Abstract
Nearly every system within the body contains an intrinsic cellular circadian clock. The circadian clock contributes to the regulation of a variety of homeostatic processes in mammals through the regulation of gene expression. Circadian disruption of physiological systems is associated with pathophysiological disorders. Here, we review the current understanding of the molecular mechanisms contributing to the known circadian rhythms in physiological function. This review focuses on what is known in humans, along with discoveries made with cell and rodent models. In particular, the impact of circadian clock components in metabolic, cardiovascular, endocrine, musculoskeletal, immune, and central nervous systems are discussed.
I. Introduction: Circadian Rhythms in Physiology
The oldest record of a circadian observation comes from Alexander the Great’s scribe Androsthenes, who in 400 B.C. noted that the leaves of certain plants displayed opening and closing patterns coincident with sunrise and sunset. It is now known that normal circadian rhythms are an integral part of healthy physiology, and that this is true across kingdoms of life, from cyanobacteria to plants to humans. Some of the oldest published data on human circadian physiology comes from the 1940s and 1950s, including the observation of diurnal patterns in lymphocyte circulation(78) as well as the landmark study from Mills and Stanbury demonstrating reproducible rhythms in renal excretory function(181). Indeed, it is now well established that the molecular machinery of the circadian clock, discussed below, is present in virtually every cell and tissue type.
Circadian rhythms are nearly as old as time, as life on Earth evolved in the presence of 24-hour periods of light and dark. Alignment of physiological processes to the light/dark cycle likely afforded organisms survival advantages, such as in relation to food availability or predator avoidance. The circadian clock mechanism contributes to the regulation of a variety of homeostatic processes in mammals, including the sleep/wake cycle, glucose metabolism, body temperature, and hormone levels(225). In humans, individual variations in preference for sleep and awakeness are classified as three different chronotypes, Morning, Evening, and Neither(187). Morning-types typically function better earlier in the day, while Evening-types are at peak functioning in the latter half of the day. Neither-types show intermediate characteristics and cannot be classified as either Morning or Evening type. The melatonin hormone, secreted in a circadian pattern by the pineal gland through photic input from the suprachiasmatic nuclei (SCN), is a determinate of sleep onset and its temporal patterns differ between the Morning and Evening chronotypes.
Loss of circadian rhythms in physiological function is associated with adverse health outcomes. Following Walter Cannon’s description of homeostasis in the 1920s, it was thought that maintaining a steady state was a reactive process. Six decades later, in 1986, on the occasion of the 30th annual Bowditch Lecture of the American Physiological Society, Martin Moore-Ede described Cannon’s great contribution to physiology as “the first systematic description of those specialized mechanisms unique to living systems which preserve internal equilibriums in the face of an inconstant world”(188). Moore-Ede went on to introduce the concept of “predictive homeostasis,” in which the circadian timing mechanism allows “an organism to predict when environmental challenges are most likely to occur and thus initiate corrective responses in advance of the challenge.” Thirty-five years later, advances in molecular biology and circadian physiology have enabled us to better understand how each tissue clock participates in predictive homeostasis, aligning physiological processes with the external environment to ensure overall health.
In this review, we present the current understanding of the molecular mechanisms by which the circadian clock controls transcription, thus contributing to genome-wide gene expression and circadian rhythms in physiological function. In subsequent sections, we review the most recent data describing the function and mechanisms of the circadian clock in the central nervous system (CNS)(see Table 1 for acronyms) as it pertains to the sleep/wake cycle, and the digestive, cardiovascular, musculoskeletal, immune, and endocrine systems. We have limited our discussion to mammalian systems, including cells cultured in vitro, rodent models, and human studies. Additionally, all animal data is assumed to be from males unless otherwise noted.
Table 1:
Listing of abbreviations and acronyms used throughout the text.
| Abbreviation | Definition |
|---|---|
| 3C, 4C, 5C | Chromosome Conformation Capture |
| ACTH | Adrenocorticotropic Hormone |
| AMPK | AMP-Activated Protein Kinase |
| BMAL1 | Aryl Hydrocarbon Receptor Nuclear Translocator-Like Protein 1 |
| CAR | Constitutive Androstane Receptor |
| CCL2 | C-C Motif Chemokine 2 |
| CCR7 | C-C Chemokine Receptor 7 |
| ChIA-PET | Chromatin Interaction Analysis with Paired-End Tag Sequencing |
| ChIP-seq | Chromatin Immunoprecipitation Followed by Sequencing |
| CLOCK | Clock Circadian Regulator |
| CNS | Central Nervous System |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CROT | Carnitine O-Octanoyltransferase |
| CRY | Cryptochrome |
| CT | Circadian Time |
| CYP | Cytochrome 450 |
| DBP | D-Element-Binding Protein |
| E-box | Enhancer Box |
| EDN1-AS | Endothelin 1-Antisense |
| ENaC | Epithelial Sodium Channel |
| GLUT | Glucose Transporter |
| GnRH | Gonadotropin-Releasing Hormone |
| GR | Glucocorticoid Receptor |
| HPA | Hypothalamic-Pituitary-Adrenal |
| HPG | Hypothalamus-Pituitary-Gonadal |
| HPT | Hypothalamic-Pituitary-Thyroid |
| ICU | Intensive Care Unit |
| IL | Interleukin |
| KO | Knockout |
| lncRNA | Long Non-Coding RNA |
| LPS | Lipopolysaccharide |
| MDSC | Myeloid-Derived Suppressor Cell |
| MED1 | Mediator Complex Subunit 1 |
| Mnase-seq | Micrococcal Nuclease Digestion with Deep Sequencing |
| mTOR | Mechanistic Target of Rapamycin |
| NCC | Sodium Chloride Cotransporter |
| NFIL3 | Nuclear Factor, Interleukin 3 Regulated |
| NF-κB | Nuclear Factor Kappa B |
| NPAS2 | Neuronal PAS Domain Protein 2 |
| NR1Dx | Nuclear Receptor Subfamily 1 Group D Member |
| OPG | Osteoprotegerin |
| Pax8 | Paired Box Gene 8 |
| PD-L1 | Programmed Cell Death-Ligand 1 |
| PER | Period |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| pT53 NCC | Phosphorylated NCC protein at Thr53 |
| RANKL | Receptor Activator of Nuclear Factor Kappa B Ligand |
| REV-ERBx | Nuclear Receptor Subfamily 1 Group D Member |
| ROR | Receptor-Related Orphan Receptor |
| SCN | Suprachiasmatic Nuclei |
| STAT | Signal Transducer and Activator of Transcription |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TAD | Topological Associatin Domain |
| TRH | Thyroid-Releasing Hormone |
| TSH | Thyroid-Stimulating Hormone |
| WNK | With No Lysine |
| ZT | Zeitgeber Time |
II. Molecular Machinery of the Clock
a. Transcription-Translation Feedback Loop
The first studies on clock gene regulation were in fruit flies (Drosophila melanogaster), including the seminal work detailing the period (PER)/timeless transcription-translation feedback loop for which Jeffrey Hall, Michael Rosbash, and Michael Young received the 2017 Nobel prize in Medicine or Physiology(239). The Drosophila per gene was cloned in 1984. Mammalian studies gained steam following cloning of the mouse circadian clock circadian regulator (Clock) gene in 1997(227). The molecular machinery of the circadian clock is evolutionarily well-conserved, with homologous genes between flies and mice(92). The expression of per, timeless, and other clock genes throughout the body paved the way for the discovery of clocks in most organs and experiments in cell culture systems(239). Although each tissue or organ regulates distinct circadian physiologies, clocks across various organs appear to share a similar mechanism at the molecular and cellular level. It should be noted, however, that validation of the detailed molecular clock framework in different cell types is ongoing.
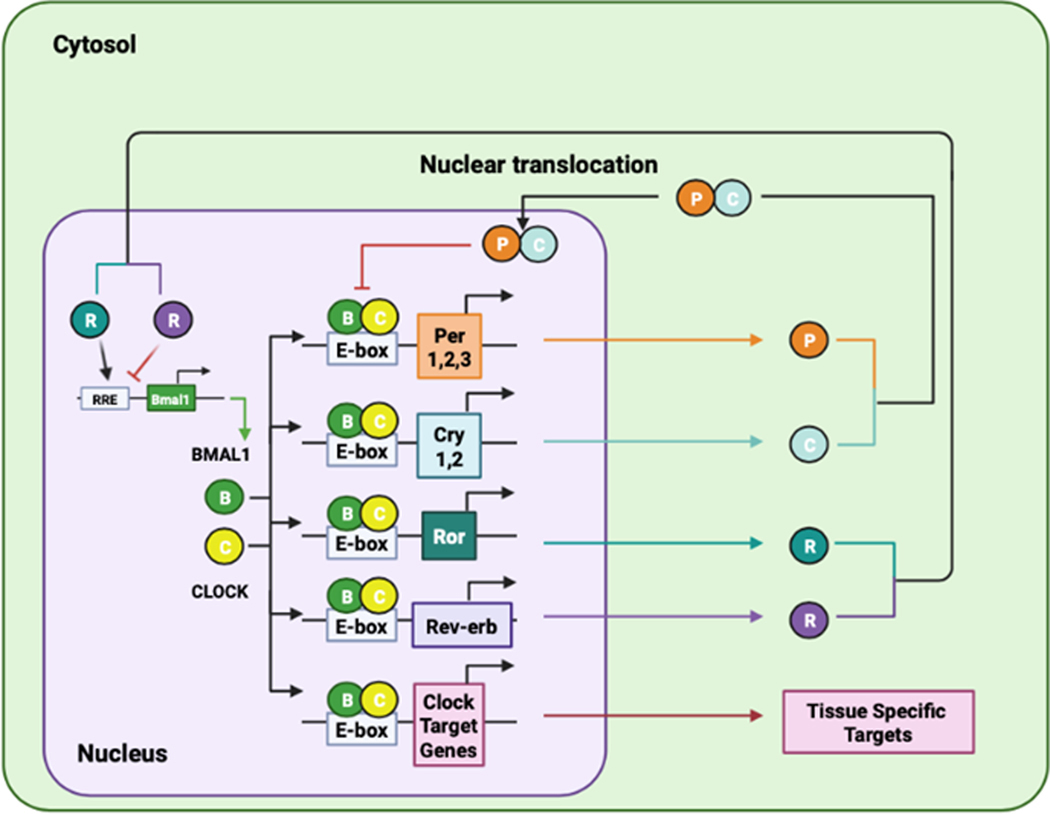
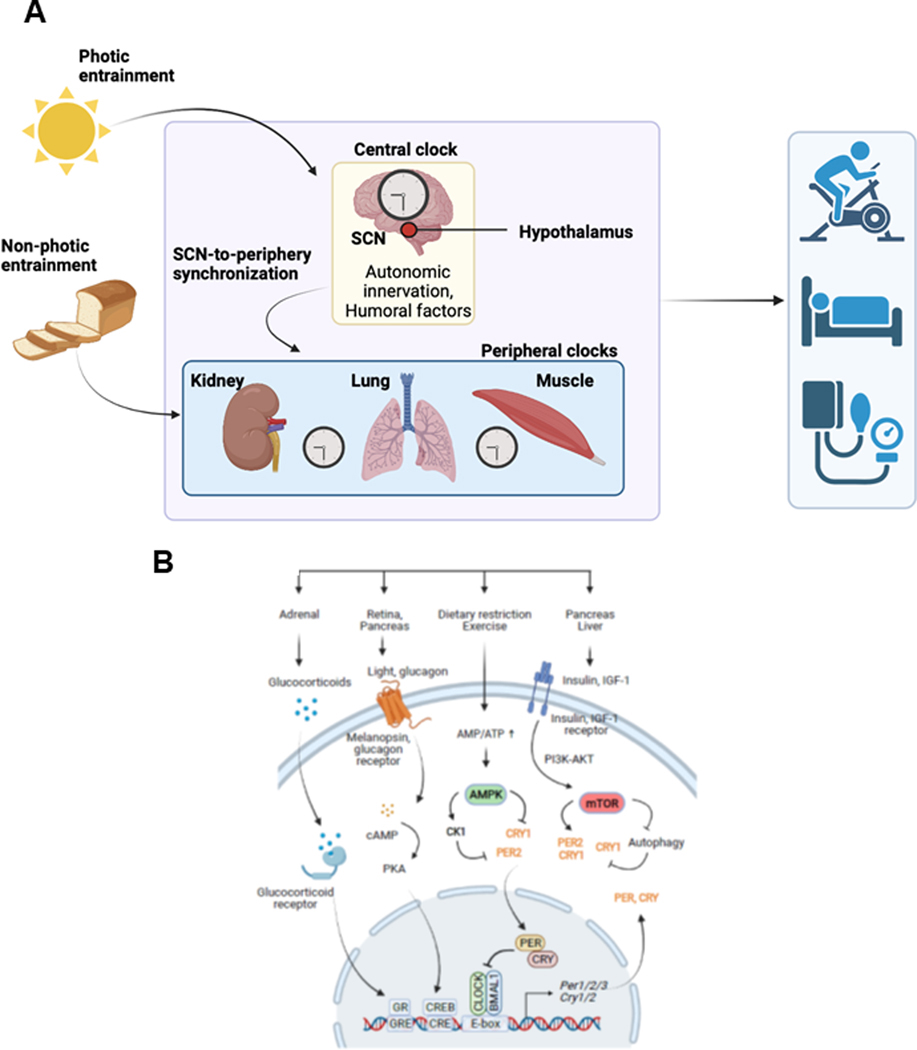
In general, the molecular machinery of the mammalian clock contains a core transcriptional negative feedback loop, within which aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL1, also known as BMAL1) and CLOCK activate the transcription of their own repressors PER1, PER2, PER3, cryptochrome circadian regulator (CRY) 1 and CRY2 through the circadian cis enhancer box (E-box) element (Figure 1). The E-box-mediated core clock loop is integrated with two known feedback loops regulated by the retinoic acid receptor-related orphan receptor (ROR)-binding element and the D-box(261, 272). The ROR-binding element, in turn, is regulated by nuclear hormone receptor transcription activators RORA, RORB, and RORC and repressors nuclear receptor subfamily 1 group D member 1 (NR1D1 also known as REV-ERBα) and NR1D2 (or REV-ERBβ). The D-box is repressed by nuclear factor, interleukin (IL)-3 regulated (NFIL3; also known as E4BP4), and activated by albumin D-element-binding protein (DBP), thyrotrophic embryonic factor, and hepatic leukemia factor. The core and secondary feedback loops, both individually and in combination, underlie the rhythmic expression of thousands of genes at several distinct phases throughout the day, and importantly, largely in a tissue-type-specific manner(129, 272, 314). They function together to introduce additional phases of clock gene expression, impart robustness against genetic perturbation, as well as offer additional nodes with which to regulate circadian outputs(21). As such, the molecular clock forms a large and complex output network to regulate various cellular and physiological processes. Over the past two decades, a number of genome-wide studies have contributed greatly to the understanding of these output gene networks(19, 104, 123, 129, 143, 210, 214, 221, 254, 271, 305, 314). The circadian clock consists of the oscillator, inputs, and outputs (Figure 2A). The central clock oscillator is located in the SCN where it is entrained by light cues traveling through the retinal hypothalamic tract. The central clock synchronizes the peripheral tissue clocks through neuronal and humoral signaling. The central and peripheral clocks are also sensitive to non-photic cues such as food intake. Rhythms in gene expression and protein function within individual tissues and across physiological systems result in rhythmic behavior, including rest/activity cycles and cardiovascular function.
Figure 1.
Transcription-Translation Feedback Loops of the Circadian Clock Mechanism. In the positive loop, BMAL1 and CLOCK heterodimerize to activate transcription of the genes encoding proteins that function in the feedback loops as well as tissue-specific target genes. In the negative loop, PER and CRY act to inhibit BMAL1/CLOCK transcriptional activity thereby decreasing their own expression. In an ancillary loop, ROR and REV-ERB mediate opposing action on the expression of the Bmal1 gene.
Figure 2.
The mammalian circadian system and its crosstalk with physiology. (A) The circadian system includes the hierarchical multi-oscillator network, the input pathways for entrainment, and rhythmic outputs in genes, metabolism, physiology and behavior. The SCN directly receives photic cues for entrainment to the light/dark cycle. In turn, the SCN functions to synchronize extra-SCN and peripheral clocks. While the SCN is responsive to photic cues, the peripheral oscillators are more responsive to non-photic cues such as feeding. (B) Several major organs and pathways that provide inputs to the circadian clock. The molecular clock is based on a transcriptional/translational negative feedback mechanism. The core loop consists of positive (BMAL1, CLOCK) and negative (PER, CRY) factors, which act on the E-box cis-element. In particular, the Per genes contain other regulatory elements including cAMP response element (CRE) and glucocorticoid response element (GRE). Melanopsin in the retina mediates photic transmission through cAMP production and the CRE binding protein (CREB) pathway. The hypothalamus (SCN to CRH neurons)-pituitary-adrenal axis regulates glucocorticoid secretion. Glucagon, insulin and IGF-1 provide metabolic and growth signal inputs to the clock via the master nutrient/energy sensors AMPK and mTOR. In the case of insulin and IGF-1, the pathways lead to the upregulation of PER2 and CRY1, thereby affecting the clock function.
Many clock modifiers have been identified in recent years(192, 219, 311). These clock modifiers affect the expression and function of the core clock components at different levels, including transcriptional, translational, and post-translational, as well as through epigenetic control. As such, these modifiers serve as inputs to the clock mechanism and affect outputs. Several best-studied clock modifiers include the nutrient/energy sensors AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR), nicotinamide adenine dinucleotide+-dependent deacetylase sirtuin 1, hypoxia-inducible factor-1α, oxidative stress-inducible factor nuclear factor erythroid 2-related factor 2, and the proinflammatory nuclear factor kappa B subunit 1 (NF-κB). For example, AMPK and mTOR function to sense metabolic and nutrient/energy levels to maintain homeostasis(96, 106). While AMPK is activated when the energy state of the cell is low and AMP/ATP is high, mTOR senses high cellular energy states through glucose, amino acids, and growth factors. As a result, AMPK inhibits catabolic consumption of ATP and induces its anabolic production, whereas mTOR increases protein synthesis and lipid synthesis, and inhibits autophagy to promote cell growth(233).
Nutrient and energy homeostasis is critical for the regulation of various metabolic and physiological processes in the CNS and peripheral tissues(112), in which the AMPK and mTOR play a central role. While AMPK is activated under low energy states, mTOR is responsive to high energy states to promote cell growth and proliferation(106). The AMPK-mTOR signaling nexus represents one of the hallmarks of aging and is often deregulated in accelerated aging(226). Recent studies provided some insights into the interplay between AMPK-mTOR signaling and clock function (Figure 2B). First, AMPK was shown to phosphorylate casein kinase 1ε, resulting in enhanced phosphorylation and degradation of PER2(231, 274), and also phosphorylate CRY1, leading to its ubiquitination and proteasomal degradation(118, 139, 231). Second, mTOR plays a critical role in regulating photic entrainment and synchronization of the SCN clock(38, 39, 156). Third, mTOR was shown to regulate the central SCN and peripheral circadian clocks, as well as circadian locomotor activity rhythms(53, 82, 154, 155, 218, 282). While mTOR activation results in shorter periods and a faster clock, its inhibition causes lengthened periods and a dampened amplitude. Mechanistically, current data support a model in which mTOR activation upregulates CRY1 through increased translation and decreased autophagic degradation(154, 218, 268). Further, key players of the AMPK-mTOR pathway (e.g., p-AMPK, p-S6, p-4EBP1) are regulated by the clock mechanism and display rhythmic activities across tissue types including the SCN(39, 102, 119, 218), providing anticipation for nutrient/energy availability. Collectively, these findings revealed a much deeper integration between the circadian clock and cellular metabolic functions. Thus, the circadian input and output gene networks are far more extensive than previously recognized and this integration is expected to play important roles in organ-specific functions such as within the liver, muscle, and adipose tissues(192, 219, 311).
b. Chromatin Dynamics and Clock Proteins
It is now well accepted that the core clock mechanism regulates nearly half of all expressed genes in a cell- and tissue-specific manner(217, 314). Understanding the mechanisms by which this regulation occurs is an ongoing area of investigation with implications for our understanding of circadian physiology and general pathophysiology. Advanced molecular methods, including Hi-C, chromatin immunoprecipitation followed by sequencing (ChIP-seq), chromatin interaction analysis with paired-end tag sequencing (ChIA-PET), as well as chromosome conformation capture (3C; and the related 4C and 5C) methods (see Inset) used in well-designed circadian biology studies have greatly increased our understanding of how the circadian clock mechanism interfaces with tissue-specific transcriptional regulatory factors to mediate widespread transcriptional control.
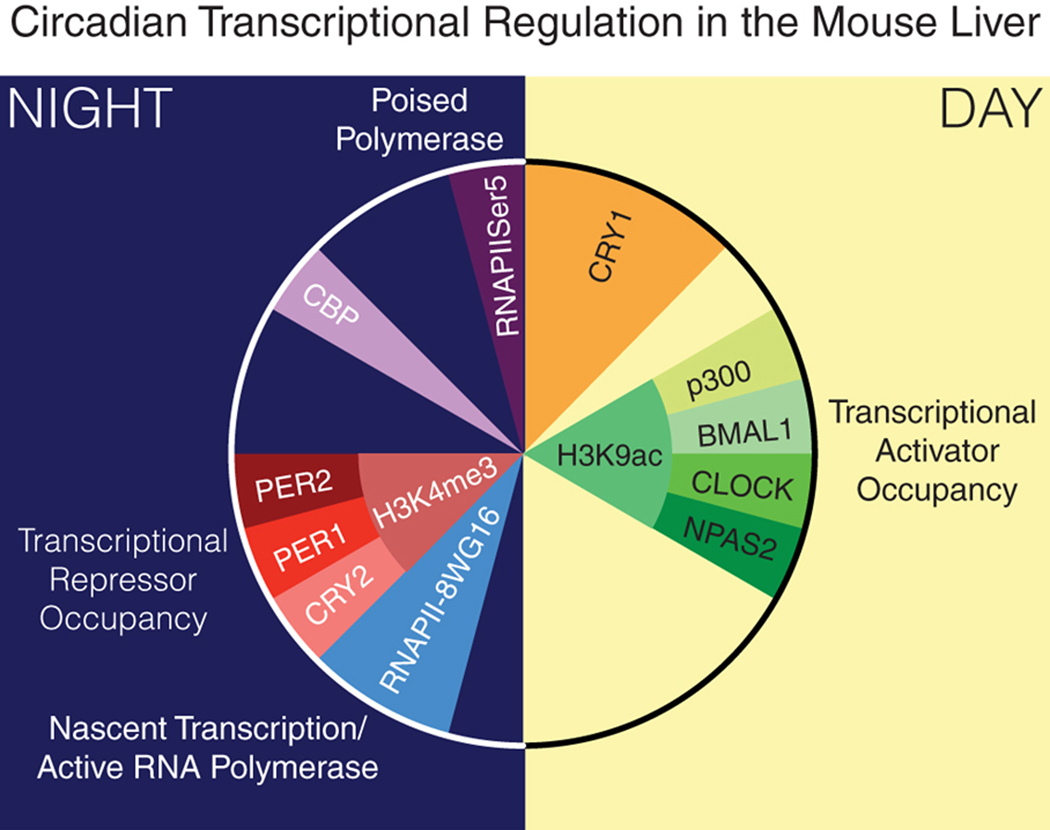
The expense of performing these highly technical molecular studies makes it difficult to assay samples collected with the frequency required for high circadian resolution. One landmark study by the Howard Hughes Medical Institute Investigator Dr. Joseph Takahashi and colleagues characterized the circadian transcriptional architecture and chromatin landscape in the liver(129). Koike et al. used ChIP-seq to characterize the binding of BMAL1, CLOCK, neuronal PAS domain protein 2 (NPAS2), PER1, PER2, CRY1, and CRY2 in chromatin samples collected every four hours over a 24 hour period. In addition to the core clock proteins, this study also performed ChIP-seq using antibodies against RNA polymerase II, histone H3 lysine 4 trimethylation, histone H3 lysine 9 acetylation, and histone H3 lysine 27 acetylation, which yielded critical information regarding chromatin conformation and histone modifications that signified whether a given gene is in an area of closed or open chromatin (Figure 3, with permission from (54)). This large-scale study was performed using tissue collected from male C57BL/6 mice between the ages of 8 and 12 weeks that had been maintained in constant darkness for 36 hours to ensure that the animals were under free-running conditions. The highlights of this study include the exciting finding that RNA polymerase II recruitment and the remodeling of chromatin occur in a circadian manner on a genome-wide scale. This study also laid the groundwork for others to build similar studies in distinct tissues, as discussed below.
Figure 3.
Transcriptional Repressors and Activators in the Mouse Liver. Correlating data from ChIP-seq using antibodies against the proteins depicted in this figure, Takahashi and colleagues developed a model demonstrating how clock target gene promoter occupancy of the activator complex (in shades of green) peaks during the light phase (day) whereas occupancy of the repressor complex (shades of red) peaks during the dark phase (night). Occupancy of the activators coincides with H3K9ac (histone 3 lysine 9 acetylation), a marker of open chromatin, in contrast to the presence of H3K4me3 (histone 3 lysine 4 trimethylation), a marker of closed chromatin, which coincides with occupancy of the repressor complex. With permission from (54).
In an important follow-up study, Rosbash and colleagues used micrococcal nuclease digestion with deep sequencing (MNase-seq; see Inset) on chromatin collected from mouse livers every four hours over a 24 hour period(174). These investigators compared this high-resolution nucleosome sequence data at these time points with the CLOCK/BMAL1 binding site data from Koike et al. to demonstrate that nucleosome signals were rhythmic at CLOCK/BMAL1 sites. Adding an additional layer of specificity to this observation, Menet et al. repeated the MNase-seq study in livers from Bmal1 knockout (KO) mice which showed loss of rhythmicity in nucleosome signals. Using this same comparison method interrogating the REV-ERBα-bound sites (from Koike et al. ChIP-seq data), REV-ERBα binding was not associated with rhythmic nucleosome signals, demonstrating a specific role of BMAL1/CLOCK in rhythmic nucleosome removal.
Based on the Koike et al. finding that maximal BMAL1 binding occurred in the liver at zeitgeber time (ZT) 6, Beytebiere et al. performed ChIP-seq for BMAL1 at this time point in the liver, kidney, and heart(25). Consistent with previous studies that showed 1000s of clock-controlled genes in these tissues, the ChIP-seq results identified >2000 BMAL1 signal peaks each in the liver, kidney, and heart samples, with minimal overlap between tissues. BMAL1 cistromes that overlapped amongst all three tissues included clock genes such as Per1 and Nr1d1. Gene ontology analysis indicated further overlap in broad pathways including glucose homeostasis, transcription factor binding, protein transport, and transcription. Of note, a kidney-specific BMAL1 target was found in the paired box gene 8 (Pax8) gene, which includes the promoter that is often used to generate Cre recombinase-mediated kidney-specific KO mice(28). Beytebiere and colleagues went on to perform ChIA-PET in liver samples collected at ZT6 and ZT18 which demonstrated stable interactions between the promoters of clock-controlled genes and enhancers(25). Using other available databases, these authors showed that rhythmic expression of BMAL1 target genes correlated with rhythmic chromatin interactions. These findings are consistent with the previous work of Menet et al. who found that BMAL1/CLOCK-dependent nucleosome signals were higher in enhancers than at transcription start sites(174). Together these results show that transcriptional regulation of tissue-specific genes by the circadian clock mechanism, at least in part, depends on changes in chromatin structure.
Further evidence for the role of chromatin conformation changes in clock-mediated control of transcription was provided by Naef and colleagues(303). Yeung et al. used an innovative approach to investigate circadian clock-dependent mechanisms of transcriptional regulation by comparing RNA sequencing data sets from several tissues, including liver and kidney, and overlaying that data with newly derived data generated by 4C. Using male wild type or Bmal1 KO mice, Yeung et al. characterized chromatin conformation at ZT8 and ZT20 in these two tissues. These animals were housed in a normal 12:12 light/dark cycle. The results revealed that tissue-specific clock target genes are regulated in part by chromatin looping which promotes tissue-specific promoter/enhancer contacts. Remarkably, the 4C results demonstrated that enhancers bound by clock transcription factors can contact promoters of clock target genes while at the same, nearby non-rhythmic promoters are looped out, thereby preventing rhythmic transcription of non-target genes. Additional support for clock proteins functioning in the chromatin looping mechanism came from Mermet et al., with their use of 4C-seq using the Cry1 transcription start site as bait(176). Samples were collected at circadian time (CT) 8 and CT20 from livers and kidneys of C57BL/6 male mice. They observed time-dependent interactions between promoters and enhancers in both tissues that were absent in the tissues from global Bmal1 KO mice. One of the confirmed interactions involved an enhancer located in a Cry1 intron. Impressively, Mermet et al. generated a mouse with a deletion of this element that exhibited a decrease in rhythmic chromatin interactions as well as a decrease in the circadian activity period. These findings demonstrate that large-scale chromatin conformation changes are of paramount importance to transcriptional regulation and have important implications for overall animal behavior.
Using mouse livers collected at 5 am (ZT22) and 5 pm (ZT10), Kim et al. performed in situ Hi-C (see Inset) to compare genome-wide chromatin organization at time points from the mouse active vs. inactive periods(127). Enhancer/promoter interactions within topological associating domains (TADs) differed between the two time points, depending on the area of chromatin in question. As an example, a focus on the region of chromosome 1 containing the Npas2 gene demonstrated that intra-TAD interactions were greater at ZT22 when Npas2 is being actively transcribed whereas, at ZT10, Npas2 is not being transcribed in the absence of enhancer/promoter contacts. To get at the mechanism of this type of effect, Kim et al. performed ChIP-seq for Rev-Erbα, the circadian clock repressor, coupled with 3C experiments at six time points throughout the circadian cycle. This finer resolution showed that Rev-Erbα inhibits the formation of promoter/enhancer loops. Subsequent experiments demonstrated that Rev-Erbα prevents loop formation through recruitment of the nuclear receptor corepressor histone deaceytylase-3 complex, histone deacetylation, and disruption of DNA/protein interactions with positive transcriptional regulators bromodomain containing 4 and mediator complex subunit 1 (MED1).
Related to clock protein interactions with enhancers, Fan et al. demonstrated a key role for long non-coding RNAs (lncRNAs) in mediating these interactions(81). Using available data from Koike et al.(129) and Zhang et al.(314), Fan et al. assembled a list of circadian lncRNAs and then combined these results with their own newly generated data from the mouse liver using samples collected every four hours over a 48 hour period. Consistent with the finding of Zhang et al.(314), Fan and colleagues found >2000 lncRNAs to exhibit circadian expression. Of these, the majority were associated with enhancers. A subset of the enhancer-associated circadian lncRNAs was confirmed to be bound by either BMAL1 or REV-ERBα. Interestingly, comparing the mouse liver data to mouse pancreas and rat liver demonstrated that the strong association of circadian lncRNAs with enhancers was observed across species and mouse tissues. Comparing the mouse and rat data revealed that the sequences of the lncRNAs themselves were not as highly conserved as the loci for the lncRNAs, demonstrating the importance of this regulatory mechanism across species. Fan et al. focused on the specific lnc-carnitine O-octanoyltransferase (Crot), which is expressed at a super-enhancer upstream of the clock-controlled Crot gene. Using the lnc-Crot locus as bait, 4C provided evidence that lnc-Crot facilitates long-range interactions between clock target genes and enhancers. This mechanism of circadian lncRNA-dependent chromatin looping likely contributes to the myriad ways the core clock mechanism acts as a master regulator of gene expression. Our own work recently identified EDN1-AS as a lncRNA antisense to the endothelin 1 gene(70). EDN1-AS exhibited circadian expression in a synchronized, immortalized cell culture model of human kidney proximal tubule cells. Preliminary results indicated that PER1 interacts with an E-box element in the region upstream of EDN1-AS. Clustered regularly interspaced short palindromic repeats (CRISPR)-mediated deletion of this area actually increased EDN1-AS expression and this effect was associated with increased expression of Endothelin 1 mRNA and excreted endothelin-1 peptide. We have recently reviewed the connections between the circadian system and endothelin signaling(68). Our data, taken together with that of Fan et al., suggests a key role for lncRNA in the clock mechanism.
III. Tissue Specific Clock Mechanisms
a. Sleep/CNS/Brain
The circadian system in mammals consists of the central SCN clock, extra-SCN clocks in the brain, and various peripheral oscillators. The SCN clock is reset daily by the light/dark cycle through photic transmission and signal transduction. Upon resetting, the SCN relays time-of-day information to extra-SCN and peripheral oscillators for internal systemic synchronization. These peripheral oscillators can run independently of the SCN, but due to lack of photoreception, they must rely on the central clock for time cues. While the SCN is largely phase-locked to the light/dark cycle, peripheral tissues are more responsive to local and systemic non-photic cues for resetting and synchronization(184). The hypothalamus functions as the master regulator of the body’s systemic homeostasis by integrating signals from energy state, food availability, as well as cognitive and emotional inputs. Within the hypothalamus, the SCN projects directly and indirectly to a host of nuclei, together regulating a multitude of homeostatic functions, including the sleep/wake cycle and associated rest/active and feeding/fasting cycles, as well as various circadian rhythms such as body temperature, blood pressure, appetite, energy balance, and the neuroendocrine system (e.g., glucocorticoids and melatonin)(43, 275). Further, recent studies have also implicated the circadian clock in the crosstalk with cognitive functions(198). For example, transcriptomic and proteomic analyses of human prefrontal cortex and mouse hippocampal tissue samples revealed age-related loss and gain of circadian expression of genes and proteins(3, 47).
The most obvious manifestation of circadian timekeeping is the sleep/wake cycle. One of the most important features of sleep is its consolidation during nighttime in humans and daytime in most rodents. Interestingly, although most clock genes are similar between mouse/rat and human, the rhythmicity of these genes does not seem to dictate whether a species is nocturnal or diurnal. Indeed, diurnality seems to involve relay neural substrates that are projected from the SCN to regulate diurnal vs. nocturnal activities(246). In mammals, the sleep/wake cycle is regulated by the combined actions of the master circadian clock in the SCN of the hypothalamus that controls the timing of sleep, and the sleep homeostatic center (whose anatomical location in the hypothalamus remains elusive) that controls the drive or need for sleep in response to low energy state(62, 232, 262). This creates an anticipatory (rather than just reactive) mechanism to coordinate behavior and physiology with the predictive light/dark cycle and nutrient availability. Disruption of this coordination causes circadian rhythm sleep disorders and sleep deficiency. Sleep supports nearly every tissue and system in the body, not just the brain(12), ranging from the heart, muscle, and kidney to metabolic, endocrine, and immune systems, as well as numerous neurological and cognitive functions(107, 166). Unfortunately, circadian- and sleep-related pathobiology is prevalent in modern society and often further influenced by diseases and chronic conditions. Sleep deficiency increases the risk of obesity, cardiovascular disease, and depression; and conversely, chronic diseases are associated with sleep deficiency. This is especially evident in the crosstalk between sleep and innate immunity, as sleep/wake disturbance causes heightened inflammatory responses(108). While it is well accepted that the SCN clock regulates the sleep/wake and rest/active cycles, much less is known about the converse relationship, that is, how sleep/wake disruption exerts adverse effects on the SCN clock to form a feed-forward dysregulation of sleep/wake homeostasis, which likely involves systemic endocrine, immune and inflammatory signaling(107).
While the importance of sleep is well recognized, we have a rather limited understanding of how sleep is regulated. A major task of sleep research is to identify the genes and pathways that regulate sleep(50, 111, 140, 240). Not surprisingly, almost all core clock genes play roles in regulating the timing of sleep(50, 240). For example, mutations in Per2, Per3, Cry1, and Cry2 cause either advanced or delayed sleep phase disorder. Advanced or delayed sleep phase disorders are common forms of insomnia caused by a misalignment between the body’s internal clock and the societal norm dictated by the light/dark cycle(279). In addition to timing, the circadian clock also regulates sleep homeostasis(50, 240). Disruption of core clock genes can cause attenuation of the sleep/wake rhythm. For example, Cry1/2-deficient mice display increased non-REM sleep drive(294) and Bmal1-deficient mice showed increased sleep fragmentation(141), indicative of a reduction of the sleep/wake rhythm. In addition to genetic factors, including mutations in the core clock genes, misalignment between the body’s internal clock and the external environment represents a common form of sleep disruption. Light-at-night (as experienced by shift-workers), high-fat diet, mistimed feeding, diabetes, and aging can all compromise physiological rhythms of sleep/wake cycles, core body temperature, plasma cortisol, and melatonin levels(64, 113, 279). Sleep timing, quantity, and quality are essential to health. Future research promises to shed light on the cellular and molecular mechanisms that underlie the mutual regulation of the circadian and sleep systems. Regimens to enhance circadian and sleep functions and improve human health and quality of life are likely to follow.
b. Digestive/Metabolic System
i. Gut/Microbiome
Recent advances have uncovered the relationship between the gut microbiome and the host circadian clock. The gut microbiome is a diverse community of commensal microorganisms in the gastrointestinal tract. The microbiome’s composition, concentration, and interaction with intestinal epithelial cells influence host homeostasis and disease(267). Gut microbiome composition is altered by diet and feeding times. Dysbiosis has been linked to obesity, inflammation, and weakened immune function(267). Circadian rhythms of the host and the host microbiota seem to have bidirectional interactions with one another, as dysfunction in host circadian genes disrupts rhythmicity in microbiota content(227), and the presence of important microbial metabolites directly affect rhythms in host liver function and metabolism(147). The gut microbiome can simultaneously influence host metabolism and lipid absorption through inducing rhythmic expression of histone deacetylase 3, which associates rhythmically with chromatin, leading to rhythmic expression of CD36, a lipid transport gene(135). This effect was associated with changes in lipid absorption and was linked to diet-induced obesity(135).
Thaiss et al. observed significant alterations in hepatic and intestinal gene oscillations in C57BL/6 background mice on 12:12 hour light/dark cycles, with dysbiosis induced by a mixture of vancomycin, ampicillin, kanamycin, and metronidazole, suggesting strong changes in liver and intestine circadian transcription upon disorganization of the gut microbiome(266). Conversely, host circadian rhythms also affected microbiome content and gene expression. The composition of the host microbiome exhibited diurnal rhythmicity which was disturbed in Per1/2-deficient mice. Although time-restricted feeding during the light phase corrected the rhythmicity of the microbiome, the antibiotic-treated mice retained their feeding rhythmicity; hence, the loss of feeding rhythmicity was ruled out as the cause of transcriptional changes(266).
Maki et al. found that sleep fragmentation impacted microbiota populations in Wistar-Kyoto rats, indicative of the circadian rhythms’ impact on the gut microbiome(164). When compared to control rats, rats with 8 hours of randomized sleep fragmentation had decreased levels of butyrate-producing bacteria, Firmicutes, and alpha diversity, indicative of induced dysbiosis; however, levels rose again after 20 days of chronic sleep fragmentation. Additionally, Proteobacteria levels were elevated in sleep fragmented rats when compared to controls, which is typically induced through a high-fat diet and is associated with disease states(164). Fecal samples collected from human patients analyzed by 16S ribosomal RNA gene amplicon sequencing linked altered microbial function to sleep-wake cycle shift, but there was little difference in microbiome composition(158). The samples were collected before and after one night of shifted sleep/wake cycle and two days after for recovery, and the effects of chronic sleep/wake cycle shift were not investigated. For a detailed review of the relationship between the gut microbiome and circadian rhythms, see Matenchuk et al.(168).
One mechanism with which the microbiome could influence the host circadian clock is through the intestinal transcription factor type-3 innate lymphoid cells-signal transducer and activator of transcription 3 (STAT3), which induces increased expression of Nfil3 in intestinal epithelial cells through REV-ERBα(289). The circadian transcription factor NFIL3 controls immune function. In intestinal cells, NFIL3 regulates lipid absorption and promotes lipid metabolism in the liver. Its expression rhythms are amplified by a healthy microbiome, and Nfil3 expression is reduced in germ-free mice. Further analysis of this pathway suggests that disturbed feeding times can decrease the function of the intestinal barrier and lead to dysbiosis via vasoactive intestinal peptide-producing neurons, which are activated during food consumption(264). Ku et al. found that C. sporogenes produced metabolites 3-(4-hydroxyphenyl) propionic acid and 3-phenylpropionic acid which increased the amplitude of PER2 and BMAL1 oscillations after their addition to Per2::LUCIFERASE knock-in and Bmal1-LUCIFERASE transgenic mouse embryonic fibroblast cells in vitro(134). Additionally, in C57BL/6J mice, the presence or absence of short-chain fatty acid butyrate caused changes in hepatic circadian rhythms independent of light cues, and hydrogen sulfide, another microbial metabolite, targets and suppresses Bmal1 expression in the liver(147). Microbiome produced short-chain fatty acid β-hydroxybutyrate has been identified as a blood pressure-regulating ligand in the kidney that attenuates hypertension, as exemplified in Dahl salt-sensitive rats, further linking the microbiome metabolites to circadian rhythm expressions(45). The connections between these metabolites and the host circadian clock are still unknown.
Cyanobacteria have a functioning circadian clock thanks to a trio of proteins, KaiA, KaiB and KaiC. Homologs of these proteins have been found in other bacterial species, but only Cyanobacteria contain all three – with KaiA seeming to be the key component for circadian cycling(114). Enterobacter aerogenes, a gut bacterium, exhibits circadian rhythms after removal from the host, but the circadian capabilities of other bacteria present in the microbiome (predominantly Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria) have not been adequately studied. Further research into bacterial circadian function within the host could help elucidate mechanisms of microbiota-host interactions(211). Additionally, methods by which to study interactions between the gut microbiome and circadian rhythms should be standardized, as timed feeding, dietary treatments, and sexes of the mice differed among experiments.
ii. Metabolism/Liver
Circadian rhythms in mammalian metabolism are well-established. The circadian clock controls a variety of physiologically critical hepatic functions in order to maintain homeostasis. Disruption of the clock’s function in the liver is linked to hepatic pathologies, including non-alcoholic fatty liver disease, alcohol-related liver disease, and hepatocarcinogenesis. Metabolic disorders such as obesity, type II diabetes, and cardiovascular disease are also more likely to occur when the internal circadian clock is chronically misaligned, as experienced during jet lag or shift work. In rodent studies, genetic perturbation of the circadian clock has been shown to exacerbate metabolic pathologies by influencing the transcriptional programs of genes involved in many pathways, including carbohydrate metabolism, lipid metabolism, and xenobiotic detoxification.
Carbohydrate metabolism occurs rhythmically and is intimately connected to the circadian clock. In chronic shift workers, circadian misalignment reduces glucose tolerance and insulin sensitivity(193). In male C57BL/6 mice, the key enzymes of glucose metabolism are dominant during the daytime, which is the rest phase for mice(290). Compared to their wild type counterparts kept in steady 24 hour light/dark cycles, chronic jet lag in male mice caused the overexpression of genes involved in glycolysis and glycogenolysis, while suppressing the expression of genes related to glycogen synthesis(126). In female mice under standard 24 hour light/dark conditions, BMAL1 was rhythmically recruited to the glycogen synthase 2 (Gys2) promoter and the glucose transporter 2 (Glut2) promoter, inducing their rhythmic expression(133). Ablation of Bmal1 abolished rhythmicity in the vast majority of carbohydrate metabolites in female mice kept under constant darkness(133) and eliminated the rhythmicity of fasting blood glucose and Glut2 expression in male mice kept at 12:12-hour light/dark cycle(14). Male mice on a 12:12 light/dark cycle with Bmal1 ablation specifically in pancreatic beta cells developed significant hyperglycemia, hypoinsulinemia, and impaired glucose tolerance compared to their wild type counterparts(214). CRY proteins, which are clock repressors, help direct carbohydrate metabolism by binding to the glucocorticoid receptor (GR) and acting on GR cistromes in male and female mice(40). In addition, the nuclear receptors REV-ERBα and REV-ERBβ bind GR with high frequency, and time-of-day dependent GR activity is dependent on REV-ERBα expression. Feeding patterns have also been shown to alter the expression of genes associated with carbohydrate metabolism. Male mice were fed under three different feeding paradigms: arrhythmic feeding, night-restricted feeding, and ad libitum. The rhythmic expression of glycogen phosphorylase (Pygl) revealed that catabolism and anabolism of glycogen were affected by rhythmic food intake, which was shown to drive the expression of rhythmic genes more potently in these mice than the cell-autonomous hepatic clock(87). When male mice were subjected to a restricted feeding treatment in which feeding was switched from the active phase to the rest phase, they developed hypoinsulinemia, which in turn initiated a metabolic reprogramming to increase levels of free fatty acids, glucagon, and glycogen synthase 3β activity(195). Increased levels of free fatty acid and glucagon activated peroxisome proliferator-activated receptor (PPAR) α and cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB), respectively. These changes in turn led to aberrant expression of Rev-Erbα, Per1, and Per2, shifting the rhythmicity of the peripheral clocks in the liver, muscle, and heart. These data reveal the role of the clock in regulating carbohydrate metabolism and the effects of disrupted feeding schedules on metabolic gene expression.
Many studies have demonstrated a significant role of the molecular clock in hepatic lipid metabolic processes. In mice, the key enzymes of lipid metabolism are dominant during the nighttime(290). Diet-induced obesity was shown to cause remodeling of circadian enhancer activity in the livers of male mice(89). Diet-induced obesity caused rhythmic expression of genes involved in de novo lipogenesis, including fatty acid synthase, acetyl coenzyme A carboxylase alpha, ATP citrate lyase, and elongation of very long chain fatty acids protein 5. Increased de novo lipogenesis was due to a high amplitude circadian rhythm of the lipogenic transcription factor, sterol regulatory element binding transcription factor 1, which triggered synchronous patterns of both fatty acid synthesis and oxidation. Diet-induced obesity also led to a high amplitude rhythm of PPARα, which is necessary for fatty acid oxidation. Pharmacological lipid-lowering was more effective at peak expression of PPARα under diet-induced obesity, pointing to a chronopharmacological approach in treating metabolic disease. In the absence of hepatic Bmal1 in male mice, m6A methylation of Pparα mRNA increased, which in turn decreased expression of Pparα and disrupted downstream lipid metabolism(319). The hormone ghrelin restored the rhythmicity of aberrantly expressed Clock and Per2 genes and their respective protein levels in diet-induced obesity in male mice with hepatic steatosis(287). Ghrelin restored the circadian rhythm in the liver through a mechanism dependent on mTOR signaling, which plays an important role in energy homeostasis. PPARγ, a regulator of adipogenesis and lipid metabolism, was cyclically recruited to chromatin in response to high-fat feeding in male C57BL/6 mice(197). The activation of PPARγ in turn led to transcriptional reprogramming in the liver to regulate lipogenesis. Furthermore, the offspring of obese female mice developed non-alcoholic fatty liver disease when fed a post-weaning obesogenic diet(194). These mice demonstrated hypermethylation at Bmal1 and Per2 promoter regions, which reduced Bmal1 expression and increased Per2 expression. Clock mRNA expression increased and Cry2 expression became arrhythmic. It is likely the misalignment of circadian transcription mechanisms contributed to the non-alcoholic fatty liver disease phenotype seen in obese mice. Bmal1 ablation in the liver caused both male and female mice to develop more severe liver steatosis and suppressed de novo lipogenesis and fatty acid oxidation pathways(310). The absence of hepatic Bmal1 in male mice reduced the levels of active cyclic adenosine monophosphate response element-binding protein H, which is required to maintain the circadian rhythmicity of genes involved in triglyceride and fatty acid metabolism(318). Liver-specific inactivation of Bmal1 increased expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein that promotes the degradation of the low-density lipoprotein receptor, and led to elevated plasma low-density lipoprotein/very low-density lipoprotein cholesterol levels(163). Further, hepatic lipogenesis induced by glucocorticoids was interrupted by loss of REV-ERBα(40). Mice with liver-specific depletion of nuclear receptors RORα and RORγ upregulated expression of lipogenic genes under fed conditions but not under fasting conditions at ZT22 and showed exacerbated hepatic steatosis(315). These studies indicate the significance of the circadian clock proteins in governing lipid metabolism.
The liver is the primary organ responsible for the metabolic pathways of ureagenesis and xenobiotic detoxification. These processes are directed via transcriptional mechanisms regulated by circadian proteins. It has been shown that CLOCK drives the circadian rhythm of ureagenesis through acetylation. Rhythmic CLOCK acetylation of argininosuccinate synthase 1, the rate-limiting enzyme of arginine biosynthesis, is facilitated by BMAL1 and inactivates the function of argininosuccinate synthase 1(153). Ablation of CLOCK/BMAL1 core clock components increased both ureagenesis and arginine biosynthesis in male mice. CLOCK and BMAL1 are also involved in regulating xenobiotic detoxification. CLOCK ablation sensitized male mice to coumarin and cyclophosphamide toxicity by downregulating the expression of CYP2A4/5 and upregulating CYP2B10 metabolism, respectively(317). The liver detoxification response also relies on input from the gut microbiota in order to maintain hepatic clock oscillations. Cyp3a11 and Cyp2b10, which encode a xenobiotic and a drug/steroid metabolizing enzyme, respectively, demonstrated very low hepatic expressions in germ-free male mice, with no or very dampened daily oscillations(186). The downregulation of genes related to xenobiotic detoxification indicates that germ-free mice may be more susceptible to oxidative stress. The pathway of bilirubin detoxification that occurs in the liver is also regulated by the core clock proteins. BMAL1 activates and generates the rhythmicity of UDP glucuronosyltransferase family 1 member A1 (Ugt1a1) and Multidrug resistance-associated protein 2 (Mrp2), the two genes primarily responsible for bilirubin metabolism(288). Bmal1 ablation in male mice abrogated the circadian time-dependent clearance of bilirubin and sensitized mice to hyperbilirubinemia. Bilirubin upregulated Bmal1 expression through antagonistic effects on Rev-Erbα, thereby inducing its own detoxification. Biliary excretion and clearance were found to be significantly higher during the dark phase of mice when Mrp2 expression is higher, and the rhythmic expression of Mrp2 disappeared in Per1/2 double KO mice(206). Furthermore, bile acid and xenobiotic metabolism were among the top deregulated pathways in the livers of wild type jet-lagged mice(126). Constitutive androstane receptor (CAR) is a liver tumor promoter that mediates toxic bile acid signaling. Loss of CAR inhibits non-alcoholic fatty liver disease-induced hepatocarcinogenesis. Chronic jet lag abolished BMAL1 binding to the xenobiotic receptor Car promoter, which dramatically increased binding of c-FOS and cyclic adenosine monophosphate response element-binding protein (CREB) to the Car promoter and strongly activated Car transcription. CAR activation is the basis for mutagen-induced hepatocarcinogenesis in mice. Taken together, these findings indicate the critical role of the circadian clock in regulating liver detoxification pathways and elucidate potential targets to prevent xenobiotic toxicity.
Irregular feeding schedules cause disruptions in metabolic pathways. Under arrhythmic feeding in which male mice were fed 1/8th of the daily food intake every 3 hours, the expression of lipogenic rate-limiting enzymes was strongly impaired(87). Mice lacking hepatic Rev-Erbα/β expression on an ad libitum diet showed reduced expression of genes involved in cholesterol metabolism and developed hypercholesteremia. Furthermore, mice with induced chronic jet lag experienced overexpression of genes promoting lipid synthesis and cholestasis(126). One potential method that has shown promise in relieving metabolic defects in animal models is time-restricted feeding, in which mice were given access to food for a 10 hour interval during their active period. Metabolic rhythms were lost in diet-induced obesity male mice but were sustained when diet-induced obesity mice were subjected to time-restricted feeding(44). However, the metabolic effects of Clock mutation in obese male mice fed a high fat diet were not rescued by time-restricted feeding, suggesting that other factors help to regulate metabolic homeostasis(177).
c. Cardiovascular System
i. Kidney
Various renal functions have been shown over the years to act in a circadian or diurnal manner. The renal excretion of sodium (Na+) and potassium (K+) exhibits a circadian rhythm(181). In humans, glomerular filtration rate peaks during the day and drops to its lowest in the night(130–132). Blood pressure in humans sees its greatest surge during the morning and drops to its lowest point at night during the sleep cycle(178). These physiological functions have been linked to the circadian molecular clock throughout different portions of the nephron(115). Circadian disruption, which may occur in shift workers or in those exposed to artificial light at night, is associated with increased incidence of chronic kidney disease(41).
Numerous studies have examined the influence of circadian rhythms in the different segments of the nephron. In the distal tubule, Susa et al. found that downstream targets of the With No Lysine (K) (WNK) signaling pathway to phosphorylate sodium chloride cotransporter (NCC), which include oxidative stress responsive kinase 1 and Ste20-related proline-alanine rich kinase, display circadian rhythms when examined in male mice(259). These rhythmic observations could be the result of a direct interaction with the circadian clock. Richards et al. demonstrated that mice lacking Per1 exhibit decreased expression of Slc12a3 (encoding NCC) and Wnk1 mRNA(224). These responses are linked to the abundance of PER1 and CLOCK on the promoter regions of these target proteins. The diurnal variation of phosphorylated NCC protein at Thr53 (pT53 NCC) is influenced by adrenal hormones, particularly glucocorticoids, as demonstrated by Ivy et al.(110). This also manifested a non-dipping blood pressure phenotype in these mice, demonstrating that glucocorticoids (corticosterone in mice) could influence blood pressure at different times of the day through the diurnal expression of phosphorylated NCC. Corticosterone also increases the mRNA expression of circadian clock genes Bmal1, Cry1, Per1, and Per2(109). In the collecting duct, our group found that Per1 mediates the ability of aldosterone to regulate α epithelial sodium channel (αENaC) expression(93). Follow-up studies examined the mechanism by which Per1 regulates αENaC in the mouse cortical collecting duct cell line, mpkCCDc14. DNA pull-down assays and ChIP experiments demonstrated the interaction of PER1 and the mineralocorticoid receptor with an E-box domain of the αENaC promoter(223). A study using an in vitro binding assay also found that PER1 and CLOCK, interact with the E-box response element in the αENaC promoter (90). Inhibiting casein kinase 1δ/ε in these cells reduce the abundance of PER1 located in the nucleus(222). As a result, PER1 and CLOCK are prevented from interacting with the E-box domain from the αENaC promoter, and αENaC expression and the number of channels are reduced. Clock KO mice exhibited impairments in water and Na+ excretion at different times of the day(322).
Johnston et al. determined that male rats deficient in functional endothelin B receptor have a delayed natriuretic response to an acute salt load that is dependent on the time of day the salt load is given(117). Furthermore, female endothelin B receptor-deficient rats are more efficient than males in excreting excess salt, suggesting a sex difference in the endothelin B receptor-dependent diurnal natriuretic response to a salt load. A high salt load also caused a 5.5 hour phase delay in Bmal1 expression in the inner medulla of control rats, unlike endothelin B receptor-deficient rats(252). These data suggest an effect on the phase of renal BMAL1 that is endothelin-1-dependent.
The regulation of blood pressure by BMAL1 has been described by many groups. Nikolaeva et al. demonstrated that an inducible tubule-specific BMAL1 KO mouse had lower blood pressure than controls despite similar solute excretion and glomerular filtration rates(201). Zhang et al. showed that male collecting duct-specific Bmal1 KO mice had lower blood pressure compared to control mice, however, this difference was not seen in female mice (309). A study by Johnston et al., utilized the Bmal1 KO rat, the first known whole-body clock gene knockout in a rat(116). In the renal cortex, male Bmal1 KO rats lost the diurnal variation in gene expression for Per1, Per2, Cry2, Rev-Erbα, and Dbp that was seen in wild type rats. Similar results were observed in the outer and inner medulla. Male Bmal1 KO rats lost the diurnal variation in urinary Na+ excretion observed in wild type rats. However, in females, the diurnal pattern of urinary Na+ excretion was conserved in both control and Bmal1 KO rats. Despite this loss of the time of day difference in urinary Na+ excretion in male Bmal1 KO rats, they maintained a diurnal variation in mean arterial pressure similar to control rats. Similarly, mice absent of BMAL1 within the distal segments of the nephron displayed sex-specific blood pressure and solute handling, with male KO mice having lower blood pressure and less Na+ retention compared to controls, a difference not seen in female mice(56). These studies also reinforced the idea of a more robust process of Na+ handling in females, and suggest a mechanism of Na+ excretion regulation that is independent of BMAL1. Future studies are warranted from this novel rat model.
Microarray studies have explored the influence of circadian clock physiology in the kidney. In a mouse renal inner medullary collecting duct cell line, our group demonstrated that genes such as Per1 and Per2 were upregulated following aldosterone treatment. Per1 saw its highest level of aldosterone-induced upregulation after 1 hour of treatment with a greater than seven-fold increase in expression, providing evidence of the molecular clock being transcriptionally responsive to aldosterone(91). In another study, RNA was examined in mouse kidneys collected every 4 hours starting at ZT0. Zuber et al. used male C57BL/6J mice to demonstrate that 356 transcripts in the distal convoluted tubule/connecting tubule and 504 transcripts in the cortical collecting duct being identified as circadian transcripts when RNA was measured in kidneys collected every 4 hours starting at ZT0(322). Transcripts expressed in both of these tubular regions included the core clock genes Bmal1, Clock, Per2/3, and Cry1/2, along with Dbp, Npas2 and Nr1d1/2. The presence of these transcripts, along with genes involved in water and electrolyte homeostasis in these nephron segments, gave credence to the ability of the renal molecular clock to regulate the circadian variation in renal tubular function.
Nikolaeva et al. performed transcriptome profiling of RNA samples from whole kidneys of wild type and Bmal1lox/lox/Pax8-rtTA/LC1 conditional KO mice(201). Transcripts that were differentially expressed when measured at ZT4 and ZT16 were linked to carboxylic acid metabolism, organic anion transport, and chemical homeostasis. Furthermore, these Bmal1 conditional KO mice displayed altered renal metabolism. BMAL1 appears to also play a role in podocyte development. A number of genes involved in podocyte development were affected by knocking out Bmal1 in the podocyte cells of mice including Transcription factor 21, N-Ethylmaleimide Sensitive Factor, G Protein Subunit Alpha 12, Cathepsin L, sulfatase 2, and G protein-coupled receptor class C, group 5, member A(18). Changes in expression of circadian clock genes Cry1, Rora, Rorc, and Npas2 were also seen. These changes in mRNA expression were associated with higher levels of creatinine excretion during the inactive period in Bmal1 conditional KO mice.
Ivy et al. shed significant light on how glucocorticoids may contribute to the regulation of rhythms in renal Na+ handling. In C57BL6J/O1a mice, clamping plasma corticosterone at ~200 nmol/L abolished the diurnal variation in pT53 NCC(110). This was due to an elevation of pT53 NCC during the inactive period. A similar result was seen in these mice after adrenalectomy, although the blunted diurnal variation in pNCC occurred due to decreased levels at night. These results were associated with non-dipping blood pressure, with systolic blood pressure increasing during the inactive period and diastolic blood pressure remaining elevated during both active and inactive periods. Hydrochlorothiazide reversed the elevation in systolic blood pressure and diastolic blood pressure during the inactive period. This study showed that glucocorticoids could influence blood pressure at different times of the day through the diurnal expression of phosphorylated NCC. These authors followed up on this result by examining GR, through which glucocorticoids regulate NCC(109). After performing adrenalectomy on male C57BL6J/O1a mice, intraperitoneal injection of corticosterone induced an increase in pNCC protein levels. This increase was observed along with an increase in the mRNA expression of serum- and glucocorticoid-inducible kinase 1, glucocorticoid-induced leucine zipper protein, which encodes for glucocorticoid-induced leucine zipper protein, and the circadian clock genes Bmal1, Cry1, Per1, and Per2. In mice with intact adrenal glands, spironolactone (a mineralocorticoid receptor antagonist) decreased total NCC protein while RU486 (a GR antagonist) had no significant effect. Chronic RU486 treatment blunted the diurnal variation in pT53 NCC and altered the diurnal mRNA expression of Per1 and Nr1d1, while leaving other clock genes unchanged. The diurnal pattern of pT53 NCC was blunted even in GR heterozygous null mice on a C57BL/6J background. This study showed the importance of both the mineralocorticoid receptor to NCC abundance and GR to NCC activity and its diurnal variation.
ii. Heart and Vasculature
Insomnia and shift work are both independent risk factors for the development of acute myocardial infarction(142, 280), with the latter explicitly influencing circadian regulation of transcriptome in peripheral blood mononuclear cells samples from female nurses at a large university hospital(220). These phenomena are likely contributed to by tissue-specific effects of circadian machinery on the heart itself, which in mice is known to be highly divergent in diurnal regulatory patterns compared to other large organs(254, 299).
Given the contribution of the autonomic system to heart rate and function, it is unsurprising that the CNS contributes a great deal to cardiac disease. Sympathovagal balance, in particular, is heavily influenced by circadian changes. These variations are associated with a particular Per3 polymorphism that results in heart rate variation equivalent to sleep deprivation in clustered patients(277). A similar network alteration in circadian synchronicity is likely a significant contributor to chronic atrial arrhythmias, according to a study that examined the molecular clock in primary atrial tissue from patients undergoing valve repair or replacement (66). Consistent with these results is the link between susceptibility to ventricular arrhythmia in mice and alterations in the circadian core clock. Rhythmic control of clock-dependent oscillator Krüppel-like factor 15 leads to changes in potassium-channel transcriptional programming, with either an increase or decrease in transcription factor activity resulting in a predisposition to cardiac arrhythmogenesis(229). Additionally, cardiomyocyte-specific deficiency of Krüppel-like factor 15 results in circadian clock disruption, suggesting a feedforward mechanism of regulating expression(313). The upstream effects of Krüppel-like factor 15 may, in part, explain why inducible cardiomyocyte-specific deletion of BMAL1 leads to increased arrhythmia secondary to electromechanical stimulus, via a Na+ channel-dependent depolarization effect(238). Redundancy in the circadian core clock is likewise demonstrated by the fact that a similar model of CLOCK-deficiency in the myocardial tissue results in profound bradycardia and mitochondrial fragmentation, with transcriptional variation of > 10% from normal(31). From these studies, we conclude that electrical syncytial activity of the heart depends both directly and indirectly upon the appropriate functioning of the central and peripheral clock.
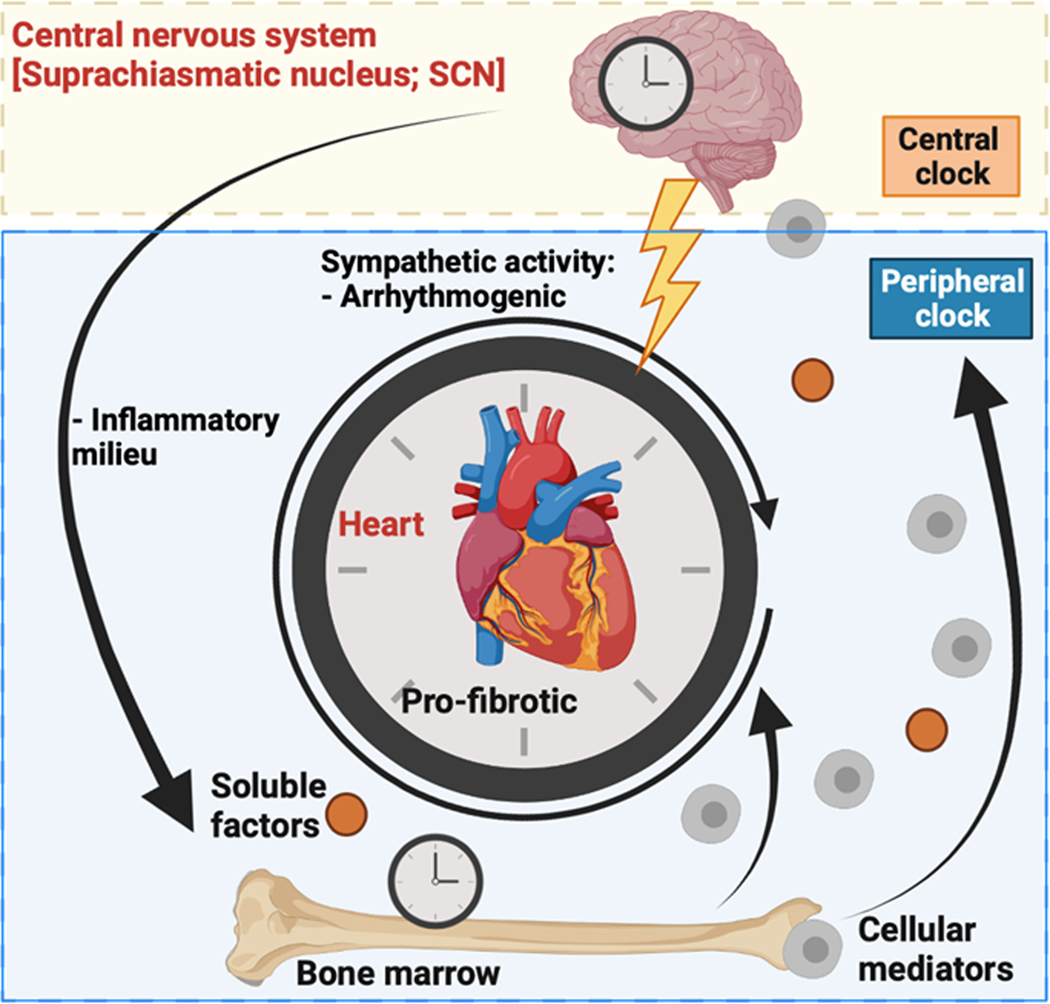
Mice globally deficient in BMAL1 die of dilated cardiomyopathy at a young age(145). Interestingly, myeloid-cell deletion of the core transcription factor alone is protective against atherosclerotic lesion formation in male and female hyperlipidemic mice(301), but is surprisingly also associated with recruitment of pro-inflammatory cellular intermediaries to the site of atherosclerosis. The mechanism for the underlying myeloid contribution to cardiac disease may be explained through the role that BMAL1 plays in neutrophil “aging” specifically, with increased chemokine receptor expression mediated throughout the roughly 24-hour lifetime of the leukocyte(5). Surprisingly, BMAL1-mediated changes in neutrophilic expression patterns (“aging”) boosted antimicrobial activity of the myeloid-derived cells, while persistence in the vascular compartment of these neutrophils promoted proliferation of a sterile thrombo-inflammatory insult, myocardial infarction, and death. These findings do not rule out a simultaneous central contribution to immune-mediated atherosclerosis, as one group demonstrated that with sleep fragmentation alone, the neuro-immune axis effects worsened vascular and cardiac injury, including heart attacks(171). Questions remain over how circadian molecular machinery coordinates this axis of signaling in chronic inflammatory states, and whether restoration of rhythmicity, either centrally or in the heart itself, will improve disease outcomes. A putative model for integration among these systems is shown in Figure 4.
Figure 4.
Model of neuro-immune axis signaling contributing to cardiovascular disease through clock-mediated signaling pathways. Soluble factors and cellular mediators contribute to the inflammatory milieu leading to pro-fibrotic signaling in the heart. Together with arrhythmogenesis due to sympathetic activity, these conditions may contribute to cardiovascular disease. Diagram was created using Biorender.com.
Of course, homing of myeloid cells to the site of vascular injury requires coordinated signaling involving many cell types, most prominently endothelial cells. Particularly, the trafficking of leukocytes to sites of vascular injury is dependent on BMAL1 expression by the affected microvascular endothelial cells(100). Likewise, trafficking of endothelial progenitor cells from the bone marrow to sites of vascular inflammation is circadian in function, with disruption in either circadian-mediated bone marrow release or homing of cells, leading to an increased risk of cardiovascular events(7). Furthermore, there is a known cell autonomous effect of circadian disruption on vascular health, with the transcriptional regulation and function of endothelial nitric oxide synthase independently contributing to vascular tone and, among other changes, hypertension(16, 230). Specifically, at a cellular level, endothelial cell loss of BMAL1 expression aggravates endothelial-to-mesenchymal transition, a predisposing factor in the promotion of atherosclerotic lesions(321), related primarily to increased oxidative stress. The same is true for CLOCK(265), influencing metabolic regulation of cells through the primordial regulator AKT protein kinase B(161), although no explicit link between the pathways in human disease has been described to date. Interestingly, circadian variability in endothelial cell function from patients with obstructive sleep apnea (a breathing disorder associated with transient hypoxemia in addition to disruption of sleep, and a common cause of increased blood pressure in the lungs, or pulmonary hypertension), is known to be linked to circadian biology via depressed melatonin levels in patients(312), where treatment with supplements decreases inflammatory milieu in the lung and subsequent disease.
Ultimately, as a common outcome, manipulation of the vascular endothelium transcriptome through clock disruption can lead to a vascularly senescent profile(284). Such “age-dependent” changes are prominently noted in the impaired rhythmicity of vascular smooth muscle cells (137), especially through alterations in Clock transcriptional activity(105). Likewise, the cell-specific effects of BMAL1 deletion are suggested by one study in which depletion of BMAL1 in smooth muscle cells led to protection against the development of abdominal aortic aneurysm via changes in the matrix remodeling protein profile(162), which is known to be exquisitely regulated by tight circadian control under homeostatic conditions(46). Similarly, ligand-activated transcription factor PPARγ, known to play a major role in vascular health via regulation of metabolic fitness, is a major drug target for diabetes and associated vascular complications in vascular smooth muscle cells. Interestingly, PPAR-expression functions directly under the control of the circadian core clock in male and female mice(95), suggesting a further refined cell autonomous link with cellular metabolism. Disruption of vascular rhythmicity of PPAR signaling in smooth muscle cells can thus result in large and small vessel disease(48, 286), affecting cardiac tissue and heart function. Similar changes have been documented in global PPARγ KO mice(300).
iii. Lung
Like the cardiovascular system, to which it is intimately intertwined, metabolic cues affect the cells in the pulmonary circulation and parenchyma. BMAL1 expression by alveolar epithelial cells mediates metabolism responses to extracellular matrix remodeling and chemokine signaling in a coordinated effort to maintain lung health after sterile and non-sterile injury(316). Accordingly, light/dark oscillations are known to tightly regulate the lung transcriptome(256), especially in relation to the lung repair apparatus. REV-ERBα in mesenchymal cells (myofibroblasts) was found to be a viable drug target in the bleomycin-induced lung fibrosis model, with supporting clinical translational data in patients with the disease(58). Of course, a common risk factor for cardiac, vascular, and lung disease is tobacco smoke exposure. Tobacco, and constituent chemicals involved in smoking and smokeless forms, dramatically alters the lung transcriptome(196), influencing the circadian profile in particular(84, 250), as reviewed recently(257, 258).
Alterations in the mouse Clock gene can also lead to drastically altered static and dynamic lung physiology in males and females(94). However, far from supporting the hypothesis that these data are due to intrinsic lung pathobiology, there is evidence that pulmonary diurnal patterns are more prominent in, and due to, infiltrating myeloid cells in the lung, specifically neutrophils in male and female mice(42). Especially under stress conditions such as lipopolysaccharide (LPS) infusion, mice display a large disruption in circadian rhythm due in large part to myeloid cells homing to sites of injury(98). Of course, epithelial cells also display a chrono-inflammatory profile during lung injury; related primary lung cells appear to affect their microenvironment in a paracrine/endocrine fashion by releasing extracellular vesicles in response to injury(85). These extracellular vesicles from the lung appear to then remotely influence the activation and release of myeloid cells from the bone marrow, a potentially pathologic coordinated effort between the lung and hematopoietic systems that is circadian in origin(67). The pulmonary-immune axis of signaling thus serves as a viable area of study to better understand the role of circadian rhythmic function in myeloid-derived cells(205).
d. Musculoskeletal System
i. Muscle
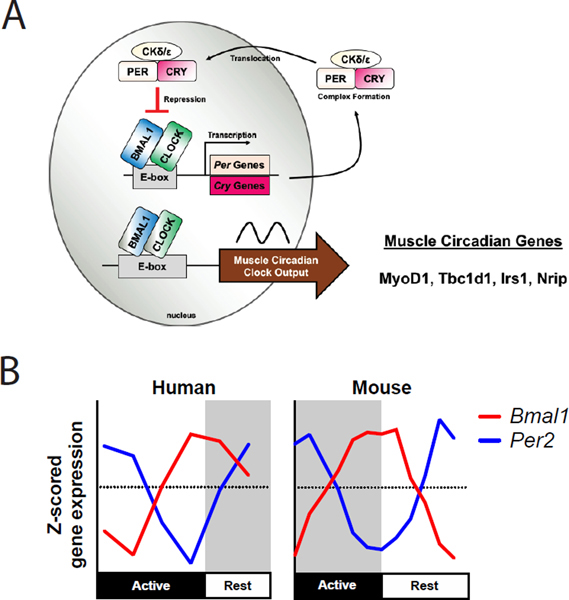
Our early understanding of the circadian clock in skeletal muscle came from studies of the circadian transcriptome in the muscle of C57BL/6J mice(179). The results of this first study confirmed that the core circadian genes were expressed in muscle and the pattern was similar to that found in the liver. In addition, while the core clock genes were in common between the muscle and liver circadian transcriptome, the rest of the circadian transcriptomes were unique to the tissue type. The next set of circadian transcriptome studies defined the circadian genes across different muscles as well as changes that occur with the muscle-specific loss of the core clock gene, Bmal1(74, 103, 172, 217). The three muscles, soleus, tibialis anterior, and gastrocnemius, represent different fiber types as well as different mechanical environments. The soleus muscle is predominantly an oxidative muscle with Type I and IIA fibers. The tibialis anterior and gastrocnemius muscles are predominantly fast Type IIB fibers, but they perform different mechanical functions in the hindlimb of female mice (35). Using MetaCycle(295) we identified 1315, 1957, and 1895 genes that were cycling in tibialis anterior, soleus, and gastrocnemius, muscles respectively. The three muscles shared 197 cycling genes (about 11%) and these were primarily circadian clock genes, the myogenic regulatory factor, Myogenic Differentiation 1 (MyoD1), and genes involved in metabolic responses including TBC1 domain family, member 1 (Tbc1d1), insulin receptor substrate 1 (Irs1) and Nuclear receptor-interacting protein 1 (Nrip) (Figure 5A). Analysis of muscles from the muscle-specific Bmal1 KO mice confirmed that the endogenous muscle clock is required for much of the circadian transcriptome in muscle(74, 237). These datasets have also helped with the identification of genes downstream from the muscle clock that are important for muscle metabolism and function such as Glut4 and Myod1. More recently, new studies have shown that the interaction of the BMAL1:CLOCK heterodimer with hypoxia-inducible factor-α in muscle is important for modulating the anaerobic glycolysis pathways(212). Thus, there are several studies that have demonstrated the importance of the muscle clock for different aspects of muscle metabolism.
Figure 5.
Skeletal Muscle Circadian Clock Signaling. A. The transcription-translation feedback loop in muscle regulates muscle specific genes including MyoD1. B. Despite differencs in diurnality in humans and nocturnality in mice, the expression patterns of Bmal1 and Per2 are quite similar between these two species.
Skeletal muscle also provides a fairly unique tissue in that it can be repeatedly biopsied over a 24 hour period from human subjects. This provides an outstanding opportunity to compare the circadian transcriptome between the diurnal human and nocturnal rodent muscle. Perrin et al. performed a genome-wide transcriptome analysis from vastus lateralis biopsies collected every 4 hours from ten healthy volunteers(216). They identified over 5000 pre mRNAs that exhibited a significant circadian oscillation which is about 50% of the total genes expressed in human muscle. Further analysis of the mature mRNAs determined that over 1000 mRNAs exhibit a circadian pattern. The difference between the numbers of pre-mRNA vs. mRNA likely reflects the influence of half-life; with those mRNAs with a longer half-life being less likely to exhibit a daily oscillation in levels. A direct comparison of the human oscillating mRNAs with previously published circadian gene expression datasets of mouse skeletal muscle(74, 237) revealed 107 common circadian oscillating genes between mouse and human skeletal muscle. These include components of the core clock (Figure 5B) as well as MyoD1 and some of the metabolic genes. Analysis of the phase of the core clock genes in human muscle compared to mouse muscle demonstrate that the phase of the clocks is aligned in reference to the sleep/wake cycles of the day, a key finding for establishing the translational framework for the use of genetic models of circadian disruption to study muscle diseases.
The importance of the muscle clock in metabolism and function has been demonstrated by preclinical research. The use of two different genetic mouse models for circadian rhythms, the Bmal1 KO mice (e.g. arrhythmic behavior) and the ClockΔ19 mice (e.g. long period length under constant conditions) provided some early insight into the role of the circadian clock and muscle phenotype in both sexes (34, 278). These studies demonstrated that muscle weakness and mitochondrial deficits were two muscle pathologies common to both genetic mouse models(15). Since that study, different groups have generated skeletal muscle-specific knockout of the core clock gene Bmal1(74, 103, 212). These studies targeted the loss of Bmal1 because it is the only non-redundant gene within the core clock. The most common outcome across slightly different models of muscle-specific Bmal1 KO is the robust change in glucose metabolism. This has been shown by glucose intolerance through a larger area under the curve in response to glucose tolerance tests, as well as through metabolomic and biochemical analysis of different skeletal muscles in males and females(74, 97, 103). Specifically, muscle-specific Bmal1 KO leads to reductions in the mRNA and protein levels of GLUT4 and TBC1D1, glucose transporter in skeletal muscle, and the translocator of GLUT4-containing vesicles, respectively. In addition, the decrease in Bmal1 in muscle causes a downregulation of the key enzymes for the use of glucose, such as hexokinase 2 and pyruvate dehydrogenase(74, 97, 103). The skeletal muscle-specific Bmal1 KO mice display normal circadian behavior under free-running conditions, unlike the global Bmal1 KO, suggesting that the changes observed in metabolism are due to effects downstream of the muscle circadian clock. These results indicate that the molecular clock generates the circadian rhythm of carbohydrate metabolism in skeletal muscle and that its disruption leads to metabolic dysfunction in skeletal muscle. While these studies have been important as foundational studies in the muscle circadian field, it is still fairly early and we propose that it is likely that many other aspects of muscle homeostasis are modulated by the intrinsic muscle clock mechanism.
ii. Bone
Circadian rhythm plays an integral role in bone development and degradation. Maintaining homeostatic conditions throughout circadian rhythm cycles can prevent metabolic issues, as well as prevent bone conditions such as osteoporosis. Bone tissue consists mainly of osteoclasts, osteocytes, and osteoblasts. These components collaborate and communicate with each other to degrade and build bone through receptor activator of NF-κB ligand (RANKL)(235). RANKL directly triggers bone resorption when it binds to osteoclasts, while osteoprotegerin (OPG) binds to RANKL to prevent bone resorption when osteoblasts secrete the hormone. By shifting light/dark cycles and adjusting circadian rhythm, Schilperoort et al. were able to determine the differences in genes related to bone function and turnover. Mice that lacked CLOCK or BMAL1 showed reduced bone mass, while double knockout mice lacking PER1/2 or CRY1/2 showed increased bone mass. There is also evidence that disruption of GR could contribute to disruptions in bone development. Precursor cells to cartilage tissue that reside in the bone marrow, called mesenchymal cells, are also thought to exhibit effects on the long bones of mice(269). BMAL1 deletion in mesenchymal cells resulted in low bone mass, reduced trabecular bone mineral density, and reduced cortical bone mineral density. However, diverging views on the reasons for changes in bone mass and how BMAL1 and other circadian genes can directly or indirectly regulate osteoclasts and osteoblast activity remain between studies.
Another factor that should be considered in tandem with circadian patterns in muscle cells is the coupling between skeletal muscle and bone. Human physical activity mostly follows a circadian rhythm, and mechanical movement and force placed on bone play a role in bone formation and development(228). Considering this when looking at BMAL1 KO populations is important and poses another instance where germline muscle-specific Bmal1 KO mice exhibited similar phenotypic features as global Bmal1 KO; however, the mice with muscle KO experienced higher calcification rates and increased skeletal growth(237). Overall, we understand that disrupting circadian rhythm and the genes that constitute reactions to light/dark cycles negatively affect homeostatic bone health and growth.
Different types of facial dysmorphisms and conditions such as skeletal mandibular hypoplasia can cause blockage of airways, immune deficiencies, as well as delayed developmental growth within bone structure(320). BMAL1 has a significant impact on osteogenic differentiation, osteoclast differentiation, and bone development in stages of embryonic as well as postnatal development. Focusing on mandibular condyle cartilage and surrounding craniofacial tissues, results from Yu et al. revealed that Bmal1 KO mice displayed thinner proliferative layer zones as well as hypertrophic layer zones; not only were these measures of bone development lower, the KO mice had overall smaller mandibular condyles as well as a lower chondrocyte count compared to the normal mice population(306). Additional evidence indicating how important the role of clock genes is within bone development can be found in the Hedgehog pathway. This pathway plays vital roles in cartilage production and chondrocyte differentiation. BMAL1 regulates this pathway through downregulating signals and binding to promoters that belong to Hedgehog activators like Indian hedgehog homolog and protein patched homolog 1(306). In correlation to the transcriptional binding abilities of BMAL1, it also interacts with genes that maintain bone homeostasis, such as OPG and RANKL(320). Much like in cases of BMAL1 deficient skeletal mandibular hypoplasia, dysrhythmia in the mice samples expressed lower levels of BMAL1 and OPG expression. Other clock genes like Per1, Per2, and Cry2 also expressed lower rates of expression in skeletal mandibular hypoplasia tissues. The mice on jet lag light/dark schedules had smaller mandibles, smaller trabecular thickness, and lower osteoblast counts.
Due to the ability of BMAL1 to bind to these promoter sites and regulate their means of transcription, we can use the information gathered on its effects on mandibular development to possibly begin curating gene therapies to treat craniofacial deformities. In alignment with these studies, it was found that BMAL1 deficiencies could be reversed during pre-puberty and puberty stages by injecting Hedgehog pathway activators Indian hedgehog homolog and protein patched homolog 1 into mandibular condylar cartilages(306). Similarly, deficiencies of BMAL1 were reversed through intraperitoneal injection of OPG into the jaw(320). These rising therapeutics shine light on possible methodologies to prevent jaw deformity and bone conditions such as osteoporosis.
Bone is formed and regulated through hormones and growth factors that induce osteoblasts to form bone and osteoclasts to resorb bone. The processes of bone formation and resorption are controlled in homeostatic conditions, and imbalances can cause diseases and disorders such as osteoporosis(263). Limited studies have been conducted on the effects of circadian rhythm genes on bone resorption, but we have evidence that connects CLOCK and BMAL1 to regulatory elements and genes within this process. Because bone resorption primarily resides in osteoclastogenic cells, BMAL1 osteoclast KO mice exemplified high bone mass and decreased differentiation. Osteoclast numbers decreased, and the femurs of mice showed significant increases in mass as well as increases trabecular thickness, indicating that BMAL1 plays a large role in osteoclasts’ ability to resorb bone(297). Taking a closer look at the mice, their calcium metabolism did not change overall due to Bmal1 deletion, showing that BMAL1 controls bone remodeling independent of its central clock expression. In particular, this study found that BMAL1 regulated osteoclastic marker and gene nuclear factor of activated T cells 1 (Nfatc1) through binding Nfatc1 promoter as a heterodimer complex with CLOCK, while proto-oncogene tyrosine-protein kinase SRC protein interacts with the complex as a coactivator. Transcriptional factors within osteoclast cells as seen in this study heavily influence osteoclastogenesis, but there is also evidence that factors within osteoblasts also affect bone resorption. BMAL1 in osteoblasts was found to regulate bone resorption through influencing RANKL-OPG expression; therefore influencing osteoclastogenesis through osteoblasts(263). Many more studies are needed to gain full understanding of these mechanisms, but these findings show that circadian genes certainly play integral roles in bone formation and degradation.
e. Immune System
The immune system is the body’s multifaceted defense mechanism against foreign agents. It is functionally divided into the innate and adaptive branches. The role of the core circadian clock in the immune system has been well described to date(59, 165, 234). We know circadian disruption in ICU patients is associated with increased severity of sepsis(52, 61). Interruption of the clock functioning through permuted sleep disturbance disrupts the transcriptome of human blood samples(185), related primarily to dysregulation of oxidative stress and metabolic pathways, particularly in individuals that perform night shift work(125). This is in line with the fact that blood transcriptome, more than any other peripheral tissue, oscillates in a tight circadian fashion during homeostasis(138). Consistent with its role in tissue-resident macrophage reprogramming in this time-of-day dependent manner, pathogen recognition and cytokine secretion (especially those associated with senescence, such as tumor necrosis factor-α and IL-6 varies widely over the course of time to influence the robustness of pathogen detection response(123). Research has also shown that antibody production is increased in response to influenza vaccination when administered in the morning compared to the afternoon in adults over 65 years old(160).
Adaptive Immune System: Circadian clock function has been well described in directly regulating elements of adaptive immune cells, such as lymphocyte trafficking to and from lymph nodes(71). In particular, the diurnal control of CD8+ T cells has been demonstrated to modulate early response to vaccination with higher levels of metabolic activity (mTOR and AKT protein kinase B activity, especially) in vaccines given midday in a murine model(203). This enhanced vaccination response is due to, in large part, changes in Toll-like receptor 9 expression throughout the day(244). Such effects appear to have a potential epigenetic effect secondary to alteration in the light/dark cycle, as transgenerational changes in the immune response have been noted with even dim light at night exposure(51). T cell variation in daily rhythm has been well demonstrated in healthy volunteers, with direct potential benefit to the utilization of life-saving therapies such as organ transplant relegation(128). To this end, it is worth noting that CD4+ T cells in both mice and humans are regulated by intrinsic cellular circadian oscillators, capable of driving complex interactions between activating and inhibitory functions(27). Thus, much work remains in informing how chronotherapy can best be utilized to balance the role of infection versus acute rejection in organ recipients.
The migratory patterns of all immune cells investigated to date show a circadian rhythm, including lymphocytes. In studies with mice entrained on a 12-hour light/dark cycle, lymphocytes were shown to be present in the lymph nodes at the highest concentration during their active phase (ZT13) and lowest at ZT5 out of the 6 time points tested(72). This migration pattern coincided with peak C-C chemokine receptor 7 (CCR7) expression. The pattern was lost in KO mouse models for both CCR7 as well as BMAL1. Along these same lines, a study with mice in total darkness following entrainment of 12-hour light/dark cycle demonstrated the greatest T-cell response following vaccination at CT6 compared to the lowest at CT18 from the 4 time points assessed(203). This indicates T-cell reaction to immune challenge via vaccination is truly a circadian process. These data taken together provide a possible explanation for temporal variation in the immune response to both vaccination and infection.
Autoimmunity is a dysfunction of the immune system, and disruption of circadian rhythms in leukocytes is associated with the development of autoimmunity in mice. T-helper 17 (Th17) cell overexpression is associated with autoimmune disorders. REV-ERBα expression in Th17 cells inhibits the development of these cells, performing a protective role against the development of experimental autoimmune encephalomyelitis in both male and female mice(11). Similarly, BMAL1 appears to be protective against T-cell based autoimmune attacks against the CNS. The CNS shows an increase in pathogenic T cell concentration following myeloid cell-specific KO of Bmal1 in mice(260). Loss of both CRY1 and CRY2 in mice leads to the development of an autoimmune phenotype with an increased number of mature B cells, indicative of overactivation of the B cell receptor pathway(37). Further exploration of core clock genes can provide important insights into the development of autoimmunity.
It should be noted that conditional KO of Bmal1 in lymphocytes disrupted rhythms of a reporter gene in those cells, but did not affect T-cell or B-cell function (101). The time-dependent increase in IL-2 upon bacterial infection was not changed by Bmal1 KO in lymphocytes, suggesting that the cell-intrinsic clock may not be required for the adaptive immune response. In contrast, cell-specific Bmal1 KO does alter the innate immune response, as discussed below.
Innate Immune System:
Much of recent research into the circadian clock in innate immune cells has focused on BMAL1 expression in mouse models. The loss of Bmal1 in myeloid cells of male mice was shown to increase lung inflammation, as well as the presence of eosinophils and IL-5, all features associated with asthma(307). In macrophages, BMAL1 has been shown to activate nuclear factor erythroid 2-related factor 2 expression, thereby inhibiting expression of inflammatory cytokines IL-1β and IL-6(75). Neutrophils also show a significant phenotypic change following loss of BMAL1. Bmal1 neutrophil-specific KO in male mice appears to inhibit the aging process, tipping the balance in favor of immature neutrophils which primarily target inflamed tissues(4). The overall purpose of BMAL1 within these innate immune cells appears to be regulation of the inflammatory response.
The injury response of the innate immune system is complicated by microenvironmental interactions with vascular endothelial cells. These interactions are regulated, in part, through myeloid-derived C-C motif chemokine 2 (CCL2), which influences leukocyte adhesion and atherogenic plaque formation in a circadian manner(292). Consistent with this mechanism, prior literature details the role of BMAL1 on Ly6Chi cell recruitment in a model of coronary disease(200). Included in these attractant signaling pathways is a direct influence on the senescence profile of the bone marrow, where Bmal1 deletion in myeloid cells specifically (utilizing the myelomonocytic cell-specific Lysozyme M (LysM) Cre-recombinase model) led to a dramatic increase in immune checkpoint programmed cell death-ligand 1 (PD-L1) expression through intermediate STAT1 activation(63). Once again, this is accomplished primarily through downstream metabolic effects on glucose utilization and lactate production in a model of severe sepsis. In a similar model, using LPS-infusion of mice, there was an associated increase in hypoxia-inducible factor stabilization due to reactive oxygen species generation in tissue, ultimately resulting in elevated circulating levels of IL-1β, and worsened outcomes(75). The circadian core clock exerts heavy influence over even the suppressive elements of the innate immune system, especially myeloid-derived suppressor cell (MDSC) response to tissue injury in linkage to adaptive immunity(215), illustrating further the complexity of leukocyte response over time.
Looking beyond BMAL1, the clock proteins DBP, NFIL3, and REV-ERBα all appear to have a role in the control of inflammation. NFIL3 and DBP show opposing action in their control of the Il12b gene, which encodes IL-12p40, a cytokine responsible for recruiting dendritic cells and macrophages to regions of inflammation(9). NFIL3 is a negative regulator of Il12b expression and Bmal1 KO in macrophages resulted in increased expression of NFIL3 and decreased expression of DBP. REV-ERBα appears to have a role in controlling neural inflammation, as Rev-Erbα KO mice showed a higher basal level of inflammation(88). At a transcriptional level, this loss in REV-ERBα appears to affect the NF-κB pathway, and REV-ERBα was shown to interact with the promoter regions of genes within this pathway. The performance of the immune system is obviously reliant on a functional circadian clock; however, further exploration into the transcriptional control mechanisms of circadian clock genes on immune function is needed.
Sepsis:
Circadian disruption has detrimental effects on the survival rates of patients with sepsis. In ICU patients, early signs of circadian disruption, determined by shifted cortisol rhythms, were associated with the development of sepsis. Patients with sepsis expressed higher levels of cortisol and had higher numbers of neutrophils and monocytes circulating in their blood(52). The mechanism of circadian control of the immune system in response to sepsis has been explored in mice. KO of Bmal1 in myeloid cells of male and female mice resulted in increased expression of PD-L1 as well as increased T-cell death thereby limiting adaptive immune response to sepsis(63). BMAL1 appears to regulate PD-L1 through transcriptional control of the Pkm2 gene which is an activator of PD-L1. For male mice with Clock KO, the survival rates of sepsis, instigated by cecal ligation and puncture, were significantly improved. Clock KO mice had lower concentrations of bacteria in their bloodstream as well as lower expression of inflammatory cytokines(283). Based on these data, maintaining circadian function in ICU patients is a possible preemptive measure to be explored for reducing the occurrence and severity of sepsis. Melatonin has been studied as a treatment in the ICU to aid with sleep and circadian disruption, but its effectiveness remains unclear(149).
Viral Infection:
The presence of a circadian clock appears to be important for the control of viral infection. Sendai and Influenza A virus infection was more severe in terms of symptoms and post-infection lung pathophysiology in male and female mice lacking Bmal1, which was not explained by viral titer (76, 242). During infection with Sendai virus, a greater number of neutrophils were seen in the lung of male and female mice along with an increase in expression of Interferon-α and Interferon-γ(76). Influenza virus infection in mice with functional BMAL1 showed variable severity depending on the time of infection, both when mice were kept in total darkness and under 12-hour light/dark conditions. Both male and female mice infected before the active phase (ZT11) showed significantly increased mortality compared to those infected before the rest phase (ZT23). Infection before the active phase was also associated with increased CD45+ and monocyte presence within the lung(242). Infection with vesicular stomatitis virus resulted in increased mortality when introduced to male mice before the rest phase. Infection before the rest phase led to increased cytokine production including CCL2 and IL-6. The same phenotype could be achieved for infection before the active phase if REV-ERBα expressed was eliminated(83). The circadian clock plays a necessary role in not only normal function of the immune system but also in the response to viral infection. In present times, a clearer understanding of immune function and viral infection is all the more urgent. SARS-CoV-2 infections present a new challenge to the medical field which could be met with a circadian-based approach(1, 173, 243).
In mice, immune challenge results in expression changes of core clock genes in the SCN(190). Additionally, disruption of circadian rhythms in neonatal mice results in aberrant immune development(183). The impact of circadian rhythms on the function of the immune system has long been known; however, the extent of circadian influence is only now starting to be fully appreciated. Circadian research in the field of immunology has come a long way in the past decade; however, there are still many exciting discoveries to be made. The transcriptional mechanisms clock proteins use to enact circadian patterns have yet to be elucidated for several of the phenomena discussed in this section(37, 83, 203, 242, 260, 307). There also appears to be a lack of consistency in the study of gender differences leaving a large gap in our knowledge about the circadian influence on the immune system.
f. Endocrine System
Physiological functions are heavily influenced by endocrine hormones(199). Upon release, hormones circulate via the bloodstream to be transported throughout the body to carry out a variety of actions. Although peripheral clocks play a role in the regulation of circadian rhythmicity, hormones are described to be the mediators for synchronization of all clocks systemically. The production of many common hormones is initially controlled by the brain. The three main axes that induce hormone production begin in the hypothalamus. These mechanisms include negative feedback signals sent from target organs back to the brain to regulate circulating levels of hormones. Given the properties of hormones, the inability to sustain normal levels and rhythms can adversely impact systemic physiological processes.
i. Adrenal Gland
The main role of the hypothalamic-pituitary-adrenal (HPA) axis is to regulate our body’s response to stress. The hypothalamus produces and releases corticotropin-releasing hormone which is delivered to the pituitary gland to stimulate the secretion of adrenocorticotropic hormone (ACTH) into circulation. Upon reaching the adrenal glands, ACTH activates the production and release of glucocorticoids(276, 281). The relationship between the HPA axis and systemic circadian rhythms is very complex and bidirectional. In fact, glucocorticoids are described as one of the primary relay signals between the SCN, or the central clock, and peripheral organs(32). The unique characteristics of hormones allow glucocorticoids to maintain systemic circadian homeostasis with peripheral organs(191, 207). Indeed, circulating glucocorticoid levels exhibit robust circadian rhythms in humans under basal conditions; demonstrating high levels during the active period and very low levels during the inactive period(255, 276). Although there are conflicting data on the presence of a rhythm in ACTH(152, 276), the sensitivity of ACTH in the pituitary has been demonstrated to be dependent on the adrenal clock(208). Additionally, there is a peak in cortisol levels, the most common glucocorticoid, during the morning (beginning of the active period). It is well documented that cortisol regulation is controlled by the SCN(33). Single nucleotide polymorphisms in the SERPINA6/SERPINA1 locus, which encodes corticosteroid-binding globulin and a1-antitrypsin, modifies circulating cortisol levels in the morning contributing to cardiovascular disease(55).
Changes in sleep schedule or engaging in night shift work leads to altered circadian rhythms in glucocorticoids, which takes at least two days of normal sleeping patterns to be restored(202). Disruption of glucocorticoid contribution to the body’s circadian systems is linked to metabolic and mental disorders(159). Similarly, symptoms resulting from altered HPA axis activity are believed to be caused by disruption of glucocorticoid regulation of circadian clocks(159, 296). As such, the circadian pattern of glucocorticoid levels is extremely important to overall health and systemic physiological rhythms. The adrenal glands are also responsible for the production and release of mineralocorticoids. The most common of this classification of hormone is aldosterone. The target organs of aldosterone are the kidney and colon for the regulation of solute handling and blood pressure control. Production is regulated by the renin-angiotensin-aldosterone system. Adrenal disorders lead to altered rhythms of aldosterone and blood pressure dysregulation(57, 80).
In rodents, corticosterone is the main glucocorticoid. Rodents see a peak in corticosterone during the early hours of their active period, similar to cortisol in humans. Animal studies have allowed the advancement in understanding of what contributes to glucocorticoid release patterns and the role of the adrenal circadian clock. Glucocorticoid production is heavily influenced by external stimuli. Unsurprisingly, the SCN has been shown to play a large role in glucocorticoid release. However, it is believed that both signals from the SCN, in addition to local circadian clocks, provide regulation of glucocorticoids. An older study demonstrated that the SCN plays a role in regulation of glucocorticoid circadian patterns by showing abolished glucocorticoid rhythms in rats with SCN lesions(189). Adrenal gland tissue collected from Per2::LUCIFERASE mice demonstrates that ACTH reset adrenal circadian clocks(304). However, rats with pituitary glands removed still demonstrate daily rhythms in corticosteroid levels(209). These studies suggest that ACTH contributes to the phase of rhythms, but not the presence of rhythms. The autonomic nervous system also plays a role in glucocorticoid release, interestingly through the adrenal medulla(273). Global Bmal1 knockout mice exhibit attenuated ACTH effectiveness on the adrenal gland and impaired glucocorticoid production(146). In another mouse study, disruption of Bmal1 exclusively in the adrenal gland attenuated the circadian rhythm of corticosterone (249). Ultimately, preclinical studies suggest that glucocorticoid release is regulated by the suprachiasmatic and hypothalamus signals, in addition to the adrenal circadian clock. Unlike the SCN where glucocorticoid signaling does not alter the central clock due to a lack of GR expression, glucocorticoids impact other peripheral clocks by altering rhythms in expression and behavior in males and females (22, 144). The rhythms of glucocorticoids play a role in the amplitude of clock gene expression in peripheral organs but are not responsible for the initiation of these clock mechanisms(251, 253). Within the promoter region of target genes, there are glucocorticoid responsive elements that are bound by cortisol. This action allows glucocorticoids to regulate the transcription of Per(169). The CLOCK/BMAL1 heterodimer inhibits this action by blocking the binding of cortisol(247).
Glucocorticoids are also implicated in regulating circadian clock mechanisms within the kidney and, in turn, Na+ handling and blood pressure. Following manipulation of corticosterone levels to eliminate fluctuations, Na+ handling factors in the kidney lose their diurnal rhythm of activity in males and females (109, 110). Similarly, the mineralocorticoid aldosterone has been demonstrated to regulate PER1-dependent actions in the kidney. Endothelin-1 is an early aldosterone target gene(69, 91). In the absence of Per1, an aldosterone analog treatment regimen combined with high salt induces a non-dipping phenotype and altered Na+ handling in mice (248). This response may be related to altered endothelin-1 levels(69).
ii. Thyroid
The hypothalamic-pituitary-thyroid (HPT) axis regulates the homeostasis of thyroid hormones in the body. The hypothalamus produces and releases thyroid-releasing hormone (TRH) which is delivered to the pituitary gland. The anterior pituitary produces and releases thyroid-stimulating hormone (TSH or thyrotropin) into the circulation. TSH travels towards the thyroid gland to stimulate the production of thyroid hormones. Triiodothyronine (T3) and thyroxine (T4) are the major hormones produced by the thyroid which also serve as negative feedback regulators for TRH and TSH production. These hormones are responsible for regulating metabolic processes and energy homeostasis. Time of day has been demonstrated to be important when measuring circulating components of the HPT axis in humans. TSH displays diurnal rhythms where levels are low during the active period compared to the inactive period in males and females(182, 276, 291). Additionally, TSH exhibits fluctuations throughout the active period with higher levels in the afternoon than in the morning(77, 285). Rhythms in circulating T3 and T4 are less conclusive but a diurnal rhythm in T3 levels has been reported(276). Dysregulation of the rhythms of these hormones is understudied. Light cues and night/day activity are major regulators of this axis, as demonstrated by TSH secretion being altered in night shift workers and following changes in sleeping patterns(8, 148). However, the link between dysregulation of these rhythms in hormone levels and health is inconclusive(148). Patients with altered HPT hormone levels demonstrate an association with worsened outcomes(6, 270). It is worth noting that melatonin and glucocorticoids have also been linked to regulating thyroid hormone levels which may complicate the ability to demonstrate the importance of the HPT axis in the health of an individual(29, 170).
The SCN regulates the rhythmic properties of the HPT in male and female rats (24, 121). As with humans, rats also demonstrate a diurnal rhythm in circulating TSH levels, with low levels during the active period and high levels during the inactive period(24). These rhythms in rats are likely stimulated by light cues(167). Within HPT, type II deiodinase accelerates the conversion of T4 into T3. This enzyme also exhibits daily variations in activity. When the SCN was disrupted, daily rhythms in type II deiodinase were attenuated, and circulating thyroid hormone levels were decreased(120). Negative feedback control of TSH secretion from the pituitary gland is controlled by a REV-ERBα-dependent mechanism when examined in mouse thyrotropic cells(17). T3 is believed to play a role in regulating pituitary gland homeostasis including the rhythmic production of TSH in male and female rats (23). The thyroid gland of male and female rats does possess a working molecular circadian clock, however, the circadian rhythms in HPT axis components are regulated by the SCN(79). Although it has been reported that rats exhibit moderate diurnal rhythms of circulating T3 and T4, human thyroid hormone levels lack this characteristic. Additionally, the regulation of circadian rhythms in the HPT axis is mainly under SCN control(121, 122). Interestingly, when rats received thyroidectomy surgery and were administered triiodothyronine continuously, circadian clock and metabolic genes were altered in cardiac cells (213), which may provide insight into why patients with hypo- and hyperthyroid diseases exhibit heart conditions.
iii. Gonads
The hypothalamus-pituitary-gonadal (HPG) axis regulates the reproductive organs. The hypothalamus produces and secretes gonadotropin-releasing hormone (GnRH) which is delivered to the pituitary gland. The anterior pituitary secretes luteinizing hormone and follicle-stimulating hormone, which activate estrogen production in the ovaries and androgen production in the testes. These sex hormones then act as a negative feedback signal to the hypothalamus to inhibit the production of GnRH. Although estradiol and follicle-stimulating hormone levels do not demonstrate any rhythms, progesterone, luteinizing hormone, and testosterone have been reported to exhibit diurnal rhythms(276). Not only do GnRH levels have a time-of-day-dependent pattern, but GnRH is also important in the HPG axis, as demonstrated by the reestablishment of testosterone diurnal rhythms in patients that lost this pattern(245). It is worth mentioning that there is an ovarian cycle that maintains a regular rhythm that lasts several weeks. Dysregulation of rhythms in the HPG axis may lead to irregular menstrual cycles, low sperm density, and fertility complications. The local circadian clock within the ovaries is largely controlled by the SCN(180, 241). Similarly, regulation of rhythms in male reproductive organs such as the reported diurnal rhythm in urinary testosterone levels(30) by local clock mechanisms is unclear. In fact, the existence of a functional peripheral clock in the testes is debated(26). However, clock genes are seen in mouse testis.
There are reports of circadian clock dysregulation in mice being associated with infertility, with BMAL1 playing a major role in females(157, 236, 298). Studies in mice and cells demonstrate the importance of BMAL1 in ovulation and fertilization(175, 298). In males, Cry1 KO mice displayed impaired spermatogenesis with no change in testosterone levels(150). Research using cell culture has provided information on the role of the molecular clock within the gonads. The use of Leydig cells, which exhibit robust circadian oscillators responsible for testosterone production, demonstrate that BMAL1 contributes to this action(65), which is consistent with male and female global Bmal1 KO mice exhibiting impaired fertility(10). Additionally, REV-ERBα contributes to testosterone synthesis, as demonstrated in Leydig cells in response to chemically-induced disruption of testosterone production(151), which is consistent with diminished fertility in REV-ERBα KO mice(49). Although clock mechanisms in the testes are important, its rhythmicity is regulated by luteinizing hormone(20). There are clear circadian rhythms in many steps of the HPG axes; however, more studies are needed to better understand the molecular mechanisms at play.
IV. Conclusion and Future Directions
As described in this review, the circadian clock affects many different physiological processes through modulation of the transcriptome, and this feedback system is critical for maintaining overall homeostasis (Figure 6). While this review highlights the known functions of the circadian clock in each organ system, it is essential to note that the organ systems discussed here do not act in isolation from each other. For example, the SCN receives light cues from the environment and transmits that information to the peripheral clocks through neuronal and hormonal signals. However, even without light cues, the peripheral clocks maintain endogenous rhythms suggesting that the SCN clock is not the sole communicator between the various organ clocks. More integrative studies on the role of clock proteins in specific tissues would shed light on the complex role of the clock in regulating gene expression and physiological function.
Figure 6.
Circadian clock effects on physiological function. Circadian proteins in parentheses represent the known proteins that are implicated in the regulation of the process examined. Additional proteins not listed may not have been tested and could also be involved. Diagram was created using Biorender.com.
Further studies are needed to understand sex differences in altering the circadian clock. Currently, most of our mechanistic knowledge comes from studies conducted in young male rodents, with much less data on the influence of clock genes in females or across the aging spectrum. Previous reports estimate that only 20% of circadian studies include females(136). The lack of female mice in circadian studies is likely due to concerns about the relationship between the estrous cycle and circadian rhythms (Figure 6). However, sex differences in circadian rhythms exist, as demonstrated by differences in male and female sleeping patterns(13). Women tend to go to sleep earlier, have an earlier phase of temperature and melatonin, and have shorter intrinsic circadian periods than men(2, 36, 73). These sex differences can be driven by gonadal hormones or by the sex chromosomes. Experimental models such as gonadectomy or the four core genotypes of mouse colony can be used to delineate the role of sex hormones and chromosomes in regulating circadian rhythms. Additionally, the effect of age is significantly understudied. Studies show that circadian rhythms dampen with age, but the mechanisms and direct consequences are not well understood(13, 86). Understanding clock mechanisms in males and females at all ages will give better insight into the impacts of circadian disruptions and possible treatments and interventions.
Rodent models have shown that mutation or loss of clock components or changes in feeding time are associated with a range of physiological effects. Some of these effects are disadvantageous, and others point to the use of chronotherapy to target disease. Previous studies have shown the harmful effects of chronic shift work and jetlag in humans. Reestablishing a natural circadian rhythm and targeting core clock proteins show promise in treating a variety of diseases. Preclinical studies have shown that the circadian clock machinery interacts with metabolic regulators, and misalignment of the clock can result in metabolic disorders (Figure 6). Time-restricted feeding, or limiting feeding time to a defined number of hours per day, results in improved insulin response, protects against a high-fat or -fructose diet, and reduces inflammatory pathway activation(308). However, the mechanisms behind the transcriptional changes induced by time-restricted feeding are not well understood. Another intervention shown to improve the transcriptional output of the circadian clock is light therapy. Bright light exposure at night during a night-shift protocol shifts the phase of the human transcriptome(124). Exposing night-shift workers to bright light during their nightly waking period may assist in work performance and sleep quality(60). Bright-light exposure is also effective in treating psychiatric disorders such as seasonal affective disorder(293). Pharmaceuticals such as Nobiletin (NOB), a ROR agonist, can modulate the clock. NOB activates ROR receptors, enhancing circadian rhythms and improving metabolic parameters in aging mice(99, 204, 302). Pharmaceuticals like NOB have the potential to minimize age-related declines in circadian rhythms and the accompanying chronic illnesses. Still, more preclinical and translational studies are needed to determine their effectiveness in humans. Chronotherapy treatments, such as those described here could improve the human quality of life by restoring circadian rhythms. The need for such treatments is becoming more apparent in the 21st century because modern lifestyles often involve blue-light emitting devices at night, night-shift work, and inconsistent feeding and fasting cycles, all of which disrupt the body’s natural circadian rhythm.
Acknowledgements
The authors thank Stuart Hesketh and Christopher Wolff for their assistance with figures and Dr. F. Rezan Sahinkaya for editorial support.
Funding sources:
National Institutes of Health Grant 1-R01-DK-109570-01A1 (to ML Gumz) and 1F32DK121424-01A1 (to GR Crislip).
References Cited
- 1.Acuna-Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, and Acuna-Fernandez C. Clinical trial to test the efficacy of melatonin in COVID-19. J Pineal Res 69: e12683, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adan A, and Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int 19: 709–720, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Adler P, Chiang CK, Mayne J, Ning Z, Zhang X, Xu B, Cheng HM, and Figeys D. Aging Disrupts the Circadian Patterns of Protein Expression in the Murine Hippocampus. Front Aging Neurosci 11: 368, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossio I, Lechuga-Vieco AV, Garcia-Prieto J, Gomez-Parrizas M, Quintana JA, Ballesteros I, Martin-Salamanca S, Aroca-Crevillen A, Chong SZ, Evrard M, Balabanian K, Lopez J, Bidzhekov K, Bachelerie F, Abad-Santos F, Munoz-Calleja C, Zarbock A, Soehnlein O, Weber C, Ng LG, Lopez-Rodriguez C, Sancho D, Moro MA, Ibanez B, and Hidalgo A. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 50: 390–402 e310, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossio I, Lechuga-Vieco AV, Garcia-Prieto J, Gomez-Parrizas M, Quintana JA, Ballesteros I, Martin-Salamanca S, Aroca-Crevillen A, Chong SZ, Evrard M, Balabanian K, Lopez J, Bidzhekov K, Bachelerie F, Abad-Santos F, Munoz-Calleja C, Zarbock A, Soehnlein O, Weber C, Ng LG, Lopez-Rodriguez C, Sancho D, Moro MA, Ibanez B, and Hidalgo A. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 51: 966–967, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Akbaş T, Deyneli O, Sönmez FT, and Akalın S. The pituitary-gonadal-thyroid and lactotroph axes in critically ill patients. Endokrynol Pol 67: 305–312, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Al Mheid I, Corrigan F, Shirazi F, Veledar E, Li Q, Alexander WR, Taylor WR, Waller EK, and Quyyumi AA. Circadian variation in vascular function and regenerative capacity in healthy humans. J Am Heart Assoc 3: e000845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan JS, and Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab 79: 508–512, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Allen NC, Philip NH, Hui L, Zhou X, Franklin RA, Kong Y, and Medzhitov R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci Signal 12: 2019. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, and Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms 23: 26–36, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amir M, Chaudhari S, Wang R, Campbell S, Mosure SA, Chopp LB, Lu Q, Shang J, Pelletier OB, He Y, Doebelin C, Cameron MD, Kojetin DJ, Kamenecka TM, and Solt LA. REV-ERBalpha Regulates TH17 Cell Development and Autoimmunity. Cell Rep 25: 3733–3749 e3738, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anafi RC, Pellegrino R, Shockley KR, Romer M, Tufik S, and Pack AI. Sleep is not just for the brain: transcriptional responses to sleep in peripheral tissues. BMC Genomics 14: 362, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson ST, and FitzGerald GA. Sexual dimorphism in body clocks. Science 369: 1164–1165, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Ando H, Ushijima K, Shimba S, and Fujimura A. Daily Fasting Blood Glucose Rhythm in Male Mice: A Role of the Circadian Clock in the Liver. Endocrinology 157: 463–469, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, and Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A 107: 19090–19095, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, and Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 111: 1157–1165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aninye IO, Matsumoto S, Sidhaye AR, and Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem 289: 17070–17077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansermet C, Centeno G, Nikolaeva S, Maillard MP, Pradervand S, and Firsov D. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Scientific reports 9: 16089, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atwood A, DeConde R, Wang SS, Mockler TC, Sabir JS, Ideker T, and Kay SA. Cell-autonomous circadian clock of hepatocytes drives rhythms in transcription and polyamine synthesis. Proc Natl Acad Sci U S A 108: 18560–18565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baburski AZ, Andric SA, and Kostic TS. Luteinizing hormone signaling is involved in synchronization of Leydig cell’s clock and is crucial for rhythm robustness of testosterone production†. Biol Reprod 100: 1406–1415, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, and Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol 7: e52, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, and Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Bargi-Souza P, Peliciari-Garcia RA, and Nunes MT. Disruption of the Pituitary Circadian Clock Induced by Hypothyroidism and Hyperthyroidism: Consequences on Daily Pituitary Hormone Expression Profiles. Thyroid : official journal of the American Thyroid Association 29: 502–512, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Bertani S, Carboni L, Criado A, Michielin F, Mangiarini L, and Vicentini E. Circadian profile of peripheral hormone levels in Sprague-Dawley rats and in common marmosets (Callithrix jacchus). In Vivo 24: 827–836, 2010. [PubMed] [Google Scholar]
- 25.Beytebiere JR, Trott AJ, Greenwell BJ, Osborne CA, Vitet H, Spence J, Yoo SH, Chen Z, Takahashi JS, Ghaffari N, and Menet JS. Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer-enhancer interactions. Genes & development 33: 294–309, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittman EL. Timing in the Testis. Journal of biological rhythms 31: 12–36, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Bollinger T, Leutz A, Leliavski A, Skrum L, Kovac J, Bonacina L, Benedict C, Lange T, Westermann J, Oster H, and Solbach W. Circadian clocks in mouse and human CD4+ T cells. PLoS One 6: e29801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchard M, Souabni A, and Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Brabant G, Brabant A, Ranft U, Ocran K, Köhrle J, Hesch RD, and von zur Mühlen A. Circadian and pulsatile thyrotropin secretion in euthyroid man under the influence of thyroid hormone and glucocorticoid administration. J Clin Endocrinol Metab 65: 83–88, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Brambilla DJ, Matsumoto AM, Araujo AB, and McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 94: 907–913, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, and Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–1047, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Buijs FN, Leon-Mercado L, Guzman-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, and Buijs RM. The Circadian System: A Regulatory Feedback Network of Periphery and Brain . Physiology (Bethesda) 31: 170–181, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Buijs RM, and Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2: 521–526, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, and Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkholder TJ, Fingado B, Baron S, and Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, and Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of biological rhythms 25: 288–296, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Q, Zhao X, Bai J, Gery S, Sun H, Lin DC, Chen Q, Chen Z, Mack L, Yang H, Deng R, Shi X, Chong LW, Cho H, Xie J, Li QZ, Muschen M, Atkins AR, Liddle C, Yu RT, Alkan S, Said JW, Zheng Y, Downes M, Evans RM, and Koeffler HP. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A 114: 12548–12553, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao R, and Obrietan K. mTOR Signaling and Entrainment of the Mammalian Circadian Clock. Mol Cell Pharmacol 2: 125–130, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao R, Robinson B, Xu H, Gkogkas C, Khoutorsky A, Alain T, Yanagiya A, Nevarko T, Liu AC, Amir S, and Sonenberg N. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron 79: 712–724, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caratti G, Iqbal M, Hunter L, Kim D, Wang P, Vonslow RM, Begley N, Tetley AJ, Woodburn JL, Pariollaud M, Maidstone R, Donaldson IJ, Zhang Z, Ince LM, Kitchen G, Baxter M, Poolman TM, Daniels DA, Stirling DR, Brocker C, Gonzalez F, Loudon AS, Bechtold DA, Rattray M, Matthews LC, and Ray DW. REVERBa couples the circadian clock to hepatic glucocorticoid action. J Clin Invest 128: 4454–4471, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carriazo S, Ramos AM, Sanz AB, Sanchez-Nino MD, Kanbay M, and Ortiz A. Chronodisruption: A Poorly Recognized Feature of CKD. Toxins (Basel) 12: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casanova-Acebes M, Nicolas-Avila JA, Li JL, Garcia-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, N AG, Ballesteros I, Devi S, Quintana JA, Crainiciuc G, Leiva M, Gunzer M, Weber C, Nagasawa T, Soehnlein O, Merad M, Mortha A, Ng LG, Peinado H, and Hidalgo A. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med 215: 2778–2795, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cedernaes J, Waldeck N, and Bass J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes & development 33: 1136–1158, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaix A, Lin T, Le HD, Chang MW, and Panda S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29: 303–319 e304, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, Yeoh BS, Mell B, Yeo JY, Vijay-Kumar M, Yang T, and Joe B. Diurnal Timing Dependent Alterations in Gut Microbial Composition Are Synchronously Linked to Salt-Sensitive Hypertension and Renal Damage. Hypertension 76: 59–72, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmers JA, Martino TA, Tata N, Ralph MR, Sole MJ, and Belsham DD. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1). Am J Physiol Regul Integr Comp Physiol 295: R1529–1538, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, and McClung CA. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A 113: 206–211, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, and Yang G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res 2014: 653017, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, and Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485: 123–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nature reviews Neuroscience 10: 549–560, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cisse YM, Russart KL, and Nelson RJ. Parental Exposure to Dim Light at Night Prior to Mating Alters Offspring Adaptive Immunity. Scientific reports 7: 45497, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coiffard B, Diallo AB, Culver A, Mezouar S, Hammad E, Vigne C, Nicolino-Brunet C, Dignat-George F, Baumstarck K, Boucekine M, Leone M, and Mege JL. Circadian Rhythm Disruption and Sepsis in Severe Trauma Patients. Shock 52: 29–36, 2019. [DOI] [PubMed] [Google Scholar]
- 53.Cornu M, Oppliger W, Albert V, Robitaille AM, Trapani F, Quagliata L, Fuhrer T, Sauer U, Terracciano L, and Hall MN. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci U S A 111: 11592–11599, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox KH, and Takahashi JS. Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol 63: R93–R102, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford AA, Bankier S, Altmaier E, Barnes CLK, Clark DW, Ermel R, Friedrich N, van der Harst P, Joshi PK, Karhunen V, Lahti J, Mahajan A, Mangino M, Nethander M, Neumann A, Pietzner M, Sukhavasi K, Wang CA, Bakker SJL, Bjorkegren JLM, Campbell H, Eriksson J, Gieger C, Hayward C, Jarvelin MR, McLachlan S, Morris AP, Ohlsson C, Pennell CE, Price J, Rudan I, Ruusalepp A, Spector T, Tiemeier H, Völzke H, Wilson JF, Michoel T, Timpson NJ, Smith GD, Walker BR, and consortium CNC. Variation in the SERPINA6/SERPINA1 locus alters morning plasma cortisol, hepatic corticosteroid binding globulin expression, gene expression in peripheral tissues, and risk of cardiovascular disease. J Hum Genet 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crislip GR, Douma LG, Masten SH, Cheng KY, Lynch IJ, Johnston JG, Barral D, Glasford KB, Holzworth MR, Verlander JW, Wingo CS, and Gumz ML. Differences in renal BMAL1 contribution to Na(+) homeostasis and blood pressure control in male and female mice. Am J Physiol Renal Physiol 318: F1463–F1477, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cugini P, Letizia C, Cerci S, Di Palma L, Battisti P, Coppola A, and Scavo D. A chronobiological approach to circulating levels of renin, angiotensin-converting enzyme, aldosterone, ACTH, and cortisol in Addison’s disease. Chronobiol Int 10: 119–122, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham PS, Meijer P, Nazgiewicz A, Anderson SG, Borthwick LA, Bagnall J, Kitchen GB, Lodyga M, Begley N, Venkateswaran RV, Shah R, Mercer PF, Durrington HJ, Henderson NC, Piper-Hanley K, Fisher AJ, Chambers RC, Bechtold DA, Gibbs JE, Loudon AS, Rutter MK, Hinz B, Ray DW, and Blaikley JF. The circadian clock protein REVERBalpha inhibits pulmonary fibrosis development. Proc Natl Acad Sci U S A 117: 1139–1147, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curtis AM, Bellet MM, Sassone-Corsi P, and O’Neill LA. Circadian clock proteins and immunity. Immunity 40: 178–186, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, and Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. The New England journal of medicine 322: 1253–1259, 1990. [DOI] [PubMed] [Google Scholar]
- 61.Davoudi A, Corbett DB, Ozrazgat-Baslanti T, Bihorac A, Brakenridge SC, Manini TM, and Rashidi P. Activity and Circadian Rhythm of Sepsis Patients in the Intensive Care Unit. IEEE EMBS Int Conf Biomed Health Inform 2018: 17–20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deboer T. Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiol Sleep Circadian Rhythms 5: 68–77, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, Cao L, Wang H, Billiar TR, Jiang J, Xie M, and Tang D. The Circadian Clock Controls Immune Checkpoint Pathway in Sepsis. Cell Rep 24: 366–378, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, and Czeisler CA. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One 7: e30037, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding H, Zhao J, Liu H, Wang J, and Lu W. BMAL1 knockdown promoted apoptosis and reduced testosterone secretion in TM3 Leydig cell line. Gene 747: 144672, 2020. [DOI] [PubMed] [Google Scholar]
- 66.Donate Puertas R, Jalabert A, Meugnier E, Euthine V, Chevalier P, and Rome S. Analysis of the microRNA signature in left atrium from patients with valvular heart disease reveals their implications in atrial fibrillation. PLoS One 13: e0196666, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dooner MS, Stewart C, Deng Y, Papa E, Pereira M, Del Tatto M, Johnson S, Wen S, Amaral A, Aliotta J, Quesenberry PJ, and Goldberg LR. Daily rhythms influence the ability of lung-derived extracellular vesicles to modulate bone marrow cell phenotype. PLoS One 13: e0207444, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douma LG, Barral D, and Gumz ML. Interplay of the Circadian Clock and Endothelin System. Physiology (Bethesda) 36: 35–43, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Douma LG, Crislip GR, Cheng KY, Barral D, Masten S, Holzworth M, Roig E, Glasford K, Beguiristain K, Li W, Bratanatawira P, Lynch IJ, Cain BD, Wingo CS, and Gumz ML. Knockout of the circadian clock protein PER1 results in sex-dependent alterations of ET-1 production in mice in response to a high-salt diet plus mineralocorticoid treatment. Canadian journal of physiology and pharmacology 98: 579–586, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Douma LG, Solocinski K, Masten SH, Barral DH, Barilovits SJ, Jeffers LA, Alder KD, Patel R, Wingo CS, Brown KD, Cain BD, and Gumz ML. EDN1-AS, A Novel Long Non-coding RNA Regulating Endothelin-1 in Human Proximal Tubule Cells. Frontiers in physiology 11: 209, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Downton P, Early JO, and Gibbs JE. Circadian rhythms in adaptive immunity. Immunology 161: 268–277, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, and Scheiermann C. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46: 120–132, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr., and Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 108 Suppl 3: 15602–15608, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dyar KA, Ciciliot S, Wright LE, Bienso RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MI, Gudiksen A, Solagna F, Albiero M, Moretti I, Eckel-Mahan KL, Baldi P, Sassone-Corsi P, Rizzuto R, Bicciato S, Pilegaard H, Blaauw B, and Schiaffino S. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Molecular metabolism 3: 29–41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE, Timmons G, Geiger SS, Fitzpatrick DJ, O’Connell D, Xavier RJ, Hokamp K, O’Neill LAJ, and Curtis AM. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A 115: E8460–E8468, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, Castro M, Bacharier LB, Wang X, Holtzman MJ, and Haspel JA. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 11: 97–111, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, Bennett ST, and Benvenga S. Circadian and Circannual Rhythms in Thyroid Hormones: Determining the TSH and Free T4 Reference Intervals Based Upon Time of Day, Age, and Sex. Thyroid : official journal of the American Thyroid Association 25: 954–961, 2015. [DOI] [PubMed] [Google Scholar]
- 78.Elmadjian F, and Pincus G. A study of the diurnal variations in circulating lymphocytes in normal and psychotic subjects. J Clin Endocrinol Metab 6: 287–294, 1946. [DOI] [PubMed] [Google Scholar]
- 79.Fahrenkrug J, Georg B, Hannibal J, and Jørgensen HL. Hypophysectomy abolishes rhythms in rat thyroid hormones but not in the thyroid clock. J Endocrinol 233: 209–216, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fallo F, Fanelli G, Cipolla A, Betterle C, Boscaro M, and Sonino N. 24-hour blood pressure profile in Addison’s disease. Am J Hypertens 7: 1105–1109, 1994. [DOI] [PubMed] [Google Scholar]
- 81.Fan Z, Zhao M, Joshi PD, Li P, Zhang Y, Guo W, Xu Y, Wang H, Zhao Z, and Yan J. A class of circadian long non-coding RNAs mark enhancers modulating long-range circadian gene regulation. Nucleic Acids Res 45: 5720–5738, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feeney KA, Hansen LL, Putker M, Olivares-Yanez C, Day J, Eades LJ, Larrondo LF, Hoyle NP, O’Neill JS, and van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532: 375–379, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gagnidze K, Hajdarovic KH, Moskalenko M, Karatsoreos IN, McEwen BS, and Bulloch K. Nuclear receptor REV-ERBalpha mediates circadian sensitivity to mortality in murine vesicular stomatitis virus-induced encephalitis. Proc Natl Acad Sci U S A 113: 5730–5735, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gebel S, Gerstmayer B, Kuhl P, Borlak J, Meurrens K, and Muller T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicological sciences : an official journal of the Society of Toxicology 93: 422–431, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, and Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature medicine 20: 919–926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibson EM, Williams WP 3rd, and Kriegsfeld LJ. Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol 44: 51–56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greenwell BJ, Trott AJ, Beytebiere JR, Pao S, Bosley A, Beach E, Finegan P, Hernandez C, and Menet JS. Rhythmic Food Intake Drives Rhythmic Gene Expression More Potently than the Hepatic Circadian Clock in Mice. Cell Rep 27: 649–657 e645, 2019. [DOI] [PubMed] [Google Scholar]
- 88.Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeno MR, Nadarajah CJ, Ezerskiy LA, Colonna M, Zhang J, Bauer AQ, Burris TP, and Musiek ES. Circadian clock protein Rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci U S A 116: 5102–5107, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guan D, Xiong Y, Borck PC, Jang C, Doulias PT, Papazyan R, Fang B, Jiang C, Zhang Y, Briggs ER, Hu W, Steger D, Ischiropoulos H, Rabinowitz JD, and Lazar MA. Diet-Induced Circadian Enhancer Remodeling Synchronizes Opposing Hepatic Lipid Metabolic Processes. Cell 174: 831–842 e812, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, and Wingo CS. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochimica et biophysica acta 1799: 622–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gumz ML, Popp MP, Wingo CS, and Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–673, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Gumz ML, Rabinowitz L, and Wingo CS. An Integrated View of Potassium Homeostasis. The New England journal of medicine 373: 1787–1788, 2015. [DOI] [PubMed] [Google Scholar]
- 93.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, and Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hadden H, Soldin SJ, and Massaro D. Circadian disruption alters mouse lung clock gene expression and lung mechanics. Journal of applied physiology 113: 385–392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, and Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab 7: 215–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hardie DG, Ross FA, and Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology 13: 251–262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, and Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6: 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haspel JA, Chettimada S, Shaik RS, Chu JH, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, Lederer JA, Englert J, Pelton A, Coronata A, Fredenburgh LE, and Choi AM. Circadian rhythm reprogramming during lung inflammation. Nat Commun 5: 4753, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, and Chen Z. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 23: 610–621, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, Bradfield P, Grenier JMP, Pelletier J, Druzd D, Chen CS, Ince LM, Bierschenk S, Pick R, Sperandio M, Aurrand-Lions M, and Scheiermann C. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity 49: 1175–1190 e1177, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hemmers S, and Rudensky AY. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep 11: 1339–1349, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henriksson E, Huber AL, Soto EK, Kriebs A, Vaughan ME, Duglan D, Chan AB, Papp SJ, Nguyen M, Afetian ME, and Lamia KA. The Liver Circadian Clock Modulates Biochemical and Physiological Responses to Metformin. Journal of biological rhythms 32: 345–358, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, and Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5: 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, and Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet 5: e1000442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Illi B, Gaetano C, and Capogrossi MC. How senescent vascular cells lose their Clock age-dependent impairment of circadian rhythmicity in smooth muscle cells. Circ Res 98: 450–452, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Inoki K, Kim J, and Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52: 381–400, 2012. [DOI] [PubMed] [Google Scholar]
- 107.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 66: 143–172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Irwin MR, and Opp MR. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 42: 129–155, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ivy JR, Jones NK, Costello HM, Mansley MK, Peltz TS, Flatman PW, and Bailey MA. Glucocorticoid receptor activation stimulates the sodium-chloride cotransporter and influences the diurnal rhythm of its phosphorylation. Am J Physiol Renal Physiol 317: F1536–F1548, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ivy JR, Oosthuyzen W, Peltz TS, Howarth AR, Hunter RW, Dhaun N, Al-Dujaili EA, Webb DJ, Dear JW, Flatman PW, and Bailey MA. Glucocorticoids Induce Nondipping Blood Pressure by Activating the Thiazide-Sensitive Cotransporter. Hypertension 67: 1029–1037, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, de Leeuw CA, Benjamins JS, Munoz-Manchado AB, Nagel M, Savage JE, Tiemeier H, White T, andMe Research T, Tung JY, Hinds DA, Vacic V, Wang X, Sullivan PF, van der Sluis S, Polderman TJC, Smit AB, Hjerling-Leffler J, Van Someren EJW, and Posthuma D. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 51: 394–403, 2019. [DOI] [PubMed] [Google Scholar]
- 112.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med 48: e245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jewett ME, Kronauer RE, and Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature 350: 59–62, 1991. [DOI] [PubMed] [Google Scholar]
- 114.Johnson CH, Zhao C, Xu Y, and Mori T. Timing the day: what makes bacterial clocks tick? Nat Rev Microbiol 15: 232–242, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston JG, and Pollock DM. Circadian regulation of renal function. Free Radic Biol Med 119: 93–107, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnston JG, Speed JS, Becker BK, Kasztan M, Soliman RH, Rhoads MK, Tao B, Jin C, Geurts AM, Hyndman KA, Pollock JS, and Pollock DM. Diurnal Control of Blood Pressure Is Uncoupled From Sodium Excretion. Hypertension 75: 1624–1634, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnston JG, Speed JS, Jin C, and Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol 311: F991–F998, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jordan SD, and Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol 366: 163–169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, and Gachon F. The circadian clock coordinates ribosome biogenesis. PLoS Biol 11: e1001455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalsbeek A, Buijs RM, van Schaik R, Kaptein E, Visser TJ, Doulabi BZ, and Fliers E. Daily variations in type II iodothyronine deiodinase activity in the rat brain as controlled by the biological clock. Endocrinology 146: 1418–1427, 2005. [DOI] [PubMed] [Google Scholar]
- 121.Kalsbeek A, Fliers E, Franke AN, Wortel J, and Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141: 3832–3841, 2000. [DOI] [PubMed] [Google Scholar]
- 122.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, and Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol 22: 362–372, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, and Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106: 21407–21412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kervezee L, Cuesta M, Cermakian N, and Boivin DB. The Phase-Shifting Effect of Bright Light Exposure on Circadian Rhythmicity in the Human Transcriptome. Journal of biological rhythms 34: 84–97, 2019. [DOI] [PubMed] [Google Scholar]
- 125.Kervezee L, Cuesta M, Cermakian N, and Boivin DB. Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proc Natl Acad Sci U S A 115: 5540–5545, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD, and Fu L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 30: 909–924, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim YH, Marhon SA, Zhang Y, Steger DJ, Won KJ, and Lazar MA. Rev-erbalpha dynamically modulates chromatin looping to control circadian gene transcription. Science 359: 1274–1277, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirsch S, Thijssen S, Alarcon Salvador S, Heine GH, van Bentum K, Fliser D, Sester M, and Sester U. T-cell numbers and antigen-specific T-cell function follow different circadian rhythms. J Clin Immunol 32: 1381–1389, 2012. [DOI] [PubMed] [Google Scholar]
- 129.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, and Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, and Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 77: 105–111, 1989. [DOI] [PubMed] [Google Scholar]
- 131.Koopman MG, Koomen GC, van Acker BA, and Arisz L. Circadian rhythm in glomerular transport of macromolecules through large pores and shunt pathway. Kidney Int 49: 1242–1249, 1996. [DOI] [PubMed] [Google Scholar]
- 132.Koopman MG, Koomen GC, van Acker BA, and Arisz L. Urinary sodium excretion in patients with nephrotic syndrome, and its circadian variation. Q J Med 87: 109–117, 1994. [PubMed] [Google Scholar]
- 133.Koronowski KB, Kinouchi K, Welz PS, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, Li W, Baldi P, Benitah SA, and Sassone-Corsi P. Defining the Independence of the Liver Circadian Clock. Cell 177: 1448–1462 e1414, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ku K, Park I, Kim D, Kim J, Jang S, Choi M, Choe HK, and Kim K. Gut Microbial Metabolites Induce Changes in Circadian Oscillation of Clock Gene Expression in the Mouse Embryonic Fibroblasts. Mol Cells 43: 276–285, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuang Z, Wang Y, Li Y, Ye C, Ruhn KA, Behrendt CL, Olson EN, and Hooper LV. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 365: 1428–1434, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, and Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154: 1501–1512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi H, Orimo M, Okada S, and Komuro I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res 98: 532–539, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Laing EE, Moller-Levet CS, Poh N, Santhi N, Archer SN, and Dijk DJ. Blood transcriptome based biomarkers for human circadian phase. Elife 6: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, and Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT, Strand LB, Winsvold BS, Wang H, Bowden J, Song Y, Patel K, Anderson SG, Beaumont RN, Bechtold DA, Cade BE, Haas M, Kathiresan S, Little MA, Luik AI, Loudon AS, Purcell S, Richmond RC, Scheer F, Schormair B, Tyrrell J, Winkelman JW, Winkelmann J, Sleep HAI, Hveem K, Zhao C, Nielsen JB, Willer CJ, Redline S, Spiegelhalder K, Kyle SD, Ray DW, Zwart JA, Brumpton B, Frayling TM, Lawlor DA, Rutter MK, Weedon MN, and Saxena R. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet 51: 387–393, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, and Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28: 395–409, 2005. [DOI] [PubMed] [Google Scholar]
- 142.Laugsand LE, Vatten LJ, Platou C, and Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation 124: 2073–2081, 2011. [DOI] [PubMed] [Google Scholar]
- 143.Le Martelot G, Canella D, Symul L, Migliavacca E, Gilardi F, Liechti R, Martin O, Harshman K, Delorenzi M, Desvergne B, Herr W, Deplancke B, Schibler U, Rougemont J, Guex N, Hernandez N, Naef F, and Cycli XC. Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLoS Biol 10: e1001442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Minh N, Damiola F, Tronche F, Schutz G, and Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20: 7128–7136, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lefta M, Campbell KS, Feng HZ, Jin JP, and Esser KA. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol 303: H475–485, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leliavski A, Dumbell R, Ott V, and Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms 30: 20–34, 2015. [DOI] [PubMed] [Google Scholar]
- 147.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, and Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leso V, Vetrani I, Sicignano A, Romano R, and Iavicoli I. The Impact of Shift-Work and Night Shift-Work on Thyroid: A Systematic Review. Int J Environ Res Public Health 17: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, and Smith AF. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev 5: CD012455, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li C, Xiao S, Hao J, Liao X, and Li G. Cry1 deficiency leads to testicular dysfunction and altered expression of genes involved in cell communication, chromatin reorganization, spermatogenesis, and immune response in mouse testis. Mol Reprod Dev 85: 325–335, 2018. [DOI] [PubMed] [Google Scholar]
- 151.Li C, Zhang L, Ma T, Gao L, Yang L, Wu M, Pang Z, Wang X, Yao Q, Xiao Y, Zhao L, Liu W, Zhao H, Wang C, Wang A, Jin Y, and Chen H. Bisphenol A attenuates testosterone production in Leydig cells via the inhibition of NR1D1 signaling. Chemosphere 263: 128020, 2021. [DOI] [PubMed] [Google Scholar]
- 152.Li SX, Liu LJ, Xu LZ, Gao L, Wang XF, Zhang JT, and Lu L. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology 38: 2789–2799, 2013. [DOI] [PubMed] [Google Scholar]
- 153.Lin R, Mo Y, Zha H, Qu Z, Xie P, Zhu ZJ, Xu Y, Xiong Y, and Guan KL. CLOCK Acetylates ASS1 to Drive Circadian Rhythm of Ureagenesis. Molecular cell 68: 198–209 e196, 2017. [DOI] [PubMed] [Google Scholar]
- 154.Lipton JO, Boyle LM, Yuan ED, Hochstrasser KJ, Chifamba FF, Nathan A, Tsai PT, Davis F, and Sahin M. Aberrant Proteostasis of BMAL1 Underlies Circadian Abnormalities in a Paradigmatic mTOR-opathy. Cell Rep 20: 868–880, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Guttler T, Davis F, Asara JM, and Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 161: 1138–1151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu D, Stowie A, de Zavalia N, Leise T, Pathak SS, Drewes LR, Davidson AJ, Amir S, Sonenberg N, and Cao R. mTOR signaling in VIP neurons regulates circadian clock synchrony and olfaction. Proc Natl Acad Sci U S A 115: E3296–E3304, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Y, Johnson BP, Shen AL, Wallisser JA, Krentz KJ, Moran SM, Sullivan R, Glover E, Parlow AF, Drinkwater NR, Schuler LA, and Bradfield CA. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci U S A 111: 14295–14300, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Z, Wei ZY, Chen J, Chen K, Mao X, Liu Q, Sun Y, Zhang Z, Zhang Y, Dan Z, Tang J, Qin L, Chen JH, and Liu X. Acute Sleep-Wake Cycle Shift Results in Community Alteration of Human Gut Microbiome. mSphere 5: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liyanarachchi K, Ross R, and Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract Res Clin Endocrinol Metab 31: 459–473, 2017. [DOI] [PubMed] [Google Scholar]
- 160.Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, and Phillips AC. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34: 2679–2685, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luciano AK, Santana JM, Velazquez H, and Sessa WC. Akt1 Controls the Timing and Amplitude of Vascular Circadian Gene Expression. Journal of biological rhythms 32: 212–221, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lutshumba J, Liu S, Zhong Y, Hou T, Daugherty A, Lu H, Guo Z, and Gong MC. Deletion of BMAL1 in Smooth Muscle Cells Protects Mice From Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol 38: 1063–1075, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ma D, Liu T, Chang L, Rui C, Xiao Y, Li S, Hogenesch JB, Chen YE, and Lin JD. The Liver Clock Controls Cholesterol Homeostasis through Trib1 Protein-mediated Regulation of PCSK9/Low Density Lipoprotein Receptor (LDLR) Axis. J Biol Chem 290: 31003–31012, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maki KA, Burke LA, Calik MW, Watanabe-Chailland M, Sweeney D, Romick-Rosendale LE, Green SJ, and Fink AM. Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiol Genomics 52: 280–292, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Man K, Loudon A, and Chawla A. Immunity around the clock. Science 354: 999–1003, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mander BA, Winer JR, and Walker MP. Sleep and Human Aging. Neuron 94: 19–36, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maroni MJ, Capri KM, Cushman AV, Monteiro De Pina IK, Chasse MH, and Seggio JA. Constant light alters serum hormone levels related to thyroid function in male CD-1 mice. Chronobiol Int 35: 1456–1463, 2018. [DOI] [PubMed] [Google Scholar]
- 168.Matenchuk BA, Mandhane PJ, and Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep medicine reviews 53: 101340, 2020. [DOI] [PubMed] [Google Scholar]
- 169.Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, and Androulakis IP. Entrainment of peripheral clock genes by cortisol. Physiol Genomics 44: 607–621, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mazzoccoli G, Giuliani A, Carughi S, De Cata A, Puzzolante F, La Viola M, Urbano N, Perfetto F, and Tarquini R. The hypothalamic-pituitary-thyroid axis and melatonin in humans: possible interactions in the control of body temperature. Neuro Endocrinol Lett 25: 368–372, 2004. [PubMed] [Google Scholar]
- 171.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, and Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566: 383–387, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, and Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Meira ECM, Miyazawa M, and Gozal D. Putative contributions of circadian clock and sleep in the context of SARS-CoV-2 infection. The European respiratory journal 55: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Menet JS, Pescatore S, and Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes & development 28: 8–13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, and Sellix MT. Conditional Deletion of Bmal1 in Ovarian Theca Cells Disrupts Ovulation in Female Mice. Endocrinology 157: 913–927, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mermet J, Yeung J, Hurni C, Mauvoisin D, Gustafson K, Jouffe C, Nicolas D, Emmenegger Y, Gobet C, Franken P, Gachon F, and Naef F. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes & development 32: 347–358, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meyer-Kovac J, Kolbe I, Ehrhardt L, Leliavski A, Husse J, Salinas G, Lingner T, Tsang AH, Barclay JL, and Oster H. Hepatic gene therapy rescues high-fat diet responses in circadian Clock mutant mice. Molecular metabolism 6: 512–523, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Millar-Craig MW, Bishop CN, and Raftery EB. Circadian variation of blood-pressure. Lancet 1: 795–797, 1978. [DOI] [PubMed] [Google Scholar]
- 179.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, and Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A 104: 3342–3347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miller BH, and Takahashi JS. Central circadian control of female reproductive function. Frontiers in endocrinology 4: 195, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mills JN, and Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol 117: 22–37, 1952. [PMC free article] [PubMed] [Google Scholar]
- 182.Mirjanic-Azaric B, Stojakovic-Jelisavac T, Vukovic B, Stojanovic D, Vujnic M, and Uletilovic S. The impact of time of sample collection on the measurement of thyroid stimulating hormone values in the serum. Clin Biochem 48: 1347–1349, 2015. [DOI] [PubMed] [Google Scholar]
- 183.Mizutani H, Tamagawa-Mineoka R, Minami Y, Yagita K, and Katoh N. Constant light exposure impairs immune tolerance development in mice. J Dermatol Sci 86: 63–70, 2017. [DOI] [PubMed] [Google Scholar]
- 184.Mohawk JA, Green CB, and Takahashi JS. Central and peripheral circadian clocks in mammals. Annual review of neuroscience 35: 445–462, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JC, Santhi N, von Schantz M, Smith CP, and Dijk DJ. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A 110: E1132–1141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Montagner A, Korecka A, Polizzi A, Lippi Y, Blum Y, Canlet C, Tremblay-Franco M, Gautier-Stein A, Burcelin R, Yen YC, Je HS, Al-Asmakh M, Mithieux G, Arulampalam V, Lagarrigue S, Guillou H, Pettersson S, and Wahli W. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Scientific reports 6: 20127, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Montaruli A, Castelli L, Mule A, Scurati R, Esposito F, Galasso L, and Roveda E. Biological Rhythm and Chronotype: New Perspectives in Health. Biomolecules 11: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol 250: R737–752, 1986. [DOI] [PubMed] [Google Scholar]
- 189.Moore RY, and Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain research 42: 201–206, 1972. [DOI] [PubMed] [Google Scholar]
- 190.Moravcova S, Pacesova D, Melkes B, Kyclerova H, Spisska V, Novotny J, and Bendova Z. The day/night difference in the circadian clock’s response to acute lipopolysaccharide and the rhythmic Stat3 expression in the rat suprachiasmatic nucleus. PLoS One 13: e0199405, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moreira AC, Antonini SR, and de Castro M. MECHANISMS IN ENDOCRINOLOGY: A sense of time of the glucocorticoid circadian clock: from the ontogeny to the diagnosis of Cushing’s syndrome. Eur J Endocrinol 179: R1–R18, 2018. [DOI] [PubMed] [Google Scholar]
- 192.Morris AR, Stanton DL, Roman D, and Liu AC. Systems Level Understanding of Circadian Integration with Cell Physiology. J Mol Biol 432: 3547–3564, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morris CJ, Purvis TE, Mistretta J, and Scheer FA. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J Clin Endocrinol Metab 101: 1066–1074, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mouralidarane A, Soeda J, Sugden D, Bocianowska A, Carter R, Ray S, Saraswati R, Cordero P, Novelli M, Fusai G, Vinciguerra M, Poston L, Taylor PD, and Oben JA. Maternal obesity programs offspring non-alcoholic fatty liver disease through disruption of 24-h rhythms in mice. Int J Obes (Lond) 39: 1339–1348, 2015. [DOI] [PubMed] [Google Scholar]
- 195.Mukherji A, Kobiita A, and Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc Natl Acad Sci U S A 112: E6683–6690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, and Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J 41: 4057–4070, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murakami M, Tognini P, Liu Y, Eckel-Mahan KL, Baldi P, and Sassone-Corsi P. Gut microbiota directs PPARgamma-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep 17: 1292–1303, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Musiek ES, and Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354: 1004–1008, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Neumann AM, Schmidt CX, Brockmann RM, and Oster H. Circadian regulation of endocrine systems. Auton Neurosci 216: 1–8, 2019. [DOI] [PubMed] [Google Scholar]
- 200.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, and Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341: 1483–1488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, Tokonami N, and Firsov D. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. J Am Soc Nephrol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Niu SF, Chung MH, Chu H, Tsai JC, Lin CC, Liao YM, Ou KL, O’Brien AP, and Chou KR. Differences in cortisol profiles and circadian adjustment time between nurses working night shifts and regular day shifts: A prospective longitudinal study. Int J Nurs Stud 52: 1193–1201, 2015. [DOI] [PubMed] [Google Scholar]
- 203.Nobis CC, Dubeau Laramee G, Kervezee L, Maurice De Sousa D, Labrecque N, and Cermakian N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci U S A 116: 20077–20086, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, Sun Y, Han L, Esser KA, Mileykovskaya E, D’Alessandro A, Green CB, Takahashi JS, Dowhan W, Yoo SH, and Chen Z. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun 10: 3923, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nosal C, Ehlers A, and Haspel JA. Why Lungs Keep Time: Circadian Rhythms and Lung Immunity. Annu Rev Physiol 82: 391–412, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Oh JH, Lee JH, Han DH, Cho S, and Lee YJ. Circadian Clock Is Involved in Regulation of Hepatobiliary Transport Mediated by Multidrug Resistance-Associated Protein 2. J Pharm Sci 106: 2491–2498, 2017. [DOI] [PubMed] [Google Scholar]
- 207.Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, and Van Cauter E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev 38: 3–45, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, and Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4: 163–173, 2006. [DOI] [PubMed] [Google Scholar]
- 209.Ottenweller JE, and Meier AH. Adrenal innervation may be an extrapituitary mechanism able to regulate adrenocortical rhythmicity in rats. Endocrinology 111: 1334–1338, 1982. [DOI] [PubMed] [Google Scholar]
- 210.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, and Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. [DOI] [PubMed] [Google Scholar]
- 211.Paulose JK, Wright JM, Patel AG, and Cassone VM. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS One 11: e0146643, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, and Bass J. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab 25: 86–92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peliciari-Garcia RA, Bargi-Souza P, Young ME, and Nunes MT. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol Int 35: 147–159, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, and Bass J. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350: aac4250, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Perfilyeva YV, Abdolla N, Ostapchuk YO, Tleulieva R, Krasnoshtanov VC, and Belyaev NN. Expansion of CD11b(+)Ly6G(high) and CD11b(+)CD49d(+) myeloid cells with suppressive potential in mice with chronic inflammation and light-at-night-induced circadian disruption. Inflamm Res 66: 711–724, 2017. [DOI] [PubMed] [Google Scholar]
- 216.Perrin L, Loizides-Mangold U, Chanon S, Gobet C, Hulo N, Isenegger L, Weger BD, Migliavacca E, Charpagne A, Betts JA, Walhin JP, Templeman I, Stokes K, Thompson D, Tsintzas K, Robert M, Howald C, Riezman H, Feige JN, Karagounis LG, Johnston JD, Dermitzakis ET, Gachon F, Lefai E, and Dibner C. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 7: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pizarro A, Hayer K, Lahens NF, and Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res 41: D1009–1013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, Cao R, and Liu AC. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet 14: e1007369, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reinke H, and Asher G. Crosstalk between metabolism and circadian clocks. Nature reviews Molecular cell biology 20: 227–241, 2019. [DOI] [PubMed] [Google Scholar]
- 220.Resuehr D, Wu G, Johnson RL Jr., Young ME, Hogenesch JB, and Gamble KL. Shift Work Disrupts Circadian Regulation of the Transcriptome in Hospital Nurses. Journal of biological rhythms 34: 167–177, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, and Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol 9: e1000595, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, and Gumz ML. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1delta/epsilon. Am J Physiol Renal Physiol 303: F918–927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Richards J, Jeffers LA, All SC, Cheng KY, and Gumz ML. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of alphaENaC in renal cortical collecting duct cells. Frontiers in physiology 4: 253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Richards J, Ko B, All S, Cheng KY, Hoover RS, and Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289: 11791–11806, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Riede SJ, van der Vinne V, and Hut RA. The flexible clock: predictive and reactive homeostasis, energy balance and the circadian regulation of sleep-wake timing. J Exp Biol 220: 738–749, 2017. [DOI] [PubMed] [Google Scholar]
- 226.Riera CE, Merkwirth C, De Magalhaes Filho CD, and Dillin A. Signaling Networks Determining Life Span. Annu Rev Biochem 85: 35–64, 2016. [DOI] [PubMed] [Google Scholar]
- 227.Rijo-Ferreira F, and Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med 11: 82, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Riley LA, and Esser KA. The Role of the Molecular Clock in Skeletal Muscle and What It Is Teaching Us About Muscle-Bone Crosstalk. Curr Osteoporos Rep 15: 222–230, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Riphaus A, and Bitter H. [Short version S3 guideline sedation for gastrointestinal endoscopy und medicolegal implications]. Z Gastroenterol 50: 407–410, 2012. [DOI] [PubMed] [Google Scholar]
- 230.Rodrigo GC, and Herbert KE. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic Biol Med 119: 115–120, 2018. [DOI] [PubMed] [Google Scholar]
- 231.Sahar S, and Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol Metab 23: 1–8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Saper CB, Scammell TE, and Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263, 2005. [DOI] [PubMed] [Google Scholar]
- 233.Saxton RA, and Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 169: 361–371, 2017. [DOI] [PubMed] [Google Scholar]
- 234.Scheiermann C, Gibbs J, Ince L, and Loudon A. Clocking in to immunity. Nat Rev Immunol 18: 423–437, 2018. [DOI] [PubMed] [Google Scholar]
- 235.Schilperoort M, Bravenboer N, Lim J, Mletzko K, Busse B, van Ruijven L, Kroon J, Rensen PCN, Kooijman S, and Winter EM. Circadian disruption by shifting the light-dark cycle negatively affects bone health in mice. FASEB J 34: 1052–1064, 2020. [DOI] [PubMed] [Google Scholar]
- 236.Schoeller EL, Clark DD, Dey S, Cao NV, Semaan SJ, Chao LW, Kauffman AS, Stowers L, and Mellon PL. Bmal1 Is Required for Normal Reproductive Behaviors in Male Mice. Endocrinology 157: 4914–4929, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, and Esser KA. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol 593: 5387–5404, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA, and Delisle BP. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol 304: C954–965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sehgal A. Physiology Flies with Time. Cell 171: 1232–1235, 2017. [DOI] [PubMed] [Google Scholar]
- 240.Sehgal A, and Mignot E. Genetics of sleep and sleep disorders. Cell 146: 194–207, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sellix MT. Circadian clock function in the mammalian ovary. Journal of biological rhythms 30: 7–19, 2015. [DOI] [PubMed] [Google Scholar]
- 242.Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, Lopez CB, and FitzGerald GA. Circadian control of lung inflammation in influenza infection. Nat Commun 10: 4107, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Silva FRD, Guerreiro RC, Andrade HA, Stieler E, Silva A, and de Mello MT. Does the compromised sleep and circadian disruption of night and shiftworkers make them highly vulnerable to 2019 coronavirus disease (COVID-19)? Chronobiol Int 37: 607–617, 2020. [DOI] [PubMed] [Google Scholar]
- 244.Silver AC, Arjona A, Walker WE, and Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36: 251–261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Simoni M, Montanini V, Fustini MF, Del Rio G, Cioni K, and Marrama P. Circadian rhythm of plasma testosterone in men with idiopathic hypogonadotrophic hypogonadism before and during pulsatile administration of gonadotrophin-releasing hormone. Clin Endocrinol (Oxf) 36: 29–34, 1992. [DOI] [PubMed] [Google Scholar]
- 246.Smale L, Lee T, and Nunez AA. Mammalian diurnality: some facts and gaps. Journal of biological rhythms 18: 356–366, 2003. [DOI] [PubMed] [Google Scholar]
- 247.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, and Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A 106: 17582–17587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, and Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta physiologica 220: 72–82, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, and Kim K. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A 105: 20970–20975, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Song P, Li Z, Li X, Yang L, Zhang L, Li N, Guo C, Lu S, and Wei Y. Transcriptome Profiling of the Lungs Reveals Molecular Clock Genes Expression Changes after Chronic Exposure to Ambient Air Particles. Int J Environ Res Public Health 14: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soták M, Bryndová J, Ergang P, Vagnerová K, Kvapilová P, Vodička M, Pácha J, and Sumová A. Peripheral circadian clocks are diversely affected by adrenalectomy. Chronobiol Int 33: 520–529, 2016. [DOI] [PubMed] [Google Scholar]
- 252.Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, and Pollock DM. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314: F89–F98, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Spencer RL, Chun LE, Hartsock MJ, and Woodruff ER. Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front Neuroendocrinol 49: 52–71, 2018. [DOI] [PubMed] [Google Scholar]
- 254.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, and Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002. [DOI] [PubMed] [Google Scholar]
- 255.Straub RH, Cutolo M, Buttgereit F, and Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med 267: 543–560, 2010. [DOI] [PubMed] [Google Scholar]
- 256.Sukumaran S, Jusko WJ, Dubois DC, and Almon RR. Light-dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. Journal of applied physiology 110: 1732–1747, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sundar IK, Yao H, Sellix MT, and Rahman I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am J Respir Cell Mol Biol 53: 285–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sundar IK, Yao H, Sellix MT, and Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol 309: L1056–1075, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Susa K, Sohara E, Isobe K, Chiga M, Rai T, Sasaki S, and Uchida S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochemical and biophysical research communications 427: 743–747, 2012. [DOI] [PubMed] [Google Scholar]
- 260.Sutton CE, Finlay CM, Raverdeau M, Early JO, DeCourcey J, Zaslona Z, O’Neill LAJ, Mills KHG, and Curtis AM. Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat Commun 8: 1923, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nature reviews Genetics 18: 164–179, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Takahashi JS, Hong HK, Ko CH, and McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature reviews Genetics 9: 764–775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S, Kodama A, Shimba S, Mieda M, Fukasawa K, Ozaki K, Iezaki T, Fujikawa K, Yoneda Y, Numano R, Hida A, Tei H, Takeda S, and Hinoi E. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 32: 872–881, 2017. [DOI] [PubMed] [Google Scholar]
- 264.Talbot J, Hahn P, Kroehling L, Nguyen H, Li D, and Littman DR. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 579: 575–580, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tang H, Zhu M, Zhao G, Fu W, Shi Z, Ding Y, Tang X, and Guo D. Loss of CLOCK under high glucose upregulates ROCK1-mediated endothelial to mesenchymal transition and aggravates plaque vulnerability. Atherosclerosis 275: 58–67, 2018. [DOI] [PubMed] [Google Scholar]
- 266.Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, and Elinav E. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 167: 1495–1510 e1412, 2016. [DOI] [PubMed] [Google Scholar]
- 267.Thursby E, and Juge N. Introduction to the human gut microbiota. Biochem J 474: 1823–1836, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, Sarparanta J, Merlo P, Botre F, Schwartz GJ, Pessin JE, and Singh R. Autophagy Regulates the Liver Clock and Glucose Metabolism by Degrading CRY1. Cell Metab 28: 268–281 e264, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tsang K, Liu H, Yang Y, Charles JF, and Ermann J. Defective circadian control in mesenchymal cells reduces adult bone mass in mice by promoting osteoclast function. Bone 121: 172–180, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tsareva YO, Mayskova EA, Fedotov EA, and Shvarts YG. [Circadian rhythms of thyroid hormones in patients with ischemic heart disease, arterial hypertension, and atrial fibrillation]. Kardiologiia 59: 23–29, 2019. [DOI] [PubMed] [Google Scholar]
- 271.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, and Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature 418: 534–539, 2002. [DOI] [PubMed] [Google Scholar]
- 272.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, and Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 37: 187–192, 2005. [DOI] [PubMed] [Google Scholar]
- 273.Ulrich-Lai YM, Arnhold MM, and Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290: R1128–1135, 2006. [DOI] [PubMed] [Google Scholar]
- 274.Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, and Chung JH. Activation of 5’-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem 282: 20794–20798, 2007. [DOI] [PubMed] [Google Scholar]
- 275.Van Drunen R, and Eckel-Mahan K. Circadian Rhythms of the Hypothalamus: From Function to Physiology. Clocks Sleep 3: 189–226, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.van Kerkhof LW, Van Dycke KC, Jansen EH, Beekhof PK, van Oostrom CT, Ruskovska T, Velickova N, Kamcev N, Pennings JL, van Steeg H, and Rodenburg W. Diurnal Variation of Hormonal and Lipid Biomarkers in a Molecular Epidemiology-Like Setting. PLoS One 10: e0135652, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Viola AU, James LM, Archer SN, and Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol 295: H2156–2163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, and Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.von Schantz M. Natural Variation in Human Clocks. Adv Genet 99: 73–96, 2017. [DOI] [PubMed] [Google Scholar]
- 280.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, and Hackam DG. Shift work and vascular events: systematic review and meta-analysis. BMJ 345: e4800, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, and Lightman SL. The origin of glucocorticoid hormone oscillations. PLoS Biol 10: e1001341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Walton ZE, Patel CH, Brooks RC, Yu Y, Ibrahim-Hashim A, Riddle M, Porcu A, Jiang T, Ecker BL, Tameire F, Koumenis C, Weeraratna AT, Welsh DK, Gillies R, Alwine JC, Zhang L, Powell JD, and Dang CV. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell 174: 72–87 e32, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang CY, Hsieh MJ, Hsieh IC, Shie SS, Ho MY, Yeh JK, Tsai ML, Yang CH, Hung KC, Wang CC, and Wen MS. CLOCK modulates survival and acute lung injury in mice with polymicrobial sepsis. Biochemical and biophysical research communications 478: 935–941, 2016. [DOI] [PubMed] [Google Scholar]
- 284.Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, and Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation 118: 2166–2173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang D, Yin Y, Yu S, Li H, Cheng X, and Qiu L. Effect of sampling time on estimates of thyroid-stimulating hormone, free thyroxine, and free triiodothyronine levels. Scand J Clin Lab Invest 79: 459–462, 2019. [DOI] [PubMed] [Google Scholar]
- 286.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, and Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8: 482–491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang Q, Yin Y, and Zhang W. Ghrelin Restores the Disruption of the Circadian Clock in Steatotic Liver. International journal of molecular sciences 19: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang S, Lin Y, Zhou Z, Gao L, Yang Z, Li F, and Wu B. Circadian Clock Gene Bmal1 Regulates Bilirubin Detoxification: A Potential Mechanism of Feedback Control of Hyperbilirubinemia. Theranostics 9: 5122–5133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, and Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357: 912–916, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, Li R, Qie J, Zhen B, Wang Y, He F, Qin J, and Ding C. A proteomics landscape of circadian clock in mouse liver. Nat Commun 9: 1553, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Weeke J, and Gundersen HJ. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol (Copenh) 89: 659–672, 1978. [DOI] [PubMed] [Google Scholar]
- 292.Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, Sperandio M, Hristov M, Zeller T, Nicolaes GAF, Weber C, Viola JR, Hidalgo A, Scheiermann C, and Soehnlein O. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab 28: 175–182 e175, 2018. [DOI] [PubMed] [Google Scholar]
- 293.Wirz-Justice A, and Benedetti F. Perspectives in affective disorders: Clocks and sleep. Eur J Neurosci 51: 346–365, 2020. [DOI] [PubMed] [Google Scholar]
- 294.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, and Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci 3: 20, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wu G, Anafi RC, Hughes ME, Kornacker K, and Hogenesch JB. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 32: 3351–3353, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wyller VB, Vitelli V, Sulheim D, Fagermoen E, Winger A, Godang K, and Bollerslev J. Altered neuroendocrine control and association to clinical symptoms in adolescent chronic fatigue syndrome: a cross-sectional study. J Transl Med 14: 121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Xu C, Ochi H, Fukuda T, Sato S, Sunamura S, Takarada T, Hinoi E, Okawa A, and Takeda S. Circadian Clock Regulates Bone Resorption in Mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 31: 1344–1355, 2016. [DOI] [PubMed] [Google Scholar]
- 298.Xu J, Li Y, Wang Y, Xu Y, and Zhou C. Loss of Bmal1 decreases oocyte fertilization, early embryo development and implantation potential in female mice. Zygote 24: 760–767, 2016. [DOI] [PubMed] [Google Scholar]
- 299.Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, and Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5: 18, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang G, Jia Z, Aoyagi T, McClain D, Mortensen RM, and Yang T. Systemic PPARgamma deletion impairs circadian rhythms of behavior and metabolism. PLoS One 7: e38117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yang G, Zhang J, Jiang T, Monslow J, Tang SY, Todd L, Pure E, Chen L, and FitzGerald GA. Bmal1 Deletion in Myeloid Cells Attenuates Atherosclerotic Lesion Development and Restrains Abdominal Aortic Aneurysm Formation in Hyperlipidemic Mice. Arterioscler Thromb Vasc Biol 40: 1523–1532, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yang X, Wang H, Li T, Chen L, Zheng B, and Liu RH. Nobiletin Delays Aging and Enhances Stress Resistance of Caenorhabditis elegans. International journal of molecular sciences 21: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yeung J, Mermet J, Jouffe C, Marquis J, Charpagne A, Gachon F, and Naef F. Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res 28: 182–191, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yoder JM, Brandeland M, and Engeland WC. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am J Physiol Regul Integr Comp Physiol 306: R387–393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yoshitane H, Ozaki H, Terajima H, Du NH, Suzuki Y, Fujimori T, Kosaka N, Shimba S, Sugano S, Takagi T, Iwasaki W, and Fukada Y. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol Cell Biol 34: 1776–1787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yu S, Tang Q, Xie M, Zhou X, Long Y, Xie Y, Guo F, and Chen L. Circadian BMAL1 regulates mandibular condyle development by hedgehog pathway. Cell Prolif 53: e12727, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zaslona Z, Case S, Early JO, Lalor SJ, McLoughlin RM, Curtis AM, and O’Neill LAJ. The circadian protein BMAL1 in myeloid cells is a negative regulator of allergic asthma. Am J Physiol Lung Cell Mol Physiol 312: L855–L860, 2017. [DOI] [PubMed] [Google Scholar]
- 308.Zeb F, Wu X, Fatima S, Zaman MH, Khan SA, Safdar M, Alam I, and Feng Q. Time-restricted feeding regulates molecular mechanisms with involvement of circadian rhythm to prevent metabolic diseases. Nutrition 89: 111244, 2021. [DOI] [PubMed] [Google Scholar]
- 309.Zhang D, Jin C, Obi IE, Rhoads MK, Soliman RH, Sedaka RS, Allan JM, Tao B, Speed JS, Pollock JS, and Pollock DM. Loss of circadian gene Bmal1 in the collecting duct lowers blood pressure in male, but not female, mice. Am J Physiol Renal Physiol 318: F710–F719, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhang D, Tong X, Nelson BB, Jin E, Sit J, Charney N, Yang M, Omary MB, and Yin L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARalpha pathway. Hepatology 68: 883–896, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhang EE, Liu AC, Hirota T, Miraglia LJ, Welch G, Pongsawakul PY, Liu X, Atwood A, Huss JW 3rd, Janes J, Su AI, Hogenesch JB, and Kay SA. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 139: 199–210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang J, Lu X, Liu M, Fan H, Zheng H, Zhang S, Rahman N, Wolczynski S, Kretowski A, and Li X. Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension. Cardiovasc Res 116: 2156–2169, 2020. [DOI] [PubMed] [Google Scholar]
- 313.Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, Liao X, Coller J, and Jain MK. KLF15 Establishes the Landscape of Diurnal Expression in the Heart. Cell Rep 13: 2368–2375, 2015. [DOI] [PubMed] [Google Scholar]
- 314.Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111: 16219–16224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhang Y, Papazyan R, Damle M, Fang B, Jager J, Feng D, Peed LC, Guan D, Sun Z, and Lazar MA. The hepatic circadian clock fine-tunes the lipogenic response to feeding through RORalpha/gamma. Genes & development 31: 1202–1211, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang Z, Hunter L, Wu G, Maidstone R, Mizoro Y, Vonslow R, Fife M, Hopwood T, Begley N, Saer B, Wang P, Cunningham P, Baxter M, Durrington H, Blaikley JF, Hussell T, Rattray M, Hogenesch JB, Gibbs J, Ray DW, and Loudon ASI. Genome-wide effect of pulmonary airway epithelial cell-specific Bmal1 deletion. FASEB J 33: 6226–6238, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhao M, Zhao H, Deng J, Guo L, and Wu B. Role of the CLOCK protein in liver detoxification. British journal of pharmacology 176: 4639–4652, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zheng Z, Kim H, Qiu Y, Chen X, Mendez R, Dandekar A, Zhang X, Zhang C, Liu AC, Yin L, Lin JD, Walker PD, Kapatos G, and Zhang K. CREBH Couples Circadian Clock With Hepatic Lipid Metabolism. Diabetes 65: 3369–3383, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM, Dolan K, Zhu X, Hubert N, Tao Y, Lin F, Martinez-Guryn K, Huang Y, Wang T, Liu J, He C, Chang EB, and Leone V. Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m(6)A mRNA Methylation. Cell Rep 25: 1816–1828 e1814, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhou X, Yu R, Long Y, Zhao J, Yu S, Tang Q, and Chen L. BMAL1 deficiency promotes skeletal mandibular hypoplasia via OPG downregulation. Cell Prolif 51: e12470, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhu M, Tang H, Tang X, Ma X, Guo D, and Chen F. BMAL1 suppresses ROS-induced endothelial-to-mesenchymal transition and atherosclerosis plaque progression via BMP signaling. Am J Transl Res 10: 3150–3161, 2018. [PMC free article] [PubMed] [Google Scholar]
- 322.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, and Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 106: 16523–16528, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]