Abstract
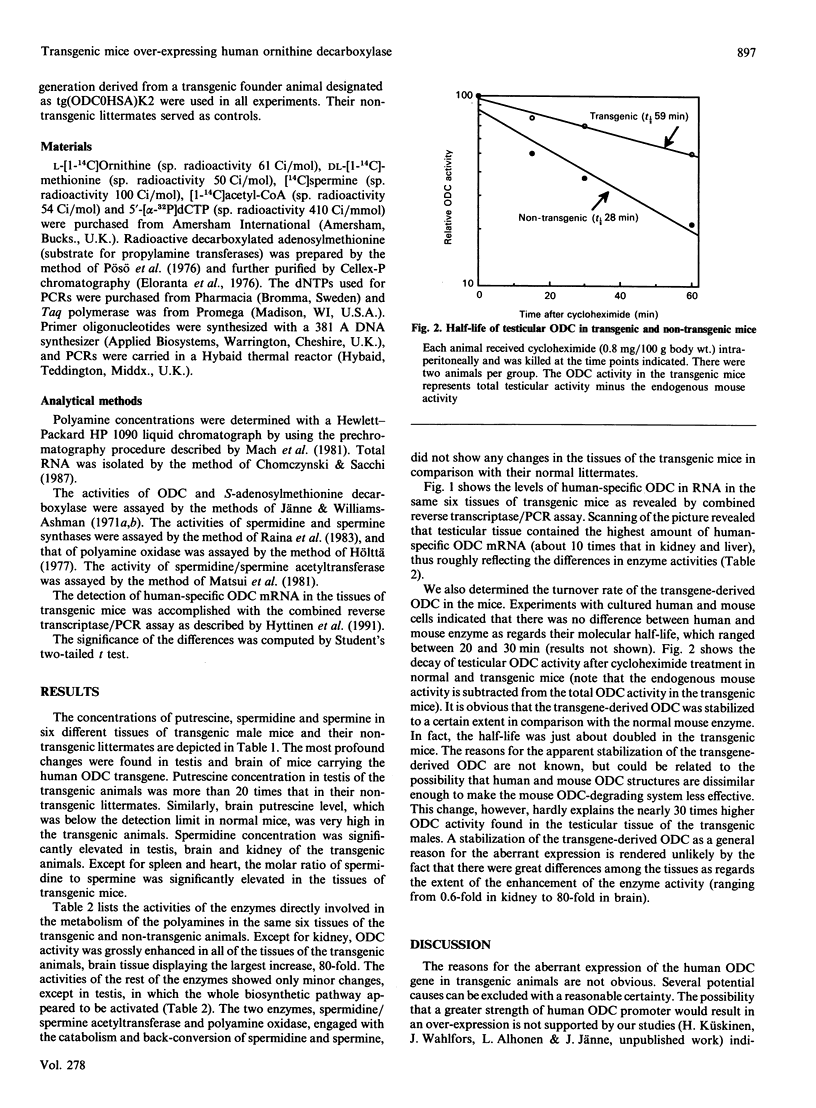
We have produced several transgenic mouse lines over-expressing the human ornithine decarboxylase (ODC) gene. We have now characterized one of the transgenic lines as regards the tissue accumulation of the polyamines and the activities of their metabolizing enzymes. Among the tissues analysed, the polyamine pattern was most strikingly changed in testis and brain of the transgenic animals. ODC activity was greatly enhanced in all tissues, except kidney, of the transgenic animals. The most dramatic increase, 80-fold, was found in brain of the transgenic mice. The activities of S-adenosylmethionine decarboxylase and spermidine and spermine syntheses were likewise significantly increased in testis of the transgenic animals. The activities of the enzymes involved in the back-conversion of the polyamines, namely spermidine/spermine acetyltransferase and polyamine oxidase, were similar in the transgenic and non-transgenic animals. As analysed by reverse transcriptase/polymerase chain reaction, all the six tissues of the transgenic animals expressed human-specific ODC mRNA. Determination of the half-life of testicular ODC revealed a stabilization of the enzyme in the transgenic males.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Kallio A., Sinervirta R., Jänne O. A., Gahmberg C. G., Jänne J. Tumourigenicity, cell-surface glycoprotein changes and ornithine decarboxylase gene pattern in Ehrlich ascites-carcinoma cells. Biochem J. 1985 Aug 1;229(3):711–715. doi: 10.1042/bj2290711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Eloranta T. O., Kajander E. O., Raina A. M. A new method for the assay of tissue. S-adenosylhomocysteine and S-adenosylmethione. Effect of pyridoxine deficiency on the metabolism of S-adenosylhomocysteine, S-adenosylmethionine and polyamines in rat liver. Biochem J. 1976 Nov 15;160(2):287–294. doi: 10.1042/bj1600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. C., Flanagan M. A. Characterization and sequence analysis of the human ornithine decarboxylase gene. DNA. 1989 Nov;8(9):623–634. doi: 10.1089/dna.1.1989.8.623. [DOI] [PubMed] [Google Scholar]
- Halmekytö M., Hirvonen A., Wahlfors J., Alhonen L., Jänne J. Methylation of human ornithine decarboxylase gene before transfection abolishes its transient expression in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):528–534. doi: 10.1016/0006-291x(89)92029-9. [DOI] [PubMed] [Google Scholar]
- Hickok N. J., Wahlfors J., Crozat A., Halmekytö M., Alhonen L., Jänne J., Jänne O. A. Human ornithine decarboxylase-encoding loci: nucleotide sequence of the expressed gene and characterization of a pseudogene. Gene. 1990 Sep 14;93(2):257–263. doi: 10.1016/0378-1119(90)90233-h. [DOI] [PubMed] [Google Scholar]
- Hyttinen I. M., Halmekytö M., Alhonen L., Jänne J. Levels of ornithine decarboxylase genomic sequences, heterogeneous nuclear RNA and mRNA in human myeloma cells resistant to alpha-difluoromethylornithine. Biochem J. 1991 Sep 15;278(Pt 3):871–874. doi: 10.1042/bj2780871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Hirvonen A., Wahlfors J., Alhonen L., Jänne J., Kallio A. Human ornithine decarboxylase(ODC)-encoding gene: cloning and expression in ODC-deficient CHO cells. Gene. 1989 Nov 15;83(1):125–135. doi: 10.1016/0378-1119(89)90410-1. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Katz A., Kahana C. Rearrangement between ornithine decarboxylase and the switch region of the gamma 1 immunoglobulin gene in alpha-difluoromethylornithine resistant mouse myeloma cells. EMBO J. 1989 Apr;8(4):1163–1167. doi: 10.1002/j.1460-2075.1989.tb03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Kersten H., Kersten W. Measurements of polyamines and their acetylated derivatives in cell extracts and physiological fluids by use of an amino acid analyzer. J Chromatogr. 1981 Apr 10;223(1):51–57. doi: 10.1016/s0378-4347(00)80067-5. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Polvinen K., Sinervirta R., Alhonen L., Jänne J. Overproduction of ornithine decarboxylase confers an apparent growth advantage to mouse tumor cells. Biochem Biophys Res Commun. 1988 Aug 30;155(1):373–378. doi: 10.1016/s0006-291x(88)81095-7. [DOI] [PubMed] [Google Scholar]
- Raina A., Eloranta T., Pajula R. L. Rapid assays for putrescine aminopropyltransferase (spermidine synthase) and spermidine aminopropyltransferase (spermine synthase). Methods Enzymol. 1983;94:257–260. doi: 10.1016/s0076-6879(83)94044-2. [DOI] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Blackshear P. J. Rat ornithine decarboxylase gene. Nucleotide sequence, potential regulatory elements, and comparison to the mouse gene. J Biol Chem. 1989 May 25;264(15):9016–9021. [PubMed] [Google Scholar]



