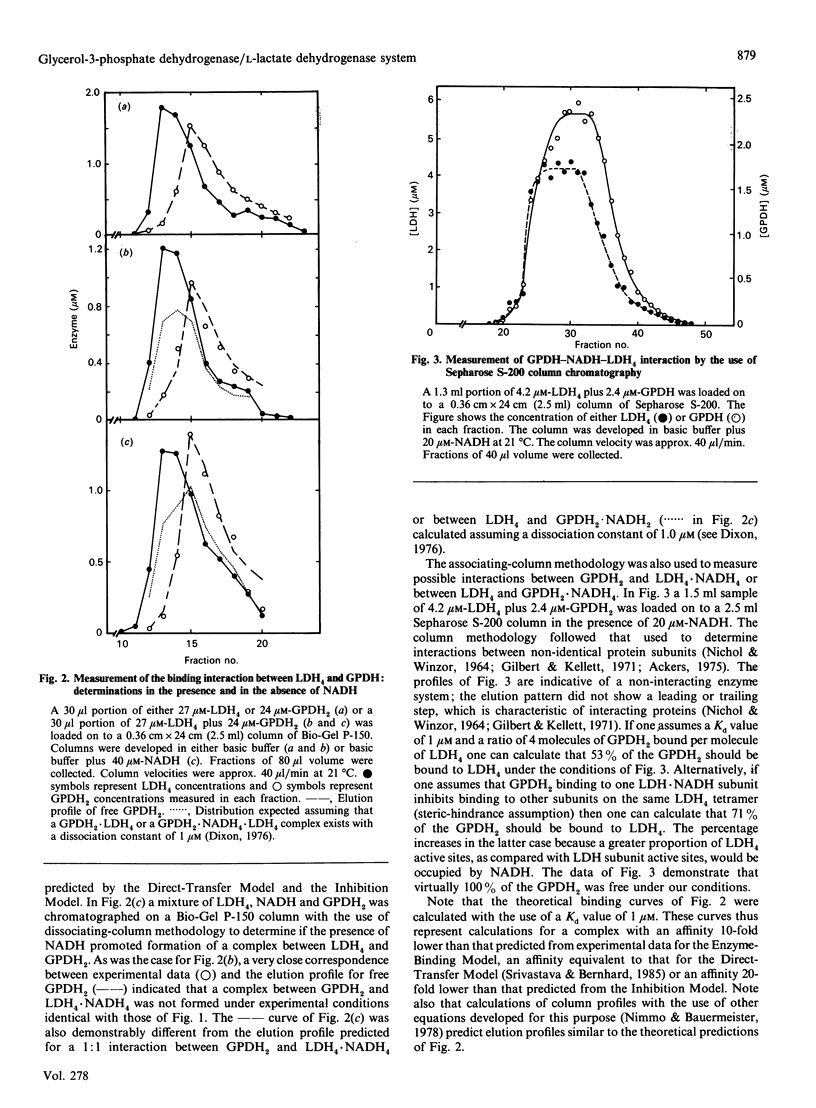
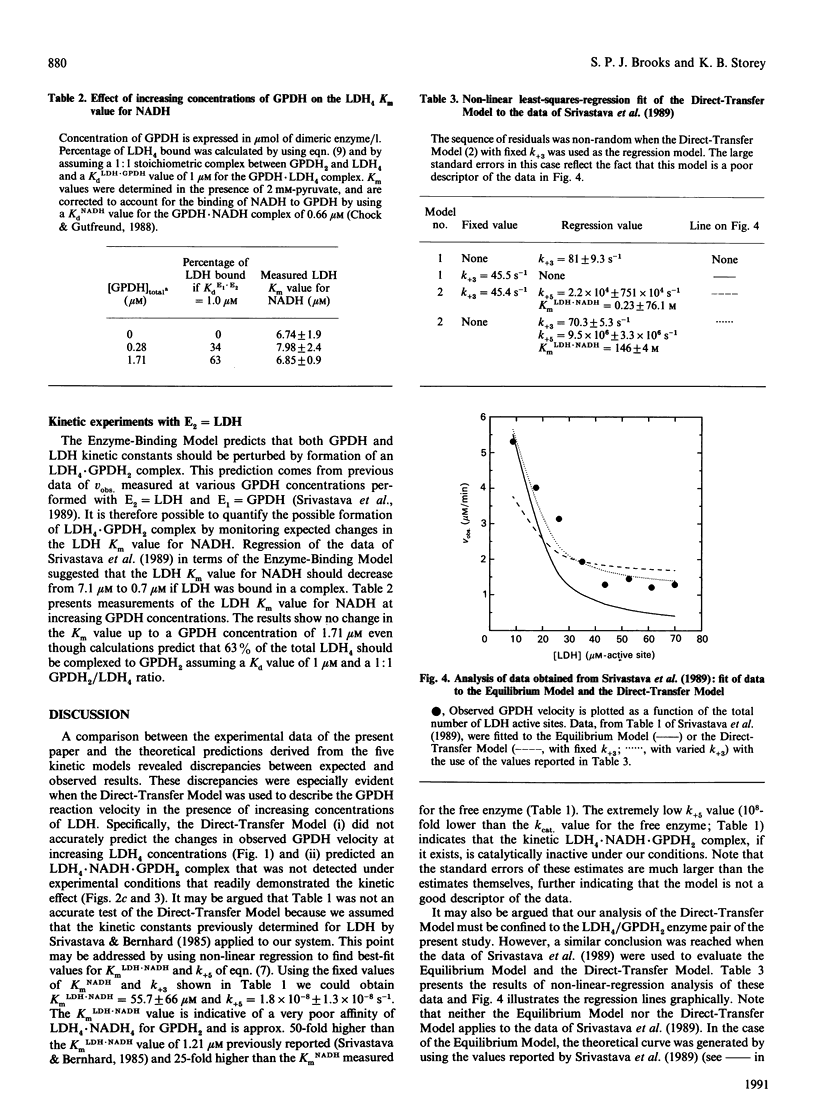
An investigation of the direct transfer of metabolites from rabbit muscle L-lactate dehydrogenase (LDH, EC 1.1.1.27) to glycerol-3-phosphate dehydrogenase (GPDH, EC 1.1.1.8) revealed discrepancies between theoretical predictions and experimental results. Measurements of the GPDH reaction rate at a fixed NADH concentration and in the presence of increasing LDH concentrations gave experimental results similar to those previously obtained by Srivastava, Smolen, Betts, Fukushima, Spivey & Bernhard [(1989) Proc. Natl. Acad. Sci. U.S.A. 86, 6464-6468]. However, a mathematical solution of the direct-transfer-mechanism equations as described by Srivastava et al. (1989) showed that the direct-transfer model did not adequately describe the experimental behaviour of the reaction rate at increasing LDH concentrations. In addition, experiments designed to measure the formation of an LDH4.NADH.GPDH2 complex, predicted by the direct-transfer model, indicated that no significant formation of tertiary complex occurred. An examination of other kinetic models, developed to describe the LDH/GPDH/NADH system better, revealed that the experimental results may be best explained by assuming that free NADH, and not E1.NADH, is the sole substrate for GPDH. These results suggest that direct transfer of NADH between rabbit muscle LDH and GPDH does not occur in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. J. Kinetic studies on the mechanism of cytoplasmic L-alpha-glycerophosphate dehydrogenase of rabbit skeletal muscle. Can J Biochem. 1966 Oct;44(10):1301–1317. doi: 10.1139/o66-150. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Chock P. B., Gutfreund H. Reexamination of the kinetics of the transfer of NADH between its complexes with glycerol-3-phosphate dehydrogenase and with lactate dehydrogenase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8870–8874. doi: 10.1073/pnas.85.23.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Subunit constitution of proteins: a table. Arch Biochem Biophys. 1975 Feb;166(2):651–682. doi: 10.1016/0003-9861(75)90432-4. [DOI] [PubMed] [Google Scholar]
- Dixion H. B. Removal of a bound ligand from a macromolecule by gel filtration. Biochem J. 1976 Oct 1;159(1):161–162. doi: 10.1042/bj1590161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert G. A., Kellett G. L. Interacting systems of the type A + B = C. J Biol Chem. 1971 Oct 10;246(19):6079–6086. [PubMed] [Google Scholar]
- Lee Y. P., Craine J. E. L-glycerol 3-phosphate dehydrogenase. I. Effects of the substrates on the catalytic properties of the hepatic nicotinamide adenine dinucleotide-linked enzyme from the rabbit. J Biol Chem. 1971 Dec 25;246(24):7616–7622. [PubMed] [Google Scholar]
- Nimmo I. A., Bauermeister A. A theoretical analysis of the use of zonal gel filtration in the detection and purification of protein-ligand complexes. Biochem J. 1978 Feb 1;169(2):437–440. doi: 10.1042/bj1690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Direct transfer of reduced nicotinamide adenine dinucleotide from glyceraldehyde-3-phosphate dehydrogenase to liver alcohol dehydrogenase. Biochemistry. 1984 Sep 25;23(20):4538–4545. doi: 10.1021/bi00315a006. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Enzyme-enzyme interactions and the regulation of metabolic reaction pathways. Curr Top Cell Regul. 1986;28:1–68. doi: 10.1016/b978-0-12-152828-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Mechanism of transfer of reduced nicotinamide adenine dinucleotide among dehydrogenases. Biochemistry. 1985 Jan 29;24(3):623–628. doi: 10.1021/bi00324a013. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Smolen P., Betts G. F., Fukushima T., Spivey H. O., Bernhard S. A. Direct transfer of NADH between alpha-glycerol phosphate dehydrogenase and lactate dehydrogenase: fact or misinterpretation? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6464–6468. doi: 10.1073/pnas.86.17.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. P., Bernhard S. A. Transfer of 1,3-diphosphoglycerate between glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase via an enzyme-substrate-enzyme complex. Biochemistry. 1982 Aug 17;21(17):4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]
- YOUNG H. L., PACE N. Some physical and chemical properties of crystalline alpha-glycerophosphate dehydrogenase. Arch Biochem Biophys. 1958 May;75(1):125–141. doi: 10.1016/0003-9861(58)90403-x. [DOI] [PubMed] [Google Scholar]


