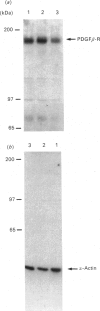
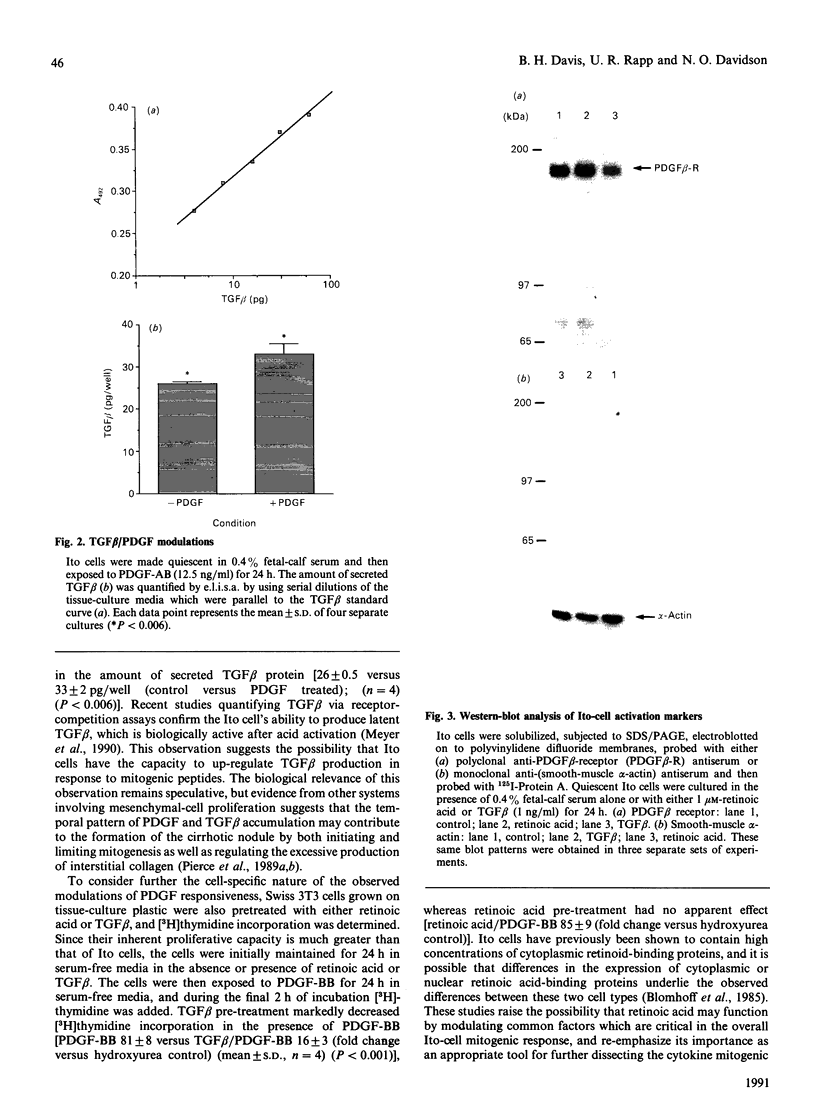
Abstract
Sinusoidal Ito cells (stellate or fat-storing cells) undergo excessive cellular proliferation before the establishment and progression of hepatic fibrosis and cirrhosis. Retinoic acid and transforming growth factor beta (TGF beta) both inhibit Ito-cell [3H]thymidine incorporation in serum-containing media. Serum-induced mitogenicity was dependent on platelet-derived growth factor (PDGF). Additionally, pre-treatment of Ito cells with retinoic acid and TGF beta blocked PDGF-induced cell proliferation. TGF beta, but not retinoic acid, diminished PDGF-receptor and smooth-muscle alpha-actin abundance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballardini G., Degli Esposti S., Bianchi F. B., de Giorgi L. B., Faccani A., Biolchini L., Busachi C. A., Pisi E. Correlation between Ito cells and fibrogenesis in an experimental model of hepatic fibrosis. A sequential stereological study. Liver. 1983 Feb;3(1):58–63. doi: 10.1111/j.1600-0676.1983.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Rasmussen M., Nilsson A., Norum K. R., Berg T., Blaner W. S., Kato M., Mertz J. R., Goodman D. S., Eriksson U. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem. 1985 Nov 5;260(25):13560–13565. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rabinovitch P. S. Platelet-derived growth factor, epidermal growth factor, and insulin-like growth factor I regulate specific cell-cycle parameters of human diploid fibroblasts in serum-free culture. J Cell Physiol. 1989 Jul;140(1):59–67. doi: 10.1002/jcp.1041400108. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Kramer R. T., Davidson N. O. Retinoic acid modulates rat Ito cell proliferation, collagen, and transforming growth factor beta production. J Clin Invest. 1990 Dec;86(6):2062–2070. doi: 10.1172/JCI114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Pratt B. M., Madri J. A. Retinol and extracellular collagen matrices modulate hepatic Ito cell collagen phenotype and cellular retinol binding protein levels. J Biol Chem. 1987 Jul 25;262(21):10280–10286. [PubMed] [Google Scholar]
- Davis B. H. Transforming growth factor beta responsiveness is modulated by the extracellular collagen matrix during hepatic ito cell culture. J Cell Physiol. 1988 Sep;136(3):547–553. doi: 10.1002/jcp.1041360323. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Vucic A. Modulation of vitamin A metabolism during hepatic and intestinal cell culture. Biochim Biophys Acta. 1989 Mar 6;1010(3):318–324. doi: 10.1016/0167-4889(89)90055-4. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1988 Jul-Aug;8(4):788–793. doi: 10.1002/hep.1840080416. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald R. G., Seifert R. A., Bowen-Pope D. F. Differential regulation of expression of two platelet-derived growth factor receptor subunits by transforming growth factor-beta. J Biol Chem. 1989 May 15;264(14):8120–8125. [PubMed] [Google Scholar]
- Harms G., van Goor H., Koudstaal J., de Ley L., Hardonk M. J. Simultaneous immunohistochemical demonstration of antigen expression and 5-bromodeoxyuridine incorporation in plastic embedded sections. Histochemistry. 1987;86(4):393–395. doi: 10.1007/BF00495000. [DOI] [PubMed] [Google Scholar]
- Hume W. J., Thompson J. Double labelling of tissue combining tritiated thymidine autoradiography with immunodetection of bromodeoxyuridine: the autoradiographic significance of inhibition of thymidine incorporation into DNA by bromodeoxyuridine given simultaneously. Cell Tissue Kinet. 1989 Sep;22(5):393–399. doi: 10.1111/j.1365-2184.1989.tb00224.x. [DOI] [PubMed] [Google Scholar]
- McGowan J. A., Strain A. J., Bucher N. L. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981 Sep;108(3):353–363. doi: 10.1002/jcp.1041080309. [DOI] [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. H., Bachem M. G., Gressner A. M. Transformed fat storing cells inhibit the proliferation of cultured hepatocytes by secretion of transforming growth factor beta. J Hepatol. 1990 Jul;11(1):86–91. doi: 10.1016/0168-8278(90)90277-x. [DOI] [PubMed] [Google Scholar]
- Minato Y., Hasumura Y., Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983 Jul-Aug;3(4):559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D., Varon S., Manthorpe M. An enzyme-linked immunosorbent assay for bromodeoxyuridine incorporation using fixed microcultures. Anal Biochem. 1990 Mar;185(2):377–382. doi: 10.1016/0003-2697(90)90310-6. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori Y., Ichida T., Geerts A., Wisse E. Modulation of collagen synthesis by fat-storing cells, isolated from CCl4- or vitamin A-treated rats. Dig Dis Sci. 1987 Nov;32(11):1281–1289. doi: 10.1007/BF01296379. [DOI] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara T., Kojima T., Miyabayashi C., Inoue K., Sasaki H., Muragaki Y., Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988 Oct;59(4):509–521. [PubMed] [Google Scholar]
- Thompson K. L., Assoian R., Rosner M. R. Transforming growth factor-beta increases transcription of the genes encoding the epidermal growth factor receptor and fibronectin in normal rat kidney fibroblasts. J Biol Chem. 1988 Dec 25;263(36):19519–19524. [PubMed] [Google Scholar]
- Thompson K. L., Rosner M. R. Regulation of epidermal growth factor receptor gene expression by retinoic acid and epidermal growth factor. J Biol Chem. 1989 Feb 25;264(6):3230–3234. [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]