Abstract
This study aimed to evaluate the effect of Astragalus polysaccharides (PG2) on reducing chemotherapy-induced fatigue (CIF) and toxicity, thereby encouraging compliance to chemotherapy. This was a randomized, placebo-controlled, phase 2 study. Patients with stage II/III early breast cancer planning to undergo adjuvant anthracycline-based chemotherapy were randomly assigned to receive PG2 500 mg or placebo on days 1, 3, and 8 every 21 days. The fatigue global score (FGS) was assessed using the brief fatigue inventory (BFI)-Taiwan. The Breast Cancer-Specific Module of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaires-Core30 evaluated the health-related quality of life during the first four cycles of adjuvant chemotherapy. Overall, 66 eligible patients were equally randomized into the PG2 and placebo groups between March 01, 2018, and March 09, 2021. The mean change in the FGS and fatigue intensity did not significantly differ between both groups. However, the FGS and fatigue intensity were less aggravated in the first four cycles in the premenopausal-PG2 group than in the placebo group. Our study concluded PG2 combined with adjuvant chemotherapy can reduce CIF, insomnia, the negative effect on future perspectives, and improve global health status, especially for premenopausal patients with breast cancer. Trial registration number: NCT03314805 registered on 19/10/2017.
Keywords: Astragalus polysaccharides, Cancer-related fatigue, Chemotherapy-related fatigue, Breast cancer, Adjuvant chemotherapy
Subject terms: Cancer, Breast cancer
Introduction
Breast cancer is the most diagnosed cancer globally and the leading cause of cancer-related deaths among women1. The cure for breast cancer becomes impossible when it develops into an advanced stage. Therefore, the key factors for enhancing clinical outcomes in breast cancer revolve around early detection, curative resection, and effective adjuvant or neoadjuvant therapy.
Substantial improvements have been made in systemic therapies for advanced breast cancer (ABC), including targeted therapy, hormonal therapy, and immunotherapy; however, chemotherapy remains the cornerstone for ABC and early breast cancers (EBC) as well. Adjuvant chemotherapy in EBC reduces recurrence risk and breast cancer mortality2. Nevertheless, chemotherapy is associated with numerous adverse events (AEs), including nausea, vomiting, mucositis, bone marrow suppression, alopecia, peripheral neurotoxicity, and fatigue.
Owing to concerns about AEs associated with chemotherapy, many patients hesitate to accept neoadjuvant or adjuvant chemotherapies. Some patients even allow their tumors to progress from EBC to ABC. Therefore, addressing and mitigating AE severity from chemotherapy constitutes crucial aspects of supportive care.
Various methods have been implemented to ameliorate AEs from chemotherapy, such as granulocyte colony-stimulating factor for leukopenia, 5-hydroxytryptamine receptor type 3 antagonist for emesis, and modifying the administration schedule from triweekly to weekly. Despite these efforts, fatigue remains a challenge with no good treatment.
A nationwide survey in Taiwan revealed that cancer-related fatigue (CRF) was among the most common symptoms for advanced cancers, including breast cancer3. Fatigue is a common AE from neoadjuvant or adjuvant therapy for ABC, either with chemotherapy alone4,5, in combination with a targeted therapy6,7, or with immunotherapy, where the incidence can exceed 35%8,9.
Astragalus polysaccharides (PG2, PhytoHealth Corp., Taiwan) injection has been approved by Taiwan Food and Drug Administration (TFDA) as a pharmacotherapy for treating CRF in patients with advanced cancer in Taiwan. In a pivotal trial, PG2 improved CRF among patients with advanced cancer10. Subsequently, a phase 4 study further validated PG2 efficacy in improving fatigue scores for patients with advanced cancer, especially those with breast cancer11.
Numerous translational and clinical studies have provided evidence of the synergistic effects of PG2 with anticancer therapies such as chemotherapy, target therapy, and immunotherapy12. Additionally, studies have highlighted PG2 immunomodulation effects13.
In our study, we aimed to evaluate the efficacy of PG2 as a complementary treatment for patients with stage II/III EBC undergoing adjuvant chemotherapy with epirubicin and cyclophosphamide (EC) regimens. The goal was to investigate whether PG2 could reduce chemotherapy-induced fatigue (CIF), minimize toxicity, and enhance compliance to chemotherapy. The primary endpoint involved evaluating the change in the fatigue global score (FGS) using the brief fatigue inventory (BFI)-Taiwan14 during the first four EC cycles. The secondary endpoints included chemotherapy dose reductions and delays, the incidence of grade 3/4 hematologic toxicities based on the Common Terminology Criteria for AE 4.03, Eastern Cooperative Oncology Group performance status, and health-related quality of life (HRQL) using the Breast Cancer-Specific Module of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaires-Core30 (EORTC QLQ-C30 & BR23)15, during the first four EC cycles.
Materials and methods
Patients
This was a double-blind, randomized, and placebo-controlled study design. Patients with stage II/III EBC scheduled for anthracycline-based adjuvant chemotherapy (including EC or EC followed by taxane regimens) underwent screening based on inclusion and exclusion criteria. Only eligible patients were enrolled in this study.
Major inclusion criteria included patients: (1) with stage II/III EBC patients, (2) aged above 20 years, (3) scheduled to receive an adjuvant EC-upfront regimen, either six cycles of EC or EC followed by taxane, (4) with adequate bone marrow function (white blood cells ≥ 3000 cells/mm3, absolute granulocyte count ≥ 1500 cells/ mm3, and platelet count ≥ 100,000 /mm mm3), and (5) with normal liver and kidney function, where serum alanine transaminase and aspartate transaminase were ≤ 2.5 times the upper limit of normal, and bilirubin was ≤ 1.5 times the upper limit of normal. The major exclusion criteria included patients who are pregnant or lactating, with a BFI score exceeding 3, chronic insomnia, chronic fatigue syndrome, uncontrolled active infection, a known severe allergy to Astragalus membranaceus or its major extracts (polysaccharides), and a history of invasive breast cancer or any other malignancy within the past 3 years.
The ethics committee of the Institutional Review Board of E-Da Cancer and Chang Gung Memorial Hospitals approved the study design and protocol. This study adheres to the principles outlined in the Declaration of Helsinki and all methods were performed in accordance with the relevant guidelines and regulations.
Treatment protocol
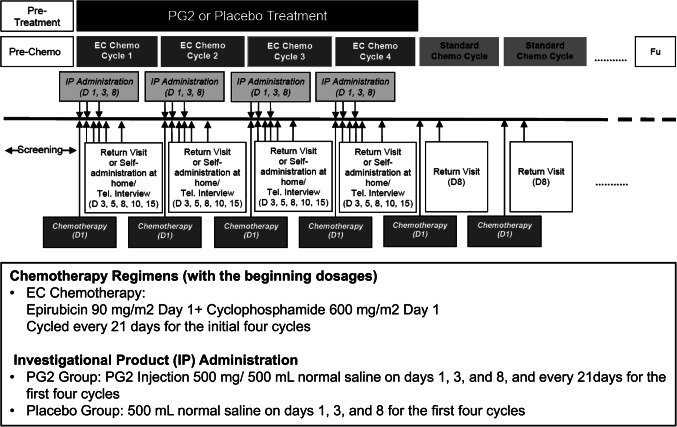
Patients who met all inclusion criteria and no exclusion criteria were assigned to the treatment or control group based on the randomization schedule. The investigational drug, PG2 (PhytoHealth Corp., Taiwan), is an active fraction of highly purified Astragalus polysaccharides (greater than 90% purity), with average molecular weights ranging from 20,000 to 60,000 daltons, obtained through the extraction, isolation, and purification of Astragalus membranaceus, a Traditional Chinese Medicine. The dominant polysaccharides in PG2 are alpha-1,4-linked glucans with branching at the 6-position of the backbone residues. The dose of PG2 used in this study was 500 mg in 500 ml normal saline, in accordance with the TFDA-approved dosage for the indication of CRF relief in advanced cancer patients, which has been demonstrated to be safe and effective in previous pivotal and phase IV clinical trials10,11. PG2 solution was clear and colourless after preparation, and normal saline was used as the placebo to maintain the blinding design. EC was prescribed a dose of 90 mg/600 mg/m^2 every 3 weeks, whereas PG2 or a placebo was administered by intravenous infusion on days 1, 3, and 8 of each cycle. Four EC cycles were designated for prescription during the elevation period (Fig. 1). The Institutional Review Board of all participating hospitals approved the study design, and it was registered at ClinicalTrails.gov with the number NCT03314805.
Figure 1.
Flow chart of screening, treatment, and follow-up.
HRQL evaluation
We used the BFI-Taiwan14 at every return visit to evaluate HRQL. Additionally, HRQL was evaluated15 on days 1 and 8 of every cycle using the EORTC QLQ-C30 & BR23 (Fig. 1). CIF was assessed through a validated questionnaire derived from the BFI-Taiwan. Two main categories were presented: FGS and fatigue intensity. Each category included several items. The “FGS” was the arithmetic mean of all nine items (score, 0–10). The “fatigue intensity” was defined as the score reflecting the worst fatigue level in the last 24 h (BFI item 3) on a 0–10 scale. EORTC QLQ-C30 & BR23 data were transformed to yield scores ranging from 0 to 100 based on the EORTC scoring manual. A higher score represented a better quality of life or a higher level of symptoms.
Efficacy of data presentation and statistical analysis methods
The baseline clinical findings, laboratory data, and performance status were evaluated at the beginning of the study before initiating treatment. Demographic and baseline characteristics were presented for each treatment group.
Summary statistics, including mean, median, standard deviation (SD), minimum, and maximum, were provided for continuous variables, such as age. A two-sample t-test or Wilcoxon rank-sum test was used for continuous variables based on whether the data follows or violates the normal distribution, respectively.
Categorical data, such as sex, were expressed using frequencies and proportions. Fisher’s exact test was utilized to compare the difference in proportions between groups when the sample size was small. Alternatively, a chi-square test was employed.
This study included three populations: the intention-to-treat (ITT), including the participants who met the eligibility criteria, took at least one study drug treatment, and were assessed using BFI at least once; the per-protocol (PP) population, including the evaluable participants who met the compliance criteria, defined as receiving a minimum of 75% of the prescribed study drug administration (nine doses) during the treatment period, and without major protocol deviations in the first four EC treatment cycles; the safety populations, comprising the participants who received at least one study drug treatment.
Safety evaluation
All AEs were evaluated using the Common Terminology Criteria for AEs 4.03 and reported throughout the study. The severity of AE and its relationship to the study drug was summarized. Additionally, changes from the baseline of all laboratory variables were summarized.
Results
Participant disposition
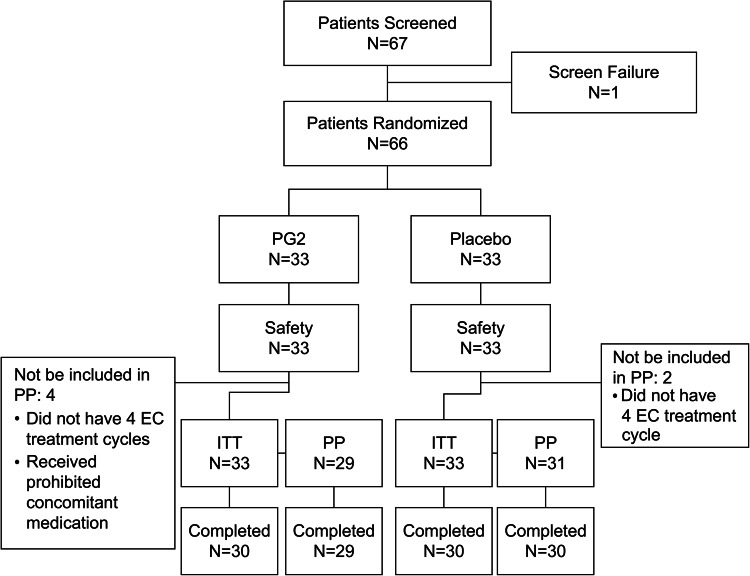
Overall, 67 patients were enrolled across five hospitals in Taiwan from March 01, 2018, to March 09, 2021. Among these, 66 were eligible and equally randomized into the PG2 and placebo groups (n = 33 in each group). At the end of the study, 30 participants (90.9%) in each group completed the study. Three participants (9.1%) from each group withdrew prematurely from the study by using the withdrawal of consent (two in the PG2 group and three in the placebo group) and by the decision of the investigator (one in the PG2 group). All 66 participants were included in the ITT and safety populations. The PP population of the PG2 and placebo groups comprised 29 and 31 participants, respectively (Fig. 2).
Figure 2.
Randomization, treatment assignments, and follow-up. ITT intention-to-treat; PG2, Astragalus Polysaccharides; PP, per-protocol.
Demographics and patients’ characteristics
Most disease characteristics were comparable among the patients, except for the menopausal status, which significantly differed between the groups. The PG2 group enrolled more menopausal participants than the placebo group, accounting for 60.6% vs. 36.4% (P = 0.049*) and 62.1% vs. 35.5% (P = 0.039*) in the ITT/safety and PP populations, respectively (Table 1). Except for this, no other significant difference in demographics was observed. In the ITT/safety population, the mean age (± SD) of the PG2 group was 53.6 ± 10.87 years, whereas that of the placebo group was 49.2 ± 10.00 years. The mean body mass index (± SD) of the PG2 group was 26.3 ± 4.51 kg/m2, while that of the placebo group was 24.4 ± 4.35 kg/m2. Similar data were observed in the PP population.
Table 1.
Patients’ characteristics.
| Characteristics | PG2a (n = 33) | Placebo (n = 33) | P-value |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 53.6 ± 10.87 | 49.2 ± 10.00 | 0.079 |
| BMI (kg/m 2 ) | |||
| Mean ± SD | 26.3 ± 4.51 | 24.4 ± 4.35 | 0.100 |
| Baseline ECOG b status | |||
| 0 | 30 (90.9) | 31 (93.9) | > 0.999 |
| 1 | 3 (9.1) | 2 (6.1) | |
| Histologic subtype, n (%) | |||
| Ductal | 27 (81.8) | 26 (78.8) | 0.201 |
| Lobular | 6 (18.2) | 3 (9.1) | |
| Unknown | 0 (0.0) | 4 (12.1) | |
| Menopausal status, n (%) | |||
| Premenopausal | 13 (39.4) | 21 (63.6) | 0.049 |
| Postmenopausal | 20 (60.6) | 12 (36.4) | |
| Molecular type, n (%) | |||
| TNBCc, n (%) | 2 (6.9) | 1 (3.0) | > 0.999 |
| HR + d/HER2e-, n (%) | 17 (51.5) | 18 (54.5) | |
| HER2 + f, n (%) | 14 (42.4) | 14 (42.4) | |
| Baseline BFI g fatigue score | |||
| Mean ± SDh | 0.3 ± 0.60 | 0.4 ± 0.63 | 0.373 |
| Surgical type, n (%) | |||
| Conservative | 3 (9.1) | 1 (3.0) | 0.420 |
| Mastectomy | 29 (87.9) | 32 (97.0) | |
| Unknown | 1 (3.0) | 0 (0.0) | |
| Radiotherapy, n (%) | 12 (36.4) | 10 (30.3) | 0.602 |
aPG2, Astragalus polysaccharides
bECOG, Eastern Cooperative Oncology Group
cTNBC, Triple-negative breast cancer
dHR+, hormone receptor-positive
eHER2-, human epidermal growth factor receptor 2-negative
fHER2+, human epidermal growth factor receptor 2-positive
gBFI, brief fatigue inventory
hSD, standard deviation
Efficacy
Primary endpoints
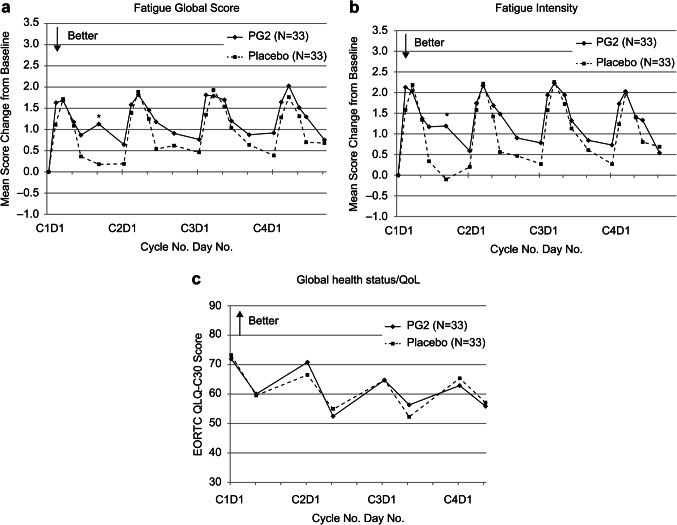
Overall, no significant difference was observed in the mean change in FGS and fatigue intensity between the ITT groups, with both groups displaying an aggravating trend in FGS and fatigue intensity, demonstrating a significant increase in both groups. In the PG2 group, the mean FGS increased by 0.6–2.0 from baseline within the initial four cycles, whereas in the placebo group, the mean FGS increased by 0.2–1.9 within the same period. Similarly, mean fatigue intensity for the PG2 group increased by 0.5–2.2 from baseline within the initial four cycles, whereas mean fatigue intensity for the placebo group increased by -0.1–2.3 within the same period (Fig. 3(a) and 3(b)). The PP population yielded results similar to those of the ITT population (data not shown).
Figure 3.
Time-dependent change in BFI fatigue scores and mean EORTC QLQ-C30 Global health status/QoL scores in the two groups at the end of cycle 4 for the ITT population. (a) Fatigue global score (b) Fatigue intensity (c) Global health status/QoL. BFI, brief fatigue inventory; PG2, Astragalus Polysaccharides. *represents the statistical significance between the groups at each time point.
We observed a lower proportion of patients experiencing grade 3/4 neutropenia from cycles 1–4 in the PG2 group compared with the placebo group (87.9% vs. 93.9% in the ITT population and 93.1% vs. 100.0% in the PP population). The incidence of grade 3/4 neutropenia was comparable between the study groups (Table 2).
Table 2.
Incidence of Grade 3/4 Neutropenia from cycle 1 to 4.
| Neutropenia, n (%) | Total Cycle 1–4 | ||
|---|---|---|---|
| PG2a | Placebo | P-value | |
| ITTb | N = 33 | N = 33 | |
| Grade 3 | 26 (78.8) | 29 (87.9) | 0.159 |
| Grade 4 | 26 (78.8) | 25 (75.8) | 0.616 |
| Grade 3/4 | 29 (87.9) | 31 (93.9) | 0.195 |
| PPc | N = 29 | N = 31 | |
| Grade 3 | 24 (82.8) | 29 (93.5) | 0.096 |
| Grade 4 | 24 (82.8) | 25 (80.6) | 0.584 |
| Grade 3/4 | 27 (93.1) | 31 (100.0) | 0.071 |
aPG2, Astragalus polysaccharides
bITT, intention-to-treat
cPP, per-protocol
Secondary endpoints
The incidence of participants with grade 3/4 hematologic toxicities, chemotherapy dose reductions, treatment delays, or granulocyte colony-stimulating factor treatment from cycles 1–4 did not significantly differ among the study groups. HRQL was evaluated using the EORTC QLQ-C30 and BR23 questionnaires and Eastern Cooperative Oncology Group. Additionally, the results revealed no significant difference when comparing the study and control groups in the ITT and PP populations. The result of HRQL is shown in the Fig. 3(c).
Subgroup analysis
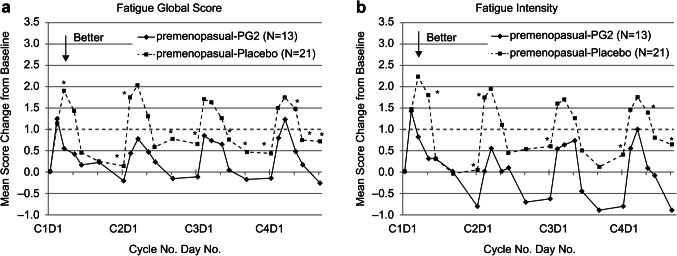
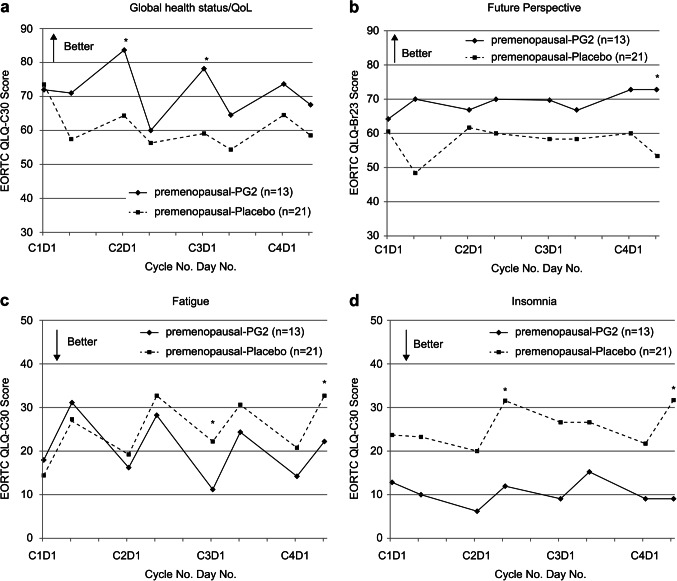
The subgroup analysis based on menopausal status revealed significant results for premenopausal patients. In the ITT population, the FGS and fatigue intensity were less aggravated in the initial four EC cycles in the premenopausal-PG2 group than in the premenopausal-placebo group (Fig. 4). Examining the results of EORTC-QLQ C30 and BR23 among premenopausal patients, those using PG2 reported a better condition of global health status and future perspective, alongside lower fatigue and insomnia burden during the first four EC cycles than those using placebo (Fig. 5). The PP population yielded similar results with the ITT population (data not shown).
Figure 4.
Time-dependent change in BFI fatigue scores in the two groups at the end of cycle 4 for the premenopausal ITT population. BFI, brief fatigue inventory, PG2, Astragalus polysaccharides. *represents the statistical significance between the groups at each time point.
Figure 5.
Time-dependent in EORTC QLQ-C30 & Br23 scores in the two groups at the end of cycle 4 for the premenopausal ITT population. (a) Global health status/QoL (b) Future perspective (c) Fatigue (d) Insomnia. PG2, Astragalus polysaccharides. *represents the statistical significance between the groups at each time point.
Safety
All reported AEs were collected and analyzed during the study. The safety outcomes between the PG2 and placebo groups were comparable. Overall, 1866 AEs were reported in 66 participants, with the majority judged as chemotherapy-related (89.6%, 1671/1866). Severe AE incidences for the PG2 and placebo groups were 22.2% (218/982) and 21.3% (188/884), respectively. Only seven AEs were causally related to PG2 treatment, including pyrexia (two participants), adverse drug reactions, cold, hypersensitivity, rash, and urticaria (one participant for each AE). No AEs led to study discontinuation or death. PG2 was considered tolerable by the participants with breast cancers.
Discussion
In this study, the primary endpoint, encompassing changes in FGS and fatigue intensity, was similar in both treatment groups. The significance of FGS and fatigue intensity change from baseline in the premenopausal groups emerged from cycles 1 through 4, implying the observable efficacy of PG2 in the premenopausal individuals. The EORTC QLQ-C30 and BR23 questionnaires surveyed the HRQL of the participants. Among the collected survey data, the deterioration of fatigue, insomnia, future perspective, and global health status were reduced during chemotherapy with the use of PG2 for premenopausal women. This result can also be supported by the fatigue domain of EORTC QLQ-C30, where the score for the placebo group increased from below 20 to over 30, whereas the score for the PG2 group remained around 20 throughout the cycles. The trend in the change in fatigue score suggests that PG2 could reduce CIF for premenopausal participants.
As menopausal status may be a factor that influences the results, compared with the 36.4% of participants in the placebo group, 60.6% in the PG2 group were postmenopausal, potentially influencing these endpoints. A positive trend was observed in the PG2 group towards the future perspective. This may be linked to the overall outcomes of fatigue, global health status, and insomnia with the PG2 complementary treatment.
PG2 is a drug therapy for relieving moderate-to-severe fatigue among patients with cancer. This study suggests that PG2 may have additional efficacy in preventing and reducing CIF severity among patients with breast cancer, particularly premenopausal patients. PG2 can assist patients in maintaining normal daily activities, caring for family, managing job responsibilities during chemotherapy, and maintaining daily activities. These factors are important for overall well-being and social-economic functions.
Our previous studies, which focused on patients with advanced cancer who might or might not have received chemotherapy at the time of the study, demonstrated that PG2 could improve patients’ CRF, especially in those with breast cancer. No definite difference existed between premenopausal and postmenopausal status in those trials. In contrast, all patients in our trial were disease-free and undergoing chemotherapy, the etiology of fatigue differed from that in the previous trials.
Furthermore, the incidence of grade 3/4 neutropenia and other hematologic toxicities increased as the cycle progressed, with no significant difference observed between both study groups. This result suggests that PG2 has no effect on the incidence of chemotherapy-induced hematologic toxicities under the current treatment schedule.
The two treatment groups exhibited comparable results in secondary outcomes such as chemotherapy dose reduction, treatment delays, and granulocyte colony-stimulating factor consumption. Based on these findings, the PG2 combination did not interfere with chemotherapy treatment or enhance toxicities in patients with breast cancer.
Additionally, PG2 was safe and well-tolerated by patients. AEs were mostly mild, manageable, and unrelated to PG2. The PG2-related AEs included pyrexia, adverse drug reactions, cold, hypersensitivity, rash, and urticaria, which were the known safety profiles of PG2.
Conclusion
Our results indicate comparable efficacy between PG2 and placebo, possibly influenced by the menopausal status of the participants. Subsequently, a subgroup analysis was conducted to evaluate the influence of menopausal status on PG2 efficacy. PG2 effects, including reduced CIF and insomnia, mitigated negative effects on future perspectives, and improved global health status, were observed in the premenopausal group compared with the placebo group.
The results suggest that PG2 may have additional efficacy in preventing and reducing the severity of CIF among premenopausal patients undergoing adjuvant anthracycline-based chemotherapy. PG2 could assist these patients in maintaining normal daily activities, caring for family, and managing job responsibilities during chemotherapy. Furthermore, patients undergoing cancer chemotherapy and treated with PG2 might be better positioned to complete their chemotherapy, alleviating concerns about their future health.
Finally, treatment with 500 mg of PG2 for 3 days per cycle for four EC chemotherapy cycles was safe and tolerable among patients with breast cancer.
Acknowledgements
We thank the patients in this study and the study coordinators who helped us complete it. We also thank Dr. Ruey-Kuen Hsieh, who helped us to design this trial.
Author contributions
Material preparation: S-C.C, C-M.H, M-T.P, C-T.L, Y-S.C, W-L.K, H-H.C, K-Y.Y, T-H.W, C-F.W, P-H.C, Y-M.H, C-C.Y, C-H.L; Conceptualization: C-H.W, K-M.R; Writing: W-C.S; Writing review and editing: K-M.R.
Funding
This work was supported by PhytoHealth Co.
Data availability
The analyzed data in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The approval was granted by the Ethics Committee of the Institutional Review Board of E-Da Cancer Hospital and Chang Gung Memorial Hospitals. This study adheres to the principles outlined in the Declaration of Helsinki and all methods were performed in accordance with the relevant guidelines and regulations.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Bines, J., Earl, H., Buzaid, A. C. & Saad, E. D. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: does the sequence matter? Ann. Oncol. 25, 1079–1085 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Rau, K. M. et al. A nationwide survey of fatigue in cancer patients in Taiwan: an unmet need. Jpn J. Clin. Oncol. 50, 693–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluz, O. et al. Nab-paclitaxel weekly versus dose-dense solvent-based paclitaxel followed by dose-dense epirubicin plus cyclophosphamide in high-risk HR+/HER2- early breast cancer: results from the neoadjuvant part of the WSG-ADAPT-HR+/HER2- trial. Ann. Oncol. 34, 531–542 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Yuan, P. et al. Effect of epirubicin plus paclitaxel vs epirubicin and cyclophosphamide followed by paclitaxel on disease-free survival among patients with operable ERBB2-negative and lymph node-positive breast cancer: a randomized clinical trial. JAMA Netw. Open. 6, e230122 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl. J. Med. 365, 1273–1283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 396, 1090–1100 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl. J. Med. 386, 556–567 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Chen, H. W. et al. A novel infusible botanically-derived drug, PG2, for cancer-related fatigue: a phase II double-blind, randomized placebo-controlled study. Clin. Invest. Med. 35, E1–E11 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Wang, C. H. et al. Karnofsky Performance Status as a predictive factor for cancer-related fatigue treatment with Astragalus polysaccharides (PG2) injection-a double blind, multi-center, randomized phase IV study. Cancers (Basel). 11, 128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du, Y., Wan, H., Huang, P., Yang, J. & He, Y. A critical review of Astragalus polysaccharides: from therapeutic mechanisms to pharmaceutics. Biomed. Pharmacother. 147, 112654 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Tsao, S. M., Wu, T. C., Chen, J., Chang, F. & Tsao, T. Astragalus polysaccharide injection (PG2) normalizes the neutrophil-to-lymphocyte ratio in patients with advanced lung cancer receiving immunotherapy. Integr. Cancer Ther. 20, 1534735421995256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, C. C. et al. Validation of the Taiwanese version of the brief fatigue inventory. J. Pain Symptom Manage. 32, 52–59 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Chie, W. C., Chang, K. J., Huang, C. S. & Kuo, W. H. Quality of Life of Breast Cancer Patients in Taiwan: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23. Psychooncology. 12, 729–735 (2003). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data in this study are available from the corresponding author upon reasonable request.