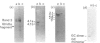Abstract
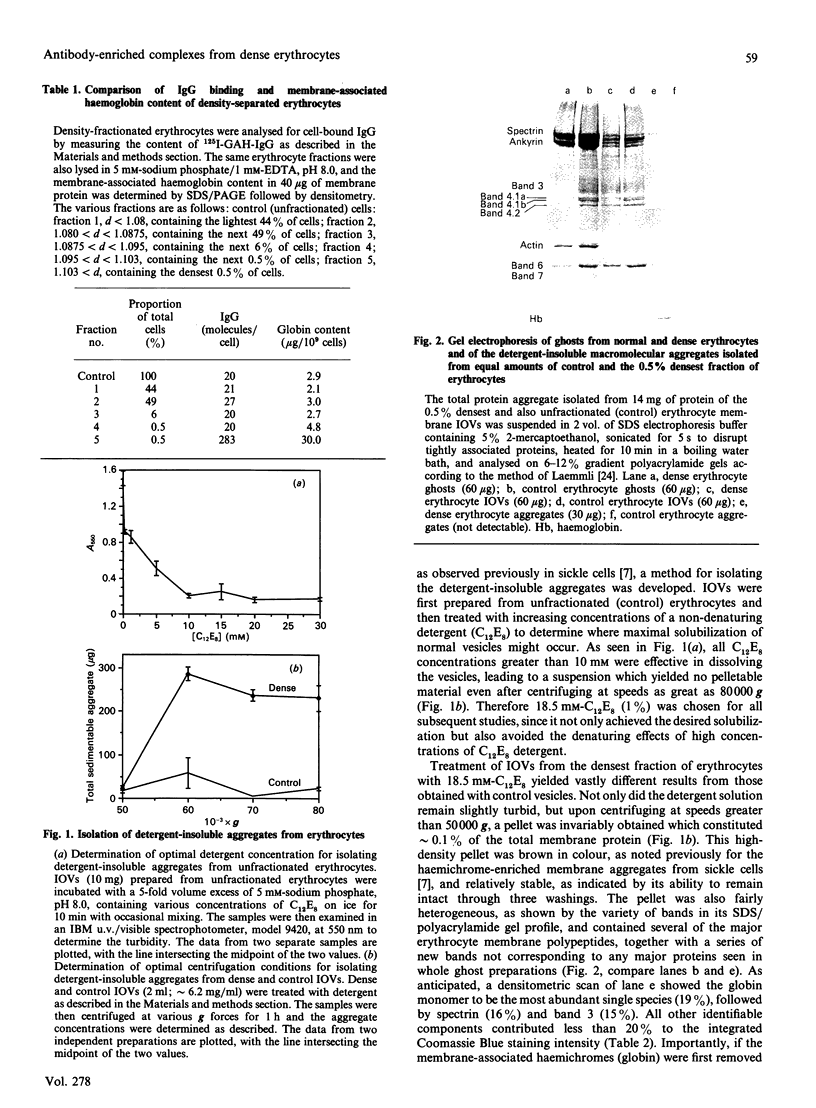
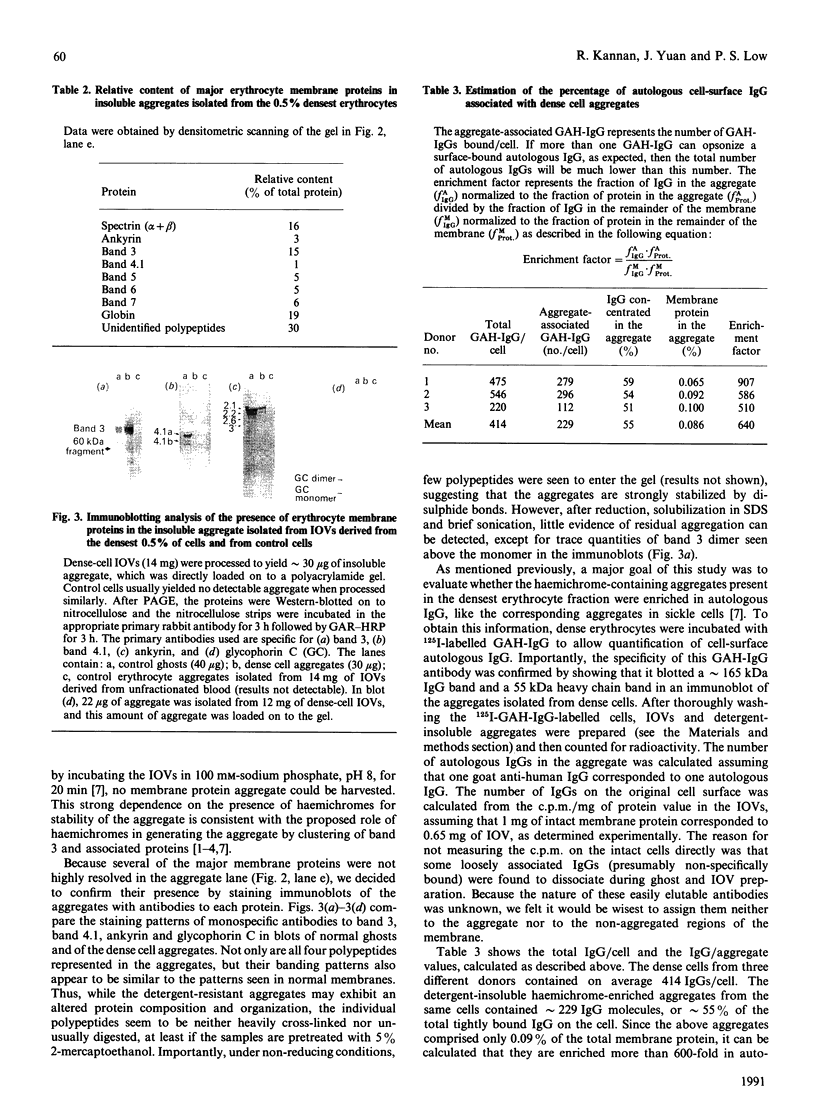
In previous studies we have described a process whereby an erythrocyte in biochemical distress can initiate its own removal by macrophages of the reticuloendothelial system. This process involves the clustering of the integral membrane protein band 3 by denatured haemoglobin and the subsequent recognition of the exofacial poles of clustered band 3 and associated proteins by autologous antibodies. To determine whether this clearance pathway might mediate normal cell turnover, the fraction of normal erythrocytes containing the 0.5% densest cells, which are known to be destined for immediate removal, was isolated and characterized biochemically. This densest fraction was found to contain 6 times more membrane-bound globin (haemichromes) and 10 times more surface-bound autologous IgG than the other fractions containing cells of lower density. To determine whether the autologous IgG was physically associated with the haemichrome-stabilized membrane protein clusters, a procedure was developed for isolation and characterization of the microscopic aggregates. The isolated aggregates were found to contain a disulphide-cross-linked mixture of several membrane proteins, predominantly haemichromes, spectrin and band 3. Although the aggregates constituted only 0.09% of the total membrane protein, they still contained approximately 55% of the total cell-surface IgG. Since in control studies anti-(blood group A) antibodies, which are distributed randomly over the surface of type A cells, could not be recovered in the aggregate, we conclude that the autologous cell-surface IgGs were physically associated with the membrane protein clusters when they were co-isolated with them in our procedure. Thus the 640-fold enrichment of autologous IgG in the aggregates compared with regions of the membrane devoid of tightly clustered protein suggests that sites of integral protein clustering either are non-specifically sticky to IgG or are viewed as foreign or 'non-self' by the immune system and aggressively opsonized with IgG.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Groat J. D., Finkel B., Rank B. H., Wood P. A., Eaton J. W. Increased adsorption of cytoplasmic proteins to the erythrocyte membrane in ATP-depleted normal and pyruvate kinase-deficient mature cells and reticulocytes. Am J Hematol. 1983 Feb;14(1):11–25. doi: 10.1002/ajh.2830140103. [DOI] [PubMed] [Google Scholar]
- Bates D. A., Winterbourn C. C. Haemoglobin denaturation, lipid peroxidation and haemolysis in phenylhydrazine-induced anaemia. Biochim Biophys Acta. 1984 Mar 22;798(1):84–87. doi: 10.1016/0304-4165(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Bensinger T. A., Gillette P. N. Hemolysis in sickle cell disease. Arch Intern Med. 1974 Apr;133(4):624–631. [PubMed] [Google Scholar]
- Brovelli A., Seppi C., Balduini C. Modification of membrane protein organization during in vitro aging of human erythrocytes. Int J Biochem. 1984;16(11):1115–1120. doi: 10.1016/0020-711x(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Campwala H. Q., Desforges J. F. Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med. 1982 Jan;99(1):25–28. [PubMed] [Google Scholar]
- Chiu D., Lubin B. Oxidative hemoglobin denaturation and RBC destruction: the effect of heme on red cell membranes. Semin Hematol. 1989 Apr;26(2):128–135. [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Franck P. F., Bevers E. M., Lubin B. H., Comfurius P., Chiu D. T., Op den Kamp J. A., Zwaal R. F., van Deenen L. L., Roelofsen B. Uncoupling of the membrane skeleton from the lipid bilayer. The cause of accelerated phospholipid flip-flop leading to an enhanced procoagulant activity of sickled cells. J Clin Invest. 1985 Jan;75(1):183–190. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaczyńska M., Bartosz G. Crosslinking of membrane proteins during erythrocyte ageing. Int J Biochem. 1986;18(4):377–382. doi: 10.1016/0020-711x(86)90044-3. [DOI] [PubMed] [Google Scholar]
- Ganzoni A. M., Barras J. P., Marti H. R. Editorial: Red cell ageing and death. Vox Sang. 1976;30(3):161–174. doi: 10.1111/j.1423-0410.1976.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Green G. A., Rehn M. M., Kalra V. K. Cell-bound autologous immunoglobulin in erythrocyte subpopulations from patients with sickle cell disease. Blood. 1985 May;65(5):1127–1133. [PubMed] [Google Scholar]
- Hebbel R. P. Auto-oxidation and a membrane-associated 'Fenton reagent': a possible explanation for development of membrane lesions in sickle erythrocytes. Clin Haematol. 1985 Feb;14(1):129–140. [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W. Pathobiology of heme interaction with the erythrocyte membrane. Semin Hematol. 1989 Apr;26(2):136–149. [PubMed] [Google Scholar]
- Kadlubowski M. The effect of in-vivo ageing of the human erythrocyte on the proteins of the plasma membrane. A comparison with metabolic depletion and blood bank storage. Int J Biochem. 1978;9(2):79–88. doi: 10.1016/0020-711x(78)90015-0. [DOI] [PubMed] [Google Scholar]
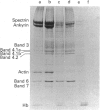
- Kannan R., Labotka R., Low P. S. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. J Biol Chem. 1988 Sep 25;263(27):13766–13773. [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Shapiro S. S., Bendich A., Bassel P. S. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2463–2467. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low P. S., Kannan R. Effect of hemoglobin denaturation on membrane structure and IgG binding: role in red cell aging. Prog Clin Biol Res. 1989;319:525–552. [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Bussolino F., Flepp R., Fasler S., Stammler P., Kazatchkine M. D., Arese P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7368–7372. doi: 10.1073/pnas.84.21.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky Y., Danon D. Electron microscope analysis of young and old red blood cells stained with colloidal iron for surface charge evaluation. J Cell Biol. 1969 Oct;43(1):1–7. doi: 10.1083/jcb.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Rheological and adherence properties of sickle cells. Potential contribution to hematologic manifestations of the disease. Ann N Y Acad Sci. 1989;565:327–337. doi: 10.1111/j.1749-6632.1989.tb24180.x. [DOI] [PubMed] [Google Scholar]
- Mueller T. J., Jackson C. W., Dockter M. E., Morrison M. Membrane skeletal alterations during in vivo mouse red cell aging. Increase in the band 4.1a:4.1b ratio. J Clin Invest. 1987 Feb;79(2):492–499. doi: 10.1172/JCI112839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer E. P., Parker J. C. Selective increase of potassium permeability in red blood cells exposed to acetylphenylhydrazine. Blood. 1977 Dec;50(6):1013–1021. [PubMed] [Google Scholar]
- Piomelli S., Lurinsky G., Wasserman L. R. The mechanism of red cell aging. I. Relationship between cell age and specific gravity evaluated by ultracentrifugation in a discontinuous density gradient. J Lab Clin Med. 1967 Apr;69(4):659–674. [PubMed] [Google Scholar]
- Platt O. S., Falcone J. F., Lux S. E. Molecular defect in the sickle erythrocyte skeleton. Abnormal spectrin binding to sickle inside-our vesicles. J Clin Invest. 1985 Jan;75(1):266–271. doi: 10.1172/JCI111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz E. A. Denaturation of the normal and abnormal hemoglobin molecule. Semin Hematol. 1974 Oct;11(4):441–462. [PubMed] [Google Scholar]
- Ravindranath Y., Brohn F., Johnson R. M. Erythrocyte age-dependent changes of membrane protein 4.1: studies in transient erythroblastopenia. Pediatr Res. 1987 Mar;21(3):275–278. doi: 10.1203/00006450-198703000-00014. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- Scott M. D., Rouyer-Fessard P., Lubin B. H., Beuzard Y. Entrapment of purified alpha-hemoglobin chains in normal erythrocytes. A model for beta thalassemia. J Biol Chem. 1990 Oct 15;265(29):17953–17959. [PubMed] [Google Scholar]
- Shalev O., Mogilner S., Shinar E., Rachmilewitz E. A., Schrier S. L. Impaired erythrocyte calcium homeostasis in beta-thalassemia. Blood. 1984 Aug;64(2):564–566. [PubMed] [Google Scholar]
- Shaw G. M., Aminoff D., Balcerzak S. P., LoBuglio A. F. Clustered IgG on human red blood cell membranes may promote human lymphocyte antibody-dependent cell-mediated cytotoxicity. J Immunol. 1980 Aug;125(2):501–507. [PubMed] [Google Scholar]
- Singer J. A., Jennings L. K., Jackson C. W., Dockter M. E., Morrison M., Walker W. S. Erythrocyte homeostasis: antibody-mediated recognition of the senescent state by macrophages. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5498–5501. doi: 10.1073/pnas.83.15.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Waugh S. M., Walder J. A., Low P. S. Partial characterization of the copolymerization reaction of erythrocyte membrane band 3 with hemichromes. Biochemistry. 1987 Mar 24;26(6):1777–1783. doi: 10.1021/bi00380a041. [DOI] [PubMed] [Google Scholar]
- Wiley J. S. Increased erythrocyte cation permeability in thalassemia and conditions of marrow stress. J Clin Invest. 1981 Apr;67(4):917–922. doi: 10.1172/JCI110140. [DOI] [PMC free article] [PubMed] [Google Scholar]